Deep Learning Model for COVID-19-Infected Pneumonia Diagnosis Using Chest Radiography Images
Abstract
1. Introduction
2. Related Literature
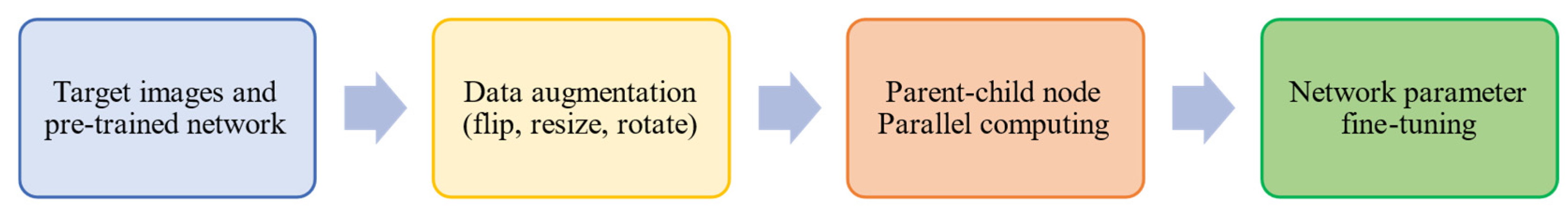
3. Proposed Methodology
3.1. Data Preprocessing
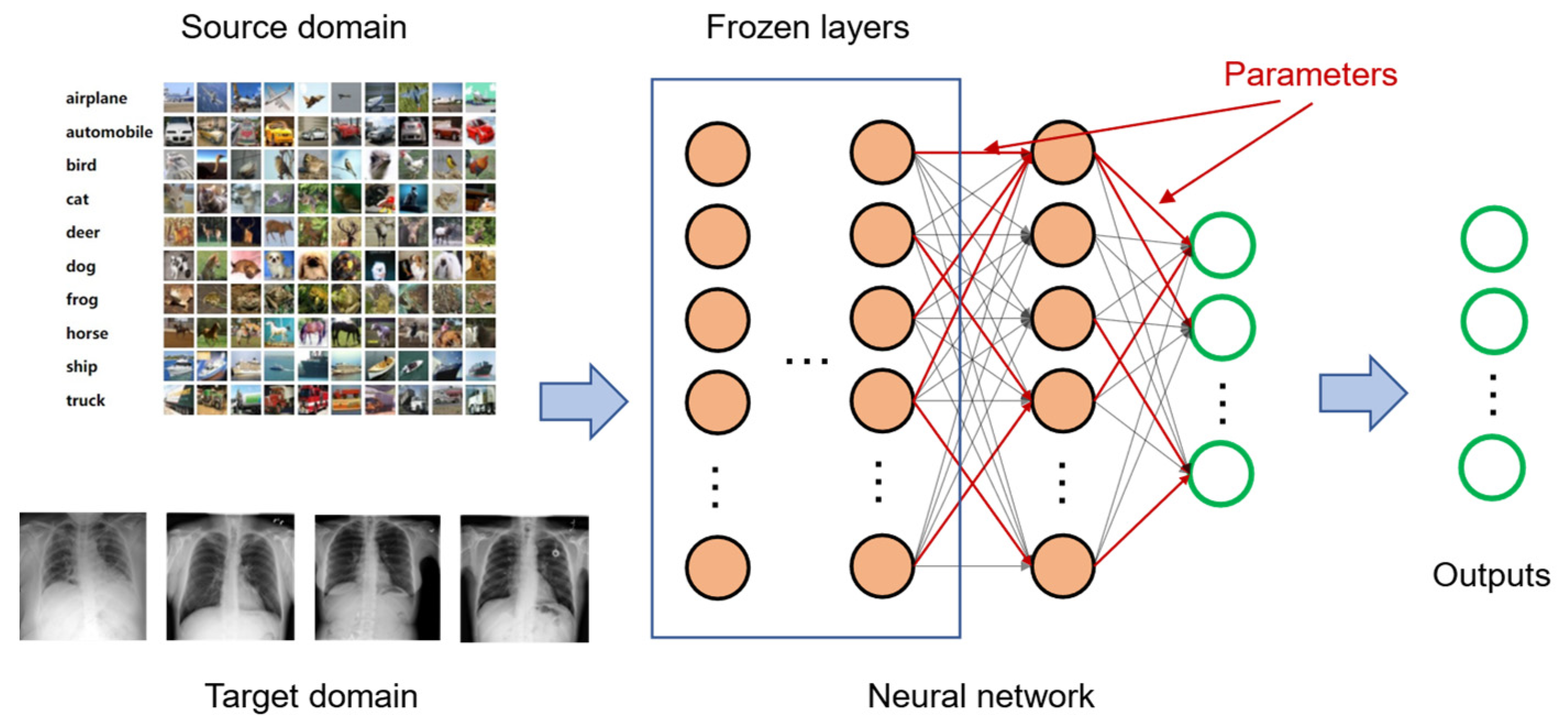
3.2. Transfer Learning
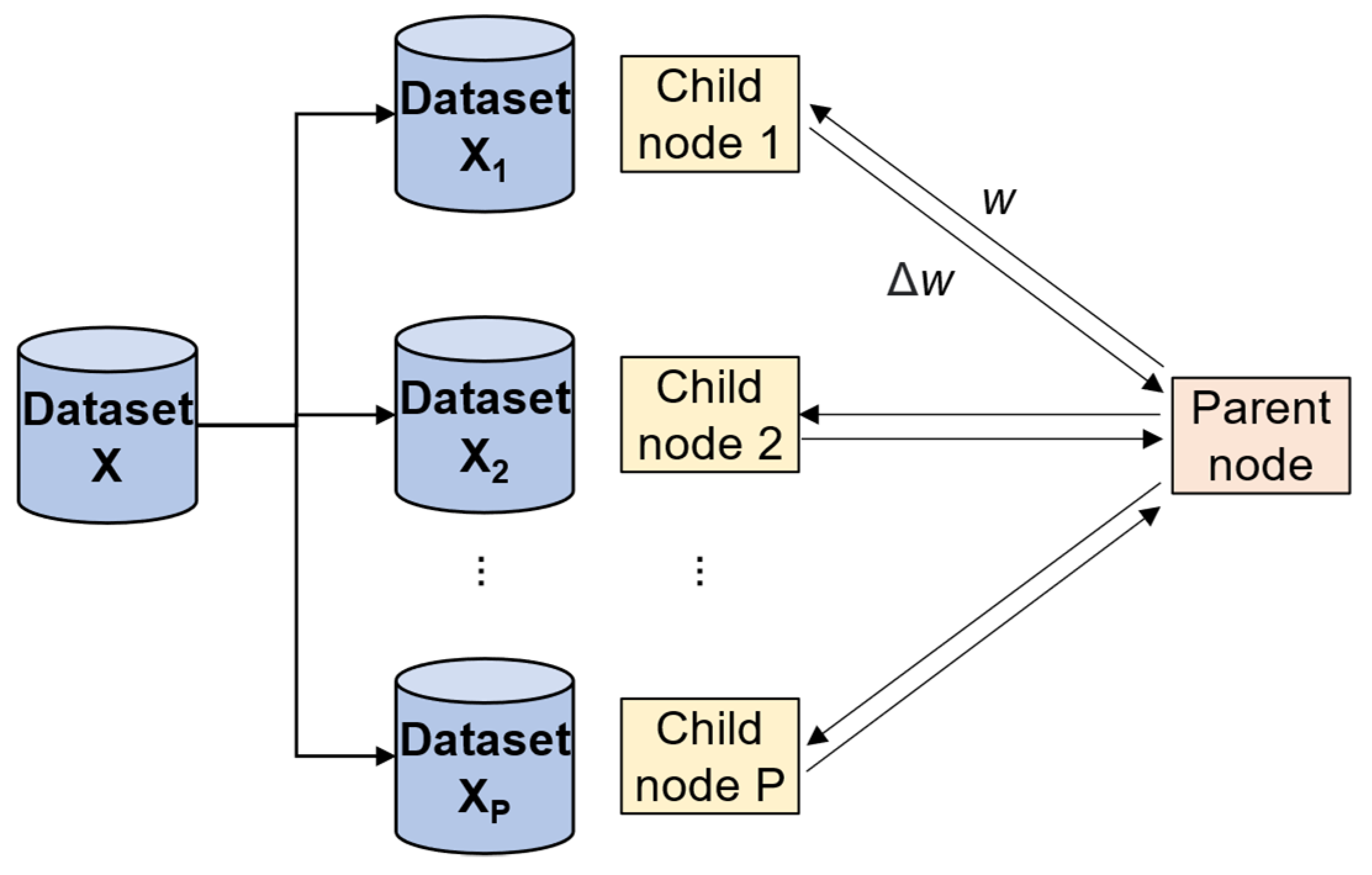
3.3. Parallel Computing
4. Experimental Environment
4.1. Performance Evaluation Metrics
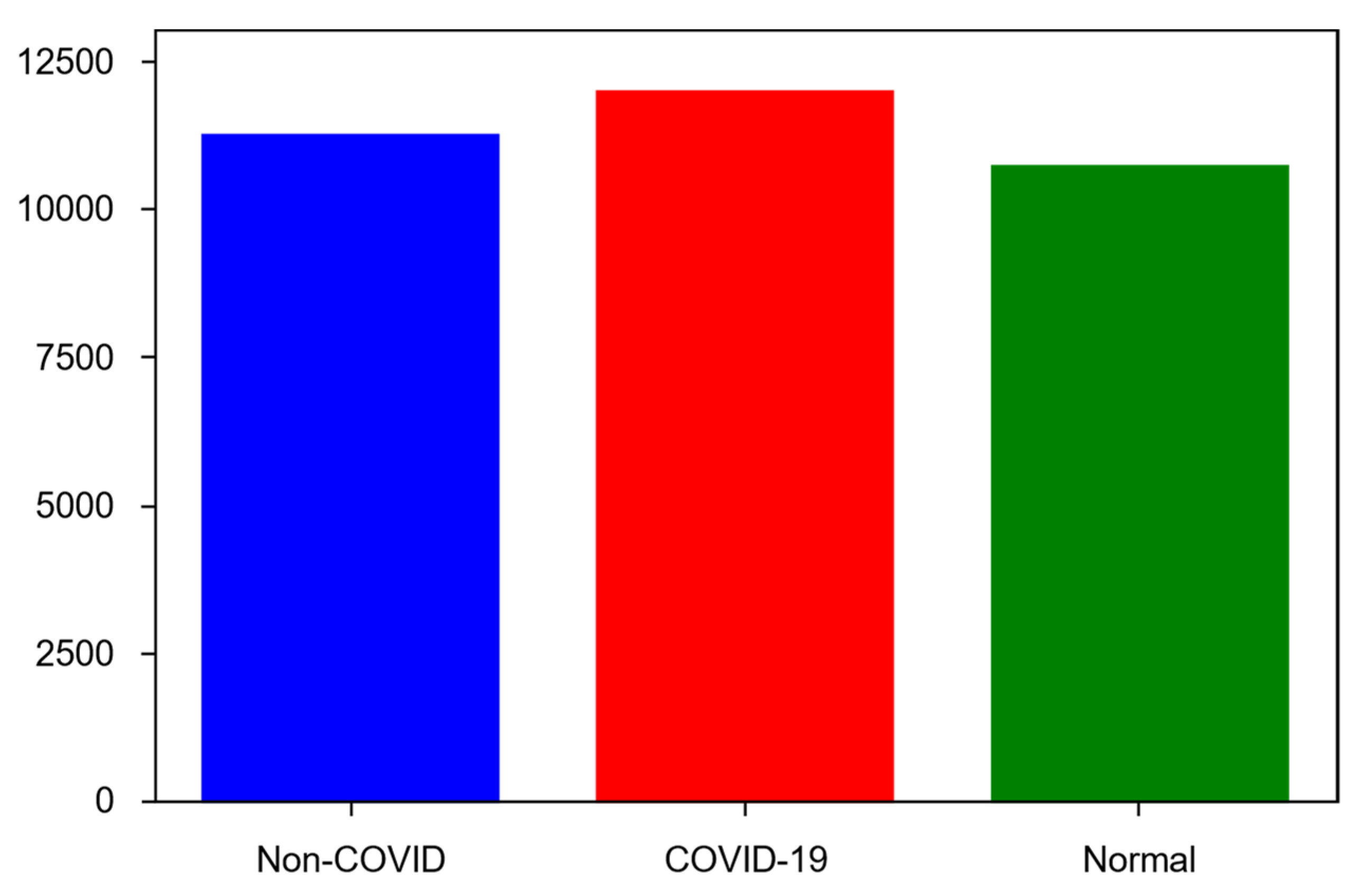
4.2. Dataset
- COVID-19—11,956 samples;
- Non-COVID infections (viral or bacterial pneumonia)—11,263 samples;
- Normal (uninfected)—10,701 samples.
4.3. Numerical Results
5. Comparison and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Worldwide Statistics. Available online: https://www.worldometers.info/coronavirus/ (accessed on 14 November 2022).
- Coronavirus and Pneumonia. Available online: https://www.webmd.com/lung/covid-and-pneumonia#1 (accessed on 14 November 2022).
