Oxidative Stress Responses in Microalgae: Modern Insights into an Old Topic
Abstract
1. Introduction
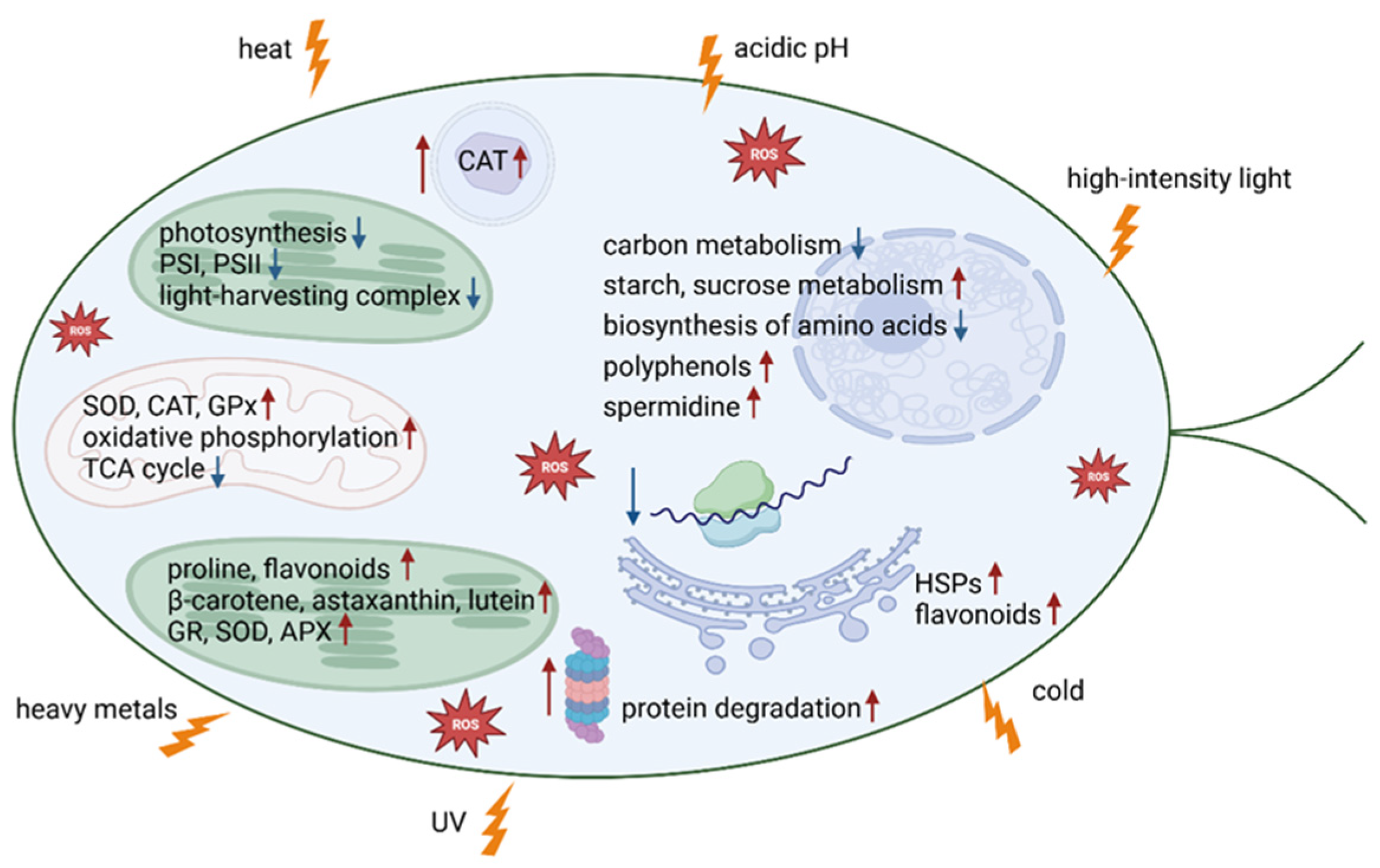
2. Oxidative Stress Mediation in Microalgae
2.1. Increase in ROS Due to Endogenous Processes
2.2. ROS Increase Due to Factors Not Related to Climate Change
2.3. ROS Increase Due to Environmental Factors Linked to Climate Change
3. Oxidative Stress Response
3.1. Oxidative Regulation via Enzymatic Response
3.2. Oxidative Regulation Through Antioxidant Compounds
3.3. Genomic Capacity and Cellular Functions Dictate Metabolic Regulation
3.4. Applications of Antioxidant Properties
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Glossary | |
| Acute stress | a short-term state of stress |
| Aerobic microorganisms | microorganisms that utilize oxygen in their metabolic processes |
| Anthropogenic contamination | pollution or environmental damage caused by human activity |
| Chronic stress | a prolonged state of stress |
| Metabolic manipulation | the process of altering or controlling the metabolism of organisms or cells to achieve a specific goal, such as enhancing energy production or synthesizing a particular compound |
| Multi-omic technologies | approaches that integrate data from various ‘omics’ fields |
| Nutrient deprivation | the condition where an organism lacks essential nutrients, leading to stress responses or altered metabolic activity |
| Obligate cold extremophiles (Psychrophiles) | organisms that thrive in extremely cold environments, and have evolved specialized mechanisms to survive in these conditions |
| Pharmaceutical contaminants | chemical substances, often derived from pharmaceutical drugs, that enter the environment and can impact ecosystems |
| Redox equilibrium | the balance between oxidation and reduction reactions in biological or chemical systems |
| Abbreviations | |
| APX | ascorbate peroxidase |
| ATPB | ATP synthase subunit beta |
| CAT | catalase |
| Cd | cadmium |
| CEP2 | cysteine endopeptidase 2 |
| CO2 | carbon dioxide |
| FABD | malonyl CoA-acyl carrier protein transacylase |
| FABF | β-ketoacyl-acyl carrier protein (ACP) synthase II |
| FABH | β-ketoacylacyl carrier protein synthase III |
| FBA3 | Fructose-bisphosphate aldolase 3 |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GRX6 | glutaredoxin 6 |
| GSH | reduced glutathione |
| GST | glutathione-S transferase |
| H2O2 | hydrogen peroxide |
| HSPs | heat shock proteins |
| HSP97 | heat shock protein 97 |
| N | nitrogen |
| OH• | hydroxyl radicals |
| O2•− | superoxide radicals |
| P | phosphorus |
| PAM | pulse-amplitude modulated |
| Pb | lead |
| PEX1 | peroxisomal biogenesis factor 1 |
| POD | peroxidase |
| PSI | photosystem I |
| PSII | photosystem II |
| psaC | photosystem I iron-sulfur center |
| psbE | cytochrome b559 subunit alpha |
| psbH | photosystem II reaction center protein H |
| RPIA | ribose 5-phosphate isomerase A |
| ROS | Reactive Oxygen Species |
| SO2 | sulfur dioxide |
| SOD | superoxide dismutase |
| TCA cycle | tricarboxylic acid cycle |
| UTEX | the University of Texas at Austin Culture Collection of Algae |
| UVR | ultraviolet radiation |
| 1O2 | singlet oxygen |
References
- Singh, J.; Saxena, R.C. An Introduction to Microalgae. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 11–24. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Yin, Z.; Wang, X.; Wang, S.; Ye, Z. Quantifying photosynthetic performance of phytoplankton based on photosynthesis–irradiance response models. Environ. Sci. Eur. 2020, 32, 24. [Google Scholar] [CrossRef]
