Finite Element Analysis of the Bearing Component of Total Ankle Replacement Implants during the Stance Phase of the Gait Cycle
Abstract
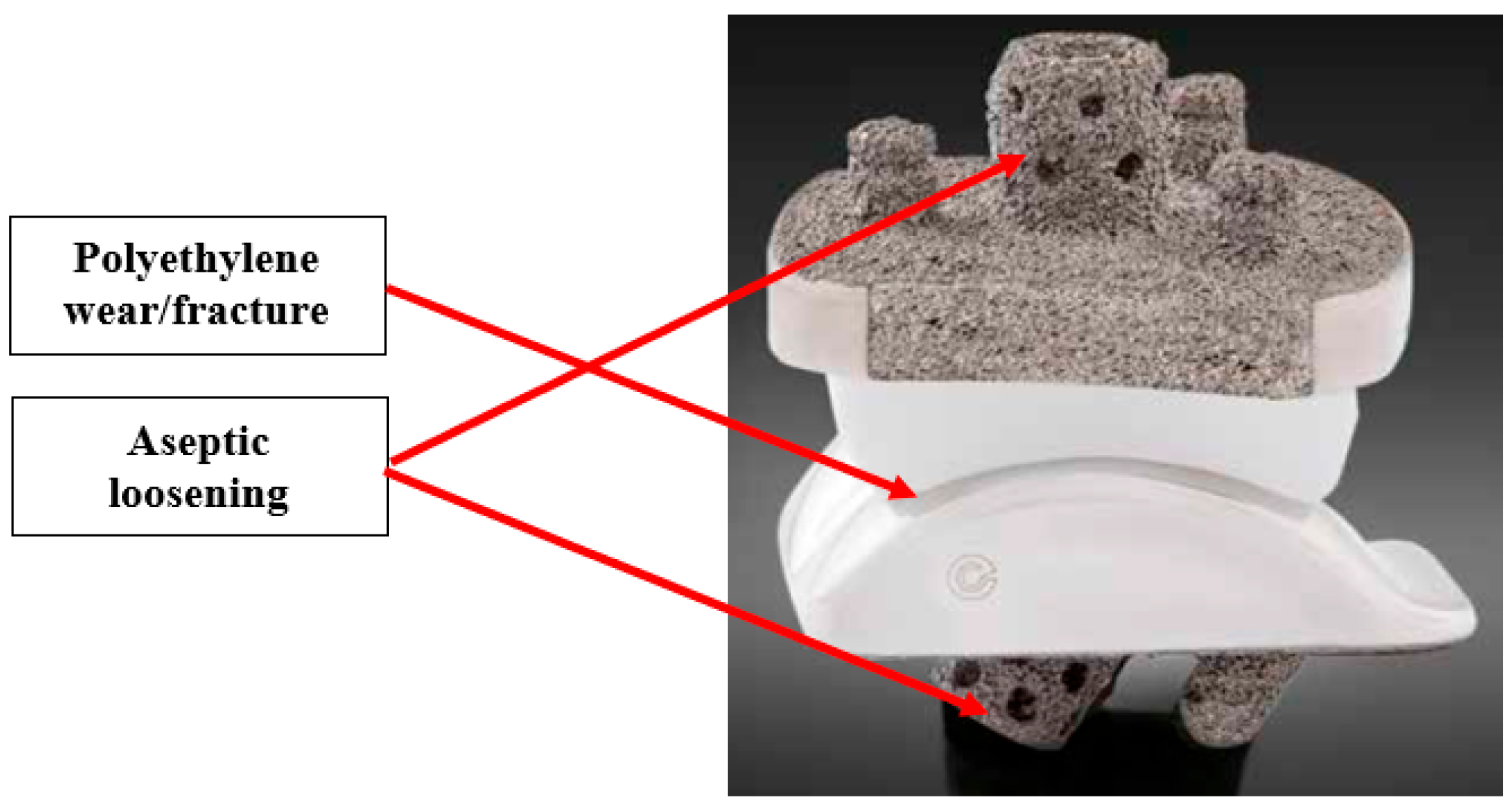
1. Introduction
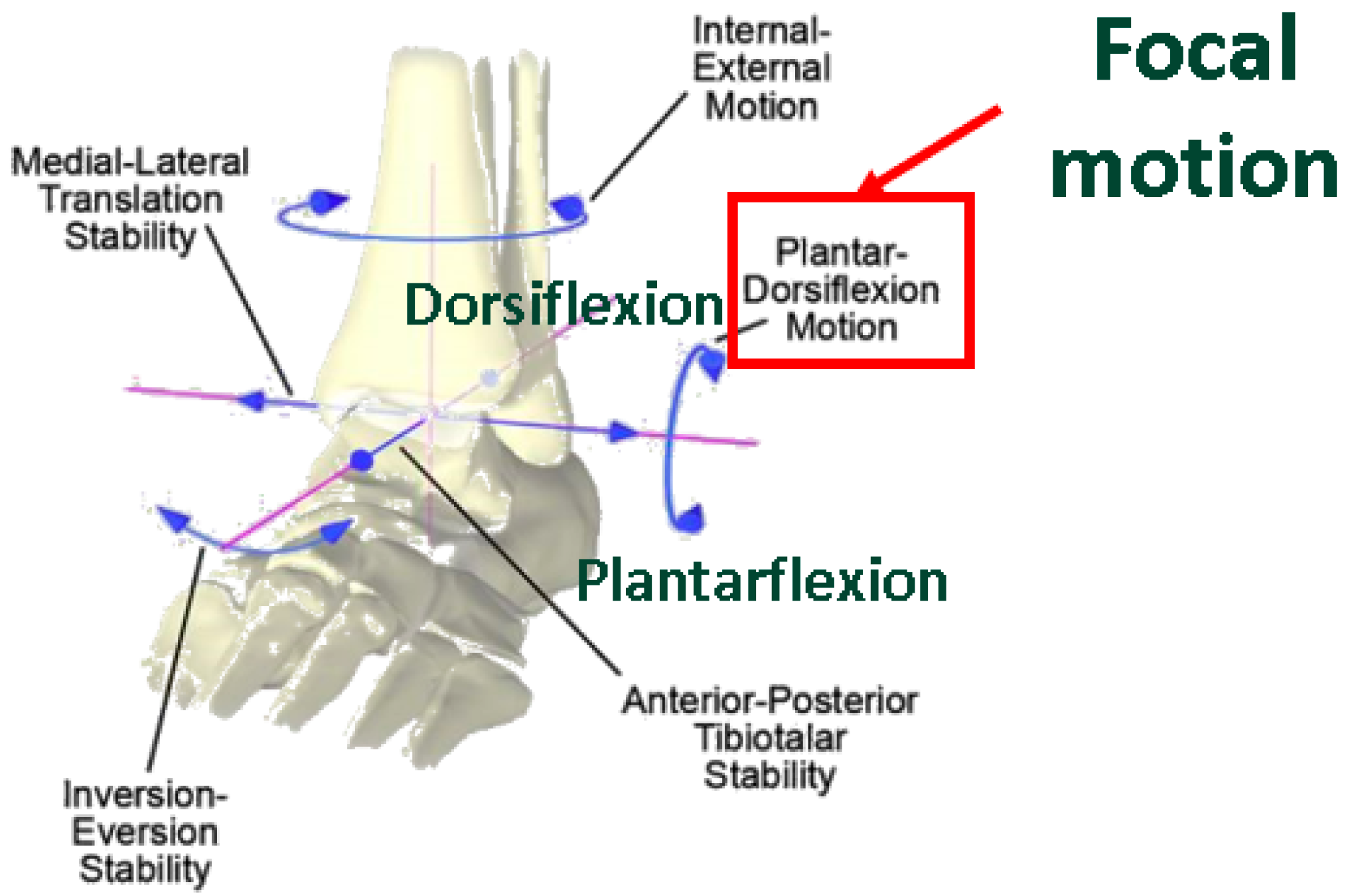
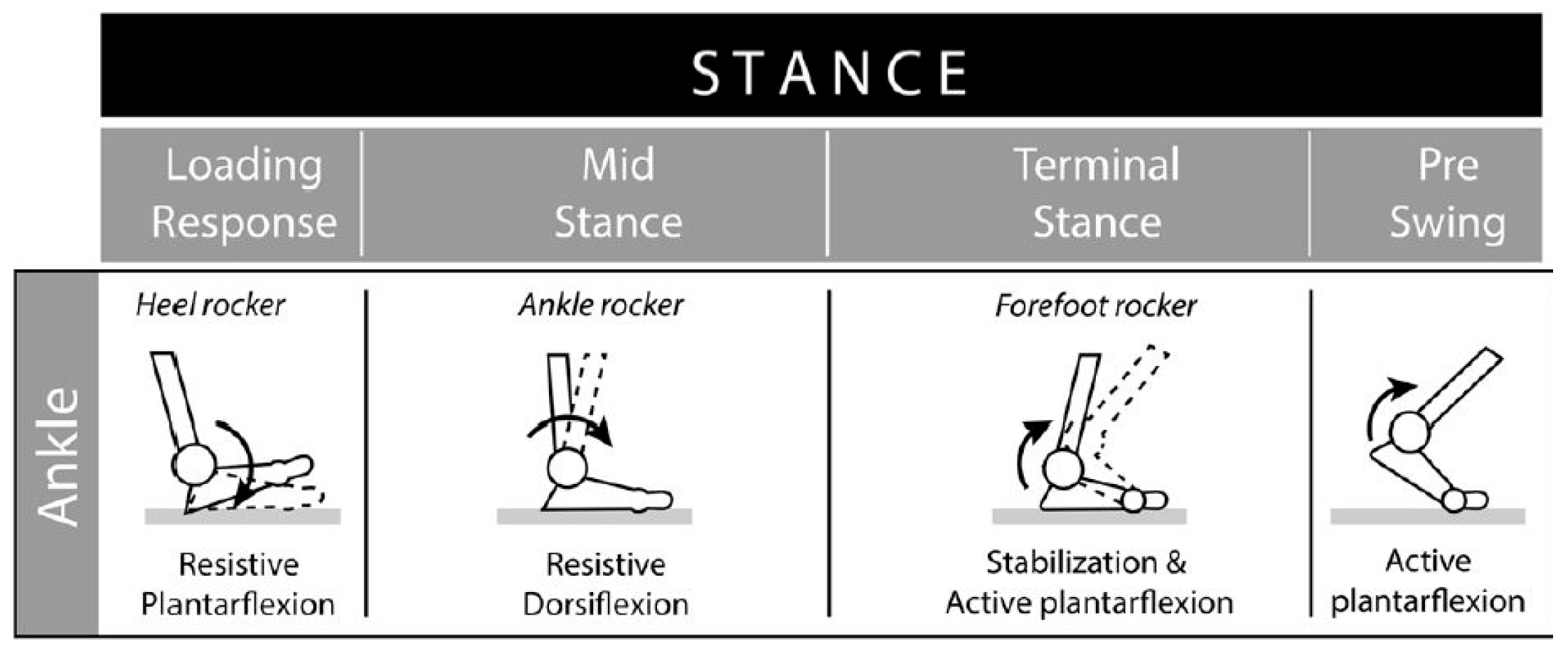
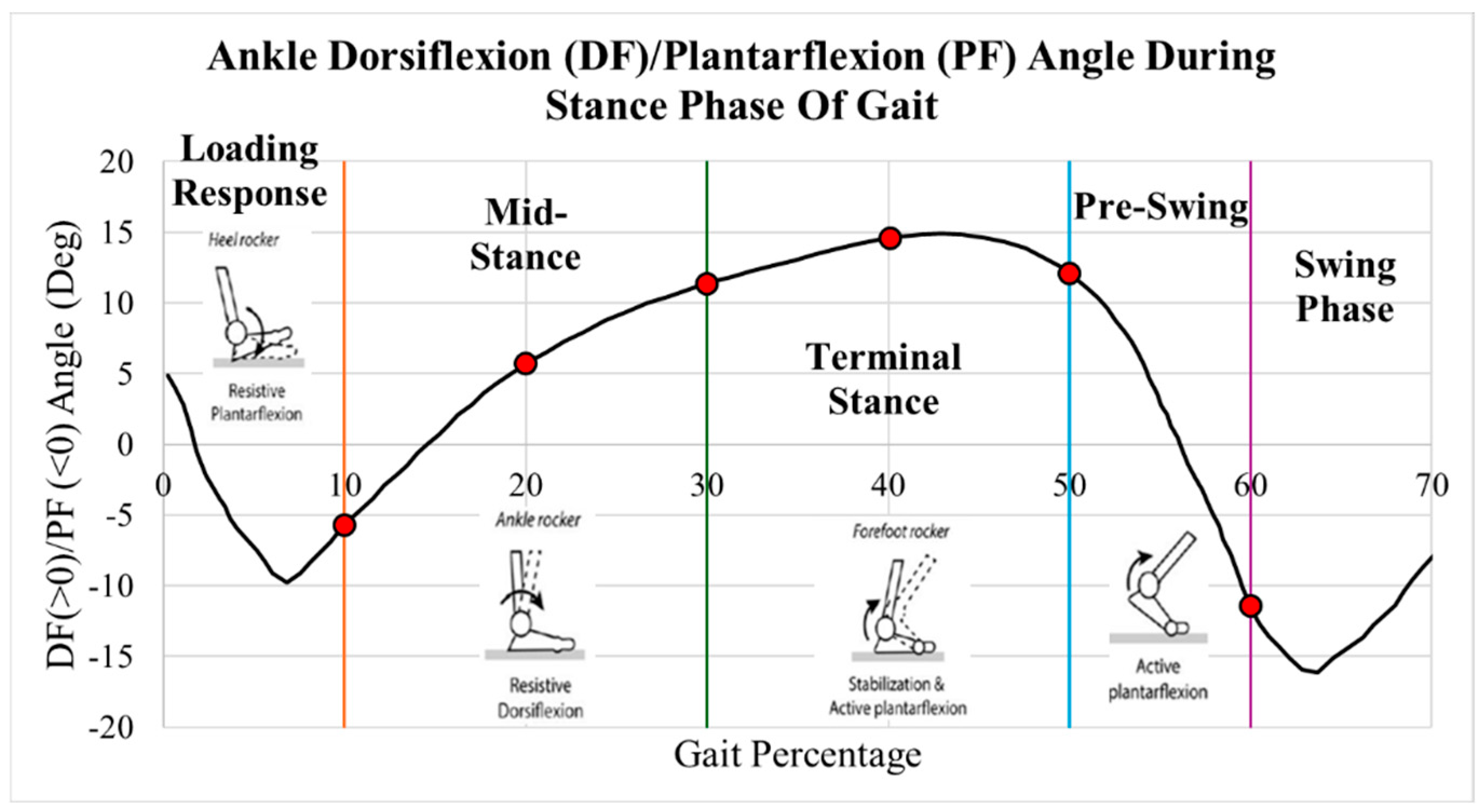
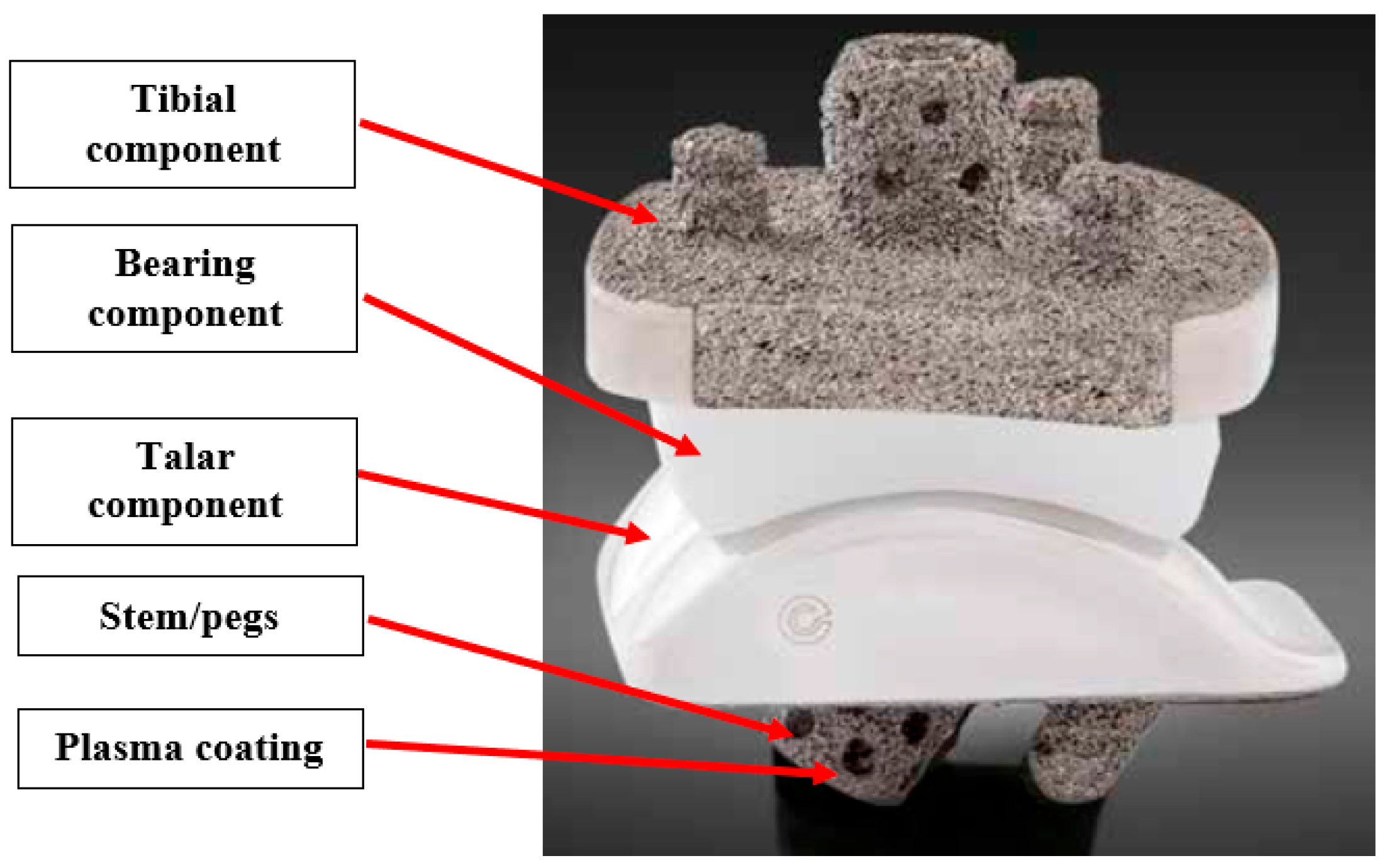
2. Methods
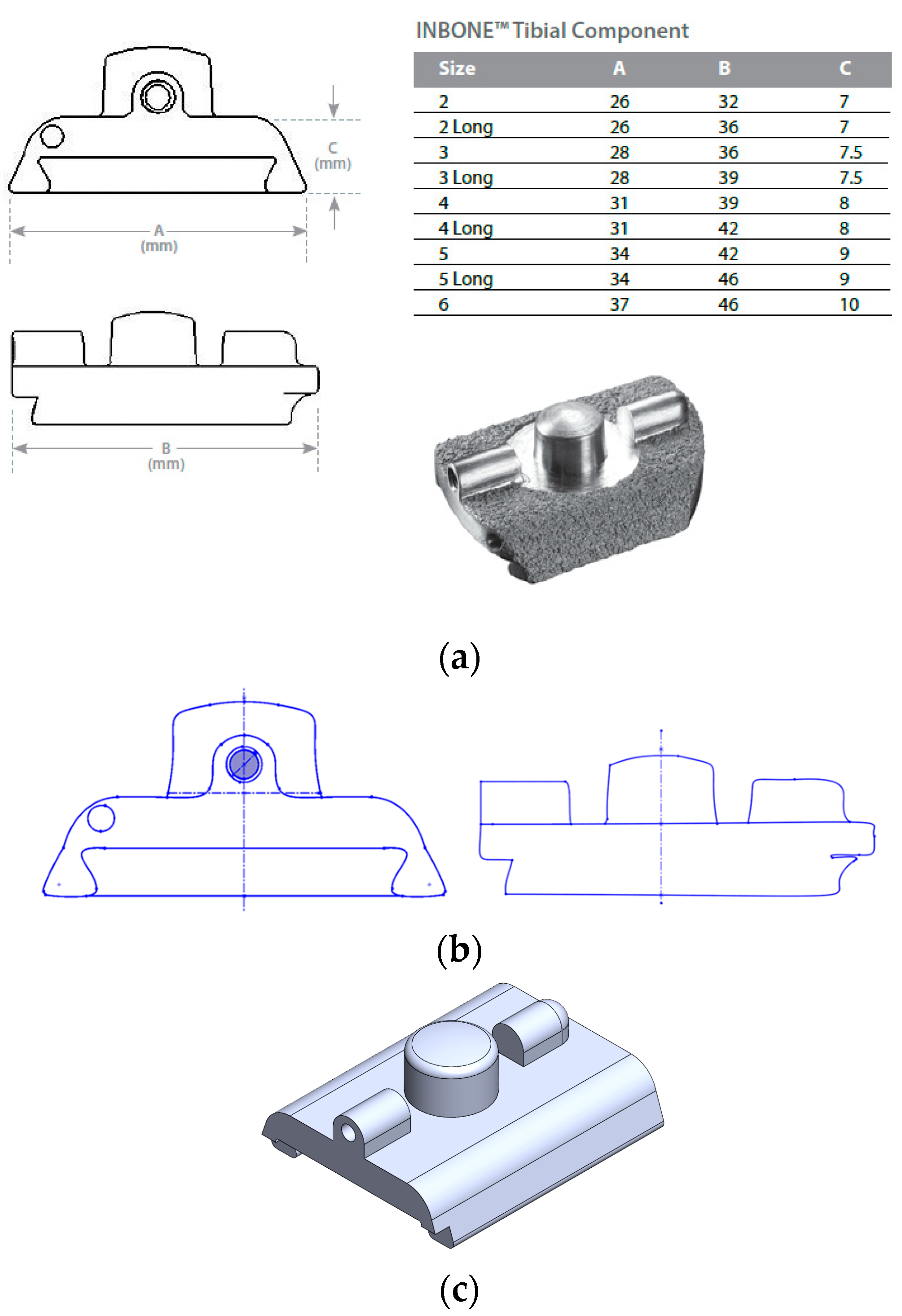
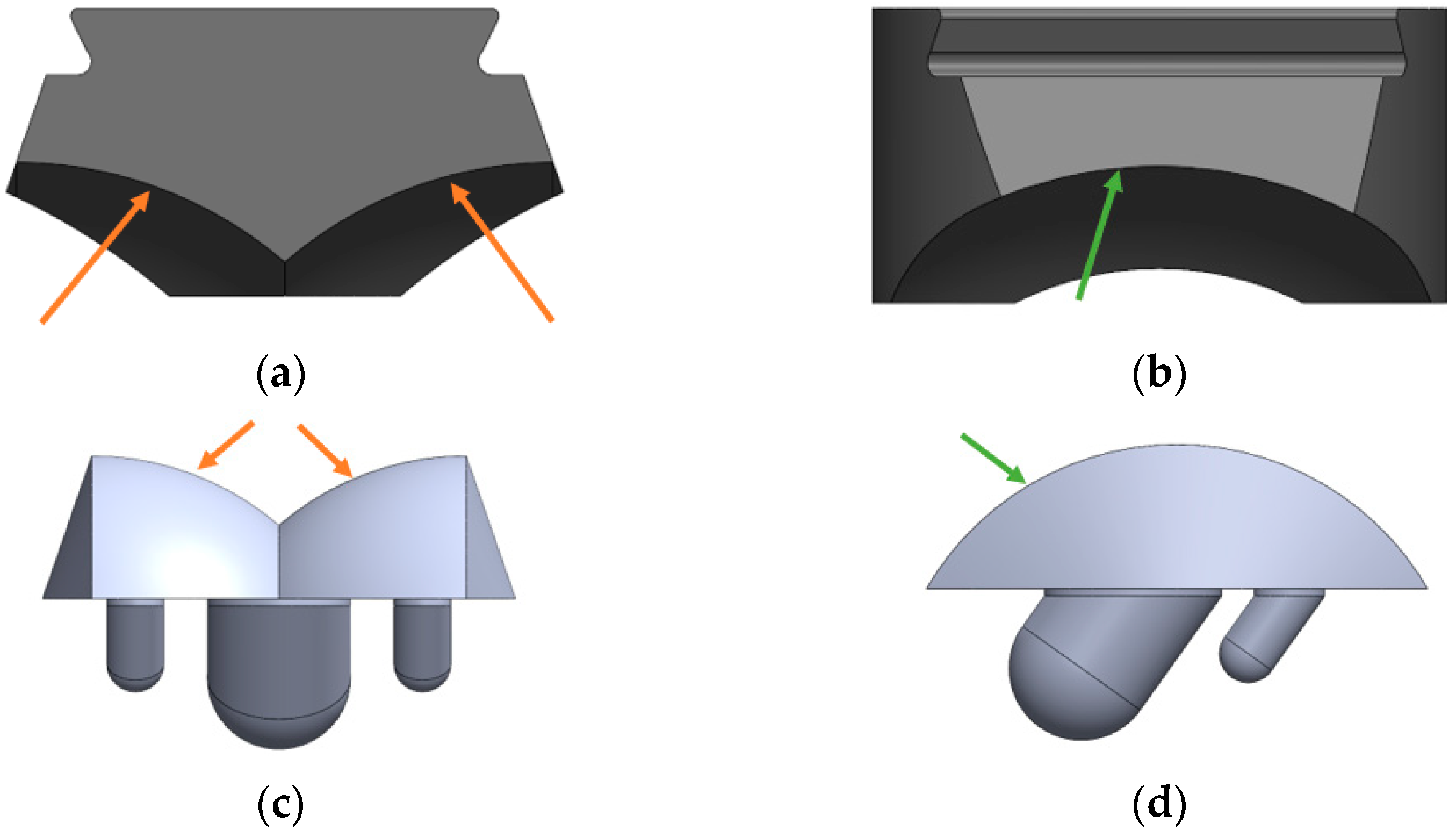
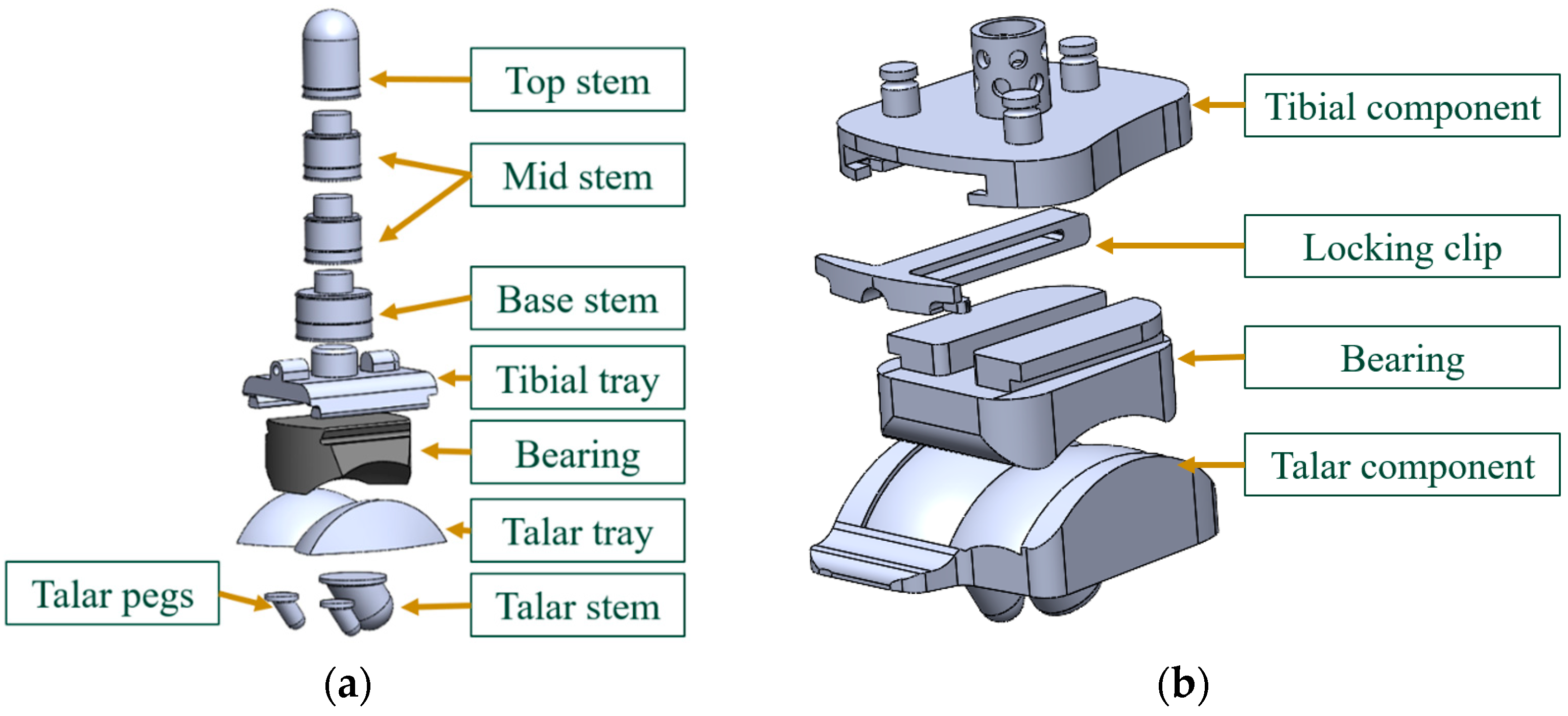
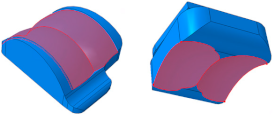
