Abstract
Hans Selye’s stress concept, first introduced in the 1930s, has undergone substantial evolution, extending beyond biology and medicine to influence diverse academic disciplines. Initially, Selye’s General Adaptation Syndrome (GAS) described nonspecific physiological responses to stressors exclusively in mammals, without addressing other biological systems. Consequently, the concept of stress developed independently in biology and medicine, shaped by distinct physiological contexts. This review provides a historical overview of stress research, highlights both parallels and divergences between the stress responses of plants and animals, and integrates insights from traditional Eastern philosophies. We propose an updated GAS framework that incorporates the dynamic balance among reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) within the broader context of oxidative stress. We highlight the ionotropic glutamate receptor (iGluR) family and the transient receptor potential (TRP) channel superfamily as minimal molecular architectures for achieving GAS. This perspective expands the classical stress paradigm, providing new insights into redox biology, interspecies stress adaptation, and evolutionary physiology.
1. Introduction
In contemporary usage, the term “stress” extends far beyond its linguistic origins, encompassing diverse contexts such as biology, physics, physiology, psychology, materials science, engineering, sociology, and other fields. In biology and medicine, stress typically refers to physiological or psychological responses to challenging stimuli. In physics and engineering, stress denotes the internal forces per unit area within a material that arise in response to external loads or deformations. In sociology and economics, stress captures societal pressures and financial strains that affect communities and individuals.
The term’s adaptability underscores its interdisciplinary importance, but its broad use across fields has led to conceptual divergence. The widespread use of the same term across different fields has led to a divergence in its meaning, often resulting in confusion and misunderstandings—especially in interdisciplinary communication, which is essential for integrating knowledge. This challenge is particularly evident in the life sciences, where the development and expansion of stress-related studies highlight the need for greater conceptual clarity and consistency.
Hans Selye, regarded as the father of stress research, introduced the term “stress” [1] to explain the General Adaptation Syndrome (GAS) in human physiology in the context of disease [2]. Since its inception, the concept of stress has been widely applied across various medical science disciplines, including cell biology [3], physiology [4], endocrinology [5], neurology [6], and psychology [7,8]. Selye defined stress as “a nonspecific response of the body to a demand” [9], and proposed that all mammals undergo a predictable sequence of reactions to stress: alarm, resistance, and exhaustion [10]. However, Selye initially presumed that stress responses require a nervous system, excluding non-animals (e.g., plants and microorganisms) from his framework. This assumption led to the independent evolution of stress concepts in medical, plant, and microbial sciences.
Recent advances in molecular biology and biochemistry have challenged this view, revealing that stress responses are not exclusive to organisms with nervous systems. In plants, microbes, and even single-celled microorganisms, stress responses involve dynamic biochemical processes, particularly those mediated by reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) [11]. The discovery of these reactive molecules has redefined oxidative stress [12] as a critical concept of cellular adaptation extending beyond mere damage to include essential regulatory functions in signaling pathways [13].
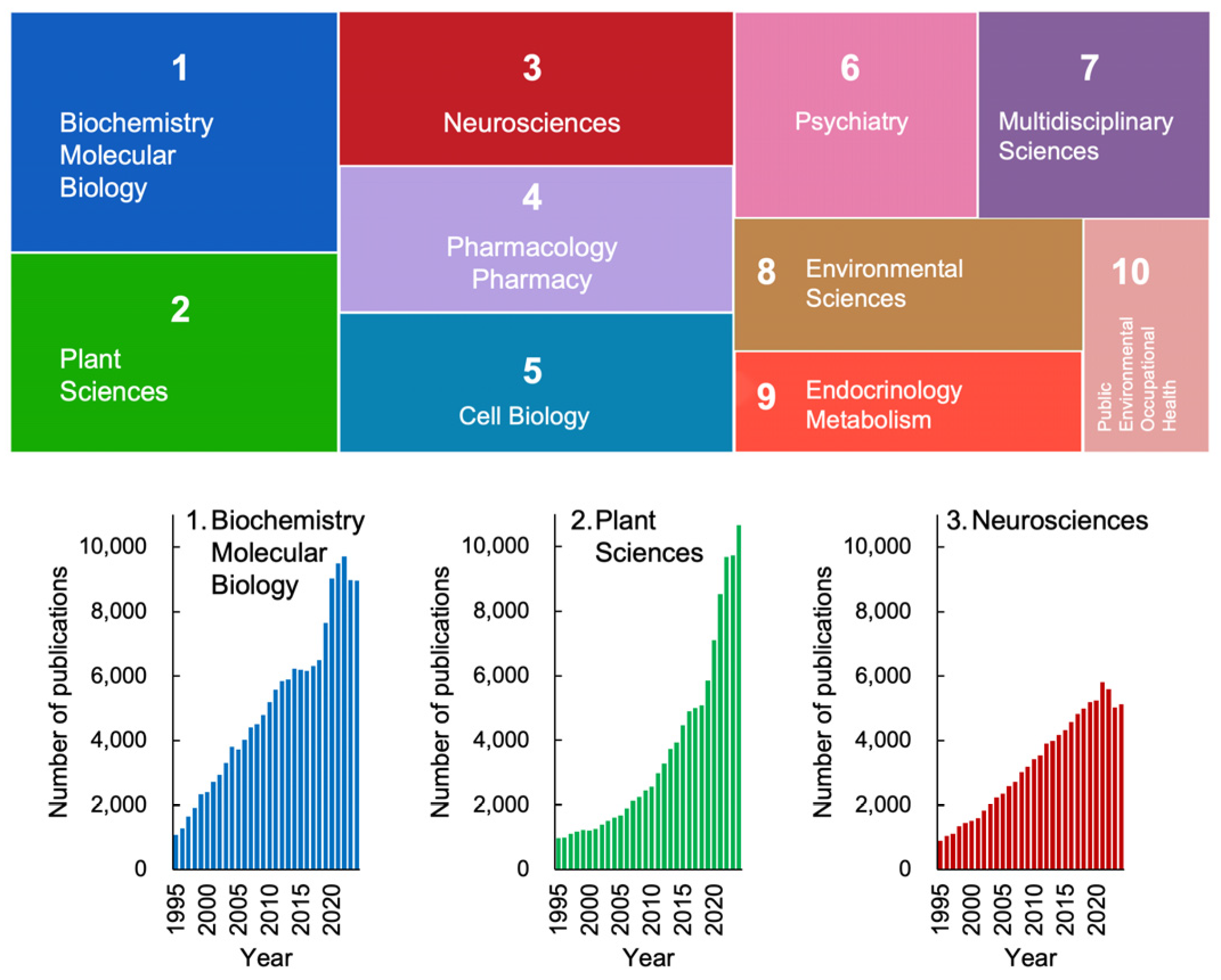
Figure 1 depicts the research trends in stress studies, excluding physics, engineering, and other fields irrelevant to life sciences, over the past 30 years. The analysis was conducted using the Web of Science database and is based on the number of publications. Articles addressing “stress” span a wide range of disciplines, even within the life sciences alone (Figure 1). Among these fields, biochemistry and molecular biology, plant sciences, and neurosciences are the top three categories in the Web of Science database.
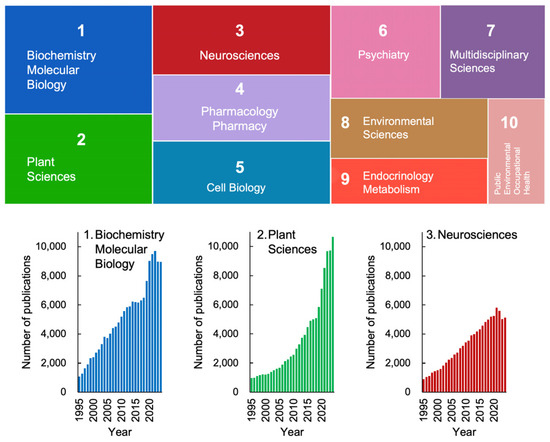
Figure 1.
Trends in stress studies in life sciences over the past 30 years. Based on annual publication numbers recorded in the Web of Science database, the number of publications (including original papers and reviews) was retrieved using “stress” as a keyword for topics. The colored boxes represent the top 10 science categories in the Web of Science. The area of each box corresponds to its proportion among these ten categories. The lower graphs show the changes in publication numbers from 1995 to 2024. Science categories not directly relevant to life sciences, such as materials science, physics, and economics, were excluded from the analysis. These trends highlight the increasing relevance of plant stress research, underscoring the need to expand Selye’s framework beyond mammalian models.
Notably, there has been a recent exponential increase in publications related to plant sciences, indicating that plant stress research is emerging as a significant trend in contemporary science (Figure 1, lower panel). This growth is particularly striking given that Hans Selye, who introduced the concept of stress, initially excluded plants from his framework. Nevertheless, it is now evident that plant sciences have become a key focus in the study of stress.
It is evident that Selye’s concept of stress has undergone significant evolution, incorporating new findings and adapting much like living organisms. This evolution has led to its diversification and the development of varied definitions across academic disciplines. Since Selye’s initial introduction, numerous refinements have been made to the discussions surrounding the concept and definition of stress. Consequently, the definitions of “stress” and “stress response” have diverged across research fields. As of 2020, more than 200 hypotheses or models addressing “stress” have been published across various disciplines [14].
Selye’s stress theory was originally developed within the context of medical science and mammalian physiology, with a primary focus on hormonal responses [10]. Therefore, adapting this concept to non-mammals, plants, and microorganisms necessitates further modifications and updates.
By reviewing the key cornerstones and the many twists and turns in the history of stress biology and medicine, we identified two critical keywords for discussion in this review: “nonspecific response” and “balance and imbalance”. The concept of a “nonspecific response” to any kind of stimulus lies at the core of Selye’s stress theory. However, to date, there is no generalized framework that fully updates his concept. The fundamental questions remain unresolved:
- (1)
- What molecular mechanisms enable a nonspecific response to diverse stressors?
- (2)
- How can the same stressor lead to two opposing outcomes—stress adaptation or stress-induced damage?
To achieve a holistic understanding of stress responses, here we propose that the multi-sensitivity of ionotropic glutamate receptors (iGluRs)—including N-methyl-D-aspartate receptors (NMDARs) in animals and glutamate-receptor-like channels (GLRs) in plants—and the transient receptor potential (TRP) channel superfamily possess the minimal molecular architectures for achieving GAS.
Beyond its physiological significance, stress embodies a fundamental principle of balance—a concept deeply embedded in Eastern philosophies, such as Yin–Yang in Traditional Chinese Medicine and Mitsudomoe in Japanese culture. These philosophical frameworks emphasize the coexistence of opposing forces, reflecting the dual role of stress in both adaptation (eustress) and dysfunction (distress).
To advance a comprehensive understanding of stress biology and medicine, we discuss how Selye’s GAS concept aligns with traditional Eastern philosophies culturally inherited in China, India, Korea, and Japan, offering valuable insights that may guide future research directions.
2. Stress and Strain in Physics and Engineering: A Foundational Perspective
Before delving into Selye’s concept of stress and the General Adaptation Syndrome, it is valuable to consider how the term “stress” is used in physics and engineering. The principles of stress and strain, which describe how materials respond to external forces, provide a useful analogy for understanding biological stress responses.
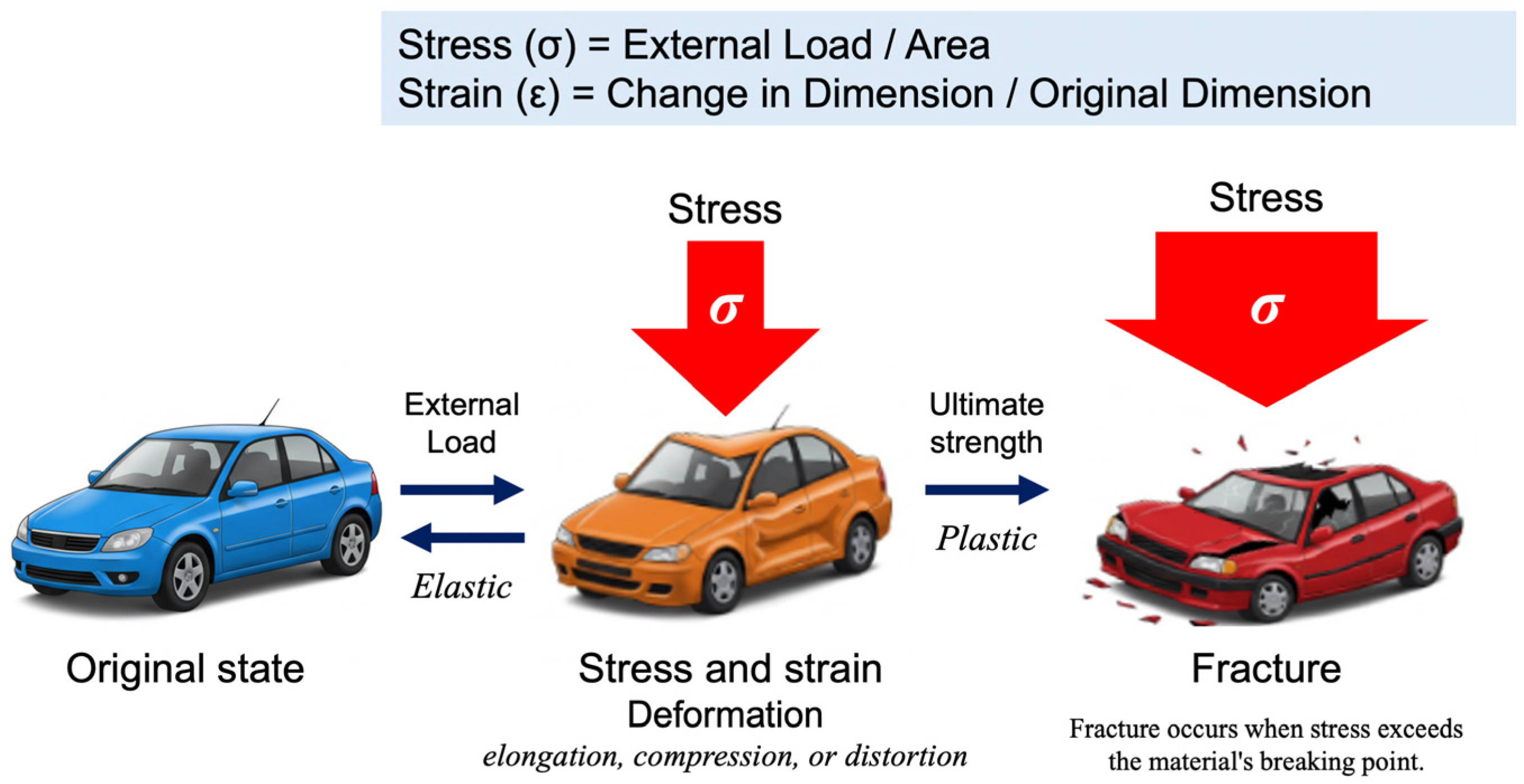
In physics and engineering, stress (σ) is defined as the internal force per unit area within a material subjected to an external load, whereas strain (ε) represents the relative deformation of the material in response to stress (Figure 2). This relationship is commonly depicted using a stress–strain curve illustrating the key phases of material behavior, including elastic deformation, plastic deformation, and eventual failure.