- Khan, A.K.; Kausar, H.; Jaferi, S.S.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. An Insight into the Algal Evolution and Genomics. Biomolecules 2020, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; An, M.; Miao, J.; Gu, Z.; Liu, C.; Zhong, B. The Antarctic sea ice alga Chlamydomonas sp. ICE-L provides insights into adaptive patterns of chloroplast evolution. BMC Plant Biol. 2018, 18, 53. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Song, Q.; Kong, F.; Liu, B.-F.; Song, X.; Ren, N.-Q.; Ren, H.-Y. Ozone oxidation of actual waste leachate coupled with culture of microalgae for efficient lipid production under different temperatures. Water Res. 2025, 277, 123305. [Google Scholar] [CrossRef]
- Behera, B.; Selvam, S.M.; Paramasivan, B. Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour. Technol. 2022, 351, 127038. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Almeida, A.C.; Gomes, T.; Langford, K.; Thomas, K.V.; Tollefsen, K.E. Oxidative stress in the algae Chlamydomonas reinhardtii exposed to biocides. Aquat. Toxicol. 2017, 189, 50–59. [Google Scholar] [CrossRef]
- Darehshouri, A.; Affenzeller, M.; Lütz-Meindl, U. Cell death upon H2O2 induction in the unicellular green alga Micrasterias. Plant Biol. 2008, 10, 732–745. [Google Scholar] [CrossRef]
- Kholssi, R.; Lougraimzi, H.; Moreno-Garrido, I. Effects of global environmental change on microalgal photosynthesis, growth and their distribution. Mar. Environ. Res. 2023, 184, 105877. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic stresses as tools for metabolites in microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, Y.; Wakisaka, M.; Yang, Z.; Yin, Y.; Fang, W.; Xu, Y.; Omura, T.; Yu, R.; Zheng, A.L.T. Mitigation of oxidative stress damage caused by abiotic stress to improve biomass yield of microalgae: A review. Sci. Total Environ. 2023, 896, 165200. [Google Scholar] [CrossRef]
- Gauthier, M.R.; Senhorinho, G.N.A.; Scott, J.A. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Pan, Y.; Shi, L.; Jin, Y.; Huang, X. The Metabolism of Reactive Oxygen Species and Their Effects on Lipid Biosynthesis of Microalgae. Int. J. Mol. Sci. 2023, 24, 11041. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghatchouli, N.; Arroussi, H. Overview of the management of heavy metals toxicity by microalgae. J. Appl. Phycol. 2022, 34, 475–488. [Google Scholar] [CrossRef]
- Ugya, A.Y.; Imam, T.S.; Li, A.; Ma, J.; Hua, X. Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production: A mini review. Chem. Ecol. 2020, 36, 174–193. [Google Scholar] [CrossRef]
- Pikula, K.S.; Zakharenko, A.M.; Aruoja, V.; Golokhvast, K.S.; Tsatsakis, A.M. Oxidative stress and its biomarkers in microalgal ecotoxicology. Curr. Opin. Toxicol. 2019, 13, 8–15. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Scott, J.A.; Ross, G.M. Management of oxidative stress by microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef]
- Nikkanen, L.; Solymosi, D.; Jokel, M.; Allahverdiyeva, Y. Regulatory electron transport pathways of photosynthesis in cyanobacteria and microalgae: Recent advances and biotechnological prospects. Physiol. Plant. 2021, 173, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Oxidative Stress-Induced Bioprospecting of Microalgae. In Systems Biology of Marine Ecosystems; Kumar, M., Ralph, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 251–276. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.; Cao, X.; Xue, S.; Li, C. Liberating photoinhibition through nongenetic drainage of electrons from photosynthesis. Nat. Sci. 2021, 1, e20210038. [Google Scholar] [CrossRef]