2.1. Implant Geometry Development
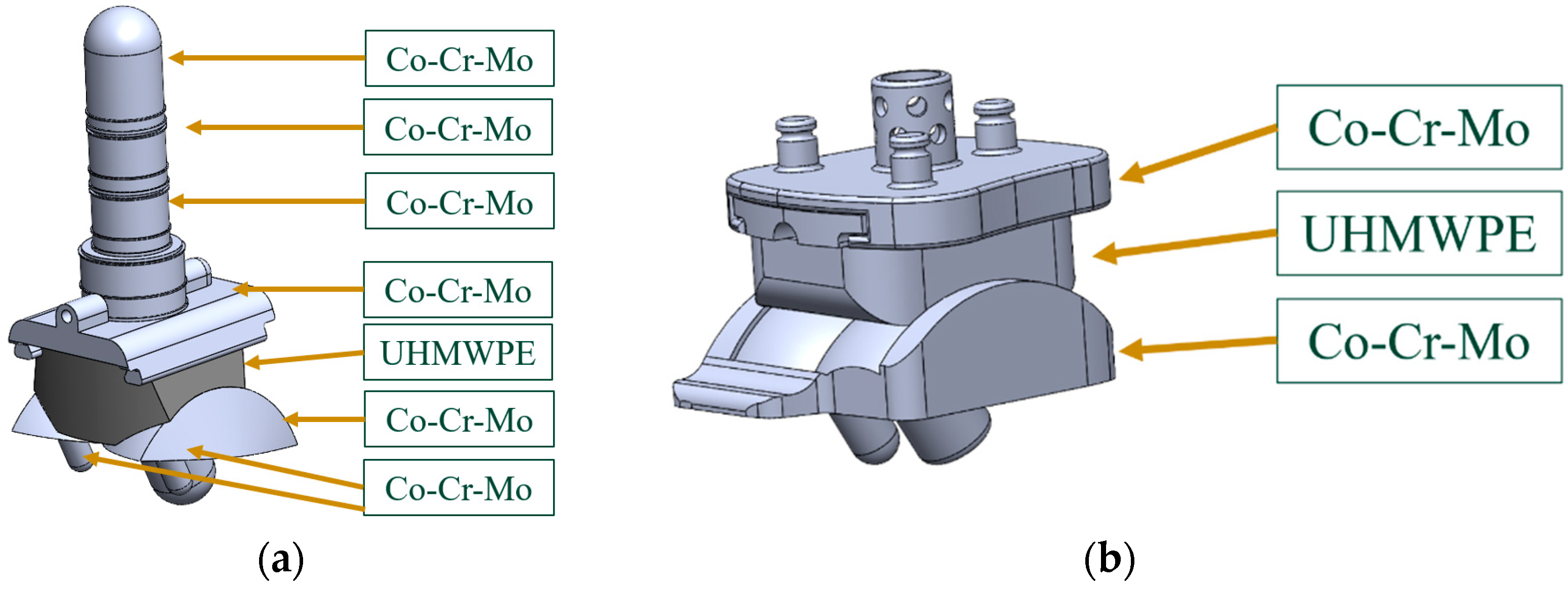
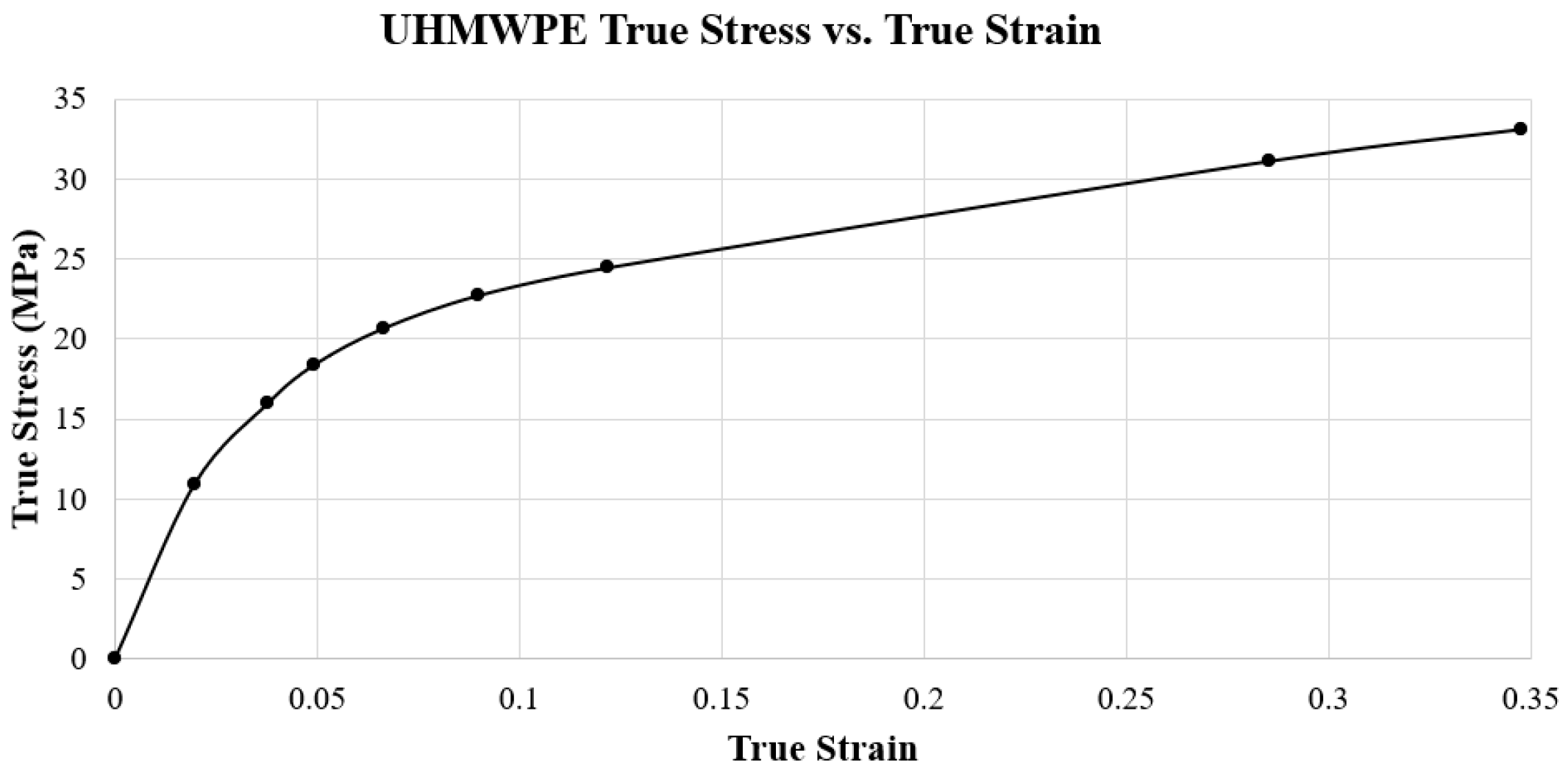
2.2. ABAQUS Material Properties
2.3. ABAQUS Step/Interactions
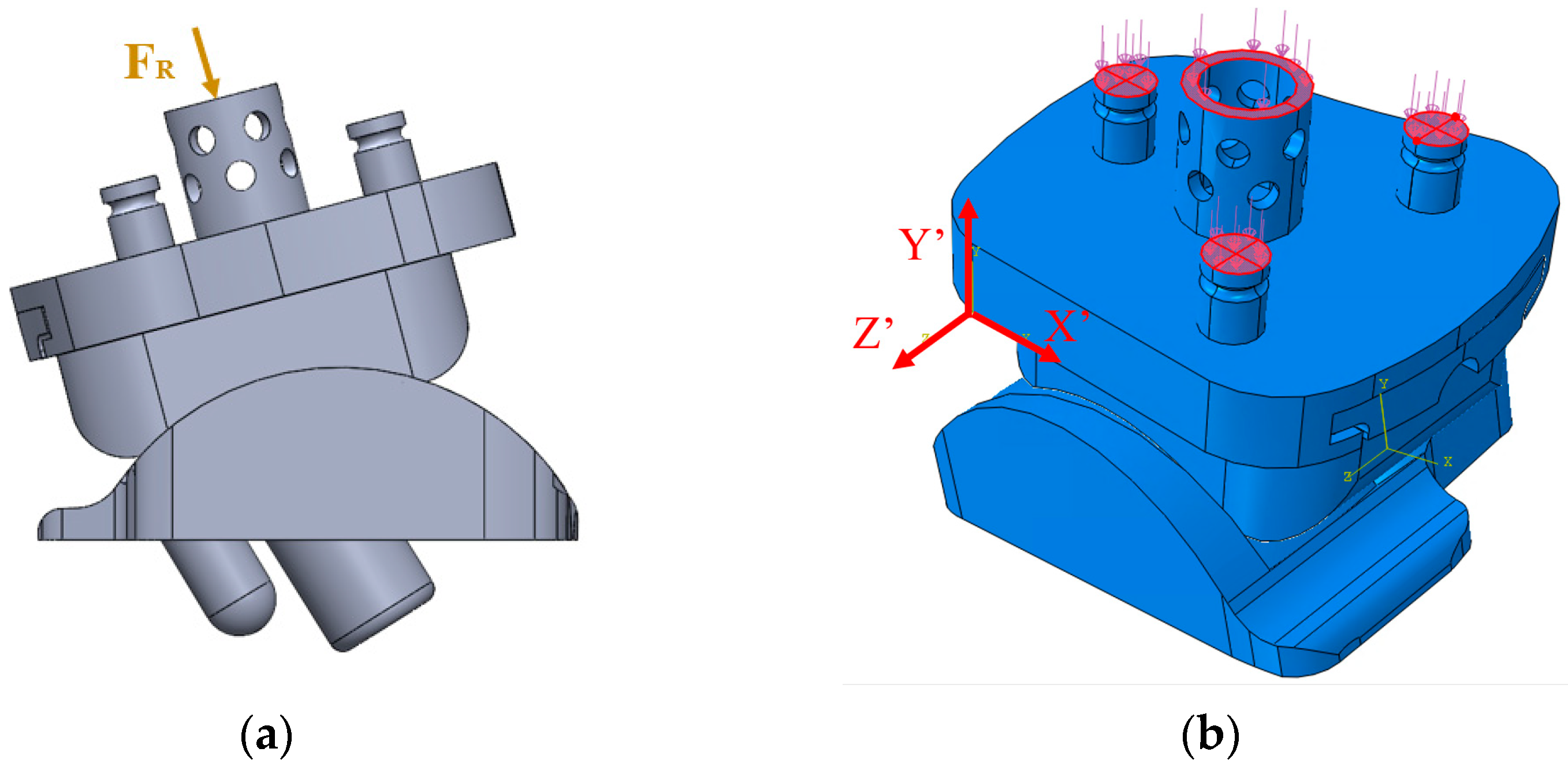
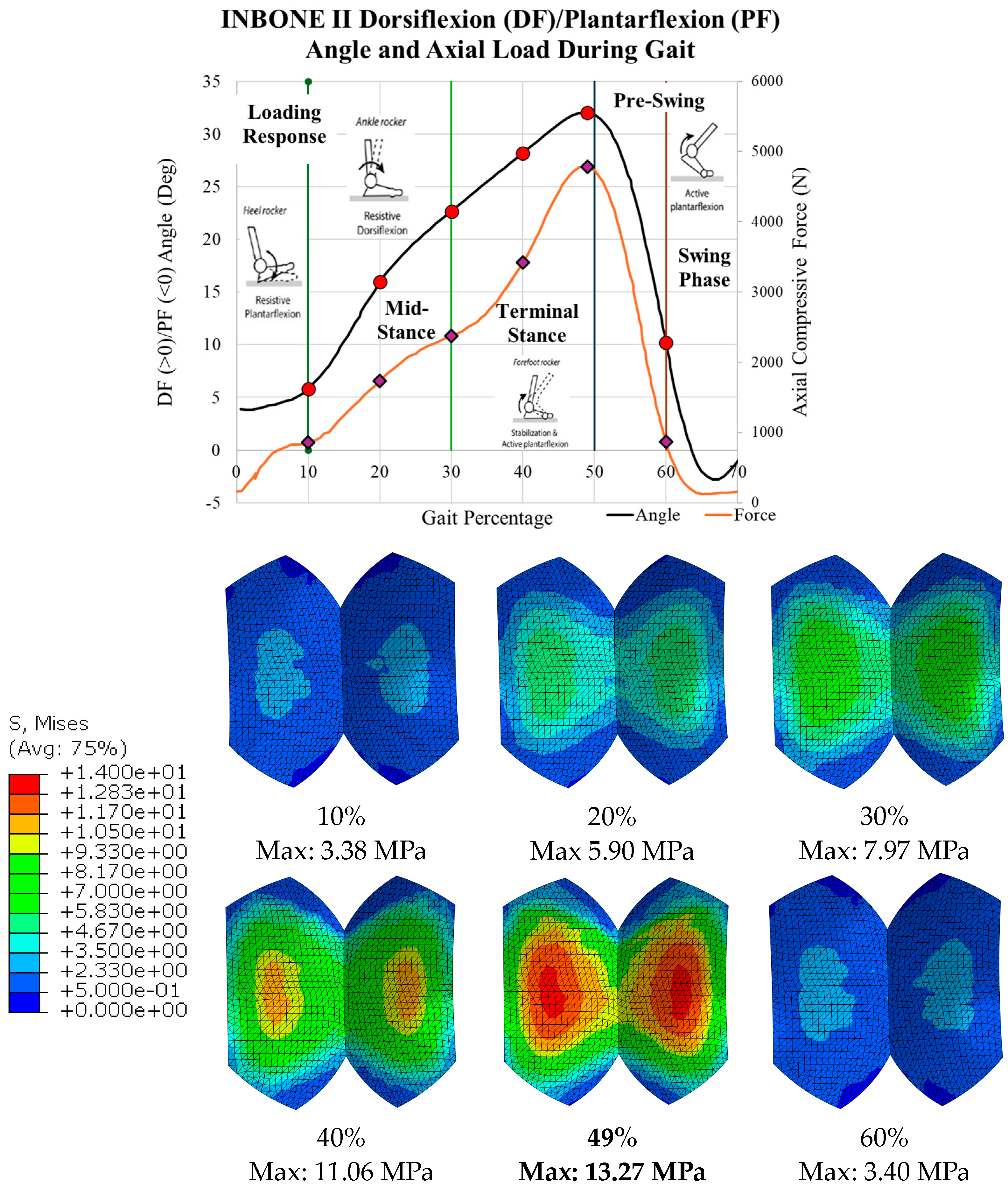
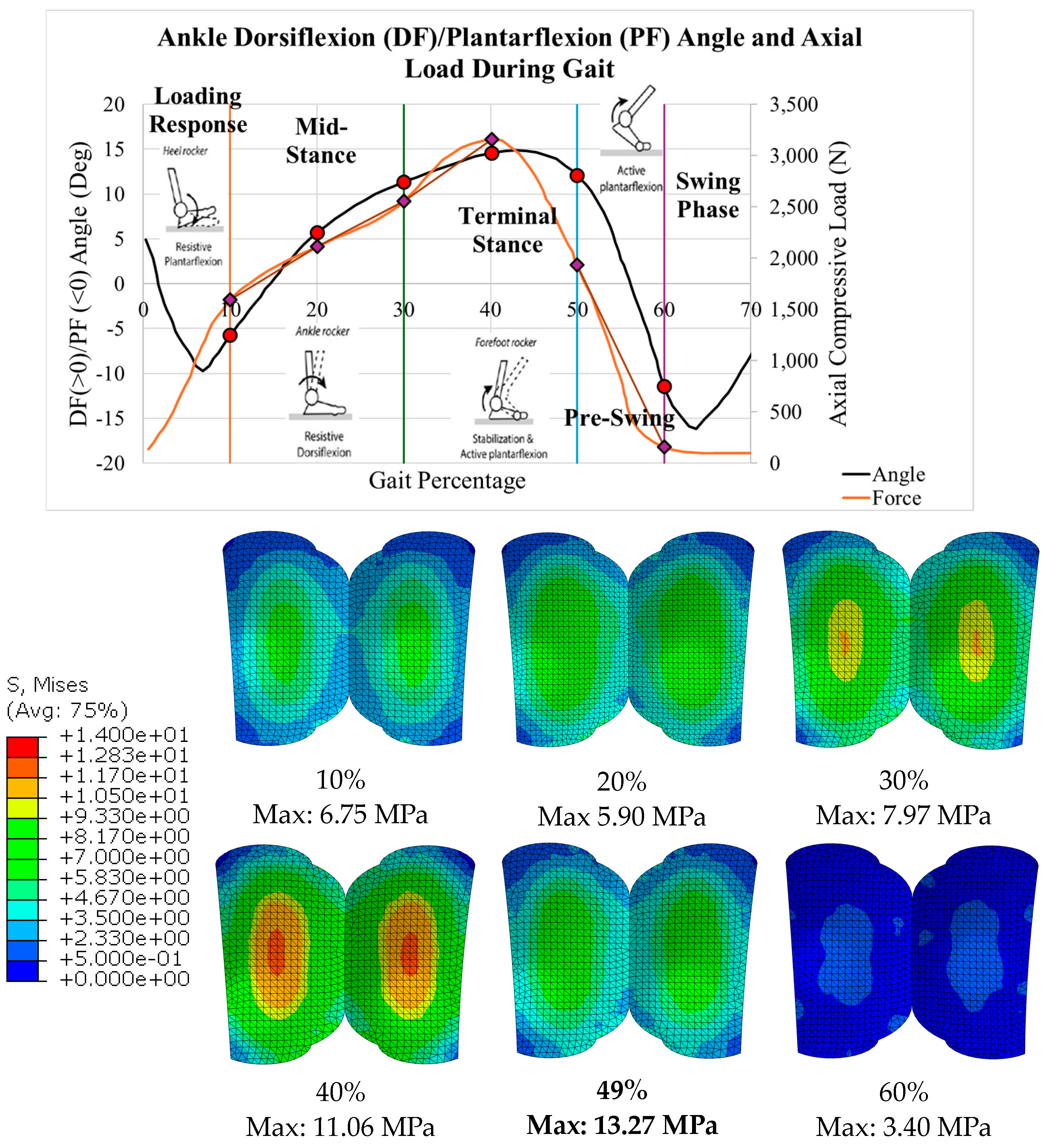
2.4. Implant Loading
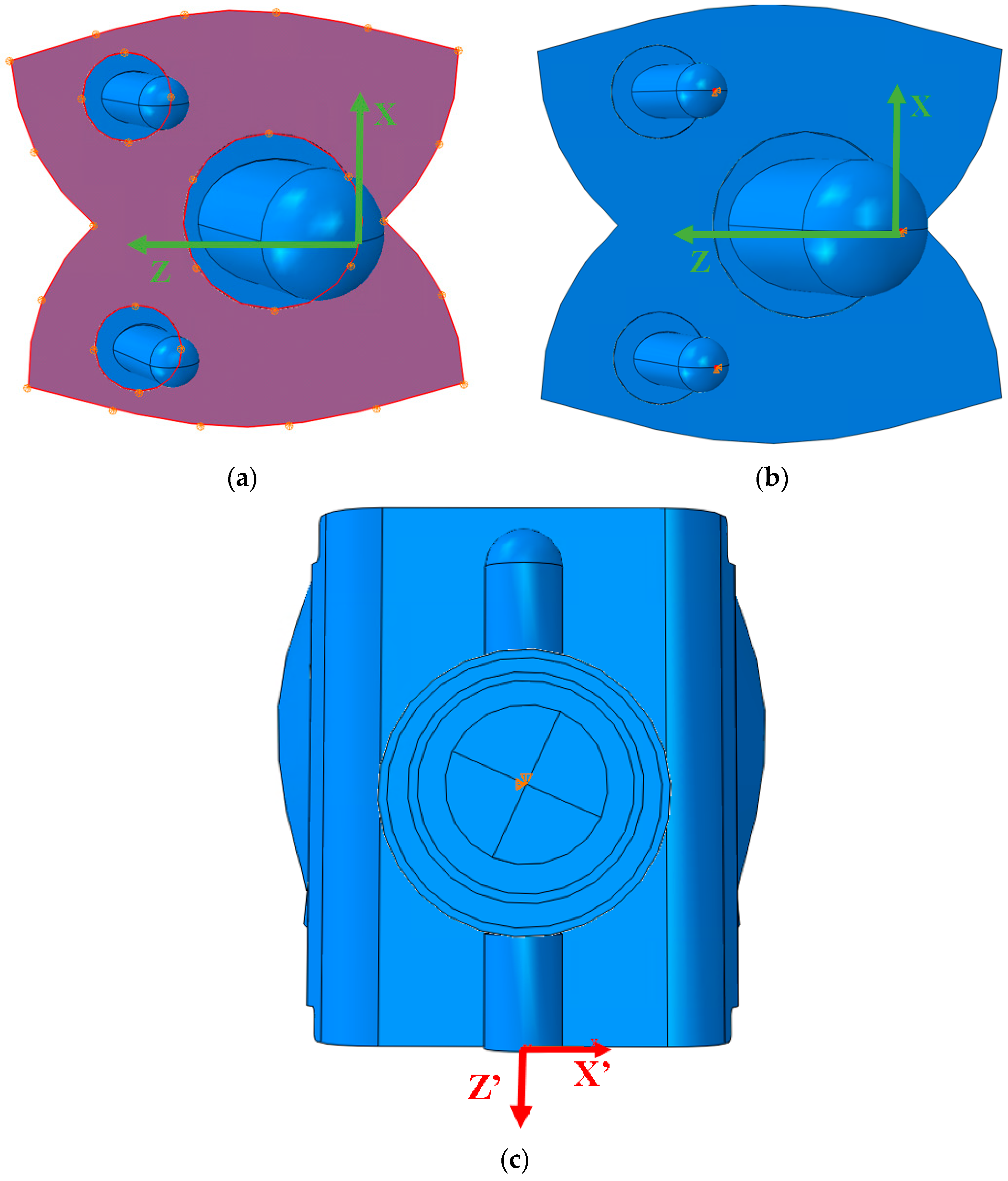
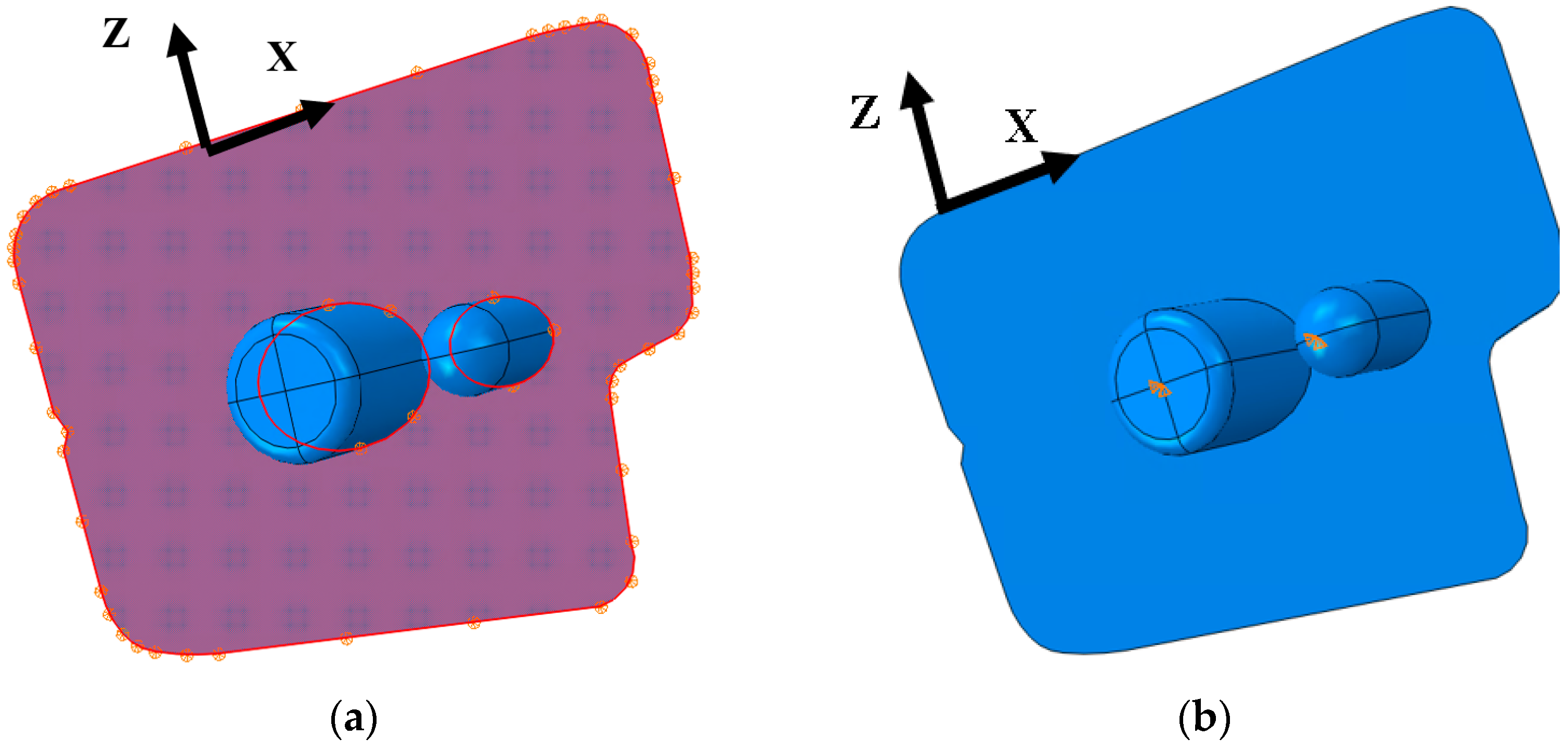
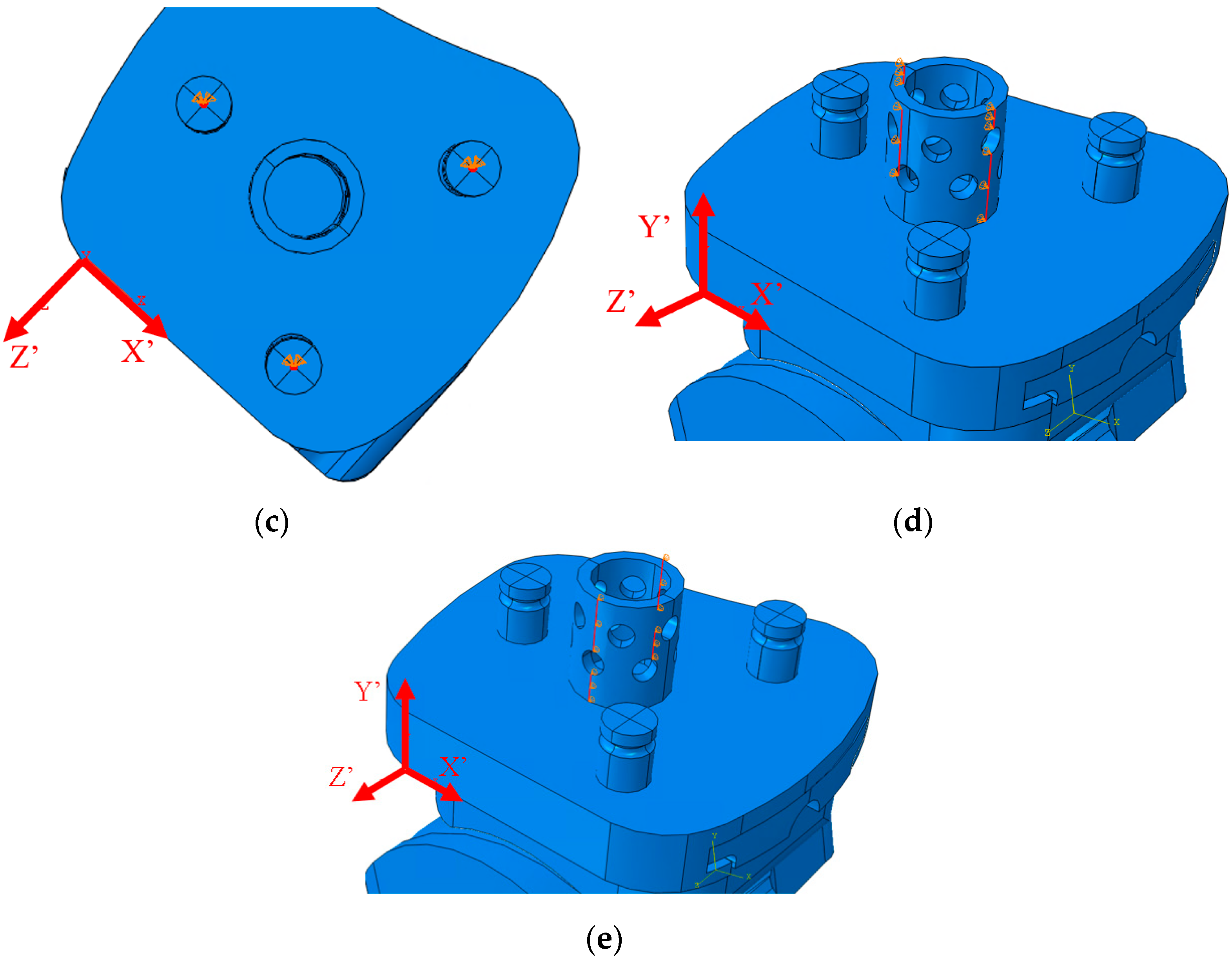
2.5. ABAQUS Boundary Conditions
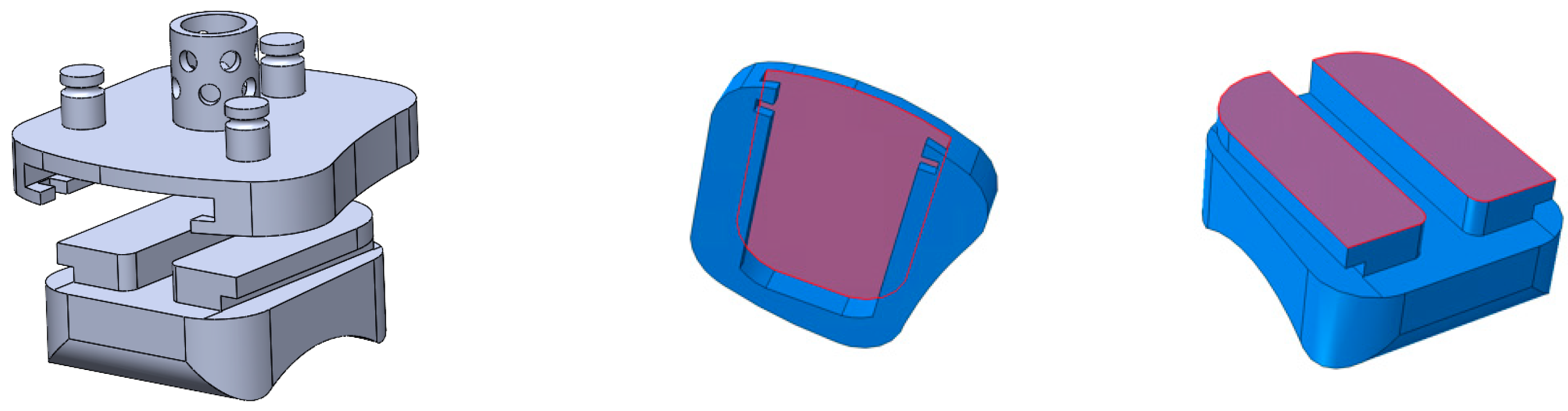
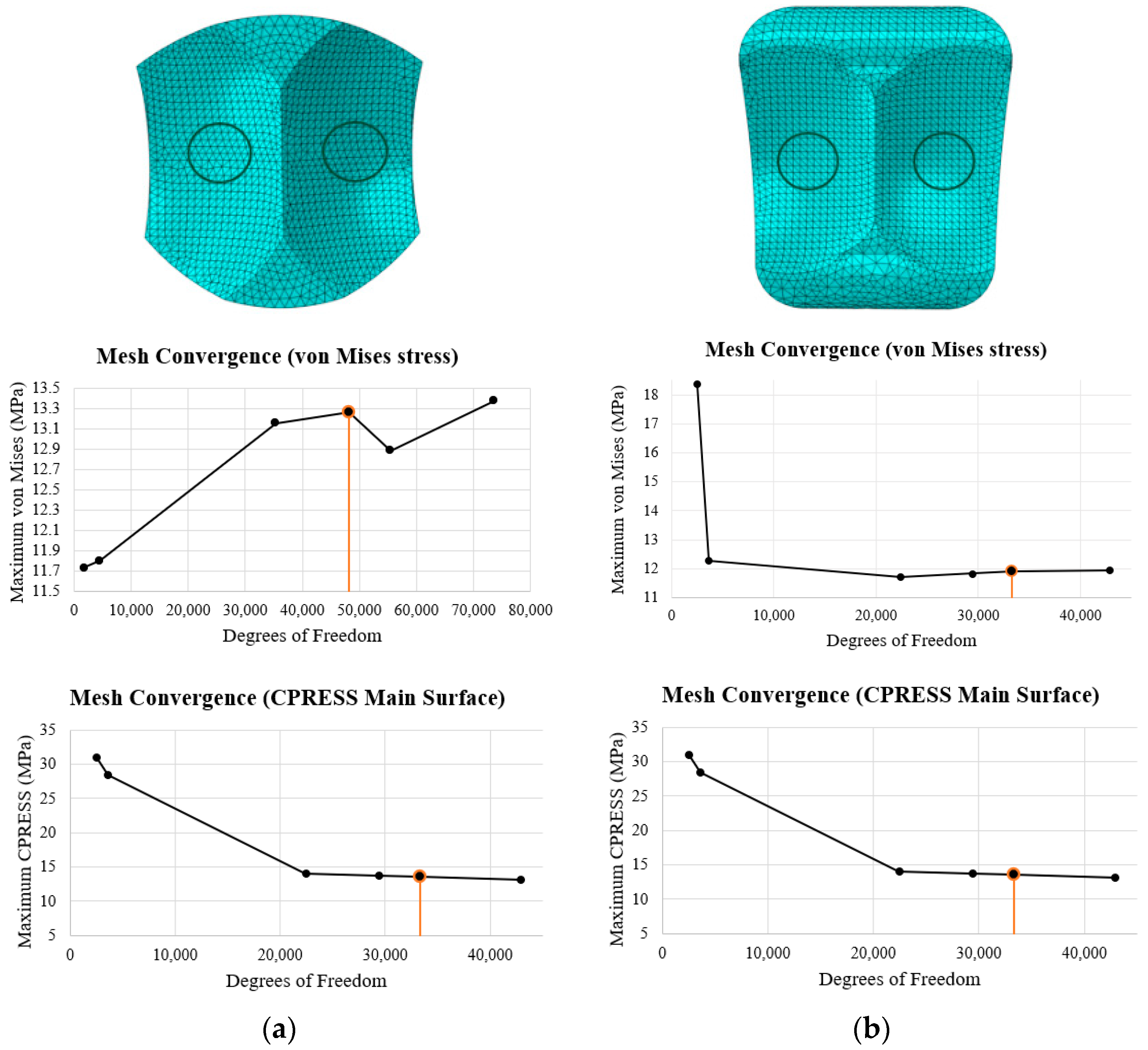