- Gray, D.; Willemse, L.; Visagie, A.; Czövek, D.; Nduru, P.; Vanker, A.; Zar, H.J. Determinants of early-life lung function in African infants. Thorax 2017, 72, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Stern, D.A.; Guerra, S.; Wright, A.L.; Morgan, W.J.; Martinez, F.D. Pneumonia in childhood and impaired lung function in adults: A longitudinal study. Pediatrics 2015, 135, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Cecilie, S.; Sunyer, J.; Plana, E.; Dharmage, S.; Heinrich, J.; Jarvis, D.; de Marco, R. Early life origins of chronic obstructive pulmonary disease. Thorax 2010, 65, 14–20. [Google Scholar]
- Zar, H.J.; Andronikou, S.; Nicol, M.P. Advances in the diagnosis of pneumonia in children. BMJ 2017, 358, j2739. [Google Scholar] [CrossRef]
- Iuri, D.; De Candia, A.; Bazzocchi, M. Evaluation of the lung in children with suspected pneumonia: Usefulness of ultrasonography. La Radiol. Med. 2009, 114, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Tomà, P.; Owens, C.M. Chest ultrasound in children: Critical appraisal. Pediatr. Radiol. 2013, 43, 1427–1434. [Google Scholar] [CrossRef]
- Shah, V.P.; Tunik, M.G.; Tsung, J.W. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013, 167, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Gorycki, T.; Lasek, I.; Kamiński, K.; Studniarek, M. Evaluation of radiation doses delivered in different chest CT protocols. Pol. J. Radiol. 2014, 79, 1. [Google Scholar]
- Sodhi, K.S.; Khandelwal, N.; Saxena, A.K.; Singh, M.; Agarwal, R.; Bhatia, A.; Lee, E.Y. Rapid lung MRI in children with pulmonary infections: Time to change our diagnostic algorithms. J. Magn. Reson. Imaging 2016, 43, 1196–1206. [Google Scholar] [CrossRef]
- Biederer, J.; Mirsadraee, S.; Beer, M.; Molinari, F.; Hintze, C.; Bauman, G.; Puderbach, M. MRI of the lung (3/3)—Current applications and future perspectives. Insights Imaging 2012, 3, 373–386. [Google Scholar] [CrossRef]
- Hirsch, W.; Sorge, I.; Krohmer, S.; Weber, D.; Meier, K.; Till, H. MRI of the lungs in children. Eur. J. Radiol. 2008, 68, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Boiselle, P.M.; Biederer, J.; Gefter, W.B.; Lee, E.Y. Expert opinion: Why is MRI still an under-utilized modality for evaluating thoracic disorders? J. Thorac. Imaging 2013, 28, 137. [Google Scholar] [CrossRef] [PubMed]
- Aboutalib, S.S.; Mohamed, A.A.; Berg, W.A.; Zuley, M.L.; Sumkin, J.H.; Wu, S. Deep learning to distinguish recalled but benign mammography images in breast cancer screening. Clin. Cancer Res. 2018, 24, 5902–5909. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kim, H.E.; Han, K.; Kang, B.J.; Sohn, Y.M.; Woo, O.H.; Lee, C.W. Applying data-driven imaging biomarker in mammography for breast cancer screening: Preliminary study. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Shariaty, F.; Mousavi, M. Application of CAD systems for the automatic detection of lung nodules. Inform. Med. Unlocked 2019, 15, 100173. [Google Scholar] [CrossRef]
- Gu, Y.; Chi, J.; Liu, J.; Yang, L.; Zhang, B.; Yu, D.; Lu, X. A survey of computer-aided diagnosis of lung nodules from CT scans using deep learning. Comput. Biol. Med. 2021, 137, 104806. [Google Scholar] [CrossRef]
- Balyen, L.; Peto, T. Promising artificial intelligence-machine learning-deep learning algorithms in ophthalmology. Asia-Pac. J. Ophthalmol. 2019, 8, 264–272. [Google Scholar]
- Kumar, A.; Fulham, M.; Feng, D.; Kim, J. Co-learning feature fusion maps from PET-CT images of lung cancer. IEEE Trans. Med. Imaging 2019, 39, 204–217. [Google Scholar] [CrossRef]
- Podnar, S.; Kukar, M.; Gunčar, G.; Notar, M.; Gošnjak, N.; Notar, M. Diagnosing brain tumours by routine blood tests using machine learning. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Ibrokhimov, B.; Kang, J.Y. Two-Stage Deep Learning Method for Breast Cancer Detection Using High-Resolution Mammogram Images. Appl. Sci. 2022, 12, 4616. [Google Scholar] [CrossRef]
- Roy, S.; Meena, T.; Lim, S.J. Demystifying Supervised Learning in Healthcare 4.0: A New Reality of Transforming Diagnostic Medicine. Diagnostics 2022, 12, 2549. [Google Scholar] [CrossRef] [PubMed]
- Meena, T.; Roy, S. Bone fracture detection using deep supervised learning from radiological images: A paradigm shift. Diagnostics 2022, 12, 2420. [Google Scholar] [CrossRef]
- Pal, D.; Reddy, P.B.; Roy, S. Attention UW-Net: A fully connected model for automatic segmentation and annotation of chest X-ray. Comput. Biol. Med. 2022, 150, 106083. [Google Scholar] [CrossRef]
- Gunjan, V.K.; Singh, N.; Shaik, F.; Roy, S. Detection of lung cancer in CT scans using grey wolf optimization algorithm and recurrent neural network. Health Technol. 2022, 12, 1197–1210. [Google Scholar] [CrossRef]
- Gangopadhyay, T.; Halder, S.; Dasgupta, P.; Chatterjee, K.; Ganguly, D.; Sarkar, S.; Roy, S. MTSE U-Net: An architecture for segmentation, and prediction of fetal brain and gestational age from MRI of brain. Netw. Model. Anal. Health Inform. Bioinform. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Tomaszewski, M.R.; Gillies, R.J. The biological meaning of radiomic features. Radiology 2021, 298, 505–516. [Google Scholar] [CrossRef]
- Mediouni, M.; Madiouni, R.; Gardner, M.; Vaughan, N. Translational medicine: Challenges and new orthopaedic vision (Mediouni-Model). Curr. Orthop. Pract. 2020, 31, 196–200. [Google Scholar] [CrossRef]
- Mediouni, M.; RSchlatterer, D.; Madry, H.; Cucchiarini, M.; Rai, B. A review of translational medicine. The future paradigm: How can we connect the orthopedic dots better? Curr. Med. Res. Opin. 2018, 34, 1217–1229. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Lång, K.; Gubern-Merida, A.; Broeders, M.; Gennaro, G.; Clauser, P.; Sechopoulos, I. Stand-alone artificial intelligence for breast cancer detection in mammography: Comparison with 101 radiologists. JNCI J. Natl. Cancer Inst. 2019, 111, 916–922. [Google Scholar] [CrossRef]
- Shen, L.; Margolies, L.R.; Rothstein, J.H.; Fluder, E.; McBride, R.; Sieh, W. Deep learning to improve breast cancer detection on screening mammography. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Latif, S.; Usman, M.; Manzoor, S.; Iqbal, W.; Qadir, J.; Tyson, G.; Crowcroft, J. Leveraging data science to combat COVID-19: A comprehensive review. IEEE Trans. Artif. Intell. 2020, 1, 85–103. [Google Scholar] [CrossRef]
- Swapnarekha, H.; Behera, H.S.; Nayak, J.; Naik, B. Role of intelligent computing in COVID-19 prognosis: A state-of-the-art review. Chaos Solitons Fractals 2020, 138, 109947. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Aslam, N. A deep-learning-based framework for automated diagnosis of COVID-19 using X-ray images. Information 2020, 11, 419. [Google Scholar] [CrossRef]
- Brima, Y.; Atemkeng, M.; Tankio Djiokap, S.; Ebiele, J.; Tchakounté, F. Transfer Learning for the Detection and Diagnosis of Types of Pneumonia including Pneumonia Induced by COVID-19 from Chest X-ray Images. Diagnostics 2021, 11, 1480. [Google Scholar] [CrossRef]
- Narin, A.; Kaya, C.; Pamuk, Z. Automatic detection of coronavirus disease (covid-19) using x-ray images and deep convolutional neural networks. Pattern Anal. Appl. 2021, 24, 1207–1220. [Google Scholar] [CrossRef]
- Minaee, S.; Kafieh, R.; Sonka, M.; Yazdani, S.; Soufi, G.J. Deep-COVID: Predicting COVID-19 from chest X-ray images using deep transfer learning. Med. Image Anal. 2020, 65, 101794. [Google Scholar] [CrossRef]
- Hemdan EE, D.; Shouman, M.A.; Karar, M.E. Covidx-net: A framework of deep learning classifiers to diagnose covid-19 in x-ray images. arXiv 2020, arXiv:2003.11055. [Google Scholar]
- Sethy, P.K.; Behera, S.K. Detection of Coronavirus Disease (COVID-19) Based on Deep Features; Preprints 2020, 2020030300. Available online: https://www.preprints.org/manuscript/202003.0300/v1 (accessed on 13 November 2022).