- Milkovic, L.; Gasparovic, A.C.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef]
- Yan, H.; Ma, H.; Li, Y.; Zhao, L.; Lin, J.; Jia, Q.; Hu, Q.; Han, D. Oxidative stress facilitates infection of the unicellular alga Haematococcus pluvialis by the fungus Paraphysoderma sedebokerense. Biotechnol. Biofuels 2022, 15, 56. [Google Scholar] [CrossRef]
- Egerton, T.A. Investigations of Phytoplankton Diversity in Chesapeake Bay. Ph.D. Thesis, Old Dominion University, Norfolk, VA, USA, 2013. [Google Scholar]
- Rzymski, P.; Klimaszyk, P.; Jurczak, T.; Poniedziałek, B. Oxidative Stress, Programmed Cell Death and Microcystin Release in Microcystis aeruginosa in Response to Daphnia Grazers. Front. Microbiol. 2020, 11, 1201. [Google Scholar] [CrossRef]
- Camacho, F.G.; Gómez, A.C.; Sobczuk, T.M.; Grima, E.M. Effects of mechanical and hydrodynamic stress in agitated, sparged cultures of Porphyridium cruentum. Process Biochem. 2000, 35, 1045–1050. [Google Scholar] [CrossRef]
- Barati, B.; Gan, S.-Y.; Lim, P.-E.; Beardall, J.; Phang, S.-M. Green algal molecular responses to temperature stress. Acta Physiol. Plant 2019, 41, 26. [Google Scholar] [CrossRef]
- Xing, C.; Li, J.; Yuan, H.; Yang, J. Physiological and transcription level responses of microalgae Auxenochlorella protothecoides to cold and heat induced oxidative stress. Environ. Res. 2022, 211, 113023. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.-H.; Jiang, J.-G.; Wang, L.; Zhu, J. Transcriptomic insights into the heat stress response of Dunaliella bardawil. Enzym. Microb. Technol. 2020, 132, 109436. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovska, M.; Hüner, N.P.A.; Smith, D.R. Chilling out: The evolution and diversification of psychrophilic algae with a focus on Chlamydomonadales. Polar Biol. 2017, 40, 1169–1184. [Google Scholar] [CrossRef]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.-S. Heat shock protein genes in the green alga Tetraselmis suecica and their role against redox and non-redox active metals. Eur. J. Protistol. 2019, 69, 37–51. [Google Scholar] [CrossRef]
- Schroda, M.; Hemme, D.; Mühlhaus, T. The Chlamydomonas heat stress response. Plant J. 2015, 82, 466–480. [Google Scholar] [CrossRef]
- Schneider, R.J.; Roe, K.L.; Hansel, C.M.; Voelker, B.M. Species-Level Variability in Extracellular Production Rates of Reactive Oxygen Species by Diatoms. Front. Chem. 2016, 4, 5. [Google Scholar] [CrossRef]
- Williamson, C.E.; Zepp, R.G.; Lucas, R.M.; Madronich, S.; Austin, A.T.; Ballare, C.L.; Norval, M.; Sulzberger, B.; Bais, A.F.; McKenzie, R.L.; et al. Solar ultraviolet radiation in a changing climate. Nature Clim. Change 2014, 4, 434–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Qiu, B. Effects of UVB Radiation on competition between the bloom-forming cyanobacterium Microcystis aeruginosa and the Chlorophyceae Chlamydomonas microsphaera. J. Phycol. 2013, 49, 318–328. [Google Scholar] [CrossRef]
- Bouchard, J.N.; Roy, S.; Campbell, D.A. UVB Effects on the Photosystem II-D1 Protein of Phytoplankton and Natural Phytoplankton Communities. Photochem. Photobiol. 2006, 82, 936–951. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy Metal–Induced Oxidative Stress in Algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Waste Water Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–87. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Jaiyeola, A.T.; Chetty, R. Bioremediation of acid mine drainage using algae strains: A review. S. Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity; Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The effect of cadmium on the growth and antioxidant response for freshwater algae Chlorella vulgaris. SpringerPlus 2016, 5, 1290. [Google Scholar] [CrossRef]