2.6. ABAQUS Meshing
3. Results
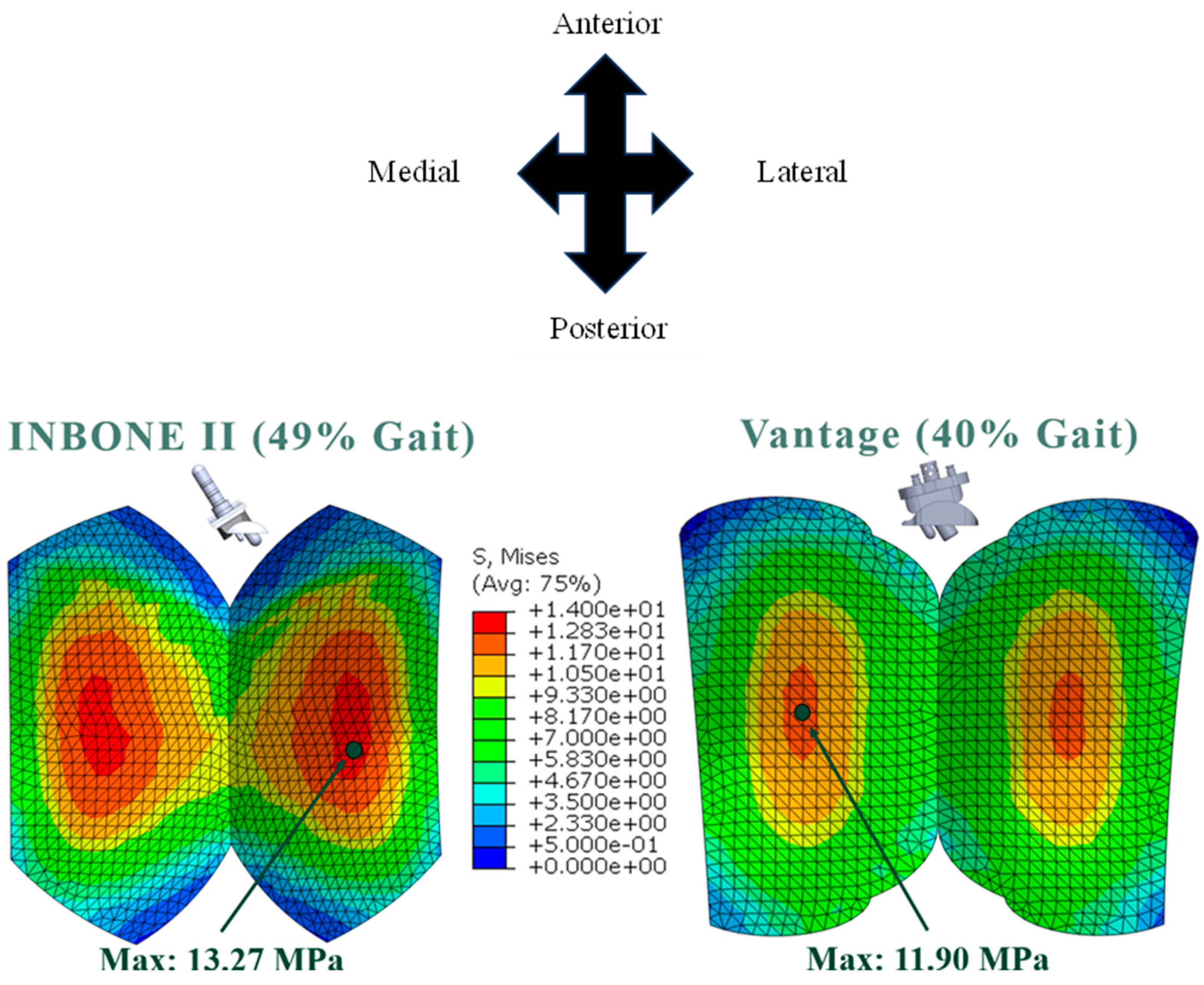
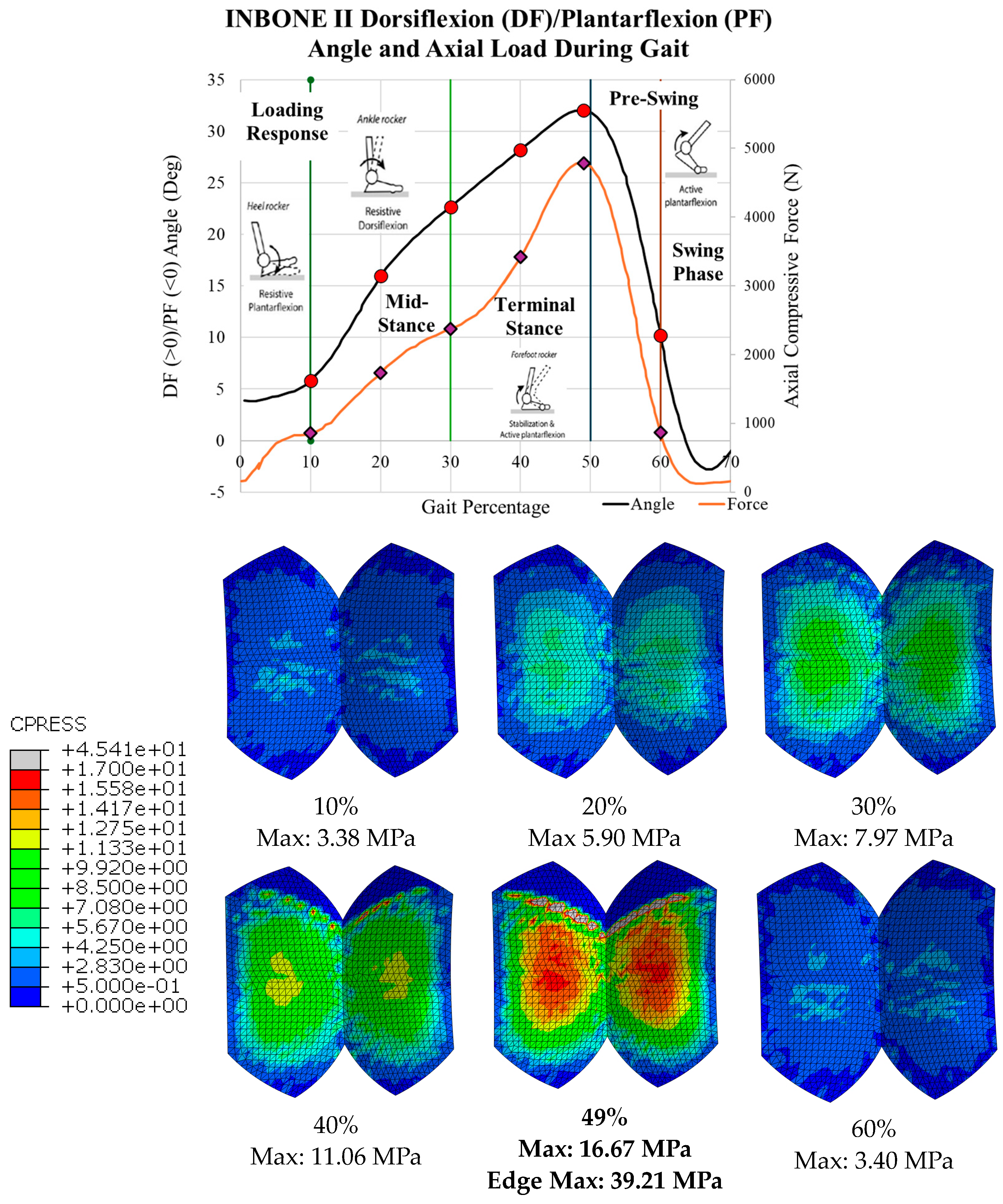
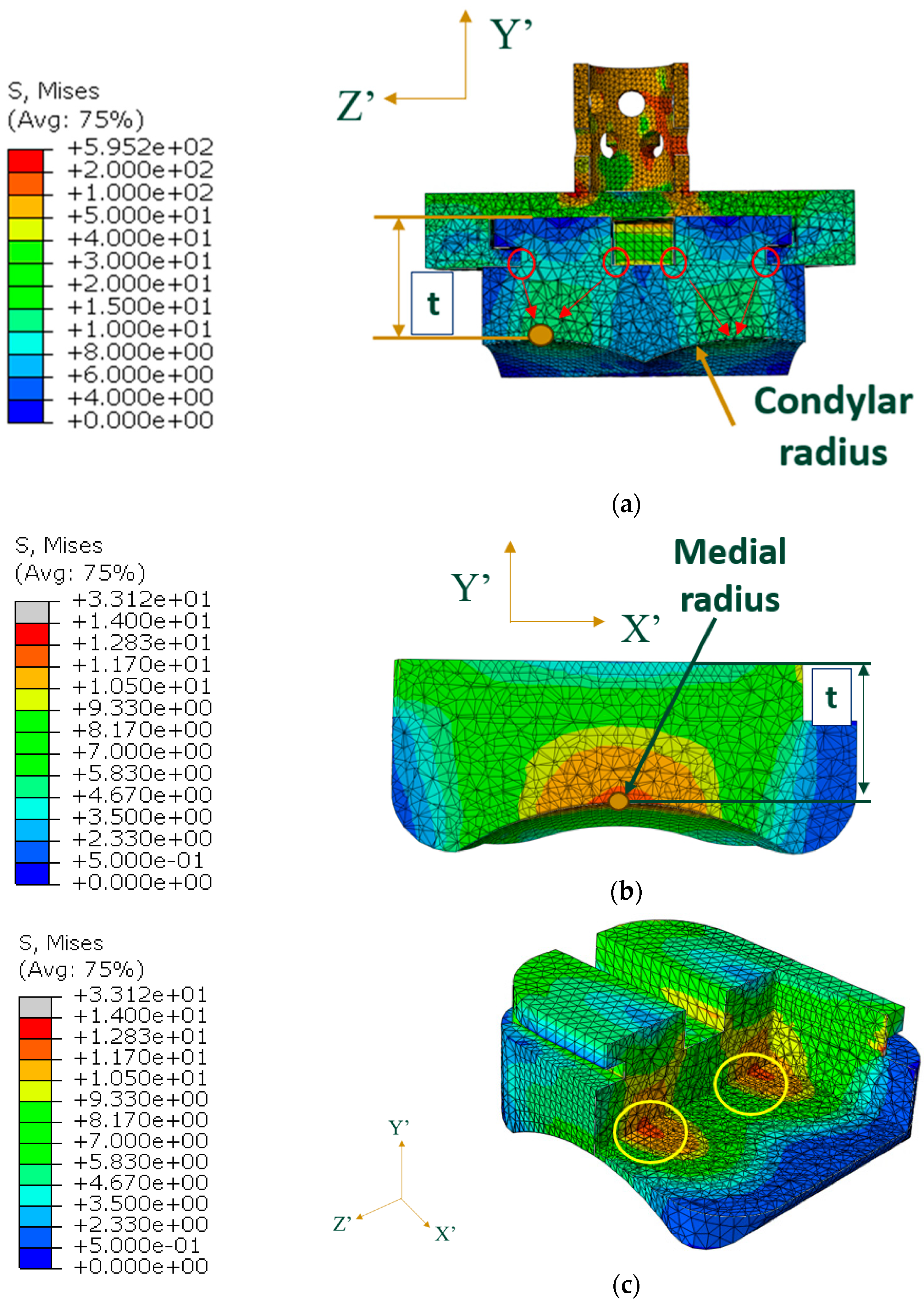
3.1. Von Mises Stress
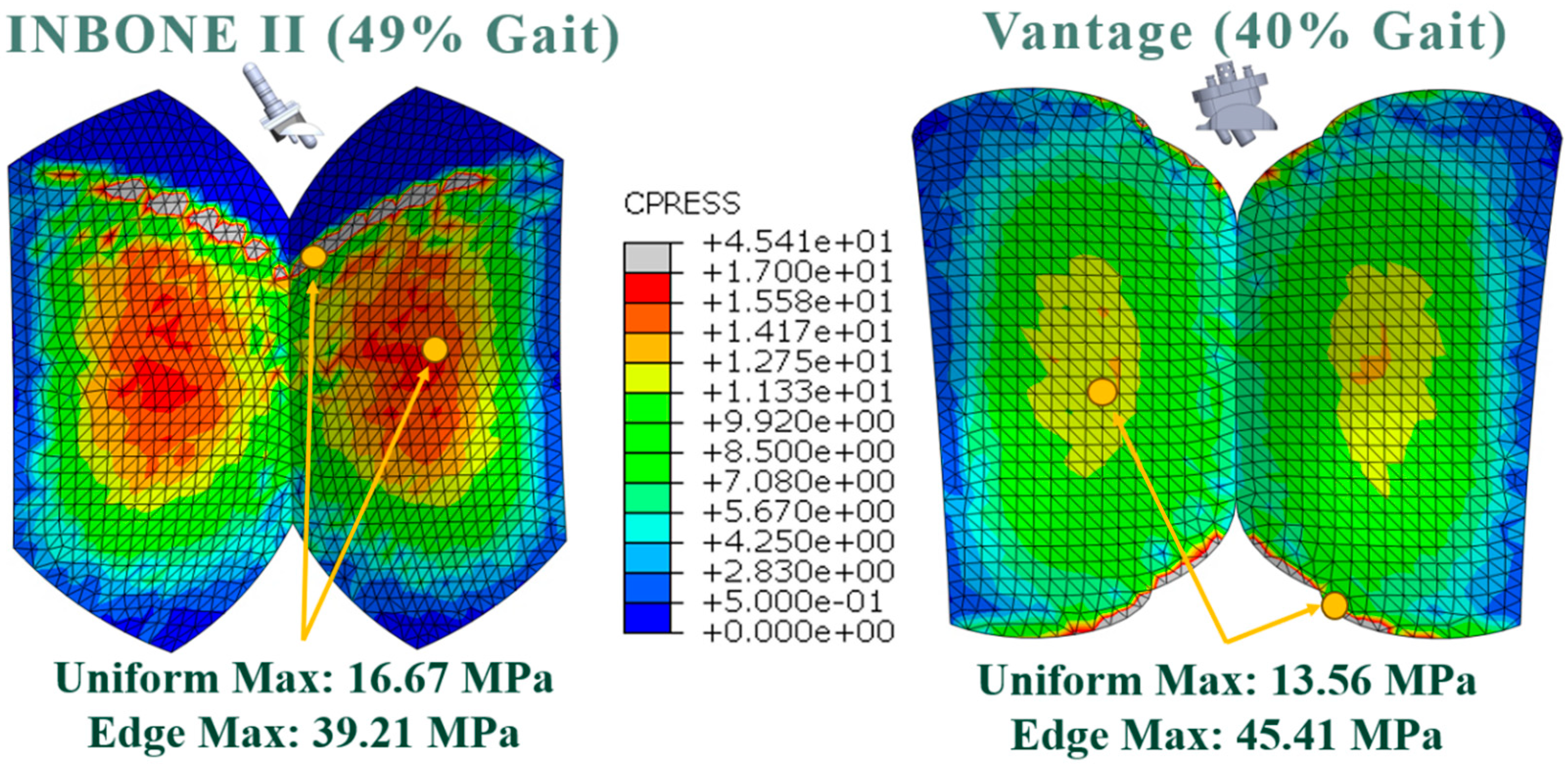
3.2. Contact Pressure
4. Discussion
4.1. Indications of Results
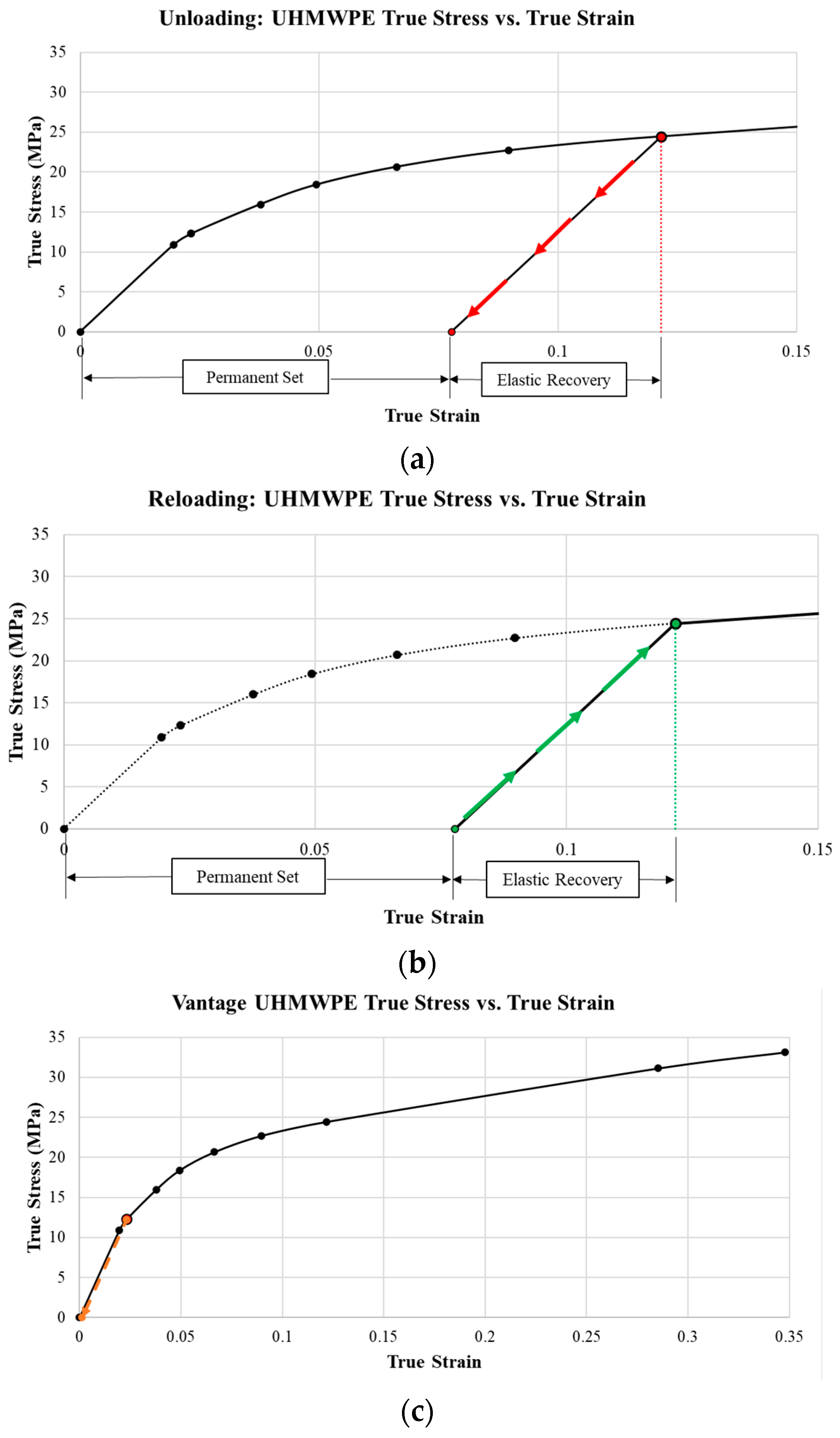
4.2. Unloading and Reloading
4.3. Comparison to Other Studies
4.4. Future Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ankle Joint. Cleveland Clinic. 2023. Available online: https://my.clevelandclinic.org/health/body/24909-ankle-joint (accessed on 28 July 2023).