- Oh, Y.; Park, S.; Ye, J.C. Deep learning COVID-19 features on CXR using limited training data sets. IEEE Trans. Med. Imaging 2020, 39, 2688–2700. [Google Scholar] [CrossRef]
- Apostolopoulos, I.D.; Mpesiana, T.A. Covid-19: Automatic detection from x-ray images utilizing transfer learning with convolutional neural networks. Phys. Eng. Sci. Med. 2020, 43, 635–640. [Google Scholar] [CrossRef]
- Ozturk, T.; Talo, M.; Yildirim, E.A.; Baloglu, U.B.; Yildirim, O.; Acharya, U.R. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput. Biol. Med. 2020, 121, 103792. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID-19 vaccine race: Challenges and opportunities in vaccine formulation. AAPS PharmSciTech 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.N.; Hardie, R.C.; Krishnaraja, V.; Karam, C.; Davuluru VS, P. Transfer-to-transfer learning approach for computer aided detection of COVID-19 in chest radiographs. AI 2020, 1, 539–557. [Google Scholar] [CrossRef]
- Tahir, A.M.; Chowdhury, M.E.; Khandakar, A.; Rahman, T.; Qiblawey, Y.; Khurshid, U.; Hamid, T. COVID-19 infection localization and severity grading from chest X-ray images. Comput. Biol. Med. 2021, 139, 105002. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Zhao, L.; Zhou, Y.; Lu, H.; Fujita, H. Parallel computing method of deep belief networks and its application to traffic flow prediction. Knowl. -Based Syst. 2019, 163, 972–987. [Google Scholar] [CrossRef]

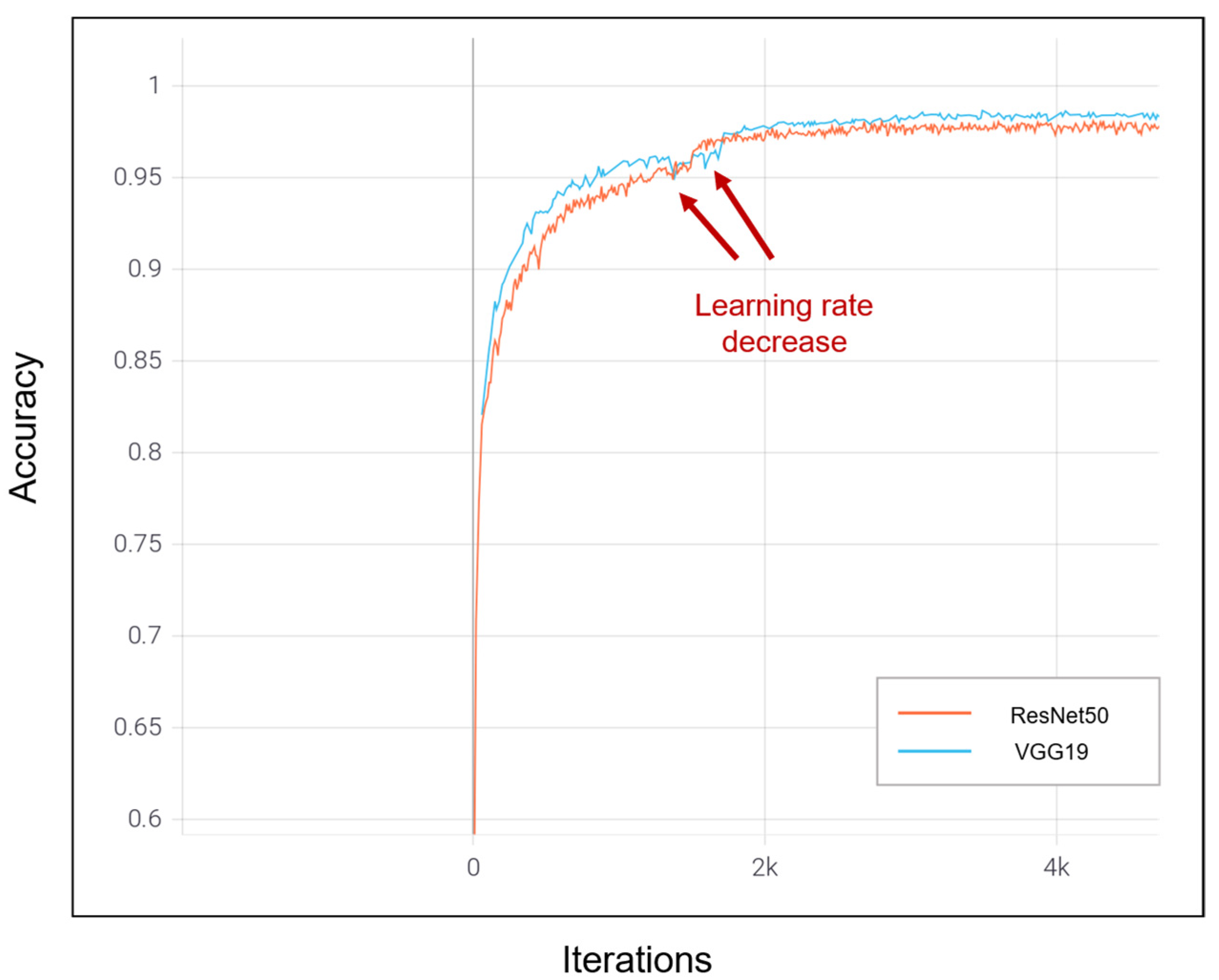
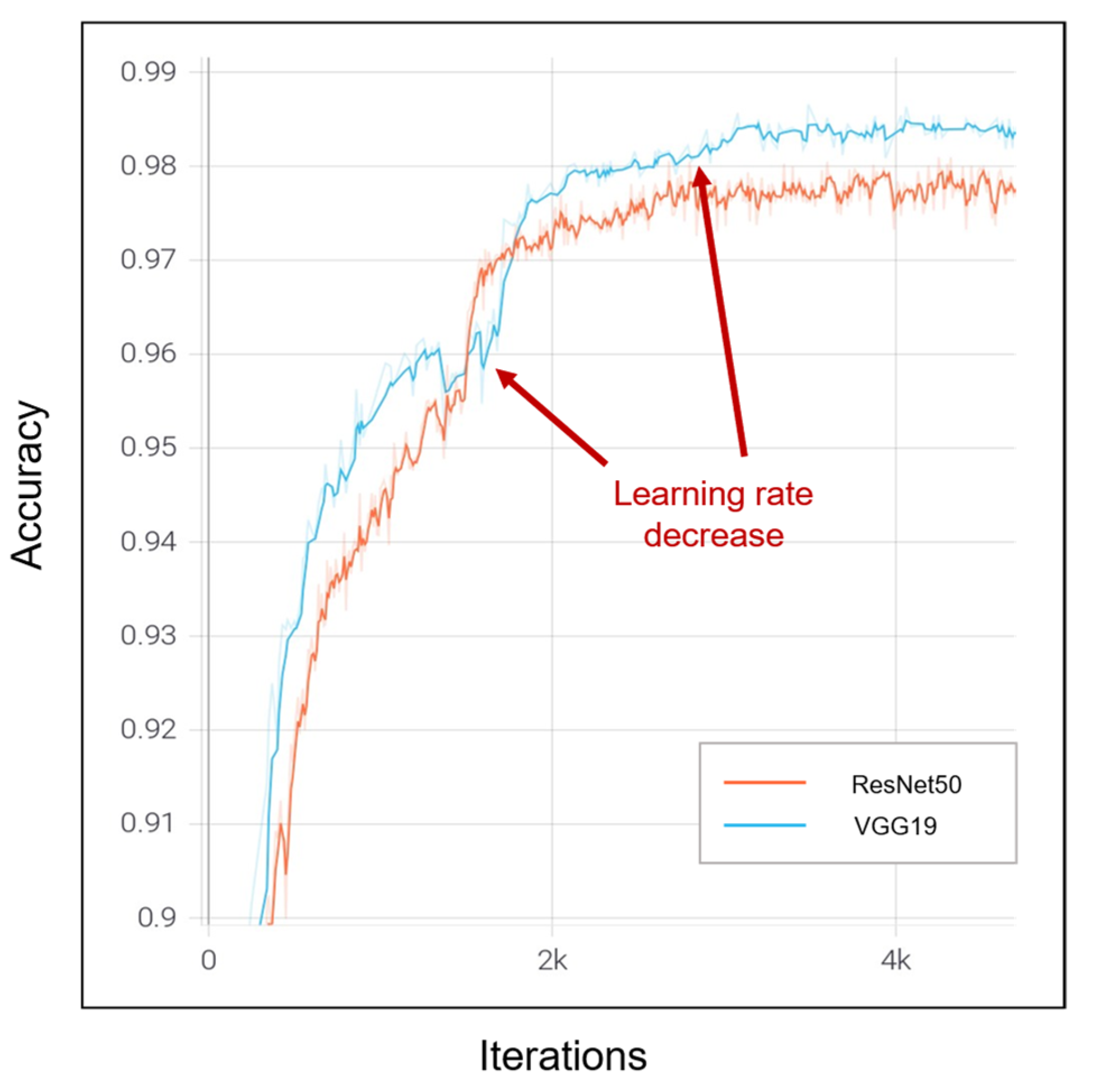