- Goiris, K.; Van Colen, W.; Wilches, I.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Procházková, G.; Brányiková, I.; Zachleder, V.; Brányik, T. Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J. Appl. Phycol. 2014, 26, 1359–1377. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Vaquero, I.; Obregón, V.; De La Morena, B.; Vílchez, C.; Vega, J.M. Lipid accumulation and antioxidant activity in the eukaryotic acidophilic microalga Coccomyxa sp. (strain onubensis) under nutrient starvation. J. Appl. Phycol. 2015, 27, 1099–1108. [Google Scholar] [CrossRef]
- Barone, M.E.; Parkes, R.; Herbert, H.; McDonnell, A.; Conlon, T.; Aranyos, A.; Fierli, D.; Fleming, G.T.A.; Touzet, N. Comparative Response of Marine Microalgae to H2O2-Induced Oxidative Stress. Appl. Biochem. Biotechnol. 2021, 193, 4052–4067. [Google Scholar] [CrossRef] [PubMed]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Ran, Y.; Sun, D.; Liu, X.; Zhang, L.; Niu, Z.; Chai, T.; Hu, Z.; Qiao, K. Chlorella pyrenoidosa as a potential bioremediator: Its tolerance and molecular responses to cadmium and lead. Sci. Total Environ. 2023, 912, 168712. [Google Scholar] [CrossRef]
- Hamid, S.; Sibi, G. Antioxidant System Response in Green Microalga Chlorococcopsis minuta Against Nutrient Stress in Growth Media. Asian J. Biol. Sci. 2018, 11, 210–216. [Google Scholar] [CrossRef]
- Aderemi, A.O.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.; Hunter, C.; Pahl, O. Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquat. Toxicol. 2018, 203, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, J.; Lei, Y.; Kanerva, M.; Li, Q.; Song, J.; Guo, J.; Sun, H. Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquat. Toxicol. 2020, 219, 105376. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Li, J.; Chen, X.; Lang, Q.; Kuang, S. Combined effects of erythromycin and enrofloxacin on antioxidant enzymes and photosynthesis-related gene transcription in Chlorella vulgaris. Aquat. Toxicol. 2019, 212, 138–145. [Google Scholar] [CrossRef]
- Fayaz, T.; Rana, S.S.; Goyal, E.; Ratha, S.K.; Renuka, N. Harnessing the potential of microalgae-based systems for mitigating pesticide pollution and its impact on their metabolism. J. Environ. Manag. 2024, 357, 120723. [Google Scholar] [CrossRef]
- Li, Z.; Gao, X.; Bao, J.; Li, S.; Wang, X.; Li, Z.; Zhu, L. Evaluation of growth and antioxidant responses of freshwater microalgae Chlorella sorokiniana and Scenedesmus dimorphus under exposure of moxifloxacin. Sci. Total Environ. 2023, 858, 159788. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Miao, R.; Deng, X.; Duan, Y.; Cheng, Y.; Zhang, W.; Shi, M.; Huang, K.; Xia, X.-Q. Transcriptomic and Physiological Responses to Oxidative Stress in a Chlamydomonas reinhardtii Glutathione Peroxidase Mutant. Genes 2020, 11, 463. [Google Scholar] [CrossRef]
- Patelou, M.; Infante, C.; Dardelle, F.; Randewig, D.; Kouri, E.D.; Udvardi, M.K.; Tsiplakou, E.; Mantecón, L.; Flemetakis, E. Transcriptomic and metabolomic adaptation of Nannochloropsis gaditana grown under different light regimes. Algal Res. 2020, 45, 101735. [Google Scholar] [CrossRef]