- Anatomy of the Ankle. Southern California Orthopedic Institute. 2023. Available online: https://www.scoi.com/specialties/ankle-doctor/anatomy-ankle (accessed on 28 July 2023).
- Brockett, C.L.; Chapman, G.J. Biomechanics of the ankle. Orthop. Trauma 2016, 30, 232–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grimston, S.K.; Nigg, B.M.; Hanley, D.A.; Engsberg, J.R. Differences in ankle joint complex range of motion as a function of age. Foot Ankle 1993, 14, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, R.N.; Chao, E.Y.; Brewster, R.C. Force and motion analysis of the normal, diseased, and prosthetic ankle joint. Clin Orthop. Relat. Res. 1977, 127, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Pappas, M.J.; Buechel, F.F. Failure Modes of Current Total Ankle Replacement Systems. Clin. Podiatr. Med. Surg. 2013, 30, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Baretta, S.; Pagano, J.; Bianchi, A.; Villa, T.; Casaroli, G.; Galbusera, F. Contact stresses, pressure and area in a fixed-bearing total ankle replacement: A finite element analysis. BMC Musculoskelet. Disord. 2017, 18, 493. [Google Scholar] [CrossRef] [PubMed]
- Gil-Castillo, J.; Alnajjar, F.; Koutsou, A.; Torricelli, D.; Moreno, J.C. Advances in neuroprosthetic management of foot drop: A review. J. NeuroEng. Rehabil. 2020, 17, 46. [Google Scholar] [CrossRef]
- Arthritis of the Foot and Ankle. American Academy of Orthopedic Surgeons. 2023. Available online: https://orthoinfo.aaos.org/en/diseases--conditions/arthritis-of-the-foot-and-ankle/ (accessed on 30 July 2023).