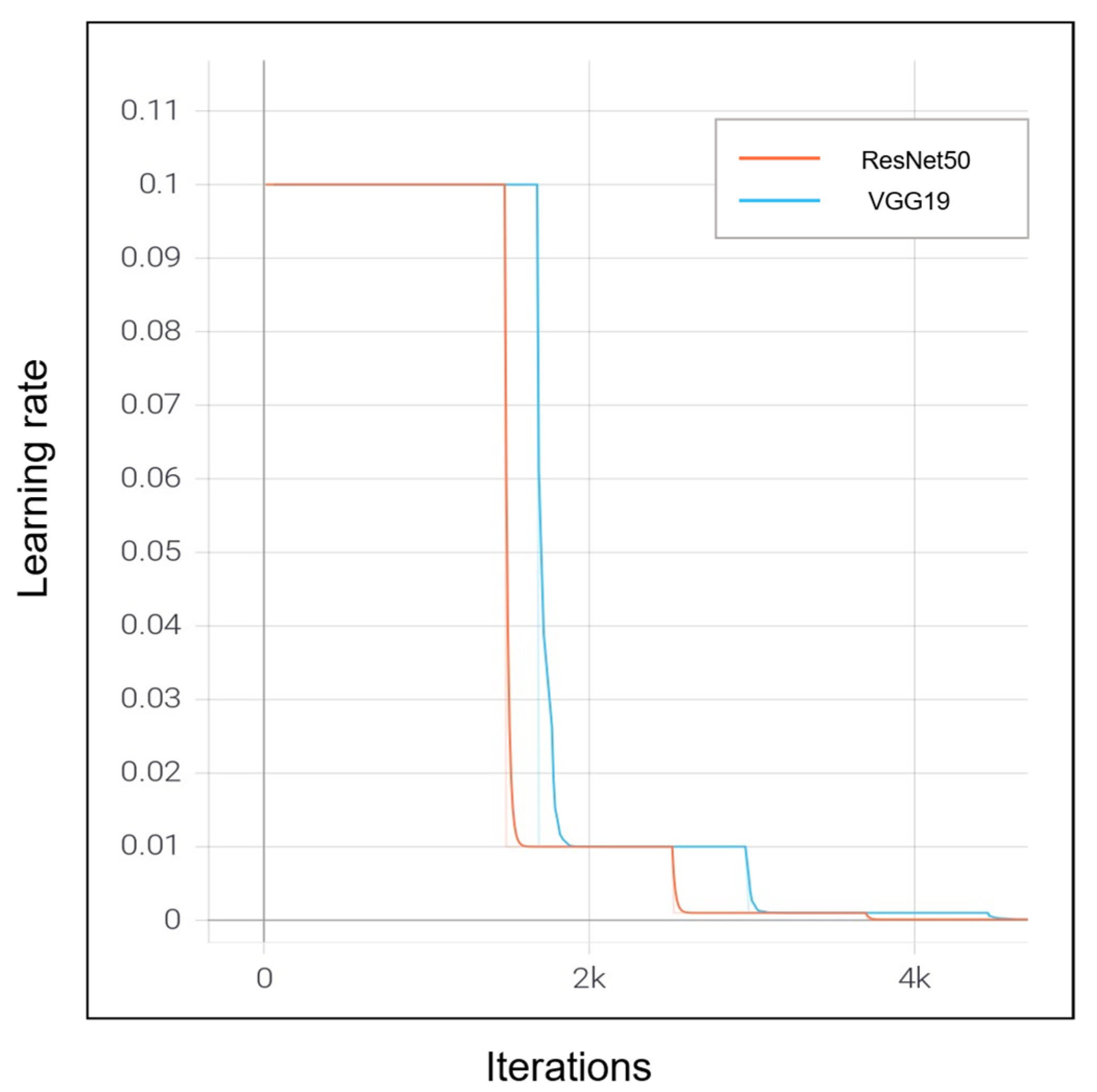
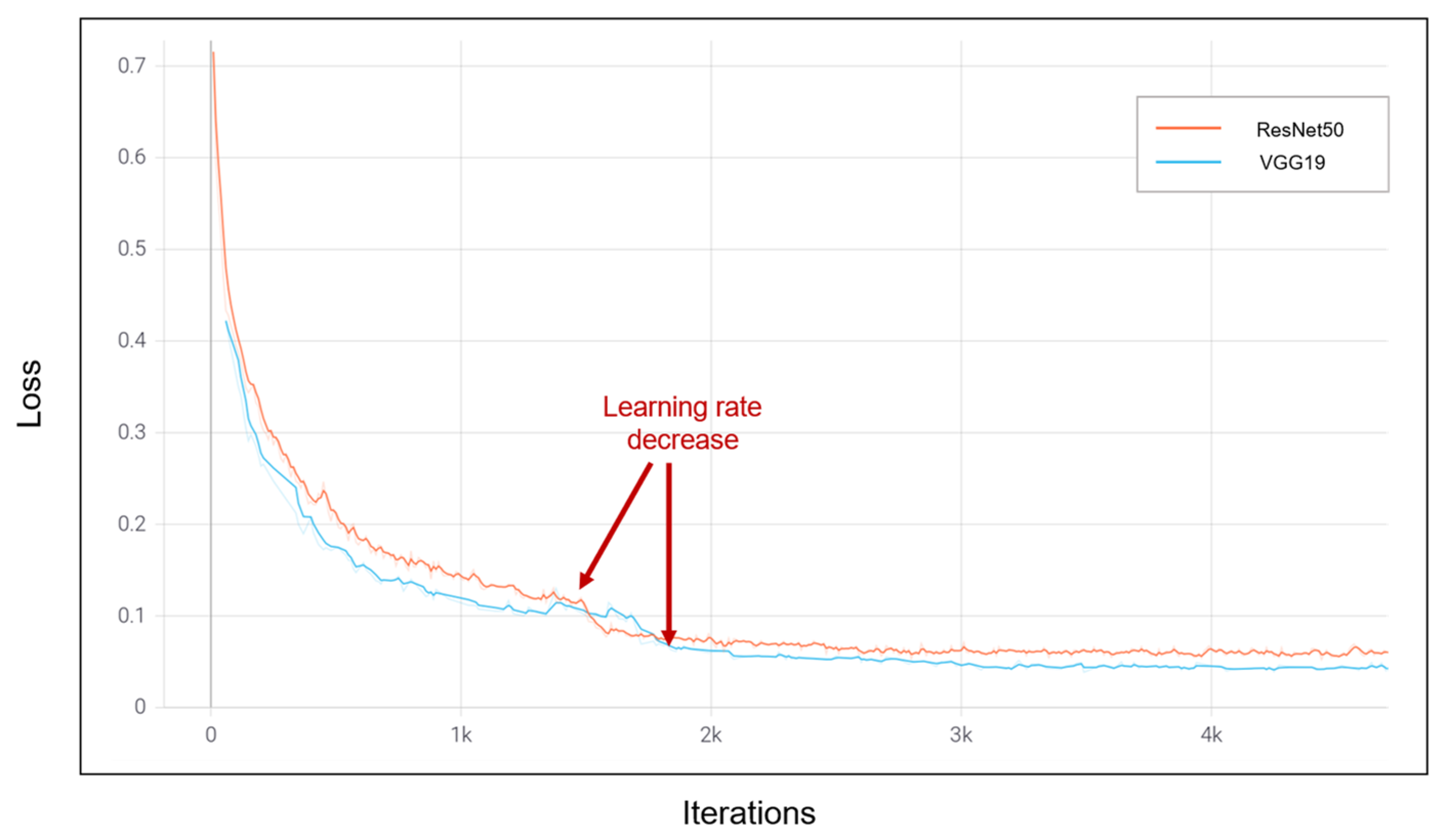

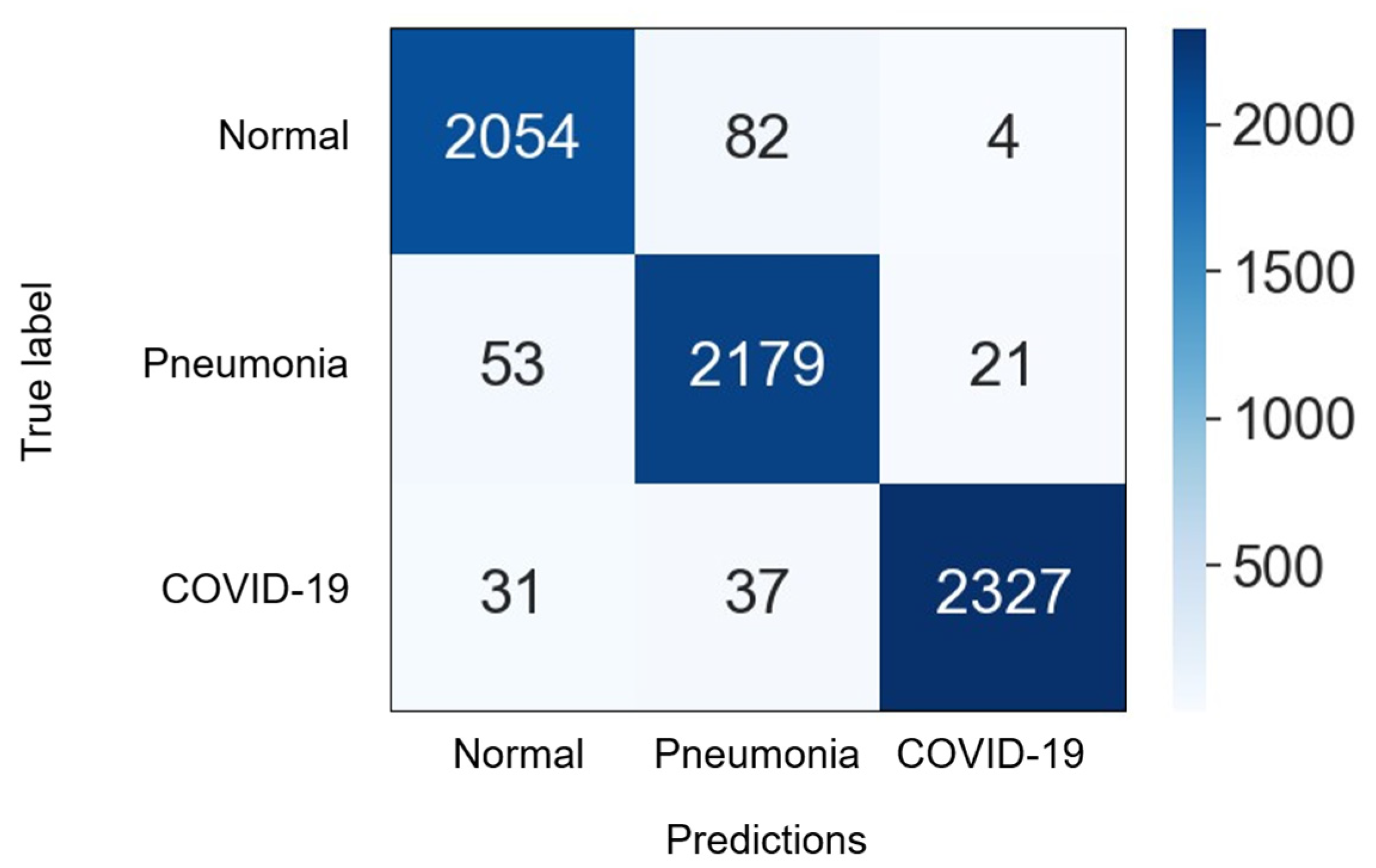
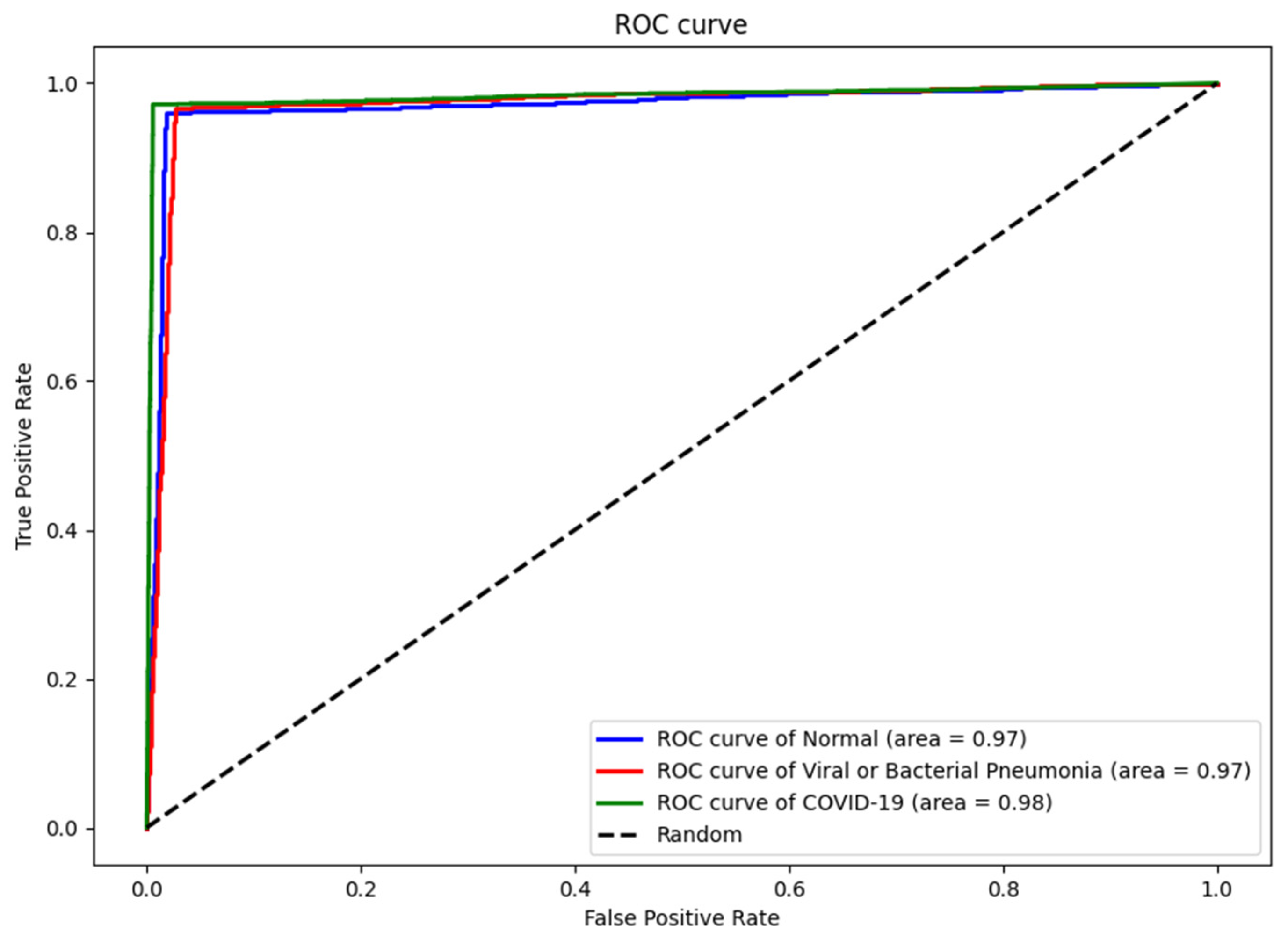
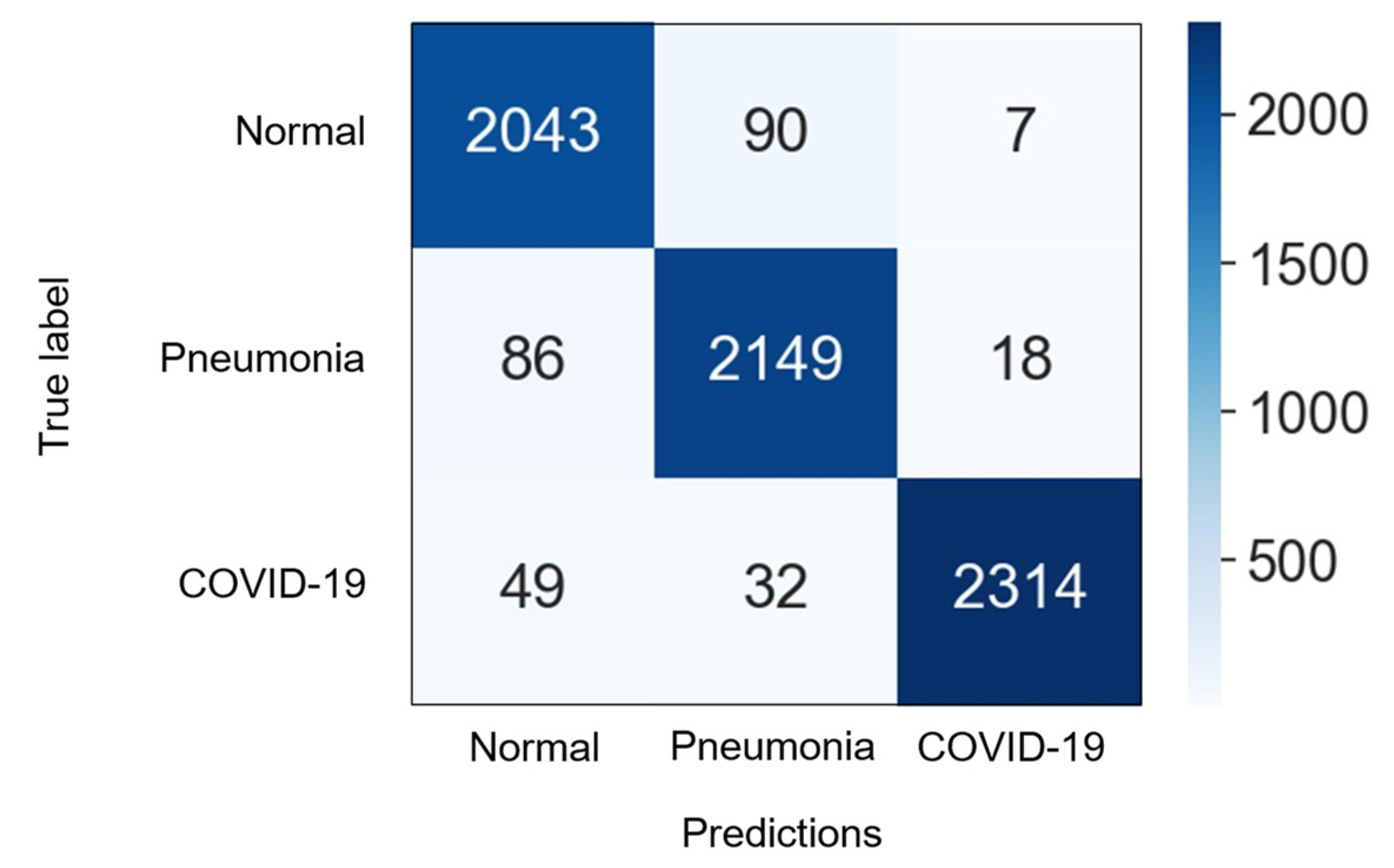
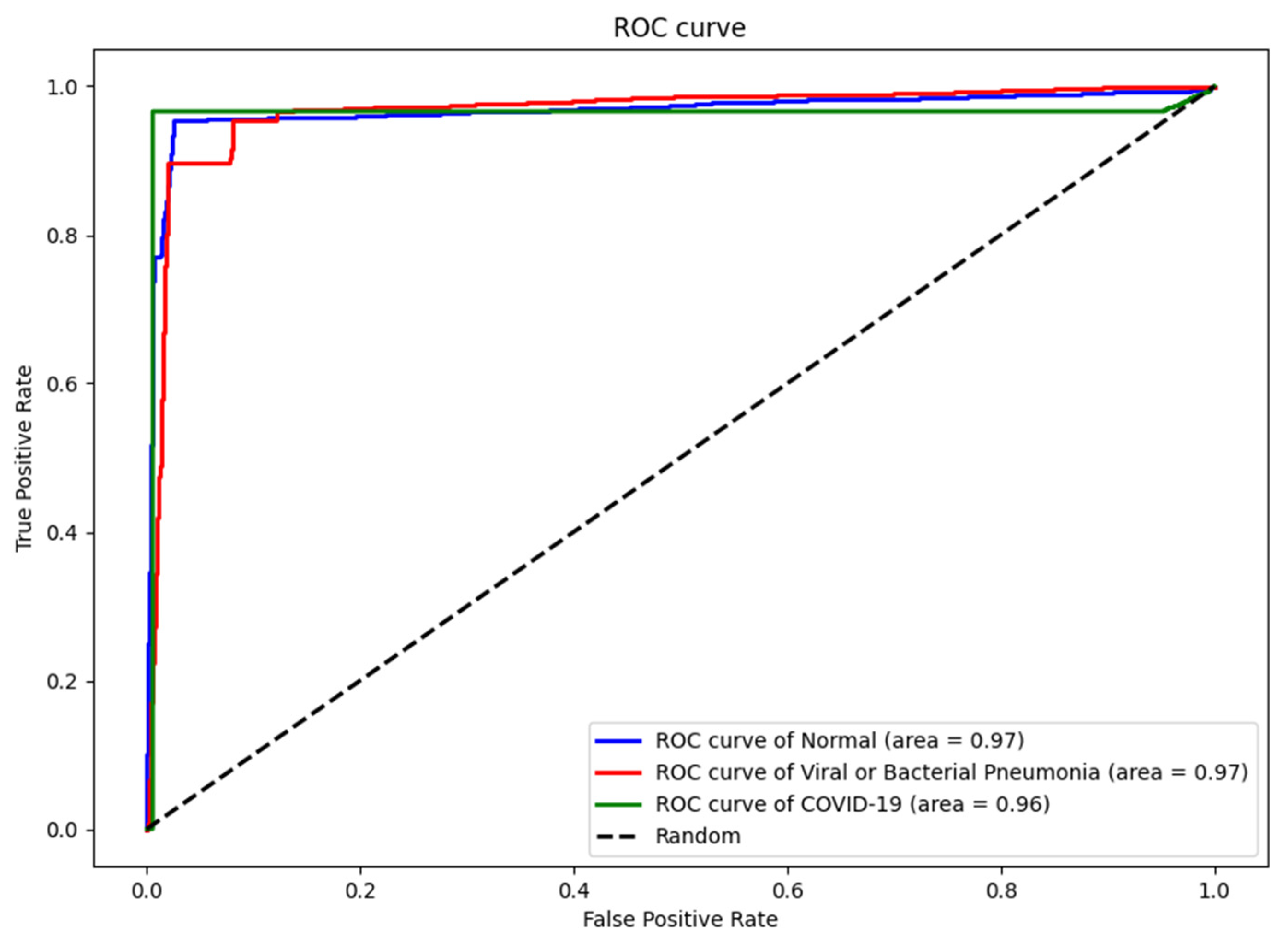
| Dataset | Normal | Non-COVID Infections | COVID-19 | Total |
|---|---|---|---|---|
| Train | 6849 | 7208 | 7658 | 21,715 |
| Validation | 1712 | 1802 | 1903 | 5417 |
| Test | 2140 | 2253 | 2395 | 6788 |
| Study | Dataset | Method | Accuracy |
|---|---|---|---|
| Oh et al. [42] | 191 Normal 74 Pneumonia 57 Tuberculosis 180 COVID-19 | ResNet18 | 88.9% |
| Ozturk et al. [44] | 1000 Normal 500 Pneumonia 125 COVID-19 | DarkCovidNet | 87% |
| Wang et al. [45] | 8066 Normal 5538 Pneumonia 358 COVID-19 | COVIDNet, VGG19, ResNet50 | 93.3% |