- Camarena-Bernard, C.; Pozzobon, V. Evolving perspectives on lutein production from microalgae—A focus on productivity and heterotrophic culture. Biotechnol. Adv. 2024, 73, 108375. [Google Scholar] [CrossRef] [PubMed]
- Couso, I.; Vila, M.; Vigara, J.; Cordero, B.F.; Vargas, M.Á.; Rodríguez, H.; León, R. Synthesis of carotenoids and regulation of the carotenoid biosynthesis pathway in response to high light stress in the unicellular microalga Chlamydomonas reinhardtii. Eur. J. Phycol. 2012, 47, 223–232. [Google Scholar] [CrossRef]
- McClure, D.D.; Nightingale, J.K.; Luiz, A.; Black, S.; Zhu, J.; Kavanagh, J.M. Pilot-scale production of lutein using Chlorella vulgaris. Algal Res. 2019, 44, 101707. [Google Scholar] [CrossRef]
- Nezafatian, E.; Farhadian, O.; Yegdaneh, A.; Safavi, M.; Daneshvar, E.; Bhatnagar, A. Enhanced production of bioactive compounds from marine microalgae Tetraselmis tetrathele under salinity and light stresses: A two-stage cultivation strategy. Bioresour. Technol. 2023, 376, 128899. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, X.; Han, B.; Zhao, Y.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Simultaneous improvement of astaxanthin and lipid production of Haematococcus pluvialis by using walnut shell extracts. Algal Res. 2021, 54, 102171. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Zhang, X.; Yang, W.; Park, J.-Y.; Kim, H.-T.; Xu, L.-H. Spermidine Protects Chlorella sp. from Oxidative Damage Caused by SO2 in Flue Gas from Coal-Fired Power Plants. ACS Sustain. Chem. Eng. 2020, 8, 15179–15188. [Google Scholar] [CrossRef]
- Koletti, A.; Skliros, D.; Kalloniati, C.; Marka, S.; Zografaki, M.-E.; Infante, C.; Mantecón, L.; Flemetakis, E. Global omics study of Tetraselmis chuii reveals time-related metabolic adaptations upon oxidative stress. Appl. Microbiol. Biotechnol. 2024, 108, 138. [Google Scholar] [CrossRef]
- Blaby, I.K.; Blaby-Haas, C.E.; Pérez-Pérez, M.E.; Schmollinger, S.; Fitz-Gibbon, S.; Lemaire, S.D.; Merchant, S.S. Genome-wide analysis on Chlamydomonas reinhardtii reveals the impact of hydrogen peroxide on protein stress responses and overlap with other stress transcriptomes. Plant J. 2015, 84, 974–988. [Google Scholar] [CrossRef]
- Koletti, A.; Dervisi, I.; Kalloniati, C.; Zografaki, M.-E.; Rennenberg, H.; Roussis, A.; Flemetakis, E. Selenium-binding Protein 1 (SBD1): A stress response regulator in Chlamydomonas reinhardtii. Plant Physiol. 2022, 189, 2368–2381. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, M.; Bao, J.; Liu, J. Physiological, metabolomic, and transcriptomic analyses reveal the dynamic redox homeostasis upon extended exposure of Dunaliella salina GY-H13 cells to Cd. Ecotoxicol. Environ. Saf. 2021, 223, 112593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zong, R.; He, H.; Huang, T. Effects of hydrogen peroxide on Scenedesmus obliquus: Cell growth, antioxidant enzyme activity and intracellular protein fingerprinting. Chemosphere 2022, 287, 132185. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Janknegt, P.J.; De Graaff, C.M.; Van De Poll, W.H.; Visser, R.J.W.; Rijstenbil, J.W.; Buma, J.A.G. Short-term antioxidative responses of 15 microalgae exposed to excessive irradiance including ultraviolet radiation. Eur. J. Phycol. 2009, 44, 525–539. [Google Scholar] [CrossRef]
- Shi, K.; Gao, Z.; Shi, T.-Q.; Song, P.; Ren, L.-J.; Huang, H.; Ji, X.-J. Reactive Oxygen Species-Mediated Cellular Stress Response and Lipid Accumulation in Oleaginous Microorganisms: The State of the Art and Future Perspectives. Front. Microbiol. 2017, 8, 793. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef]