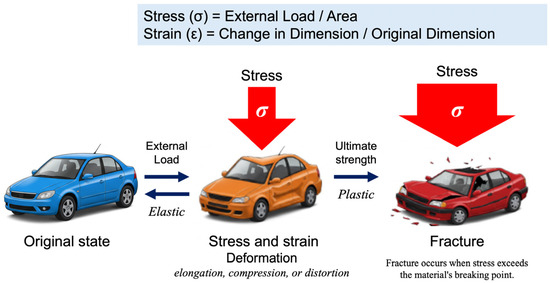
Figure 2.
The fundamental relationship between stress and strain, first formalized through Hooke’s Law in 1660, remains a cornerstone of materials science and engineering. When materials are subjected to external forces, their deformation behavior provides critical insights into their mechanical properties and structural integrity. Stress (σ) is defined as the force applied per unit area (σ = load/area), while strain (ε) represents the relative deformation, expressed as the ratio of the change in dimension to the original dimension (ϵ = change in dimension/original dimension). These fundamental parameters form the basis of the stress–strain curve, which characterizes a material’s mechanical response. If a material returns to its original shape upon removal of stress, the deformation is classified as elastic. In contrast, irreversible deformation or fracture indicates plastic behavior.
This fundamental concept—in which external forces induce measurable changes in a system—has expanded beyond its origins in physics to inform our understanding of cellular and physiological adaptations. Recent advances in biomaterials and tissue engineering have revealed remarkable parallels between engineered materials and biological systems, particularly regarding their capacity to sense, respond, and adapt to environmental pressures. These discoveries have deepened our understanding of cellular mechanotransduction [15,16] and tissue remodeling processes [17], significantly contributing to advancements in regenerative medicine and bioengineering applications [18].
Although stress–strain relationships in physics offer useful analogies, biological stress includes biochemical, molecular, and systemic adaptations that extend beyond mechanical deformation. Nevertheless, the stress–strain framework remains a valuable model for exploring biological materials, including plant structures such as wood [19], providing essential insights into the mechanical properties of living organisms.
3. Cannon’s Homeostasis: Introducing the Principle of Balance to Life Sciences
This review synthesizes a conceptual framework built upon two foundational concepts: Hans Selye’s notion of stress and Walter Bradford Cannon’s principle of homeostasis. Walter Bradford Cannon (1871–1945), a distinguished physiologist, is renowned for his groundbreaking contributions to our understanding of the autonomic nervous system and for establishing the principle of homeostasis [20,21]. His research demonstrated that organisms maintain internal stability through complex regulatory networks, emphasizing a holistic rather than a reductionist approach. Cannon also introduced the term “fight or flight response”, describing the physiological changes that prepare organisms to confront or escape perceived threats [22].
In his seminal work The Wisdom of the Body (1932) [23], Cannon coined the term “homeostasis” to describe the coordinated physiological processes that sustain steady states within organisms, which is distinct from equilibrium in the physical sense. This concept built upon Claude Bernard’s earlier notion of the “milieu intérieur” (internal environment) [21].
Through extensive experimental research conducted at Harvard Medical School, Cannon demonstrated that survival depends on maintaining internal stability via intricate regulatory mechanisms [20]. He showed that multiple physiological systems act in concert to regulate critical parameters—such as blood glucose levels, oxygen concentration, blood pressure, and body temperature—keeping them within narrow physiological ranges [24].
Cannon’s work was revolutionary for the life sciences, providing a unifying principle that explains diverse physiological processes. His studies highlighted the interplay among the autonomic nervous system, endocrine glands, and various organ systems that occurs in maintaining homeostatic balance. Furthermore, Cannon bridged physiology and psychology by demonstrating the intimate connection between emotional states and physiological responses, laying the foundation for subsequent research in psychosomatic medicine and stress physiology [20].
Importantly, Cannon’s insights profoundly influenced Selye’s development of the General Adaptation Syndrome and the concept of the stress response, as well as subsequent studies on stress responses in plants and at the cellular level, topics that will be explored in subsequent sections.
4. Hans Selye’s Original Concept
4.1. Selye’s Concept of Stress
As illustrated in Figure 2, the term “stress” in physics refers to the interaction between an applied force and the resistance counteracting that force. Hans Selye (1907–1982) was the first to introduce this term into the medical lexicon, defining stress as the “nonspecific response of the body to any demand” [25]. Often referred to as the “Einstein of medical research” and recognized as the “father of stress research” [25,26], Selye departed from the traditional approach of studying the specific signs and symptoms associated with particular diseases, instead focusing on universal physiological reactions to diverse stressors.
In 1926, as a second-year medical student, Selye posed two fundamental questions: “Why do patients suffering from the most diverse diseases have so many signs and symptoms in common?” and “How could different agents produce the same physiological response?” [27]. These inquiries evolved into a broader hypothesis: “Is there a nonspecific adaptive reaction to change?” Observing that patients with various illnesses often displayed common physiological symptoms, such as loss of appetite and weight loss, Selye termed these reactions “nonspecific responses” [28]. His groundbreaking insights laid the foundation for modern diagnostic approaches, particularly through recognizing the “alarm reaction”, exemplified by fever during infectious diseases.
Because living organisms must continuously adapt to environmental fluctuations, Selye’s work provided a unifying framework for understanding bodily responses to stress—whether physical, emotional, or environmental. Over time, this concept was refined, leading to the clear distinction between the “stressor” (the triggering factor) and the “stress response” (the body’s adaptive reaction) [9,29]. His theory emphasized that the body’s response follows a consistent pattern, irrespective of the type of stressor involved (Figure 3).
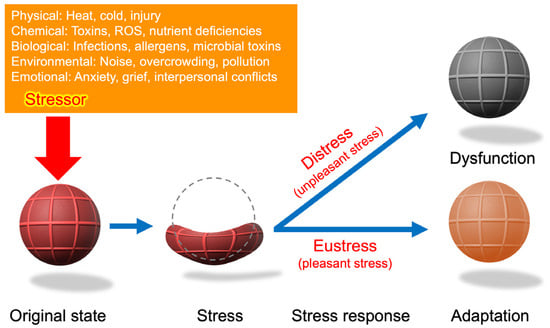
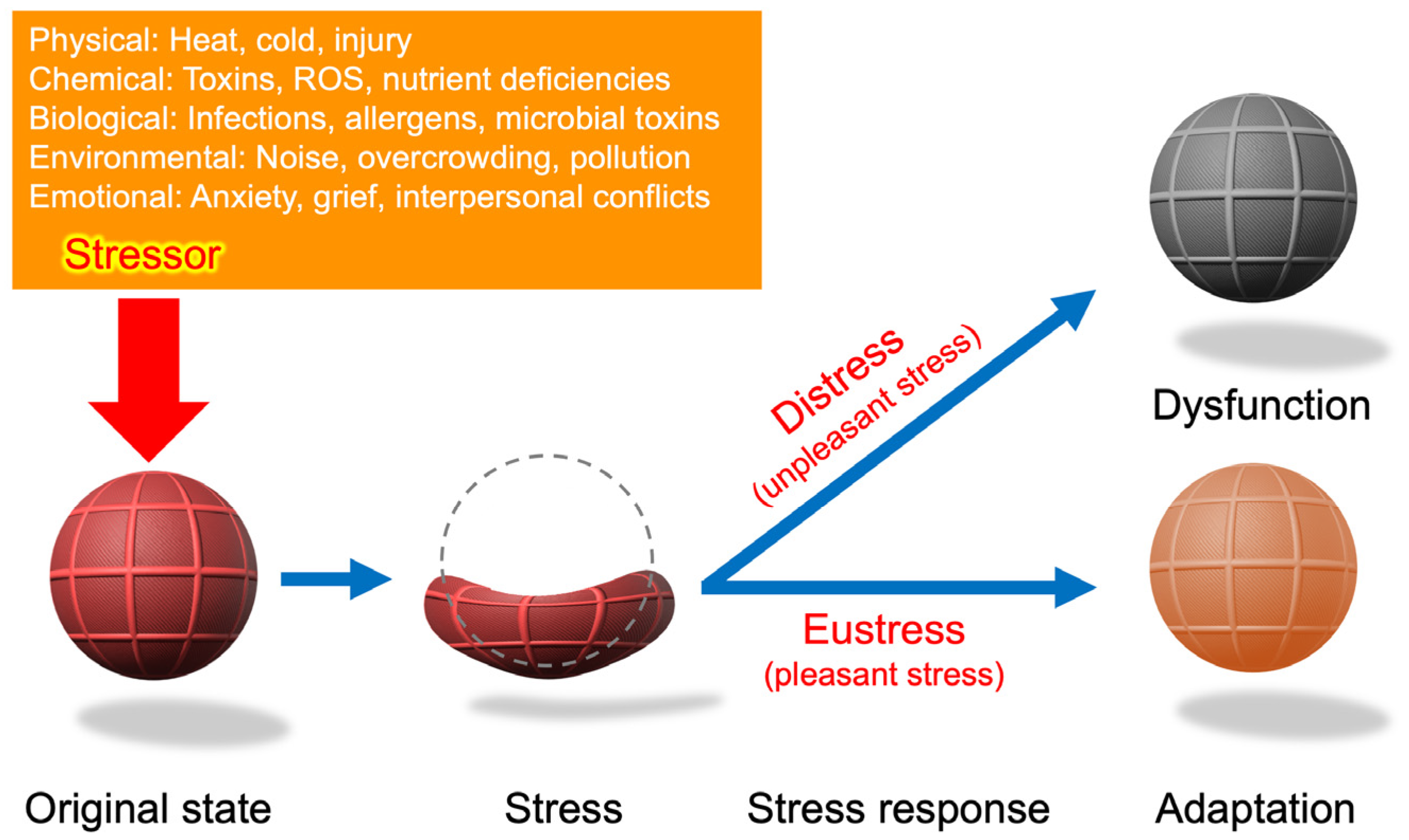
Figure 3.
A schematic representation of Hans Selye’s stress concept. The rubber ball symbolizes the organism, drawing an analogy between mechanical stress (strain as deformation) and biological stress (adaptation or breakdown). The stressor (red arrow) represents an external force that triggers a nonspecific response. While the stress–strain relationship provides a useful conceptual framework, biological stress responses involve dynamic biochemical and systemic processes that extend beyond mechanical deformation. Eustress is not merely the ball returning to its original state but may also involve adaptive responses, where the organism develops greater resilience or strength. Conversely, distress entails more than the failure to recover; it includes systemic changes leading to long-term dysfunction, which are not explicitly represented here. Although the rubber ball analogy provides a simplified model, biological stress responses incorporate complex hormonal, cellular, and systemic adaptations. Biological adaptation often involves biochemical and molecular changes beyond mechanical resilience. Moreover, biological and chemical stressors frequently overlap, as seen in oxidative stress induced by pathogen infections.
The following hold according to Selye’s original concept [27]:
- (1)
- Stress is the nonspecific response of the body to any demand placed upon it.
- (2)
- Stress is inevitable. To be entirely without stress is to be dead!
- (3)
- Stress is not the nonspecific result of damage.
- (4)
- Stress is not something to be avoided.
These fundamental principles illustrate that stress, as Selye conceptualized it, extends beyond pathological states. For example, astronauts experiencing microgravity often exhibit cardiovascular deconditioning, bone demineralization, muscle atrophy, and other physiological disruptions due to the absence of gravitational stress. This clearly demonstrates that gravitational stress is essential for maintaining physiological homeostasis, underscoring stress’s fundamental role in maintaining normal bodily function [30].
4.2. Stress as a Biological State: Refinements
Selye emphasized the “nonspecific” nature of stress, highlighting that the body activates a similar set of general adaptive mechanisms regardless of the type of stressor—whether physical injury, emotional distress, or infection (Figure 4). This adaptive process underpins the General Adaptation Syndrome (GAS), described by Selye in three distinct stages [1]:
- (1)
- The Alarm Stage, characterized by an initial acute response, often exemplified by symptoms like fever during infections.
- (2)
- The Resistance Stage, where adaptation mechanisms stabilize physiological functions.
- (3)
- The Exhaustion Stage, where prolonged or excessive stress exceeds the body’s adaptive capacity, potentially leading to dysfunction or disease.
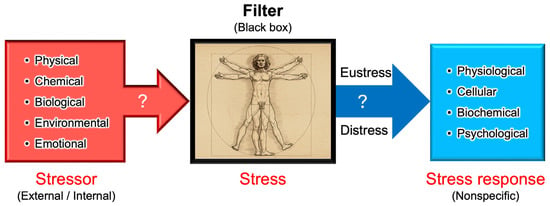
Figure 4.
Stressor–stress–stress response model. One of the unique aspects of Selye’s stress concept is his distinct definitions for the terms stressor, stress, and stress response. Unlike its usage in physics, Selye defined stress as a state of biological conditions. Regardless of the type of stressor (stimulus), the body exhibits a nonspecific and common stress response. Selye introduced the concept of a “filter” in the conversion of stressor signals into stress response mechanisms [9]. However, the specific nature of this hypothesized “filter” remains unidentified to date.
Figure 4.
Stressor–stress–stress response model. One of the unique aspects of Selye’s stress concept is his distinct definitions for the terms stressor, stress, and stress response. Unlike its usage in physics, Selye defined stress as a state of biological conditions. Regardless of the type of stressor (stimulus), the body exhibits a nonspecific and common stress response. Selye introduced the concept of a “filter” in the conversion of stressor signals into stress response mechanisms [9]. However, the specific nature of this hypothesized “filter” remains unidentified to date.
Although Selye’s original research primarily addressed human physiology and medicine, his concept of stress reveals a broader biological phenomenon intrinsic to all living organisms. Biological stress is defined by biochemical, molecular, and systemic adaptations rather than purely mechanical deformation. Unlike physical stress, which commonly implies deformation or damage, biological stress encompasses complex adaptive responses that support organismal survival.
Research advances have expanded the concept of stress beyond mammals to include plants, bacteria, and ecological interactions, demonstrating that stress responses are fundamental and evolutionarily conserved across biological kingdoms. For example, in butterflies, wing color patterns are influenced by pupal temperature stress, a phenomenon linked to a humoral factor [31,32]. In plants, drought stress activates hormonal signaling pathways, such as the abscisic acid (ABA) pathway [33], which is functionally analogous to stress hormone regulation in animals. Similarly, bacteria exhibit adaptive responses to environmental stressors [34,35], further highlighting the universality of stress response mechanisms across diverse biological systems.
According to Selye’s original concept, stress is not inherently harmful; rather, it represents the body’s nonspecific response to any demand. Thus, stress responses can be adaptive (eustress) or maladaptive (distress), and distinguishing between these forms is crucial for a holistic understanding of stress in biological systems. This fundamental principle has profoundly influenced modern research, particularly in studies of oxidative stress, where the dynamic balance among reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) is critical. Maintaining this redox balance is essential for optimal physiological functioning. Consequently, Selye’s foundational ideas continue to shape contemporary research, significantly influencing studies of oxidative stress and its impact across biological systems [36], topics that will be explored in subsequent sections.