| Narayanan et al. [46] | 1583 Normal 1493 Viral Pneumonia 2780 Bacterial Pneumonia | ResNet50, Inceptionv3, Xception, DenseNet201 | 98% |
| Apostolopoulos et al. [43] | 504 Normal 714 Pneumonia 224 COVID-19 | VGG19, Inception, Xception, MobileNet | 98.75% |
| Brima et al. [37] | 10,192 Normal 1345 Pneumonia 6012 Lung opacity 3616 COVID-19 | VGG19, DenseNet121, ResNet50 | 94% |
| Khan et al. [36] | 802 Normal 790 COVID-19 | VGG16, VGG19 | 99.38% |
| Our method | 10,701 Normal 11,263 Pneumonia 11,956 COVID-19 | VGG19, ResNet50 | 96.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrokhimov, B.; Kang, J.-Y. Deep Learning Model for COVID-19-Infected Pneumonia Diagnosis Using Chest Radiography Images. BioMedInformatics 2022, 2, 654-670. https://doi.org/10.3390/biomedinformatics2040043
Ibrokhimov B, Kang J-Y. Deep Learning Model for COVID-19-Infected Pneumonia Diagnosis Using Chest Radiography Images. BioMedInformatics. 2022; 2(4):654-670. https://doi.org/10.3390/biomedinformatics2040043
Chicago/Turabian StyleIbrokhimov, Bunyodbek, and Justin-Youngwook Kang. 2022. "Deep Learning Model for COVID-19-Infected Pneumonia Diagnosis Using Chest Radiography Images" BioMedInformatics 2, no. 4: 654-670. https://doi.org/10.3390/biomedinformatics2040043
APA StyleIbrokhimov, B., & Kang, J.-Y. (2022). Deep Learning Model for COVID-19-Infected Pneumonia Diagnosis Using Chest Radiography Images. BioMedInformatics, 2(4), 654-670. https://doi.org/10.3390/biomedinformatics2040043