- Pick, U.; Zarka, A.; Boussiba, S.; Davidi, L. A hypothesis about the origin of carotenoid lipid droplets in the green algae Dunaliella and Haematococcus. Planta 2019, 249, 31–47. [Google Scholar] [CrossRef]
- Shi, T.-Q.; Wang, L.-R.; Zhang, Z.-X.; Sun, X.-M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Ota, S.; Morita, A.; Ohnuki, S.; Hirata, A.; Sekida, S.; Okuda, K.; Ohya, Y.; Kawano, S. Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis. Sci. Rep. 2018, 8, 5617. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Dewanjee, S.; Riaz, M. (Eds.) Carotenoids: Structure and Function in the Human Body; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Nair, A.; Ahirwar, A.; Singh, S.; Lodhi, R.; Lodhi, A.; Rai, A.; A Jadhav, D.; Harish; Varjani, S.; Singh, G.; et al. Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Mar. Drugs 2023, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Telfer, A. What is β–carotene doing in the photosystem II reaction centre? Phil. Trans. R. Soc. Lond. B 2002, 357, 1431–1440. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef]
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Rausch, T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth. Res. 2005, 86, 459–474. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Han, B.; Yu, X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Bioresour. Technol. 2019, 274, 549–556. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins—Evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef]
- Sörenson, E.; Capo, E.; Farnelid, H.; Lindehoff, E.; Legrand, C. Temperature Stress Induces Shift From Co-Existence to Competition for Organic Carbon in Microalgae-Bacterial Photobioreactor Community—Enabling Continuous Production of Microalgal Biomass. Front. Microbiol. 2021, 12, 607601. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnology 2023, 13, 100205. [Google Scholar] [CrossRef]
- El-Moustaqim, K.; Mabrouki, J.; Benchrifa, M.; Azdem, D.; Hmouni, D. Microalgae-Based Food Additives for Improved Shelf Life and Nutritional Value. Biol. Life Sci. Forum 2024, 40, 42. [Google Scholar] [CrossRef]
- Kaur, M.; Bhatia, S.; Gupta, U.; Decker, E.; Tak, Y.; Bali, M.; Gupta, V.K.; Dar, R.A.; Bala, S. Microalgal bioactive metabolites as promising implements in nutraceuticals and pharmaceuticals: Inspiring therapy for health benefits. Phytochem. Rev. 2023, 22, 903–933. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, A.; Bala, S.; Satheesh, N.; Nile, A.S.; Nile, S.H. Microalgae in the food-health nexus: Exploring species diversity, high-value bioproducts, health benefits, and sustainable market potential. Bioresour. Technol. 2025, 427, 132424. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.-H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae Bioactive Compounds to Topical Applications Products—A Review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef] [PubMed]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sun, J.; Fa, Y.; Liu, X.; Lindblad, P. Enhancing microalgal lipid accumulation for biofuel production. Front. Microbiol. 2022, 13, 1024441. [Google Scholar] [CrossRef]
| Microalgae | Stressors | Antioxidant Responses | Omics Study | References | |
|---|---|---|---|---|---|
| Chlorella pyrenoidosa | Cd, Pb | upregulation/accumulation | PEX1, SOD upregulation | transcriptomics | [57] |