5. Levitt’s Concepts of Stress and Strain in Plants
5.1. Plant Growth and Environment
Plants are fundamental to sustaining life on Earth, and their growth is strongly influenced by environmental factors. Historically, farmers recognized the intrinsic connection between environmental conditions and plant development, relying primarily on observational knowledge. Early agricultural practices identified key factors such as water availability, temperature, and nutrient levels as major determinants of plant growth patterns. While these early insights were largely observational, scientific research into the precise mechanisms governing plant–environment interactions gained momentum in subsequent centuries, leading to a deeper understanding of plant physiology and adaptation.
The concept of “stress” in plant science began gaining prominence only in the late 20th century (Figure 5); however, systematic research into plant responses to environmental factors had already begun. Early studies, primarily observational, evolved into comprehensive explorations of plant adaptation mechanisms. In this context, Jacob Levitt’s influential work provided a clear conceptualization of stress and strain in plants, laying foundational principles for contemporary plant stress physiology [37].
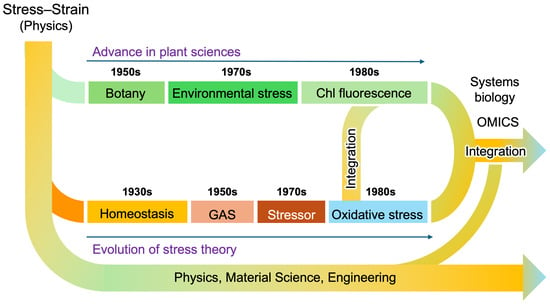
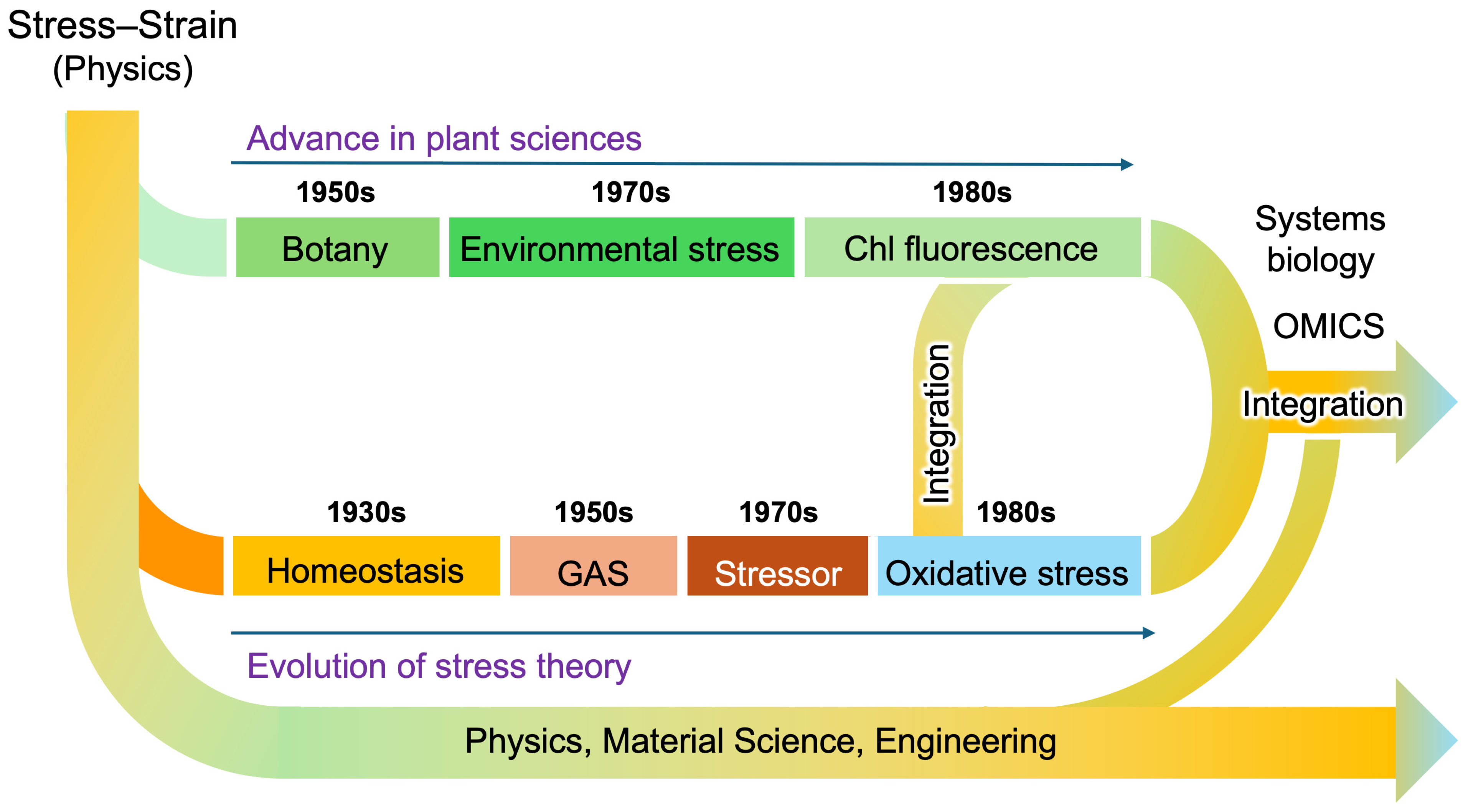
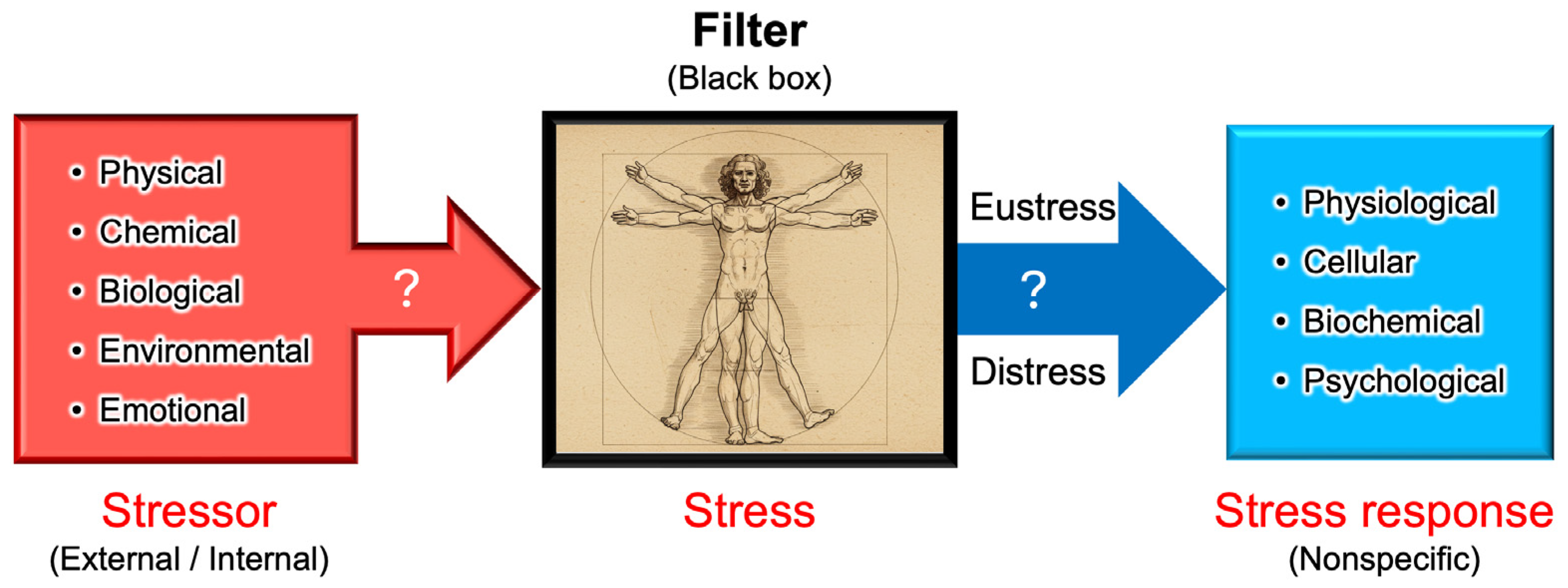
Figure 5.
A diagram illustrating the progression of stress research in plant and animal sciences. Although the principle of the stress–strain relationship was originally established in physics, the terminology has evolved independently across multiple scientific disciplines. The integration of stress research has accelerated since the emergence of the oxidative stress concept, alongside technological advancements such as omics (i.e., genomics, transcriptomics, proteomics, metabolomics, etc.) technologies and systems biology. Due to these historical developments, the definitions of stress and related terminology remain divergent even today, often leading to misunderstandings and confusion.
5.2. Introduction of Stress and Strain to Plant Science
In his influential work published in 1972, Levitt introduced the concept of “stress” to plant science, defining two main categories: abiotic stress (e.g., drought, salinity, and extreme temperatures) and biotic stress (e.g., pathogens and herbivory) [37]. Levitt also introduced the concept of “strain”, describing it as the physical or chemical damage resulting from stress [37]. Specifically, he defined strain as “the physical or chemical damage produced by stress” [37]. Furthermore, Levitt clearly differentiated stress from strain, defining strain explicitly as the measurable effects of stress on plants. He introduced critical terminology such as “environmental stress” and “stress tolerance”, and clarified strain as “the physical or chemical damage resulting from stress”.
Levitt’s conceptual framework significantly contributed to the development of ecophysiology and environmental science, fields dedicated to understanding how plants perceive, respond to, and adapt to environmental stress within their natural habitats. However, while Selye’s theory of stress focuses on “dynamic” physiological responses, Levitt’s framework is more “static”, emphasizing mechanical perspectives similar to those in physics. Advances in molecular biology have shown that plant stress responses are dynamic, leading to a conceptual shift from Levitt’s mechanical framework to a physiological model akin to Selye’s General Adaptation Syndrome. This shift reflects the growing understanding of oxidative stress and its role in plant adaptation, as discussed later.
While recent research has expanded our understanding of stress responses—especially regarding oxidative stress and its critical role in plant adaptation—Levitt’s terminology continues to be foundational. Terms such as “environmental stress”, “abiotic and biotic stress”, and “stress tolerance” remain central concepts in contemporary plant research [38], reflecting his enduring impact on the field.
6. Lichtenthaler’s Application of Chlorophyll Fluorescence as a Marker of Plant Stress
6.1. Four Stages of Plant Stress Responses
One advantage of adopting Levitt’s stress–strain theory in plant research is that “strain” can be quantitatively measured and easily compared in experiments. In contrast, Selye’s concept of stress lacks precise experimental tools for direct measurement in plants. Hartmut Lichtenthaler, a pioneer in plant stress research and an expert in photosynthesis, made a significant breakthrough by introducing chlorophyll fluorescence measurement as a method to detect plant stress.
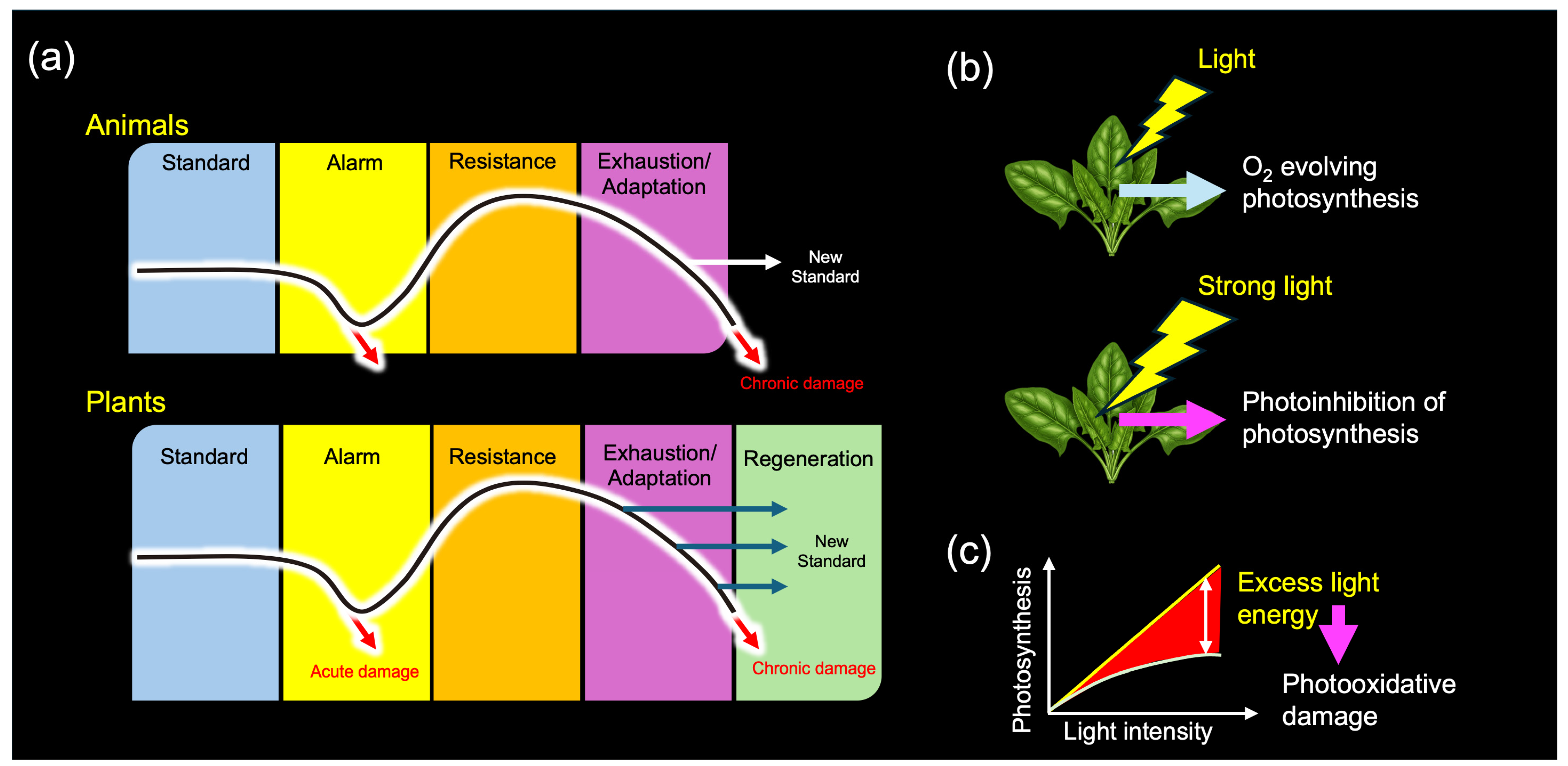
In his influential book, The Stress Concept in Plants, Lichtenthaler revisited Selye’s foundational work from 1936 and elaborated on its relevance to plant biology. He established a generalized stress framework specifically for plants, categorizing stressors and stress responses in alignment with Selye’s theory [39,40,41]. Notably, Lichtenthaler expanded upon Selye’s three-stage model (alarm, resistance, and exhaustion) by introducing a plant-specific fourth stage, “regeneration” (Figure 6a). He defined regeneration as the phase in which plants recover and establish new physiological baselines if stressors are removed at an appropriate time, before senescence becomes dominant [39].