- Coester, L.M.; Saltzman, C.L.; Leupold, J.; Pontarelli, W. Long-term results following ankle arthrodesis for post-traumatic arthritis. J. Bone Jt. Surg. Am. 2001, 83, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, H.; Stürup, J. Comparison of ankle arthroplasty and arthrodesis. A prospective series with long-term follow-up. Foot 1994, 4, 6–9. Available online: https://api.semanticscholar.org/CorpusID:71193582 (accessed on 30 July 2023). [CrossRef]
- Glazebrook, M.; Burgesson, B.N.; Younger, A.S.; Daniels, T.R. Clinical outcome results of total ankle replacement and ankle arthrodesis: A pilot randomised controlled trial. Foot Ankle Surg. 2021, 27, 326–331. [Google Scholar] [CrossRef]
- Dekker, T.J.; Hamid, K.S.; Federer, A.E.; Steele, J.R.; Easley, M.E.; Nunley, J.A.; Adams, S.B., Jr. The Value of Motion: Patient-Reported Outcome Measures Are Correlated with Range of Motion in Total Ankle Replacement. Foot Ankle Spec. 2018, 11, 451–456. [Google Scholar] [CrossRef] [PubMed]
- DeOrio, J.K.; Nunley, J.A.; Easley, M.E.; Valderrabano, V. Vantage Total Ankle Replacement, Published in Primary and Revision Total Ankle Replacement, pp 151–163. 2021. Available online: https://link.springer.com/chapter/10.1007/978-3-030-69269-8_13 (accessed on 30 July 2023).
- A New Perspective in Total Ankle: Vantage Total Ankle. Exactech Extremities. Available online: https://it.exac.com/wp-content/uploads/sites/33/2022/04/721-00-11-Rev-B_Vantage_Ankle-Product_Sheet_OUS.pdf (accessed on 30 July 2023).
- Baena, J.C.; Wu, J.; Peng, Z. Wear performance of UHMWPE and reinforced UHMWPE composites in arthroplasty applications: A review. Lubricants 2015, 3, 413–436. [Google Scholar] [CrossRef]
- Patil, N.A.; Njuguna, J.; Kandasubramanian, B. UHMWPE for biomedical applications: Performance and functionalization. Eur. Polym. J. 2020, 125, 109529. [Google Scholar] [CrossRef]
- Hauer, G.; Hofer, R.; Kessler, M.; Lewis, J.; Leitner, L.; Radl, R.; Leithner, A.; Sadoghi, P. Revision Rates After Total Ankle Replacement: A Comparison of Clinical Studies and Arthroplasty Registers. Foot Ankle Int. 2022, 43, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Anil, U.; Singh, V.; Schwarzkopf, R. Diagnosis and Detection of Subtle Aseptic Loosening in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.B.; Ouyang, J.Y.; Noori, M.; Altabey, W.A. A Review Study on Total Ankle Replacement. Appl. Sci. 2023, 13, 535. [Google Scholar] [CrossRef]
- Jones, M.D.; Buckle, C.L. How does aseptic loosening occur and how can we prevent it? Orthop. Trauma 2020, 34, 146–152. [Google Scholar] [CrossRef]
- Frequently Asked Questions about Total Ankle Replacement. Washington University Orthopedics. Available online: https://www.ortho.wustl.edu/content/Education/2915/Patient-Education/Educational-Materials/Total-Ankle-Replacements-FAQs.aspx#:~:text=While%20results%20at%205%20and,years%20after%20the%20original%20surgery (accessed on 13 March 2024).
- Yu, J.; Zhao, D.; Chen, W.-M.; Chu, P.; Wang, S.; Zhang, C.; Huang, J.; Wang, X.; Ma, X. Finite element stress analysis of the bearing component and bone resected surfaces for total ankle replacement with different implant material combinations. BMC Musculoskelet. Disord. 2022, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Zhao, H.; Zhao, D.; Zhang, X.; Ma, X.; Jin, Z. Comparison of joint load, motions and contact stress and bone-implant interface micromotion of three implant designs for total ankle arthroplasty. Comput. Methods Programs Biomed. 2022, 223, 106976. [Google Scholar] [CrossRef]
- Saad, A.P.B.M.; Syahrom, A.; Harun, M.N.; Kadir, M.R.A. Wear of Total Ankle Replacement (TAR). In Wear Prediction on Total Ankle Replacement; SpringerBriefs in Applied Sciences and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- INBONE Total Ankle System. Wright Medical Total Ankle Institute. 2023. Available online: http://www.totalankleinstitute.com/inbone-products/inbone-ankle/ (accessed on 22 August 2023).
- Timothy, S. Jain, Finite Element Analysis of The Bearing Component of Total Ankle Replacement Implants during the Stance Phase of Gait, California Polytechnic State University, San Luis Obispo, CA, USA. Available online: https://digitalcommons.calpoly.edu/theses/2766/ (accessed on 10 March 2024).
- Zhang, Y.; Chen, Z.; Zhao, D.; Yu, J.; Ma, X.; Jin, Z. Articular geometry can affect joint kinematics, contact mechanics, and implant-bone micromotion in total ankle arthroplasty. J. Orthop. Res. 2023, 41, 407–417. [Google Scholar] [CrossRef]
- Miller, M.C.; Smolinski, P.; Conti, S.; Galik, K. Stresses in polyethylene liners in a semiconstrained ankle prosthesis. J. Biomech. Eng. 2004, 126, 636–640. [Google Scholar] [CrossRef]
- Malito, L.G.; Arevalo, S.; Kozak, A.; Spiegelberg, S.; Bellare, A.; Pruitt, L. Material properties of ultra-high molecular weight polyethylene: Comparison of tension, compression, nanomechanics and microstructure across clinical formulations. J. Mech. Behav. Biomed. Mater. 2018, 83, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Godest, A.C.; Beaugonin, M.; Haug, E.; Taylor, M.; Gregson, P.J. Simulation of a knee joint replacement during a gait cycle using explicit finite element analysis. J. Biomech. 2002, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Zhao, H.; Liang, X.; Sun, C.; Jin, Z. Musculoskeletal modeling of total ankle arthroplasty using force-dependent kinematics for predicting in vivo joint mechanics. Proc. Inst. Mech. Eng. H 2020, 234, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Nunley, J.M.M.; Valderrabano, V.M.P.; DeOrio, J.M.; Easley, M.M. Vantage Ankle Design Rationale. Exactech Extremities. Available online: https://www.exac.com/wp-content/uploads/2019/04/721-00-40_Vantage_Ankle_Design_Rationale_Web_6835600.pdf (accessed on 30 July 2023).
- National Heart, Lung, and Blood Institute. Calculate Your Body Mass Index. National Institute of Health. 2024. Available online: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi-m.htm (accessed on 10 March 2024).