| S. obliquus | Pb | SOD, CAT, POD, GR activity increase, polyphenol accumulation | - | [50] | |
| C. minuta | N, P deprivation | SOD, CAT activity increase | - | [58] | |
| C. vulgaris, R. subcapitata | erythromycin and clarithromycin | SOD, CAT, GPx activity increase | RT-PCR | [59,60,61] | |
| C. vulgaris | glufosinate | APX, SOD, CAT activity increase | - | [62] | |
| C. sorokiniana | moxifloxacin | chlorophyll a, b, carotenoid accumulation | - | [63] | |
| C. reinhardtii | rose bengal | heat shock protein and ubiquitin–proteasome pathway gene upregulation | transcriptomics | [64] | |
| A. protothecoides | high temperature | SOD, hsp97 upregulation | RT-PCR | [34] | |
| D. bardawil | high temperature | genes coding for chloroplast membrane upregulation | transcriptomics | [35] | |
| A. protothecoides | low temperature | proline accumulation, heat shock protein genes upregulation | RT-PCR | [34] | |
| Nannochloropsis gaditana | green filtered light | GPx1, SOD upregulation | RT-PCR, metabolomics | [65] | |
| high-intensity light | lutein accumulation | - | [66] | ||
| C. reinhardtii, C. vulgaris | high-intensity light | lutein accumulation | RT-PCR | [67,68] | |
| Chromochloris zofingiensis | light, salinity | carotenoid accumulation | - | [69] | |
| H. pluvialis | salinity | astaxanthin and lipids accumulation | RT-PCR | [70] | |
| Chlorella sp. | SO2, CO2 | spermidine accumulation | - | [71] | |
| T. chuii | H2O2 | peroxisome function, endocytosis, starch and sucrose metabolism, biosynthesis of secondary metabolites, galactose metabolism, protein degradation, and heat shock protein genes upregulation | transcriptomics, metabolomics | [72] | |
| C. reinhardtii | H2O2 | protein degradation and heat shock protein genes upregulation | transcriptomics, metabolomics | [73,74] | |
| D. bardawil | high temperature | glycolytic metabolism, ascorbate–glutathione cycle, heat shock protein genes upregulation | transcriptomics | [35] | |
| D. salina | Cd | downregulation | TCA cycle genes upregulation | transcriptomics, metabolomics | [75] |
| C. reinhardtii | H2O2 | central carbon metabolism and photosynthesis genes upregulation | transcriptomics, metabolomics | [73] | |
| T. chuii | H2O2 | energy flow, carbon fixation, photosynthesis, fatty acids metabolism, biosynthesis of amino acids, ribosome structural proteins, purine and porphyrin metabolism downregulation | transcriptomics, metabolomics | [72] | |
| S. obliquus | H2O2 | psaC, psbH, psbE, atpB downregulation | proteomics | [76] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koletti, A.; Skliros, D.; Dervisi, I.; Roussis, A.; Flemetakis, E. Oxidative Stress Responses in Microalgae: Modern Insights into an Old Topic. Appl. Microbiol. 2025, 5, 37. https://doi.org/10.3390/applmicrobiol5020037
Koletti A, Skliros D, Dervisi I, Roussis A, Flemetakis E. Oxidative Stress Responses in Microalgae: Modern Insights into an Old Topic. Applied Microbiology. 2025; 5(2):37. https://doi.org/10.3390/applmicrobiol5020037
Chicago/Turabian StyleKoletti, Aikaterini, Dimitrios Skliros, Irene Dervisi, Andreas Roussis, and Emmanouil Flemetakis. 2025. "Oxidative Stress Responses in Microalgae: Modern Insights into an Old Topic" Applied Microbiology 5, no. 2: 37. https://doi.org/10.3390/applmicrobiol5020037
APA StyleKoletti, A., Skliros, D., Dervisi, I., Roussis, A., & Flemetakis, E. (2025). Oxidative Stress Responses in Microalgae: Modern Insights into an Old Topic. Applied Microbiology, 5(2), 37. https://doi.org/10.3390/applmicrobiol5020037