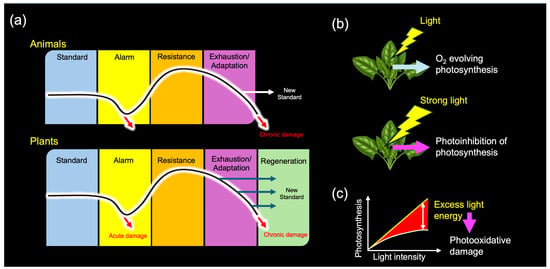
Figure 6.
(a) Stress responses in animals and plants. (b) Light stress in plants. (c) Light-response curve of photosynthesis. Based on Selye’s original framework, Lichtenthaler initially distinguished three phases of plant stress responses and later introduced a fourth, plant-specific regeneration phase (a). Although light energy is essential for photosynthesis, it can also function as a stressor (b). When the input of light energy exceeds the maximum operational capacity of the photosynthetic apparatus (c)—such as under intense illumination—photosynthesis becomes inhibited, a phenomenon known as “photoinhibition”. Prolonged photoinhibition can lead to photooxidative damage, ultimately resulting in plant death. This dual nature of light exemplifies the stress concept, clearly distinguishing between eustress (pleasant stress) and distress (unpleasant stress).
6.2. Chlorophyll Fluorescence as a Measure of Stress in Plants
In 1931, Kautsky and Hirsch discovered light-induced changes in red chlorophyll fluorescence from green leaves, which correlated with photosynthetic activity—a phenomenon now known as the “Kautsky effect” [42]. In his seminal work, Murata (1969) demonstrated reversible, light-induced changes in chlorophyll fluorescence under physiological conditions [43], thereby helping to establish a conceptual framework for photosynthetic regulation in response to light stress. This work served as an early example of the idea that the photosynthetic apparatus is not merely a passive energy collector but an actively regulated system optimized for dynamic light environments [44].
Lichtenthaler demonstrated that changes in chlorophyll fluorescence parameters could effectively reveal physiological alterations induced by environmental stressors such as drought, salinity, and extreme temperatures [39]. This technique provided a non-invasive, highly sensitive approach for studying photosynthetic efficiency and assessing plant stress status, significantly improving upon traditional destructive biochemical methods [45]. Chlorophyll fluorescence thus became a groundbreaking tool for gaining insights into photosynthesis and assessing the functionality of the photosynthetic apparatus, particularly Photosystem II (PSII) [46]. Environmental stressors, including drought [47], salinity [48], extreme temperatures [49], and pollutants [50], disrupt photosynthetic processes, and these disruptions can be sensitively detected by fluorescence measurements.
The impact of this method extends far beyond plant stress research. Satellite-based chlorophyll fluorescence measurements are now widely employed in remote sensing to monitor large-scale vegetation health and global carbon cycles [51]. In ecology, chlorophyll fluorescence has become invaluable for studying plant responses to ecosystem changes driven by global climate change [52]. In crop breeding programs, chlorophyll fluorescence facilitates the identification of stress-tolerant cultivars by efficiently evaluating photosynthetic performance under stress conditions [47]. By offering a non-invasive and rapid screening method, this technique has accelerated the development of more resilient crop varieties.
6.3. Broad Applications and Its Limitations
The evaluation of stress using chlorophyll fluorescence has been applied not only to plants but also to symbiotic invertebrate animals. Technological advancements—such as the development of pulse-amplitude modulation (PAM) chlorophyll a fluorometers—have enabled the monitoring of stress in reef-building corals, which rely on symbiotic photosynthetic dinoflagellates for energy production [53]. This application is particularly important in coral research [54], as fluorescence measurements allow researchers to assess stress levels and species-specific susceptibility to coral bleaching [55]—a phenomenon increasingly exacerbated by global warming, climate change, and anthropogenic eutrophication [56,57,58].
Despite its transformative impact, chlorophyll fluorescence measurement has notable limitations. Primarily, the method is effective at detecting distress but requires supplementary approaches to evaluate eustress adequately. Additionally, it specifically targets photosynthetic activity, potentially overlooking other stress responses unrelated to photosynthesis. This limitation also extends to non-photosynthetic organisms, such as animals and microbes, where understanding stress necessitates mechanistic insights at the cellular and molecular levels. Addressing these gaps is essential to achieving a more comprehensive understanding of stress responses across all biological systems.
7. Sies’s Concept of Oxidative Stress and Redox Biology
7.1. Oxygen Toxicity
Older textbooks often emphasized molecular oxygen (O2), primarily for its essential role in aerobic respiration, which enables organisms to generate energy efficiently. However, in 1969, McCord and Fridovich [59] made a groundbreaking discovery by identifying superoxide dismutase (SOD)—the first enzyme known to neutralize radical oxygen species—marking a major advance in redox biology [60]. Another historical milestone in early redox biology research was reached in 1970 by Sies and Chance [61], who were the first to describe hydrogen peroxide (H2O2) as a normal physiological metabolite in eukaryotic cells and organs. This was detected as a steady-state level of catalase compound I [62]. These findings laid the foundation for further investigations into “oxygen toxicity” [63,64], revealing the potentially harmful effects of oxygen at the molecular level [64]. Subsequently, highly reactive oxygen-derived molecules, such as superoxide radicals (O2•−) and hydrogen peroxide (H2O2), were initially classified as “active oxygens” or “reactive oxygen intermediates (ROI)” and are now commonly referred to as reactive oxygen species (ROS) [65]. It soon became evident that, without effective neutralization, ROS could induce substantial cellular damage [66,67].
7.2. Oxidative Stress as an Imbalance Between Oxidants and Antioxidants
In 1985, Helmut Sies introduced the concept of “oxidative stress” in his influential book, Oxidative Stress [68], profoundly transforming our understanding of stress responses across biological kingdoms. Sies shaped the field of redox biology by elucidating the intricate balance between ROS generation and antioxidant defense mechanisms, fundamentally reshaping our understanding of stress responses at the molecular level [62]. Importantly, his definition of oxidative stress highlighted not only its role in pathological processes but also its function as a regulatory mechanism within cellular signaling networks.
Sies’s most significant contribution was defining “oxidative stress” as “an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage” [68,69]. This refined definition underscores oxidative stress as a double-edged sword—not merely a cause of molecular damage but also a key modulator of physiological signaling processes [13].
Helmut Sies was among the first to identify H2O2 as a signaling molecule, demonstrating its function as a second messenger in cellular signal transduction pathways [70]. His pioneering research laid the foundations of redox biology, emphasizing the dual role of ROS—harmful when excessive (oxidative distress) but essential at physiological levels for adaptive responses (oxidative eustress) [13,36]. For example, ROS generation is not inherently detrimental; it plays a crucial role in cellular signaling cascades, regulating transcription factors and enzymatic activities. However, sustained ROS overproduction can lead to oxidative damage, resulting in cellular dysfunction, aging, and disease pathogenesis. This dual concept has been validated in diverse biological systems, including plants [71,72] and bacteria [73], reinforcing the evolutionary significance of oxidative stress adaptation.
Recent research has provided extensive insights into cellular defense mechanisms against ROS-induced damage, particularly through the characterization of antioxidant systems [74]. However, Sies’s work clearly distinguishes the dual nature of ROS—damaging at high concentrations yet indispensable at physiological levels for cellular homeostasis, signaling, and adaptation [36]. This perspective has driven advancements in antioxidant-based therapeutic strategies [75], biomarker development for oxidative stress monitoring [76], and preventive approaches in medicine [13,36] and bridging the theoretical gap between psychosocial work stress and coronary heart disease (CHD) [77].
Thus, Helmut Sies’s pioneering contributions established the foundations of modern redox biology, profoundly enhancing our understanding of oxidative stress, cellular homeostasis, and stress adaptation across diverse organisms.
8. Photooxidative Stress in Plants: The Origin of Oxygen Toxicity
8.1. Oxygenic Photosynthesis as the Origin of Oxygen Toxicity
The Great Oxygenation Event (GOE) approximately 2.4 billion years ago is widely recognized as a pivotal moment in biological evolution [78,79]. This event dramatically transformed Earth’s atmosphere, facilitating the emergence of aerobic organisms dependent on molecular oxygen (O2) for respiration. A critical biological innovation during this period was the evolution of oxygenic photosynthesis in ancestral cyanobacteria, which utilized water (H2O), instead of hydrogen sulfide (H2S), as an electron donor for light-driven electron transport [79]. This fundamental transition drastically changed Earth’s atmosphere by increasing the O2 concentration and simultaneously introduced oxidative stress, profoundly influencing the physiology of modern-day plants and animals.
Even oxygen-evolving plants themselves face significant challenges from photooxidative stress [80], which occurs when the absorption of light energy exceeds the photosynthetic regulatory capacity (Figure 6c). Such an imbalance between energy supply and metabolic demand leads to excessive production of ROS [81].
In 1951, Mehler demonstrated that O2 acts as an electron acceptor (Hill oxidant) in the photosynthetic electron transport chain of chloroplasts. This reaction, involving the one-electron reduction of O2 to form O2•−, was measured as light-induced O2 uptake in isolated thylakoid membranes or disrupted chloroplasts [82]—a process now known as the Mehler reaction [83]. Since chlorophylls are known to undergo rapid photobleaching when extracted from thylakoid membranes using organic solvents, it is evident that thylakoid-embedded chlorophylls are protected by internal mechanisms that mitigate ROS generation during light exposure [84].
In 1975, Takahama and Nishimura—in a pioneering study in the field of photooxidative damage in chloroplasts—demonstrated that singlet oxygen (1O2) is formed within photosystems, initiating lipid peroxidation [85,86]. They further showed that photosynthetic pigments such as carotenoids act as internal quenchers of 1O2, thereby protecting the photosystems from oxidative damage [87].
In the 1970s, Kaiser elucidated that H2O2, the reaction product of the SOD reaction of O2•−, reversibly inhibits photosynthetic enzymes, particularly those containing thiol groups. He also showed that this inhibition could be reversed by the thiol-reducing agent dithiothreitol (DTT) [88,89].
These pioneering studies provided crucial insights into the photooxidative damage (photooxidative distress) and regulatory roles of ROS generated during photosynthesis (photooxidative eustress) in plants.
8.2. Plant Antioxidant Systems and Human Health
In 1976, Foyer and Halliwell proposed that ascorbate (vitamin C) functions as an ROS scavenger in conjunction with glutathione (GSH) [90]. Subsequently, Asada et al. identified ascorbate peroxidase (APX), an enzyme that removes H2O2 using ascorbate, functionally analogous to glutathione peroxidase (GPX) in animals. They also characterized the associated enzymes involved in regenerating ascorbate using NADPH [91]. The chloroplast ROS detoxification pathway, originally termed the “Halliwell–Asada cycle” [92], is now more commonly known as the ascorbate–glutathione cycle [84,93].
While it has long been recognized that consuming fruits and vegetables prevents scurvy—a disease caused by vitamin C deficiency [64]—a fundamental question remained: Why do plants accumulate high levels of vitamin C in their tissues? The oxidative stress concept provides a compelling explanation, bridging plant biology and human health sciences. It offers scientific validation for the health benefits of a plant-based diet, highlighting the protective effects of abundant plant-derived antioxidants against oxidative distress and related diseases in humans [64,94,95,96,97,98,99].
In plants, these antioxidants are crucial for protecting the photosynthetic machinery from photooxidative damage [84], thereby enhancing survival in their natural habitats [100]. According to the stress concept, plant species that evolved under high levels of photooxidative stress, including ultraviolet (UV) radiation [97], are more likely to have enhanced nutritional and medicinal properties. However, this aspect has been largely overlooked in nutritional science and herbal medicine.
8.3. Unification of Plant and Animal Stress Responses by Oxidative Stress
Helmut Sies’s oxidative stress concept has had a profound impact on plant biology, particularly in understanding plant responses to environmental and physical stressors [101]. His work demonstrated that oxidative stress is a universal principle applicable across biological kingdoms [102], thus providing a unified framework for understanding cellular stress responses in both plants and animals. This concept has significantly advanced our understanding of ROS signaling networks in plants [84,103], particularly in response to environmental stresses [101], and has deepened our comprehension of photooxidative stress in chloroplasts and its role in photosynthetic regulation.
9. The Expanding Universe of Redox Biology
9.1. Updating Oxidative Stress: Integration of Reactive Nitrogen Species (RNS) and Reactive Sulfur Species (RSS)
The concept of oxidative stress has effectively bridged plant biology and human health sciences, particularly regarding disease prevention and stress adaptation, thereby unifying diverse biological systems within the framework of redox biology. Over time, this field has expanded significantly beyond its initial emphasis on ROS.
Historically, oxidative stress research primarily focused on ROS-mediated cellular responses. However, recent advancements have substantially broadened this perspective by recognizing the crucial roles of other reactive species, including reactive nitrogen species (RNS) and reactive sulfur species (RSS), in cellular redox homeostasis. These discoveries have fundamentally reshaped our understanding of redox regulation, highlighting a more complex and interconnected network of reactive molecules—one that cannot be fully explained by ROS or oxidative stress alone.