- Bell, C.J.; Fisher, J. Simulation of polyethylene wear in ankle joint prostheses. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 162–167. [Google Scholar] [CrossRef]
- Rodrigues, D.S.S. Biomechanics of the Total Ankle Arthroplasty: Stress Analysis and Bone Remodeling. 2013. Available online: https://api.semanticscholar.org/CorpusID:53547308 (accessed on 12 February 2024).
- Mulcahy, H.; Chew, F.S. Current Concepts in Total Ankle Replacement for Radiologists: Features and Imaging Assessment. Am. J. Roentgenol. 2015, 205, 1038–1047. [Google Scholar] [CrossRef]
- Elliot, B.J.; Gundapaneni, D.; Goswami, T. Finite element analysis of stress and wear characterization in total ankle replacements. J. Mech. Behav. Biomed. Mater. 2014, 34, 134–145. [Google Scholar] [CrossRef]
- National Joint Registry Steering Committee. 16th Annual Report 2019: National Joint Registry for England, Wales, Northern Ireland and the Isle of Man; 20th National Joint Registry Annual Report. 2019. Available online: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf (accessed on 5 February 2024).
- Shaffrey, I.; O’Malley, E.; Henry, J.K.; Rajan, L.; Deland, J.T.; O’Malley, M.; Ellis, S.J.; Demetracopoulos, C.A. Midterm Clinical Outcomes, Radiographic Outcomes, and Survivorship of the Infinity Total Ankle Arthroplasty. Foot Ankle Int. 2023, 44, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.; Almeida, J.F.; Pereira, B.; Andrade, R.; Espregueira-Mendes, J.; Gomes, T.M.; Oliva, X.M. Complications after total ankle arthroplasty- A systematic review. Foot Ankle Surg. 2023, 29, 32–38. [Google Scholar] [CrossRef] [PubMed]
- van der Plaat, L.W.; Hoornenborg, D.; Sierevelt, I.N.; van Dijk, C.N.; Haverkamp, D. Ten-year revision rates of contemporary total ankle arthroplasties equal 22%. A meta-analysis. Foot Ankle Surg. 2022, 28, 543–549, Erratum in Foot Ankle Surg. 2023, 29, 177. https://doi.org/10.1016/j.fas.2022.10.005. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.; Teehan, E.; Ellis, S.J.; Deland, J.; Demetracopoulos, C. Lessons from Revision Total Ankle Replacement: Tibias Fail Early, and Taluses Fail Late (And Fail Again). Foot Ankle Orthop. 2024, 9, 10711007241255112. [Google Scholar] [CrossRef]
- Millstein, I.D.; Koneru, M.; Dibato, J.E.; Gentile, P.; Mahjoub, A.; Freeland, E. Comparing Rates of Radiographic Baseplate Loosening Between Cement and Cementless INFINITY Total Ankle Prostheses. Foot Ankle Spec. 2024, Epub ahead of print. 19386400241247456. [Google Scholar] [CrossRef] [PubMed]
- Jennison, T.; Spolton-Dean, C.; Rottenburg, H.; Ukoumunne, O.; Sharpe, I.; Goldberg, A. The outcomes of revision surgery for a failed ankle arthroplasty: A systematic review and meta-analysis. Bone Jt. Open 2022, 3, 596–606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]





| Material | Poisson’s Ratio | Young’s Modulus (MPa) |
|---|---|---|
| CoCrMo | 0.3 | 210 × 106 * |
| UHMWPE | 0.46 | 557 |
 |  |
| Main Component | Secondary Component |
| Talar tray | Bearing |
| Normal Behavior | Tangential Behavior |
|
|
| Discretization Method | Sliding Formulation |
| Surface-to-surface | Finite sliding |
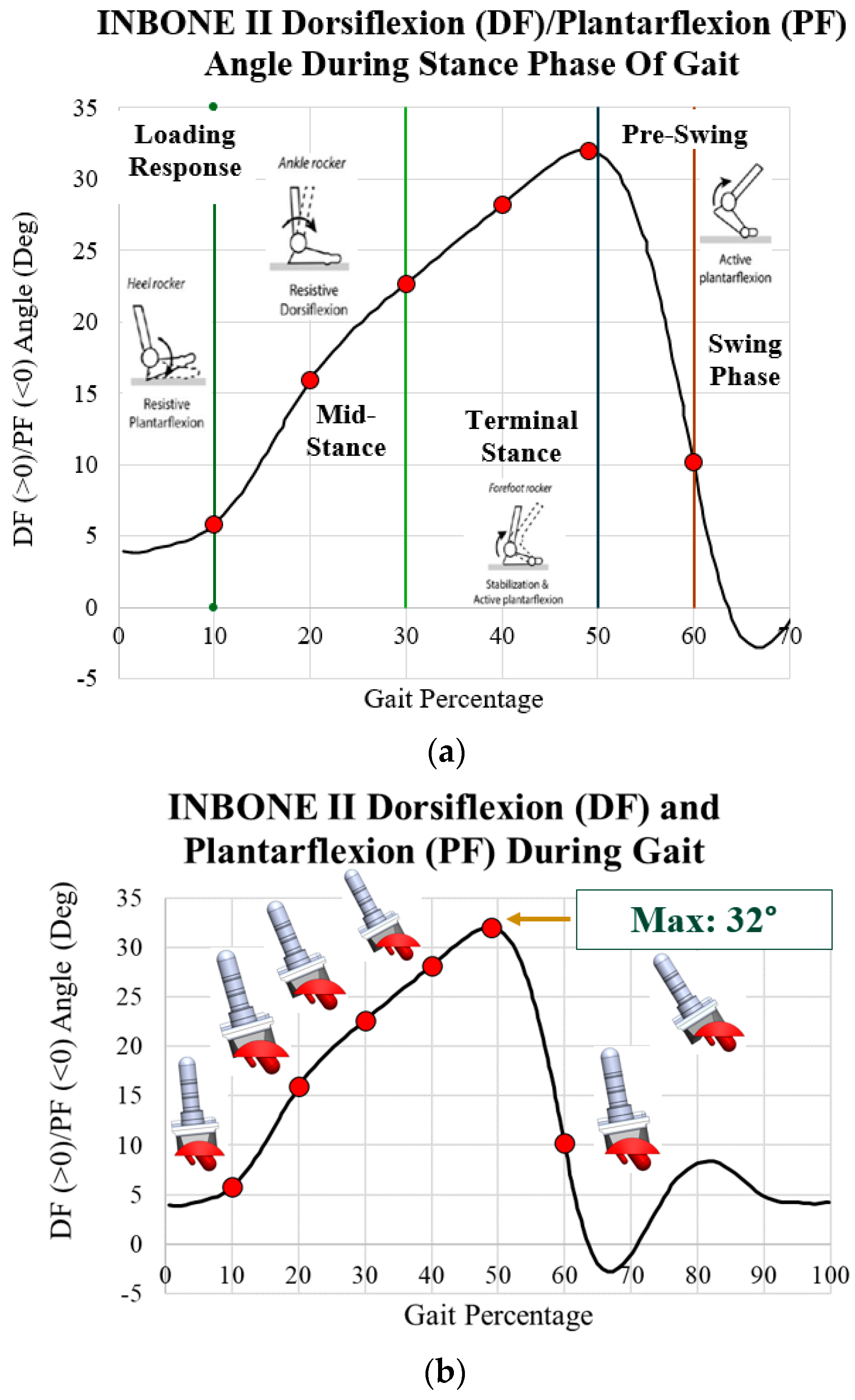
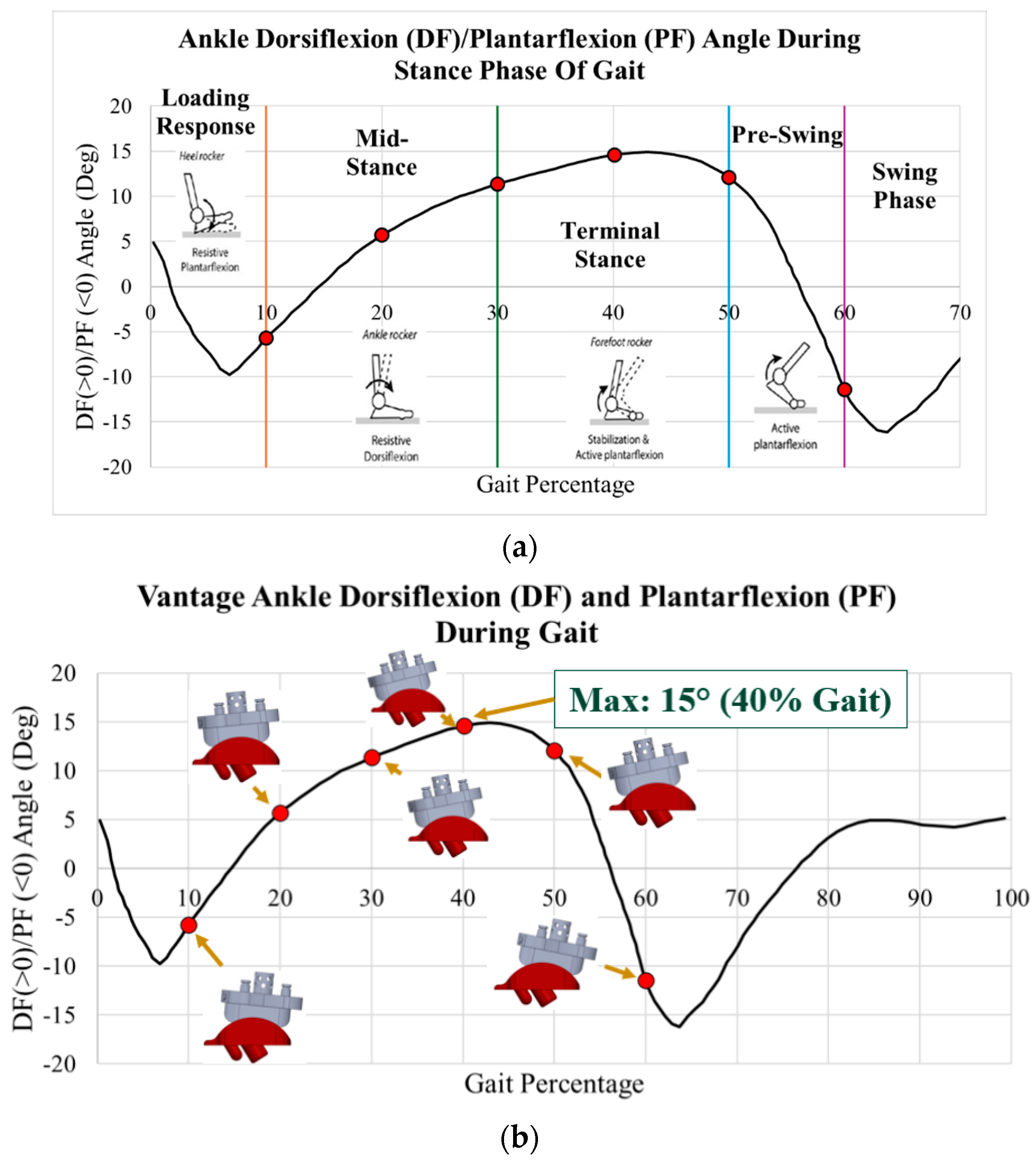
| Gait Pct | Angle | Pressure Load (N/mm2) | Von Mises Max (MPa) | Safety Factor (Max) |
|---|---|---|---|---|
| 10 | 5.8 | 1.38 | 3.38 | 3.22 |
| 20 | 16.0 | 2.79 | 5.90 | 1.84 |
| 30 | 22.7 | 3.82 | 7.97 | 1.36 |
| 40 | 28.2 | 5.50 | 11.06 | 0.98 |
| 49 | 32.0 | 7.69 | 13.27 | 0.82 |
| 60 | 10.2 | 1.40 | 3.40 | 3.20 |
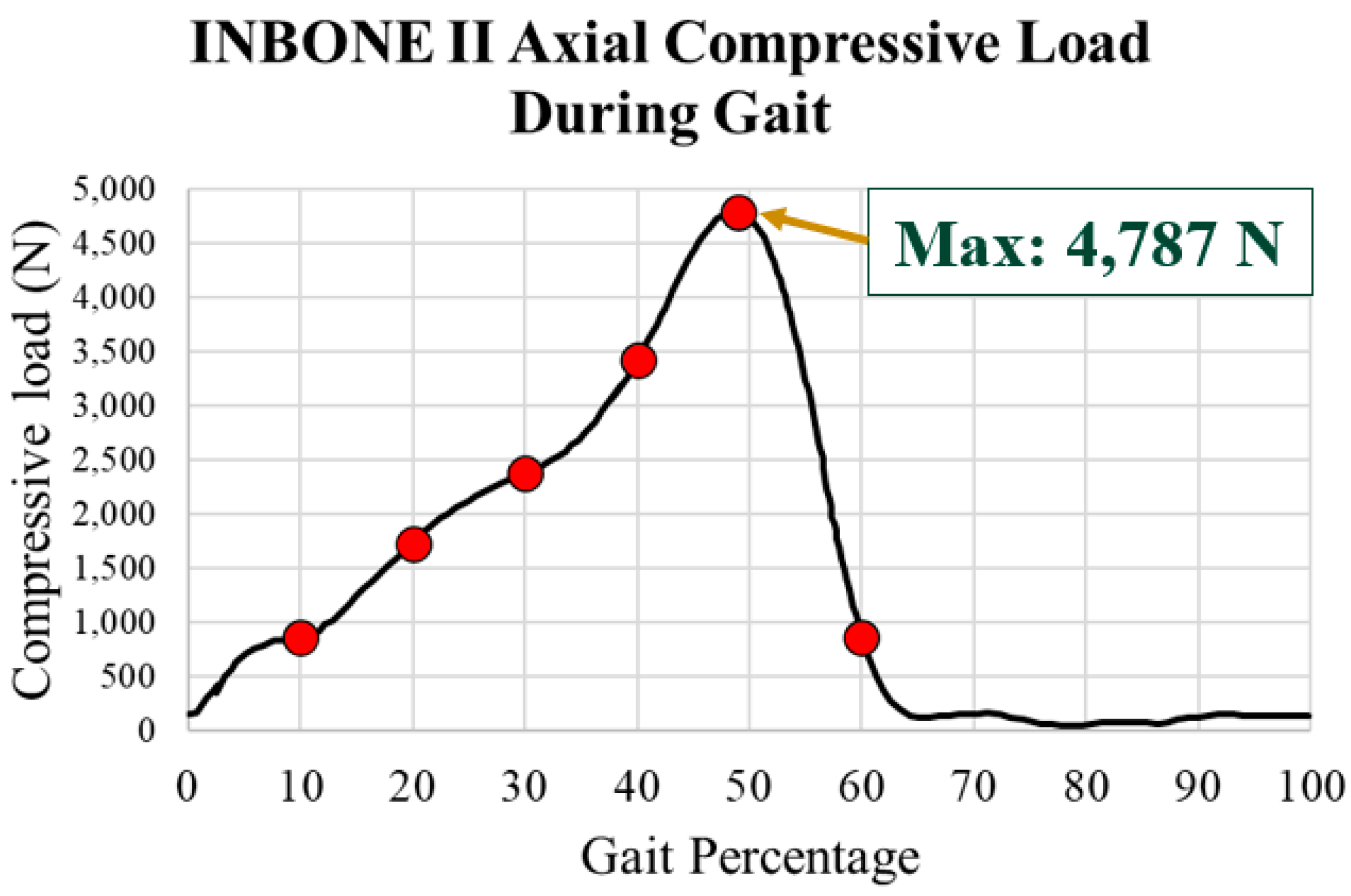
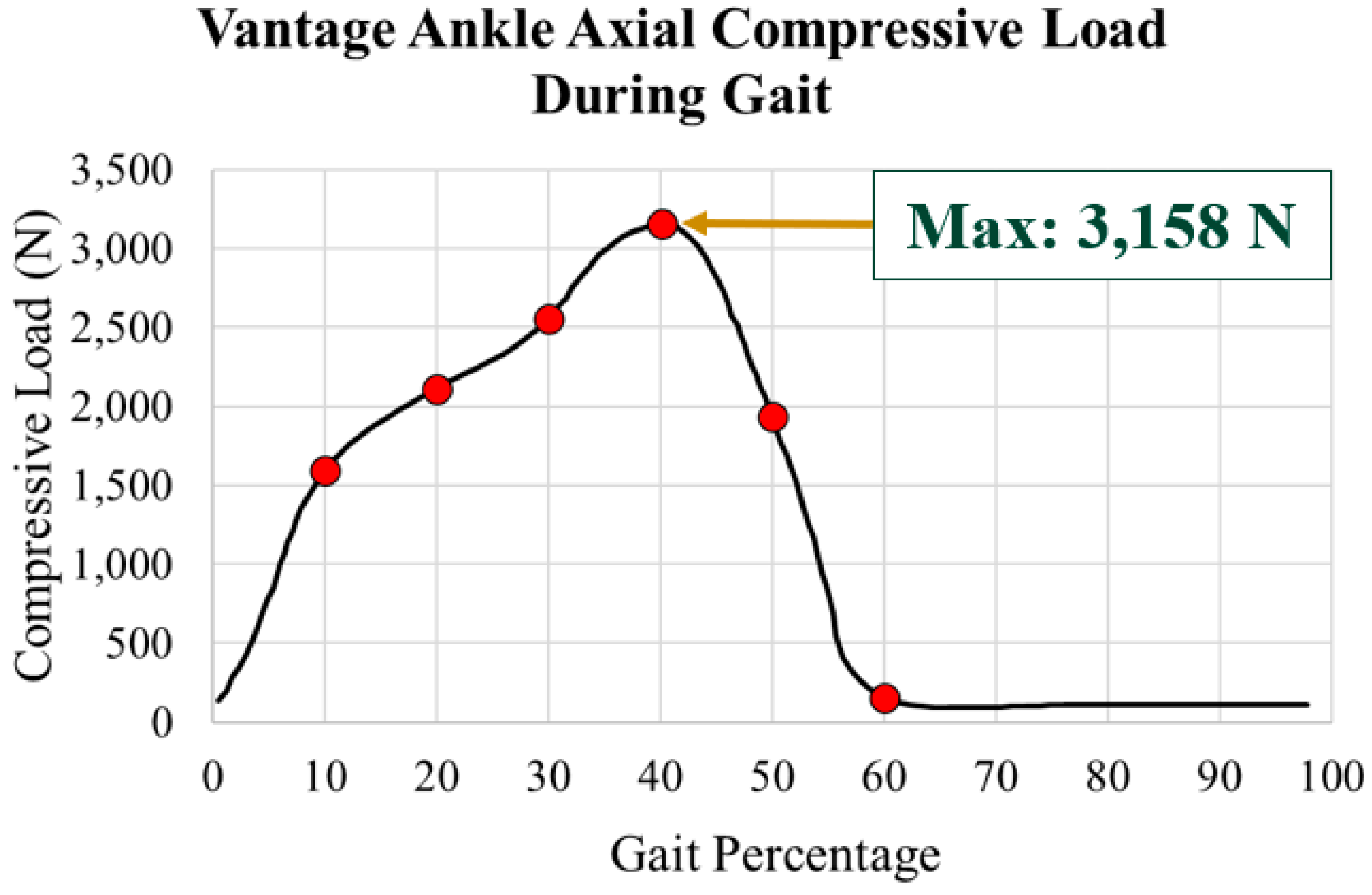
| Gait Pct | Angle | Compressive Load (N) | Von Mises Max (MPa) | Safety Factor (Max) |
|---|---|---|---|---|
| 10.0 | −5.7 | 1593.95 | 6.75 | 1.61 |
| 20.0 | 5.7 | 2111.65 | 9.03 | 1.20 |
| 30.0 | 11.4 | 2555.13 | 10.66 | 1.02 |
| 40.0 | 14.6 | 3158.26 | 11.90 | 0.91 |
| 50.0 | 12.1 | 1933.46 | 8.15 | 1.33 |
| 60.0 | −11.5 | 156.28 | 1.44 | 7.56 |
| Gait Pct | Angle | Pressure Load (N/mm2) | CPRESS Max Non-Edge (MPa) | CPRESS Max Edge (MPa) |
|---|---|---|---|---|
| 10 | 5.8 | 1.38 | 5.54 | |
| 20 | 16.0 | 2.79 | 7.73 | |
| 30 | 22.7 | 3.82 | 9.46 | |
| 40 | 28.2 | 5.50 | 12.49 | 22.14 |
| 49 | 32.0 | 7.69 | 16.67 | 39.21 |
| 60 | 10.2 | 1.40 | 5.64 |
| Gait Pct | Angle | Compressive Load (N) | CPRESS Max Non-Edge (MPa) | CPRESS Max Edge (MPa) |
|---|---|---|---|---|
| 10.0 | −5.7 | 1593.95 | 7.04 | 28.28 |
| 20.0 | 5.7 | 2111.65 | 9.39 | 26.02 |
| 30.0 | 11.4 | 2555.13 | 11.03 | 34.85 |
| 40.0 | 14.6 | 3158.26 | 13.56 | 45.41 |
| 50.0 | 12.1 | 1933.46 | 8.58 | 30.09 |
| 60.0 | 11.5 | 156.28 | 2.64 | 5.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, T.S.; Noori, M.; Rencis, J.J.; Anderson, A.; Noori, N.; Hazelwood, S. Finite Element Analysis of the Bearing Component of Total Ankle Replacement Implants during the Stance Phase of the Gait Cycle. BioMedInformatics 2024, 4, 1949-1978. https://doi.org/10.3390/biomedinformatics4030107
Jain TS, Noori M, Rencis JJ, Anderson A, Noori N, Hazelwood S. Finite Element Analysis of the Bearing Component of Total Ankle Replacement Implants during the Stance Phase of the Gait Cycle. BioMedInformatics. 2024; 4(3):1949-1978. https://doi.org/10.3390/biomedinformatics4030107
Chicago/Turabian StyleJain, Timothy S., Mohammad Noori, Joseph J. Rencis, Amanda Anderson, Naudereh Noori, and Scott Hazelwood. 2024. "Finite Element Analysis of the Bearing Component of Total Ankle Replacement Implants during the Stance Phase of the Gait Cycle" BioMedInformatics 4, no. 3: 1949-1978. https://doi.org/10.3390/biomedinformatics4030107
APA StyleJain, T. S., Noori, M., Rencis, J. J., Anderson, A., Noori, N., & Hazelwood, S. (2024). Finite Element Analysis of the Bearing Component of Total Ankle Replacement Implants during the Stance Phase of the Gait Cycle. BioMedInformatics, 4(3), 1949-1978. https://doi.org/10.3390/biomedinformatics4030107