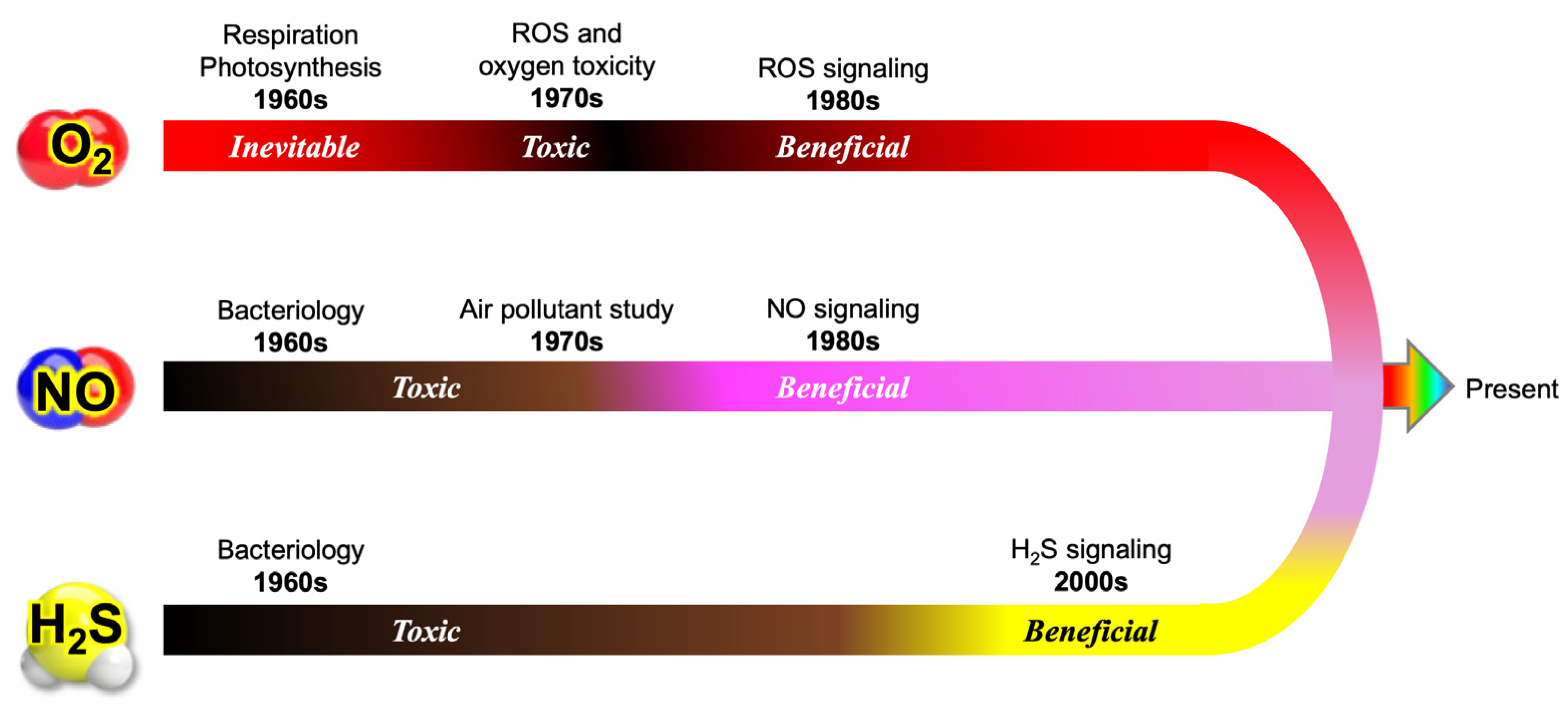
This evolution has provided a nuanced understanding of how these diverse reactive species interact to regulate cellular physiology, underscoring the inherent complexity of redox biology. Figure 7 summarizes the evolution of our perception of three key gaseous molecules—molecular oxygen (O2), nitric oxide (NO), and hydrogen sulfide (H2S)—that has occurred as our knowledge has advanced within redox biology.
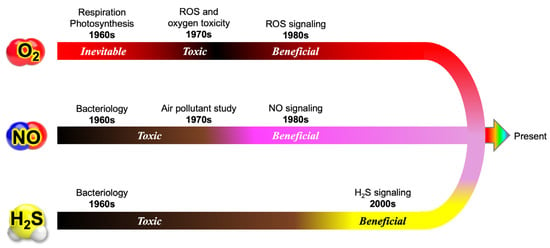
Figure 7.
Paradigm shifts in the understanding of O2, NO, and H2S in biology. In earlier textbooks, molecular oxygen (O2) was primarily described as an essential molecule required by aerobic organisms. However, the discovery of reactive oxygen species (ROS) and oxygen toxicity initiated a major paradigm shift, revealing that O2 has dual roles—as both a vital component of cellular respiration and a potentially harmful oxidant. Similarly, nitric oxide (NO) was widely regarded as a toxic air pollutant for animals and plants. Subsequent groundbreaking research, however, demonstrated that NO serves critical functions as a signaling molecule, mediating processes such as vascular regulation, neurotransmission, and immune responses. Likewise, hydrogen sulfide (H2S) was long recognized as a microbial metabolite, but its biological significance remained largely underestimated until the late 1990s. These shifts highlight how perceptions of these gaseous molecules have evolved from viewing them merely as toxic compounds to recognizing their essential functions in biology. This transformation underscores the dynamic nature of scientific progress, where ongoing research continuously reshapes our understanding of fundamental biological processes.
9.2. Integration of Reactive Nitrogen Species (RNS)
9.2.1. Nitric Oxide (NO) as a Signaling Molecule
Initially, nitric oxide (NO), along with nitrogen dioxide (NO2), was recognized primarily as a harmful air pollutant (collectively referred to as NOx) anthropogenically produced from fossil fuel combustion by industries and automobiles and cigarette smoke. Consequently, early NO research emphasized its cytotoxic effects on animals [104] and plants [105]. A major turning point occurred when Furchgott and Zawadzki (1980) discovered that endothelial cells produce an unknown factor responsible for vascular relaxation, termed “endothelium-derived relaxing factor” (EDRF) [106]. It took several years before this unknown factor was identified as the previously known air pollutant NO.
Pioneering research by Ignarro [107], Furchgott [108], Murad [109], and Moncada [110] ultimately revealed that EDRF was indeed NO. Their discoveries led to the identification of the L-arginine-dependent NO synthase (NOS) enzyme and its associated cyclic guanosine monophosphate (cGMP) signaling pathway, fundamentally altering our understanding of cellular signaling [111]. This finding challenged prior assumptions and established NO as a key biological messenger. Recognized as one of the most significant biomedical discoveries of the 20th century, NO was named “Molecule of the Year” in 1992 [112], and its role in biology was further celebrated with the awarding of the Nobel Prize in Physiology or Medicine in 1998.
Since then, NO has transitioned from having a reputation as a toxic air pollutant to being recognized as a crucial biological signaling molecule profoundly influencing cellular communication and leading to diverse therapeutic applications. Examples include sildenafil (Viagra) for treating erectile dysfunction [113], NO therapy for neonatal pulmonary hypertension [114], and its therapeutic use in infectious diseases [115,116].
9.2.2. Alternative Mechanisms of NO Production
Early research on NO in plants paralleled studies in animals, initially focusing on the inhibitory effects of NOx gases on plant growth [105]. Although plant researchers initially presumed the existence of a mammalian-type NOS enzyme in plants [117,118], extensive studies demonstrated that neither mammalian-like NOS [117], the proposed plant-specific inducible NOS (“plant iNOS”) [119], nor the putative “AtNOS1” protein [120] displayed true NOS enzymatic activity [119,121,122,123,124,125].
In 1999, we reported an alternative enzymatic pathway for NO production in plants, showing that assimilatory nitrate reductase (NR) possesses nitrite reductase (NiR) activity and can catalyze the NADH-dependent reduction of nitrite (NO2−) to NO [126]. This NR-mediated pathway [127] was later confirmed in unicellular algae [128] and has been shown to play critical roles in various plant physiological processes, including stomatal movement [129,130], root development [131], and stress responses [132], particularly under hypoxic conditions such as flooding [133,134]. A similar NiR-mediated NO-generating mechanism operates in bacteria during denitrification [135].
In mammals, an alternative nitrite-dependent NO production pathway has also been identified. Gladwin et al. demonstrated that deoxyhemoglobin can reduce nitrite to NO under low-oxygen conditions, and evaluated the NiR activity of heme-globins [136,137]. Other mammalian enzymes, including xanthine oxidoreductase (XOR), a molybdenum-containing enzyme, also contribute to NO formation from nitrite [138]. This nitrite-based pathway [139], termed the “nitrate–nitrite–NO pathway” [140], complements the classical oxidative mechanism of NOS, which requires oxygen and L-arginine [141]. The alterative pathway becomes especially important under hypoxic or inflammatory conditions where NOS activity is compromised.
The physiological relevance of nitrate (NO3−) and nitrite (NO2−) as NO precursors has been well established, particularly in stress-related diseases [137,139,142,143]. NO generated via the alternative pathway reversibly inhibits cytochrome oxidase (COX or complex IV) [144], thereby reducing mitochondrial oxygen consumption and preserving oxygen availability in tissues. NO also stabilizes hypoxia-inducible factor 1α (HIF-1α) by inhibiting prolyl hydroxylases (PHDs), promoting the expression of genes that enhance hypoxia tolerance [145].
The alternative NO-producing mechanisms allow animals to maintain redox balance and physiological function under stress, underscoring the central role of the nitrate–nitrite–NO pathway in stress biology [140,146,147]. As humans are incapable of synthesizing inorganic nitrite or nitrate (except as oxidation products of endogenous NO), these findings underscore the nutritional importance of consuming nitrate-rich vegetables (plants) as dietary sources of NO precursors [148,149,150]. Such a dietary intake is essential for maintaining vascular health [151] and may also play a role in protecting against infectious diseases [152].
9.3. The Expanding Roles of Reactive Sulfur Species (RSS)
9.3.1. H2S as the Third Gasotransmitter
Alongside the paradigm shift in our understanding of NO and RNS, our perception of the biological significance of sulfur-containing molecules—particularly hydrogen sulfide (H2S) and its derivatives—has undergone a dramatic transformation in recent decades [153,154]. The perception of H2S, previously regarded primarily as a toxic gas and occupational hazard, has shifted profoundly with increasing recognition of its critical biological roles [155].
Historically, research on H2S focused predominantly on its toxicity, especially in occupational settings, leading many countries to establish strict safety guidelines (e.g., limiting exposure to 10 ppm for up to 10 min). However, perceptions of the biological significance of H2S changed dramatically following discoveries in recent decades.
A major breakthrough in the field came in 1996, when Abe and Kimura demonstrated that H2S is endogenously produced from L-cysteine in mammalian brain homogenates [156]. This finding marked a paradigm shift, establishing H2S as the third recognized gasotransmitter, alongside NO and carbon monoxide (CO) [157].
9.3.2. Endogenous H2S Production in Plants and Animals
Under physiological conditions, H2S exists in multiple forms, including fully protonated hydrogen sulfide (H2S), hydrosulfide anion (HS−), and polysulfides (HSSn−). Endogenous production of H2S in animals primarily involves enzymes such as cystathionine β-synthase (CBS) [156], cystathionine γ-lyase (CSE) [154], and 3-mercaptopyruvate sulfurtransferase (3-MST) [158]. Research in RSS biology and medicine has continued to expand, revealing numerous physiological roles in both plants [159,160] and animals [36], highlighting significant therapeutic potential [153,161].
9.3.3. Plant-Derived Sulfur Compounds and Human Health
Recent research has highlighted that, in addition to plant-derived antioxidants such as ascorbate and polyphenols [98], sulfur-containing compounds play a crucial role in mitigating oxidative-stress-related disorders in humans. Sulfur-rich vegetables (e.g., garlic, onions, and cruciferous vegetables) contain organosulfur compounds such as allicin ((2E)-prop-2-ene-1-sulfinothioic acid S-2-propenyl ester) and sulforaphane (1-isothiocyanato-4-(methylsulfinyl) butane), which regulate redox balance and have been implicated in cancer prevention [162,163], cardiovascular health [164], and neuroprotection [165]. Understanding plant redox adaptations can thus provide valuable insights into dietary interventions and pharmacological strategies for combating oxidative-stress-related diseases in humans [159].
9.4. The Interplay Among ROS, RNS, and RSS
9.4.1. O2-NO-H2S (ONS)
Table 1 lists representative ROS, RNS, and RSS derived from O2, NO, and H2S. It is important to note that ROS, RNS, and RSS—collectively referred to as RONSS [166]—exist as neutral radicals (e.g., •OH and NO), radical ions (e.g., O2•− and ONOO−), and non-radical species (e.g., H2S and H2O2) [68]. In principle, ionic forms of RONSS, such as O2•− and other charged molecules, cannot diffuse across membranes without the assistance of ion transporters, the characteristic determining their roles and cellular impacts [166]. We suggest that these abbreviations (ROS, RNS, and RSS) serve primarily as a reference to their original molecular sources—O2, NO, or H2S (ONS)—rather than an indication of specific chemical properties or cellular functions.

Table 1.
Representative reactive molecules derived from O2, NO, and H2S.
9.4.2. Cysteine Thiol at the Crossroad of Redox Interactions
Integrating RNS and RSS into the oxidative stress paradigm has provided a more comprehensive understanding of cellular redox biology [11]. Importantly, these reactive species do not function independently; rather, they engage in extensive chemical crosstalk, modulating signaling pathways and stress responses. The diverse array of reaction products arising from interactions among ROS, RNS, and RSS further complicates both in vivo chemical reactions and their physiological implications.
For example, the reaction between O2•− and NO produces ONOO−, one of the most cytotoxic RNS, implicated in both “nitrosative (R–NO) stress” [167] and “nitrative (R–NO2) stress” [168]. Among critical redox modifications, S-nitrosylation (Cys–SNO)—a post-translational modification involving the covalent addition of NO to cysteine thiol (–SH) groups—has emerged as a significant regulatory mechanism in redox biology extensively studied in both plants [169] and animals [170,171,172,173,174]. This process underscores the intersection between ROS and RNS, demonstrating their interconnected roles in cellular regulation [175,176,177].
Subsequent research identified RSS, specifically persulfides (RSSH) and polysulfides (RSSn−), as endogenously produced molecules that form glutathione persulfide (GSSH) and cysteine persulfide (Cys–SSH) [178], along with various polysulfide derivatives in mammalian cells [161]. These RSS compounds interact readily with H2O2, further highlighting their biological significance [178]. Moreover, hybrid reactive species arising from NO–H2S interactions have been identified as pivotal regulatory molecules [179], underscoring the intricate chemical interplay among multiple reactive species and their diverse physiological outcomes.
Table 2 shows representative thiol modifications mediated by ROS, RNS, and RSS. Cysteine thiols (–SH) within glutathione and proteins occupy a central position in redox biology, crucially influencing stress responses and cellular homeostasis. Increasing evidence indicates that cysteine thiol modulation serves as a molecular switch mechanism, dynamically regulating redox-sensitive cellular processes [180]. Consequently, the diversity of cysteine thiol modifications represents a molecular signature or “snapshot” of dynamic redox interactions between RONSS and antioxidants.

Table 2.
Cysteine thiol modifications mediated by ROS, RNS, and RSS.
10. The Three-Body Problem in Stress Biology
10.1. The Dynamic Interplay Among ROS, RNS, and RSS
Thiol modifications by ROS, RNS, and RSS represent dynamic processes with diverse functional outcomes under in vivo conditions. Protein thiols modified by NO to form S-nitrosothiols (–SNO) are reversible and function as signal-activating modifications regulating vasodilation, neurotransmission, and immune response [170,181]. H2S can convert SNOs to persulfides (–SSH) or directly modify thiols, particularly under reducing conditions [182].
Under oxidative stress, SNOs undergo further oxidation to sulfenic (–SOH) or sulfinic (–SO2H) acids, eventually reaching irreversible sulfonation (–SO3H), resulting in protein inactivation [183]. The persulfidation pathway mediated by RSS protects against irreversible oxidation by enhancing thiol nucleophilicity while preserving or augmenting protein function [184,185].
These chemical conversions are traditionally studied as “pairwise” redox interactions or binary “crosstalk”, but the ultimate modification state of regulatory proteins depends on the dynamic balance among all three species—ROS, RNS, and RSS—each with distinct kinetic rate constants and redox potentials [186]. Together, they form a nonlinear, interdependent system with complex biochemical outcomes [187,188]. This integrated redox interplay perspective is essential for understanding how cells maintain redox homeostasis while utilizing reversible thiol modifications for signaling purposes [178].
10.2. Analogy to the Three-Body Problem in Physics
In classical mechanics, the three-body problem describes a system in which gravitational interactions among three celestial bodies lead to nonlinear, unpredictable motion [189]. Unlike simpler two-body systems, three-body interactions often generate chaotic, emergent behaviors, making long-term predictions highly complex.
This conceptual framework offers a compelling analogy for the biochemical interplay among ROS, RNS, and RSS in redox biology. Figure 8 presents a schematic model in which the redox interactions of ROS, RNS, and RSS are interpreted through the lens of the classical three-body problem, which describes gravitational interactions among three celestial bodies.
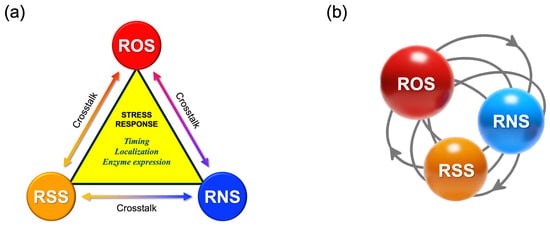
Figure 8.
The redox triad: a dynamic three-body system of ROS, RNS, and RSS. (a) Triadic interactions among reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) illustrated in a symmetrical, linear configuration. (b) A conceptual illustration of the three-body problem. We propose that redox biology involving ROS, RNS, and RSS should not be viewed merely as a series of binary redox interactions or pairwise “crosstalk” (e.g., ROS–RNS or ROS–RSS), but rather as a triadic, interdependent system. Its biological significance emerges from context-dependent and spatiotemporally dynamic interactions. This concept parallels the classical three-body problem in Newtonian mechanics, in which no general analytical solution exists due to the nonlinear and mutual influences among the three interacting bodies.
Unlike conventional binary models of “oxidant–antioxidant” interactions, this triad introduces context-dependent and nonlinear dynamics that can lead to highly variable stress responses across different organisms and environmental conditions. While ROS, RNS, and RSS each play unique roles in redox signaling, their chemical cross-reactivity can produce emergent effects not easily predicted from their individual functions.
By applying the three-body problem analogy to redox biology, we gain deeper insight into how cellular redox homeostasis dynamically adjusts to fluctuating physiological and environmental stressors.
10.3. Broader Implications of the Redox Triad in Stress-Related Diseases
The dynamic interplay among ROS, RNS, and RSS—conceptualized as a redox “three-body system”—has broad implications across a wide spectrum of stress-related diseases (Table 3). Numerous studies have demonstrated that altered levels of O2, NO, and H2S—the principal precursors of ROS, RNS, and RSS, respectively—are associated with the onset, progression, and prognosis of various pathological conditions. Notably, reduced oxygen availability or hypoxic microenvironments are common features in many of these disorders.

Table 3.
Overview of human diseases involving O2, NO, and H2S imbalance.
11. Ecological Perspectives: Stress and Neurodegenerative Diseases
11.1. Environmental Stressors and Neurodegeneration
Chronic exposure to environmental stressors is implicated in various neurodegenerative diseases through mechanisms involving oxidative damage, inflammation, and protein aggregation [223,224,225]. Hans Selye’s General Adaptation Syndrome (GAS) provides a foundational framework in physiology and psychiatry; however, a significant gap remains in our understanding of how diverse stressors trigger generalized adaptive responses, particularly in psychiatric and neurodegenerative conditions. This unresolved question was one that Selye himself pondered, especially regarding how different stressors could lead to similar pathological outcomes.
A striking historical example is the unusually high incidence of the amyotrophic lateral sclerosis/Parkinsonism–dementia complex (ALS/PDC), also known as Lytico–Bodig disease, among the Chamorro population in Guam during the mid-20th century [226]. Epidemiological studies from the 1950s and 1960s indicated that the incidence of ALS/PDC in Guam was up to 100 times higher than global averages [227]. Despite decades of research, the definitive cause of ALS-PDC in Guam remains elusive.
11.2. Natural Neurotoxin BMAA: Linking Disease and Environment
The prevalence of ALS/PDC in Guam has declined dramatically, coinciding with rapid westernization. This trend supports the involvement of environmental, rather than purely genetic, factors as the primary cause. A 2003 island-wide prevalence study revealed that all identified cases involved individuals born before 1940, consistent with a disappearing syndrome resulting from a point-source exposure in the 1940s [228]. The sharp decline in incidence beginning in the 1950s corresponds with the cessation of traditional cycad use and broader changes in lifestyle and diet [229].
The Chamorro people traditionally consumed seeds of the cycad plant (Cycas micronesica), which were later found to contain high concentrations of β-N-methylamino-L-alanine (BMAA), a natural neurotoxin. In addition, the traditional Chamorro diet included flying foxes (Pteropus mariannus), which fed on cycad seeds, leading to further BMAA bioaccumulation in brain tissues through trophic magnification [230].
Among several proposed etiological hypotheses—including the methylazoxymethanol (MAM) hypothesis and the mineral deficiency hypothesis [231]—the BMAA hypothesis was the first to provide a compelling biochemical mechanism linking environmental exposure to neurodegeneration [227].
11.3. BMAA and Cyanobacterial Blooms: Global Health Implications
Initially, BMAA was thought to be exclusively produced by certain cyanobacteria (Nostoc spp.) living symbiotically with cycads [232] and the water-floating fern Azolla [233,234]. Subsequent research, however, revealed that various cyanobacteria can produce BMAA [235], and its bioaccumulation has been detected in multiple organisms, including seafood [236], vegetables [237], and even drinking water [238].
A recent study reported BMAA accumulation in the brains of wild dolphins exhibiting abnormal behavior before death. Postmortem analyses revealed an increased number of β-amyloid+ plaques and dystrophic neurites in the auditory cortex, reinforcing a potential link between chronic environmental BMAA exposure and neurodegenerative pathology [239]. These findings underscore the global health risks posed by cyanobacterial blooms, suggesting a potential impact of environmental BMAA exposure on human neurodegenerative diseases [236].
Lytico–Bodig disease has drawn attention to the link between environmental stress and human health. Cyanobacteria are primitive organisms with no direct evolutionary relationship to humans, the most highly evolved animals. Although the biological function of BMAA in cyanobacteria remains unclear [240,241,242], and its neurotoxicity remains a matter of debate [226,227,243], numerous studies have suggested that BMAA interacts with N-methyl-D-aspartate (NMDA) receptors in the neural cells of animals [227,244,245].
12. The Minimum Machinery for Nonspecific Stress Response
12.1. Multi-Sensitivity of NMDARs
N-methyl-d-aspartate receptors (NMDARs) are essential glutamate receptors (GluRs) that mediate the majority of excitatory neurotransmission in the mammalian central nervous system (CNS) [246]. They play a pivotal role in synaptic plasticity, learning, and memory formation, while also contributing to excitotoxic neuronal cell death in various acute and chronic neurological disorders [247].
NMDARs belong to the ionotropic glutamate receptor (iGluR) family, which is further subdivided into α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), kainate, and NMDA receptor channels [246]. These iGluRs are widely distributed throughout the nervous system, with particularly high concentrations in human brain synapses.
A defining feature of NMDARs is their role in coincidence detection, requiring both glutamate binding and membrane depolarization to remove the Mg2+ block, thereby permitting Ca2+ influx [248]. This dual gating mechanism enables NMDARs to integrate pre- and postsynaptic activity, making them essential for synaptic plasticity and long-term potentiation (LTP) [249,250].
However, a well-documented paradox exists: NMDARs can either promote neuronal survival or trigger excitotoxic cell death, depending on their activation context [251]. The dysfunction of NMDARs has been implicated in various neurological disorders, including epilepsy [252], schizophrenia [253], and neurodegenerative diseases [251,252].
Unlike traditional neurotransmitter receptors that respond solely to ligand binding, NMDARs exhibit high sensitivity to diverse external and internal factors. Their activity is tightly regulated by pH (protons) [254], Mg2+ [255], Zn2+ [256], D-serine [257], polyamines [258], and histamine [248]. Additionally, NMDAR function is influenced by redox states, particularly via the oxidation of cysteine thiol (–SH) groups in receptor subunits. Consequently, NO [259], H2S [222,260], and H2O2 [261] regulate NMDAR activity via thiol modifications [161].
Although further validation is necessary [262], BMAA was identified as a partial agonist at the glycine-binding site of NMDARs [263]. In the presence of bicarbonate ions (HCO3−), BMAA enhances NMDAR activation, presumably leading to excessive Ca2+ influx and subsequent excitotoxic neuronal damage [264].
12.2. GLRs in Plants
Despite lacking a nervous system, plants have evolved systemic signaling mechanisms to coordinate whole-body responses to environmental challenges. These long-distance signaling pathways include electrical, Ca2+, ROS, and glutamate signals [265]. Interestingly, plants possess glutamate-receptor-like channels (GLRs), which are homologous to NMDARs [266]. Similar to their neuronal counterparts, plant GLRs regulate Ca2+ signaling in response to d-amino acids [267], pH changes [268], and redox modifications [269,270].
In plants, BMAA has been reported to induce hypocotyl elongation and inhibit cotyledon separation in light-grown seedlings [271]. This evolutionary parallel implies that iGluRs, including their ancestral bacterial prototypes, function as sensors for environmental stressors across biological kingdoms [266], potentially providing a universal mechanism for integrating diverse stress signals.
12.3. TRP Superfamily
The transient receptor potential (TRP) superfamily, comprising seven subfamilies, represents another cation channel prototype [272] that is evolutionarily distinct from the iGluRs but has members that are similar in responsiveness to ONS.
TRP channels function as “multiple signal integrators”, responding to chemical, thermal, osmotic, mechanical, and oxidative stimuli [272]. Although first discovered in Drosophila (TRPC) as photoreceptor-associated channels, TRP channels are conserved across biological kingdoms, including unicellular algae, fungi, and eukaryotes [273].
The ability of TRP channels to integrate diverse environmental and intracellular signals highlights their essential role in cellular homeostasis and adaptation. In humans, TRP channels are involved not only in pain perception, vascular tone regulation, neuronal excitability, and inflammatory responses but also in various pathophysiological processes, making them critical pharmacological targets [274,275,276,277,278].
Notably, plant-derived natural compounds, such as coumarin osthole from traditional medicinal herb Cnidium monnieri [279], cannabinoids from Cannabis (Cannabis sativa) [280], and the phytoestrogen genistein (4′,5,7-trihydroxyisoflavone) from soybeans (Glycine max) [281], have recently been shown to inhibit certain TRP channels [278]. These findings further support the link between plant adaptation mechanisms and human health, emphasizing the potential for plant-derived compounds to modulate TRP channel activity.
12.4. TRP Channels in Response to ONS
TRP channel activity has been reported to be regulated by O2, NO, and H2S. In Drosophila, TRP channel activity is enhanced by classical mitochondrial uncouplers (chemical H+ transporter) or anoxic conditions [282]. In the free-living nematode Caenorhabditis elegans, TRP channels are required for O2 sensing and movement behavior [283].
In in vitro studies, certain TRP families have been shown to induce Ca2+ entry into cells in response to NO, through either direct S-nitrosylation of specific cysteine residues [274,275] or indirect mechanisms [272]. Similarly, H2S has been demonstrated to activate TRP channels in the brain via polysulfide formation [161,284]. Additionally, many TRP channel families exhibit redox sensitivity, with functional cysteine residues modulated by ROS, RNS, and RSS, further emphasizing their role as key molecular sensors of oxidative stress [285].
12.5. The Minimum Machinery for Selye’s “Filter” Function
To maintain homeostasis, organisms must detect and respond to environmental changes. Early single-celled organisms likely encountered pH fluctuations, temperature shifts, and osmolarity stress as primary stressors. As multicellularity evolved, cell–cell communication mechanisms became essential for coordinating responses to biotic and abiotic stressors.
Since neural communication is a specialized form of cell–cell signaling [286,287], it is unsurprising that naturally occurring compounds from plants and algae—such as BMAA—act as neurotoxins. This suggests that neurodegenerative diseases may arise from dysfunctions in conserved cellular communication mechanisms shared across cyanobacteria, plants, and humans.
13. Updating Selye’s General Adaptation Syndrome (GAS) Model
13.1. O2–NO–H2S (ONS) Homeostasis Model
To address the question posed in the introduction—“What molecular mechanisms enable a nonspecific response to diverse stressors?”—one may consider the possibility that the multi-sensitivity of iGluRs and TRP channels to O2, NO, and H2S may constitute a minimal essential system for translating diverse stress signals into Ca2+-mediated physiological responses.
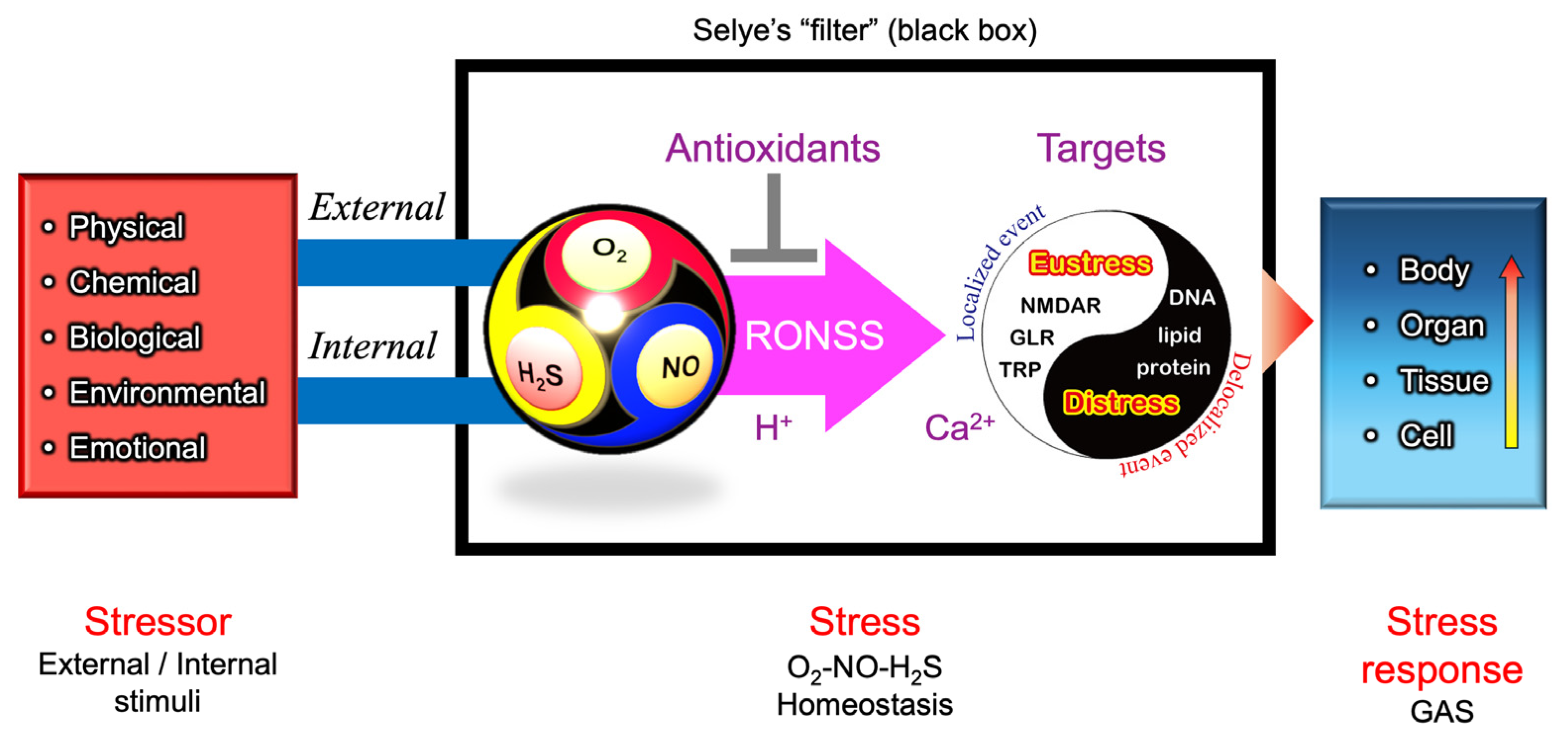
In conjunction with other specialized mechanisms, these Ca2+-permeable channels may serve as the molecular basis of Selye’s hypothesized “filter” (Figure 4)—a mechanism that integrates various stress signals into a unified, nonspecific adaptation response.
Figure 9 presents a hypothetical O2–NO–H2S (ONS) homeostasis model, refining Selye’s original stress “filter” concept (Figure 4). To align with the core principles of Selye’s stress theory, a simple and universal mechanism applicable across biological kingdoms is needed. In natural environments, the relative ratios of O2, NO, and H2S fluctuate due to both internal metabolic activities and external environmental factors including light intensity (e.g., diurnal cycles of UV and visible light), water availability (e.g., rainfall events), and temperature variations (e.g., daily and seasonal fluctuations). Additionally, biological interactions—including pathogenic, parasitic, and symbiotic relationships—further modulate the balance of these three gases.
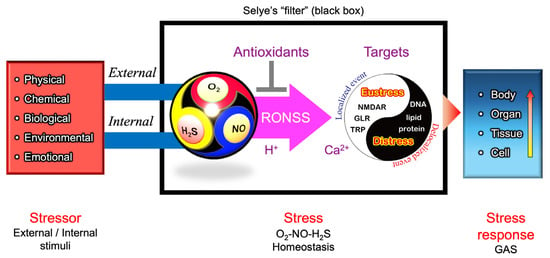
Figure 9.
A conceptual model for an updated version of Selye’s General Adaptation Syndrome (GAS). O2, NO, and H2S are dynamically produced and consumed through various biological processes. Their reactive derivatives—reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) (collectively referred to as RONSS)—interact in a pH-dependent manner, shaping redox homeostasis. The multi-sensitivity of thiol-containing ionotropic glutamate receptors (iGluRs) and transient receptor potential (TRP) channels suggests that they may function as signal integrators through thiol modifications, translating external and internal stimuli into cellular and physiological stress responses via Ca2+-dependent signaling pathways. Under localized conditions, RONSS-associated signaling promotes adaptive (eustress) responses. However, excessive delocalized RONSS generation, such as in pathogen responses, leads to nonspecific biomacromolecule modifications, resulting in oxidative distress, cellular damage, dysfunction, and, ultimately, disease or death [166].
Thiol (–SH) residues in cysteine react with ROS, RNS, and RSS (Table 1), generating a diverse array of reaction products (Table 2). These products function as molecular switches, transducing stimuli into physiological responses and contributing to the nonspecific stress response.
Changes in O2–NO–H2S balance, along with local pH or proton availability, influence chemical interactions among ROS, RNS, and RSS (collectively, RONSS). This leads to modifications of functional thiol groups in TRP channels and iGluRs, including NMDARs in animals, and GLRs in plants. These modifications can initiate Ca2+-dependent signaling pathways, triggering localized redox reactions (eustress).
In contrast, delocalized RONSS generation may lead to delocalized redox reactions, causing oxidative distress, physiological dysfunction, and pathological conditions [166]. These processes are not independent but are interconnected within a delicate adaptive balance.
Unlike Selye’s original model, the updated GAS framework offers testable experimental approaches—such as loss-of-function analyses using CRISPR-Cas9 or gene knockout methodologies targeting key components. These approaches allow researchers not only to evaluate the minimal machinery required for stress responses across biological kingdoms, but also to elucidate how living organisms transmit external and internal stimuli to cellular signaling systems through redox mediators such as ROS, RNS, and RSS.
13.2. Hypoxia: A Distinctive O2–NO–H2S Balance
In the context of the O2–NO–H2S balance, most contemporary plant and animal species thrive in oxygen-biased environments (normaxia), where oxygenic respiration supports survival and development. However, a wide range of taxa have evolved remarkable adaptations to hypoxic conditions.
High-resistance animals, such as certain fish species, turtles, and fossorial mammals, have developed specialized adaptations that enable survival during prolonged oxygen limitation [288]. These adaptations include metabolic depression, enhanced glycolytic capacity, and sophisticated oxygen-sensing mechanisms involving NMDA receptors [288]. In contrast, low-resistance animals—including humans—rapidly experience tissue damage and functional impairment under hypoxic conditions [288,289].
Among mammals, subterranean species such as the Spalax ehrenbergi superspecies exhibit high constitutive expression of hypoxia-inducible factor-1 (HIF-1) and vascular endothelial growth factor (VEGF), enabling survival in chronically hypoxic burrow environments [290]. In invertebrate model organisms such as Caenorhabditis elegans, hypoxic stress triggers transcriptional reprogramming via HIF-1-dependent pathways [266], along with additional regulatory mechanisms [291].
Although O2 is a by-product of oxygenic photosynthesis, plants lack a circulatory system like that of animals to distribute O2 to heterotrophic tissues such as roots, tubers, meristems, germinating pollen, and developing seeds. These organs—particularly roots under flooding—are vulnerable to oxygen deficiency. Recent advances in high-throughput technology have facilitated genome-wide transcriptomic analyses of plant responses to hypoxia, including root waterlogging and complete submergence [292]. In rice (Oryza sativa), submergence tolerance is conferred by traits like aerenchyma formation and the SUBMERGENCE1 (SUB1A) gene, which modulates gibberellin signaling to suppress shoot elongation under flooding conditions [293]. Aquatic ferns like Azolla exhibit root abscission in response to ROS, RNS, and RSS [294], allowing detachment from stressed sites and relocation to more oxygenated environments.
13.3. Involvement of NO and H2S Productions in Hypoxic Adaptation
NO and H2S function through interconnected signaling networks to modulate cellular responses to hypoxia. Under low-oxygen conditions, NO production via NOS is impaired due to their oxygen dependency, leading to reliance on the nitrate–nitrite–NO pathway for reductive NO generation [140,295,296]. This alternative pathway is also implicated in hypoxia and anoxia tolerance in plants [297].
In parallel, hypoxia-tolerant animals often show increased expression of H2S-producing enzymes such as cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) in response to low-oxygen environments [298,299]. H2S mitigates hypoxia-induced oxidative stress through multiple mechanisms: directly scavenging ROS, activating antioxidant defenses via Nrf2 signaling, and preserving mitochondrial function [300].
In mammals, crosstalk between NO (or nitrite) and H2S leads to the formation of nitrosopersulfide (SSNO−), a more stable NO donor under hypoxia [186]. Under normoxia, HIF-1α is hydroxylated by prolyl hydroxylase domain (PHD) enzymes and targeted for degradation, while H2S can enhance PHD2 activity via persulfidation, thereby modulating HIF-1α stability during stress [301].
Thus, understanding hypoxia in the context of the O2–NO–H2S triad is not only key to our understanding of ecological adaptation but also to our understanding of its physiological and pathological roles in human diseases [298,302], as outlined in Table 3.
14. The Concept of Balance Behind the Opposing Nature of Contributors to the Stress Response: Philosophical Implications
14.1. The Dynamic Harmony of Two Opposites
To address the second question posed in the introduction—“How can the same stressor lead to two opposing outcomes?”—here, we introduce the benefits of incorporating Asian philosophies into the holistic perspective underlying the principle of balance.
The coexistence of the opposing natures within a single entity is frequently described in the scientific literature using metaphors such as “two sides of the same coin” or a “double-edged sword”. Traditionally, this dual nature has been illustrated as a balance scale with two arms (Figure 10a). Recently, however, many researchers in stress studies have adopted the Yin–Yang symbol (Figure 10b), rather than a balance scale, to effectively represent the simultaneous presence and dynamic harmony of opposing extremes.
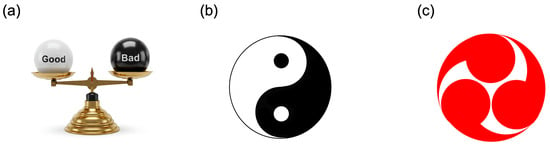
Figure 10.
Graphical representations of balance. (a) Balance scale model. (b) Yin–Yang symbol. (c) Mitsudomoe. The balance scale has often been used as a graphical metaphor in scientific debates centered on binary oppositions, such as good vs. bad, beneficial vs. harmful, protective vs. toxic, or oxidant vs. antioxidant. However, most natural phenomena do not conform to such simplifications. Instead, they are continuous, reversible, and dynamic. The Yin–Yang (shadow–light) symbol, based on the Chinese Taiji philosophy, represents the dynamic balance between two opposites. This concept has been incorporated into quantum physics, plant science, and, more recently, nitric oxide (NO) research. The Mitsudomoe (spinning threefold comma) is rooted in the concept of the threefold Taiji, a design commonly found on the roof tiles of Japanese shrines and temples, as well as on Japanese drums. Korean culture has inherited both the Taiji and the threefold Taiji (Samtaegeuk). The philosophy of dynamic harmony among three elements is also reflected in the Tri-Dosha concept of Ayurveda in India. These ancient philosophies form the foundation of traditional medicine in China, India, Korea, and Japan.
The Yin–Yang (light and shadow in its English translation) symbol, often associated with ancient Chinese philosophy, is a graphical representation of the concepts of dualism, balance, and interdependence in the natural world. It reflects the harmony of opposites and the idea that seemingly contradictory forces are interconnected and mutually dependent. The Yin–Yang symbol serves as a reminder of the harmony and interconnectivity of all aspects of life and the universe. It encourages the acceptance of duality and the pursuit of harmony within opposing forces, aligning well with the characteristics of key molecules in oxidative stress and the definitions of terminology in Selye’s stress concept.
14.2. The Yin–Yang Principle in Modern Science
The concept of Yin–Yang has influenced numerous prominent figures in science and philosophy, leading to profound insights and applications across various disciplines. Following a visit to China in 1937, Niels Bohr, a theoretical physicist who made foundational contributions to our understanding of atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922, evidently recognized the philosophical relevance of Yin and Yang to his theory of complementarity [303]. Carl Jung’s analytical psychology incorporated Yin–Yang-like dualities, such as “anima” and “animus”, to explore the balance between the unconscious and conscious elements of the human psyche [304]. Fritjof Capra explicitly linked modern physics with the Eastern philosophies underlying Taoism, Buddhism, Hinduism, and Shintoism, highlighting Yin–Yang as a bridge between science and Eastern thought in his book The Tao of Physics (1975) [303].
In the 1990s, Barry Osmond, a specialist in photosynthesis, introduced the Yin–Yang symbol into photosynthetic research to represent the dynamic coexistence of two opposing forces [305]. His philosophical influence is evident in the former logo of the Research School of Biological Sciences (RSBS) at the Australian National University, as well as in the logo of the Journal of Experimental Botany [306]. Inspired by his philosophy, we have applied the Yin–Yang symbol to illustrate the dual nature of flavonoids (antioxidant/prooxidant) [98], vitamin C (antioxidant/prooxidant) [152], and NO (signaling function/cytotoxicity) [152,307] (nitrite/NO) [149]. Lundberg, Weitzberg, and Gladwin also apply the Yin–Yang symbol representation to account for the coexistence of oxidative and reductive mechanisms for NO synthesis [140].
By framing their immunological observations within the Yin–Yang paradigm, Santambrogio and Franco provided a conceptual model to illustrate how self-tolerance and immune responses are interconnected, emphasizing the importance of maintaining a dynamic equilibrium to prevent diseases such as autoimmunity and chronic inflammation [308]. More recently, Uvnäs-Moberg et al. discussed the interaction between the hormonal and neuronal arms of both the hypothalamic–pituitary–adrenal (HPA) axis and the oxytocinergic system using the Yin–Yang framework [309]. Collectively, these recent studies demonstrate the enduring relevance of Yin–Yang as a unifying principle across disciplines.
14.3. The Balance of Threefold Elements: Evolution of the Yin–Yang Principle
As shown in Figure 7, research on O2 (ROS), NO (RNS), and H2S (RSS) has historically progressed in parallel. It has become evident that each of these reactive molecules competes for the same targets, such as heme and cysteine thiols (–SH), in cellular regulation. At the same time, they can cause oxidative modifications to proteins, lipids, DNA, RNA, and polysaccharides, leading to metabolic dysfunction, mutations, and cell death. Each of these molecules exhibits a Yin–Yang-like continuous duality, namely, a signaling function (positive) and oxidative damage (negative).
The Yin–Yang symbol is also referred to as Taiji. As an advanced concept of the Yin–Yang principle, the threefold Taiji emphasizes the significance of dynamic interactions among three elements. Since 2005, we have advocated the need for balance between ROS, RNS, and RSS—an aspect not widely recognized at the time—for a deeper understanding of the physiological functions of these reactive molecules, adopting the Japanese Mitsudomoe [310]. Mitsudomoe (Figure 10c), a family crest that was once popular among samurai families, is frequently found on the roof tiles of Japanese shrines and temples. In Korea, Samtaegeuk, which evolved from Taiji philosophy, represents a triadic balance of heaven, earth, and humanity. In India, Tri-Dosha is a fundamental concept in Ayurveda (Indian traditional medicine) that explains human health and constitution through three vital energies (Doshas). The balance of these three dynamic elements remains central to both traditional Indian and Korean medicine even today. It appears that Asian countries have culturally shared a very similar philosophical concept regarding the balance of three dynamic forces—an idea that resonates with the principles underlying the three-body problem (Figure 8b).
15. Bridging Modern Science and Traditional Medicines While Emphasizing Balance
15.1. Traditional Eastern Medicines
Traditional medicine in China, Korea, Japan, and India shares ancient roots but has evolved into distinct systems. Traditional Chinese Medicine (TCM) emphasizes herbal medicine, acupuncture, and Yin–Yang theory, while Traditional Korean Medicine (TKM) incorporates Sasang Constitutional Medicine. Kampo (Japanese traditional medicine) focuses on simplified herbal formulas and integration with modern medical practices, whereas Ayurveda in India is based on the balance of three doshas (Vata, Pitta, and Kapha) and incorporates herbal treatments, diet, and yoga. These systems continue to coexist alongside modern Western medicine, with specialized professionals ensuring their continued practice.
A notable moment in the global recognition of TCM occurred in 1971, preceding President Nixon’s historic visit to China in 1972. New York Times journalist James Reston, while in Beijing, underwent an emergency appendectomy and received acupuncture for post-operative pain management. His subsequent newspaper article describing the experience helped generate widespread interest in acupuncture in the United States, marking a significant moment of “East meets West” in medical history [311].
15.2. Acupoints and Meridians
Acupuncture, acupressure, and moxibustion have been integral to TCM for thousands of years. Based on clinical trials evaluated by modern medical standards, the World Health Organization (WHO) has endorsed acupuncture for specific medical applications [79].
Acupoints, which are central to acupuncture, acupressure, and moxibustion, are believed to possess distinct physiological properties that mediate their therapeutic effects. Surprisingly, Traditional Chinese Veterinary Medicine (TCVM) has documented the use of acupuncture in animals for over 3000 years, not only in domesticated species such as horses, cattles, pigs, dogs, and cats, but also in exotic animals such as elephants and tigers [312].
Recent research has demonstrated the presence of acupoints in frogs, lizards, birds, and even giant pandas [312]. This suggests that animals share similar physiological responses to acupoint stimulation, further supporting the potential universality of acupuncture’s mechanisms across species.
TCM proposes that acupoints are connected through “meridians”, conceptual pathways through which the vital energy “qi” flows. Despite its demonstrated efficacy, the underlying mechanisms of acupuncture remain largely unknown, and no definitive anatomical evidence has been found to support the physical existence of acupoints or meridians. However, recent research suggests that NO may play a crucial role in these enigmatic points.
15.3. NO Generation at Acupoints
A number of studies have shown that acupoint stimulation via acupuncture enhances NO production [313]. Su et al. investigated the role of acupuncture in modulating ROS and RNS in the context of oxidative stress and proposed that acupuncture functions as a multi-target antioxidant therapy in ischemic stroke [314]. Ma et al. demonstrated NO generation at skin acupoints using a non-invasive device in humans [315], while Tang et al. conducted real-time monitoring of NO release from acupoints in vivo and observed L-arginine-dependent local NO generation [316].
Collectively, these studies suggest that NO levels at acupoints are significantly higher than those in brain regions and blood vessels. It is intriguing to hypothesize that NO may represent the physiological basis of qi (or “ki” in Japanese pronunciation), generated at acupoints in response to external stimuli, including mechanical and thermal stress. Although further validation is required, the application of recent technologies, such as acupuncture needles equipped with NO microsensors [317], may elucidate the dynamic NO response at acupoints, offering the first solid evidence to bridge modern science with traditional medicine and the philosophy of balance.
15.4. Stimulation to a Minimum Machinery
From a biophysical perspective, acupuncture, acupressure, and moxibustion exert mechanical and thermal stresses on tissues, potentially activating NMDARs and TRP channels to transmit Ca2+ signals that elicit whole-body responses. Recent advancement in lipid structural analysis has concluded that compartmentalization of such signal transduction systems is associated with the formation of special lipid domains.
The plasma membrane in eukaryotic cells contains microdomains enriched with sterols (such as cholesterol), forming membrane/lipid rafts (MLR) [318,319,320,321]. These regions may exist as caveolae, which appear morphologically as flask-like invaginations, or in a less observable planar form [322,323,324]. MLR include O2•− generators such as NAD(P)H oxidase (Nox) in animals [325] and respiratory burst oxidase homologs (RBOHs) in plants [326], as well as NO generators such as eNOS [325,327,328]. Additionally, signaling receptor [329] and ion channels [330]—including NMDARs [331] and TRP channels [332]—within MLR facilitate the communication of extracellular stimuli to the intracellular milieu [322,333]. One may hypothesize that cell membranes at acupoints are particularly rich in lipid rafts or lipid nano-environments [334]—specialized membrane microdomains that serve as a “toolkit” for signal transduction [166]. The dynamic response of these microdomains to mechanical and thermal stimuli may provide a compelling explanation for the physiological effects observed in acupuncture therapy.
16. Future Perspectives
16.1. O2–NO–H2S (ONS) Dynamics from Physiological, Ecological, and Evolutionary Perspectives
Figure 11 presents a conceptual O2–NO–H2S (ONS) balance meter, inspired by the Mitsudome symbol (Figure 10c), illustrating how living organisms on Earth have adapted to varying proportions of O2, NO, and H2S (ONS) [159]. This ONS gradient is observed across diverse natural environments, ranging from the surface to deeper layers of the atmosphere, soils, and oceans.
At the surface of land and aquatic natural environments, where solar energy is available, photosynthetic organisms contribute to O2 production through oxygenic photosynthesis, shaping the upper layers of the biosphere rich in O2. However, O2 availability becomes lower in deeper layers of these environments due to microbial respiration. In these hypoxic or anoxic environments, sulfur-oxidizing, sulfate-reducing, nitrifying, and denitrifying bacteria establish microbial communities [38,335,336], reflecting the biochemical diversity along the ONS gradient [159].
In addition to these macroscopic landscapes, shifts in the ONS gradient can also be observed at microscopic, cellular, tissue, or organ levels within the body, such as in the gastrointestinal tracts of animals under normal conditions, as well as in organs affected by disease or pathological conditions [166] such as inflammatory bowel disease (Table 3).
The ONS gradient is present in stromatolites, fossilized microbial structures that provide insights into early life on Earth [159]. This gradient reflects not only ecological distributions but also the evolutionary development of energy conversion systems (H2S → NO3− → O2). Interestingly, our research progress appears to trace the history of evolution, moving from the present to ancient times (O → N → S) (Figure 11).
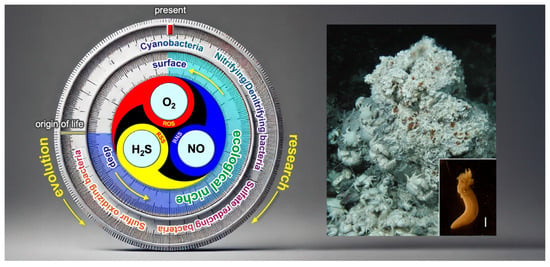
Figure 11.
A graphical representation of the O2–NO–H2S (ONS) balance meter. The left illustration presents a conceptual O2–NO–H2S (ONS) balance meter, depicting how living organisms on Earth have adapted to varying proportions of oxygen (O2), nitric oxide (NO), and hydrogen sulfide (H2S). This ONS gradient exists across different environments, ranging from surface habitats to deeper layers of soils and oceans, and even in the gastrointestinal tracts of animals. In these environments, sulfur-oxidizing, sulfate-reducing, nitrifying, and denitrifying bacteria form microbial communities, reflecting the biochemical diversity along the gradient. This gradient not only represents ecological distributions but also illustrates the biological evolution of energy conversion systems. To fully understand universal stress response mechanisms across biological kingdoms, it is essential to investigate not only mechanistic insights using model organisms but also the diversity of adaptation mechanisms in non-model organisms that have evolved in distinct ONS environments. The right image shows a photograph of a deep-sea hydrothermal vent at Hatoma Knoll [337], located at a depth of 1500 m in the Okinawa Trough, taken during the JAMSTEC NT07-12 cruise [338]. The inset image depicts one of the polychaete worms Paralvinella spp. assembled near the vent. White bar = 2 mm.
Figure 11.
A graphical representation of the O2–NO–H2S (ONS) balance meter. The left illustration presents a conceptual O2–NO–H2S (ONS) balance meter, depicting how living organisms on Earth have adapted to varying proportions of oxygen (O2), nitric oxide (NO), and hydrogen sulfide (H2S). This ONS gradient exists across different environments, ranging from surface habitats to deeper layers of soils and oceans, and even in the gastrointestinal tracts of animals. In these environments, sulfur-oxidizing, sulfate-reducing, nitrifying, and denitrifying bacteria form microbial communities, reflecting the biochemical diversity along the gradient. This gradient not only represents ecological distributions but also illustrates the biological evolution of energy conversion systems. To fully understand universal stress response mechanisms across biological kingdoms, it is essential to investigate not only mechanistic insights using model organisms but also the diversity of adaptation mechanisms in non-model organisms that have evolved in distinct ONS environments. The right image shows a photograph of a deep-sea hydrothermal vent at Hatoma Knoll [337], located at a depth of 1500 m in the Okinawa Trough, taken during the JAMSTEC NT07-12 cruise [338]. The inset image depicts one of the polychaete worms Paralvinella spp. assembled near the vent. White bar = 2 mm.
To fully understand dynamic redox reactions in cells, it is essential to monitor local concentrations of O2, NO, and H2S, which are the primary sources of ROS, RNS, and RSS, with high spatiotemporal resolution in a non-invasive manner. Although oxygen-enhanced magnetic resonance imaging (OE-MRI) has advanced as a tool for mapping O2 distribution and is now applied in cancer diagnosis [339,340], there is currently no definitive methodology that allows for simultaneous, non-invasive monitoring of local O2, NO, and H2S concentrations.
Given that intrinsic chlorophyll fluorescence is an effective tool for studying plant stress, fluorescence-based techniques—when combined with recent synergistic advancements in imaging technologies—hold great potential for in situ detection of ONS dynamics. Recently, Dong et al. developed an NO-H2S dual-responsive probe, SAB-NH-SC, which generates distinct fluorescence signals in response to both H2S and NO, enabling visualization of intracellular fluctuations in their levels [70].
Future research should prioritize the development of novel methodologies for real-time monitoring of ONS dynamics with a high spatiotemporal resolution, which will provide deeper insights into stress responses across biological systems.
16.2. Special Solutions to the Three-Body Problem in Stress Biology
In classical physics, the three-body problem lacks a general solution due to its nonlinear and chaotic dynamics, yet special solutions—such as those of Euler and Lagrange—can be derived under simplified or symmetrical conditions [189]. A similar approach may help uncover predictable patterns in the complex redox interactions among ROS, RNS, and RSS in biological systems.
Among diverse species, ROS fluctuations are particularly sensitive to oxygen levels, which vary widely across environmental and physiological contexts—from anoxia and normoxia to the supersaturation observed in photosynthesizing plants. By identifying systems with constrained oxygen availability or simplified redox interactions, it may be possible to isolate biologically meaningful “special solutions”.
Comparative studies of hypoxia- and anoxia-resistant animals provide valuable opportunities to investigate simplified redox dynamics under extreme conditions [341]. Freshwater turtles from the genera Trachemys and Chrysemys can tolerate prolonged periods of severe hypoxia [342,343]. In these species, NO, H2S, and their associated RNS and RSS play central roles in coordinating physiological responses to oxygen deprivation [143].
Interestingly, anoxia-tolerant turtles appear to have evolved NO-independent mechanisms to maintain cerebral blood flow (CBF), potentially involving NMDAR signaling [343]. In contrast, mammals rely on NO-mediated vasodilation during hypoxia, which can exacerbate glutamatergic excitotoxicity and trigger DNA fragmentation in neural tissue [343].
These uniquely constrained redox environments—whether arising from evolutionary adaptations or specific ecological niches—offer promising models for dissecting the nonlinear roles of O2, NO, and H2S. Investigating such systems may help identify biologically grounded “special solutions” to the three-body problem in stress biology, with potential applications in developing therapies for hypoxia-related conditions such as stroke and cardiac ischemia [341].
These interspecies differences reflect distinct redox dynamics shaped by ecological and evolutionary pressures. Comparative analysis of such models may help define boundary conditions under which ROS, RNS, and RSS interactions can be simplified or amplified, thus providing biologically grounded “special solutions” to the three-body problem in redox biology.
16.3. The Search for Missing Links
Systems biology and omics-based approaches employ network-level analyses to integrate known components and elucidate complex biological interactions. However, these methods may produce misleading interaction maps if key, yet unidentified, elements are missing. Research in stress biology and medicine has predominantly focused on a limited number of major model organisms. To uncover missing links and complete the broader picture, greater attention should be directed toward non-model species—as demonstrated in studies involving freshwater turtles.
It has been proposed that cell–cell signaling by amino acids in animal brains may have evolved from a primitive signaling mechanism that existed before plants and animals diverged [344]. In fact, chemical sensors, including those detecting small molecules such as purines, have been identified in prokaryotes [345]. For a better understanding of neuronal brain functions and disease, it is crucial to evaluate the broader role of iGluRs in non-model animals, plants, and prokaryotes, as universal stress sensors across evolutionary lineages.
Just as studies of turtles have advanced our understanding of NO- and H2S-associated hypoxia tolerance mechanisms, identifying missing links in the evolution of such adaptive systems requires examining organisms that inhabit extreme environments characterized by a distinct ONS balance. Deep-sea hydrothermal vents represent particularly strong candidates for this purpose [300] (Figure 11). These environments support a unique, solar-independent ecosystem that relies not on photosynthesis but on chemosynthesis—driven by H2S emitted from thermal vents.
A major scientific breakthrough occurred in 1977 when large deep-sea animals, such as the giant tube worm Riftia pachyptila, were discovered at the Galápagos Rift hydrothermal vents [346]. Near these high-temperature zones, where vent water temperatures exceed 300 °C and are rich in H2S, specialized organisms such as polychaete worms thrive (Figure 11).
The exploration of deep-sea hydrothermal vents—closely linked to the origin of life hypothesis [347]—not only contributes to our understanding of universal stress response mechanisms but also holds promise for novel drug discovery [348] and may provide insights into the potential existence of extraterrestrial life forms [349].
17. Conclusions
Hans Selye’s theory of stress laid the conceptual foundation for our understanding of how organisms respond to internal and external challenges. Over the past century, this concept has been adapted across multiple disciplines in biology and medicine, but such broad application has led to divergent definitions. One reason may be the absence of a universal indicator of stress that is applicable across biological kingdoms.
A major milestone in the evolution of stress biology research was the introduction of the concept of oxidative stress, which highlights the cellular actions of ROS. This was followed by the discoveries of NO as an RNS and H2S as an RSS, both of which expand the redox landscape of stress signaling. While these species serve as critical signaling molecules, they also have the potential to cause cellular damage and death. This duality underscores the central dilemma that Selye left unresolved: what determines whether a stress response results in adaptation (eustress) or in pathology (distress)?
Despite major advances in redox biology, including growing insights into ROS, RNS, and RSS, we still lack a unifying framework to explain their collective and context-dependent effects on stress outcomes. These reactive species do not function independently, but interact in nonlinear and dynamic ways that influence survival, inflammation, repair, and degeneration. Their interdependent behavior—stochastic and often unpredictable—parallels the complexity of the three-body problem in physics, which famously lacks a general analytical solution. However, just as physics has progressed through simplified models under defined boundary conditions, biological insights can emerge from studying redox systems under specific constraints, such as hypoxia or redox-limited environments.
In his 1967 book In Vivo: The Case for Supramolecular Biology [28], Selye described two types of scientists: “problem solvers”, who begin with established knowledge and dissect it methodically, and “problem finders”, who rely on intuition, pattern recognition, and a broad sense of significance. The latter, he argued, often struggle for recognition—dismissed in their time as mystics or pseudoscientists [28]. Selye himself, despite being nominated for the Nobel Prize ten times, never received the award [26].
Together with Walter Cannon, who introduced the principle of homeostasis, Selye can be viewed as a quintessential problem finder—an originator of holistic thinking whose influence continues to shape life sciences today. Although we still lack a complete molecular account of stress, the foundational concepts they advanced—balance, regulation, and system integration—remain central to biology and medicine.
Werner Heisenberg, awarded the Nobel Prize in Physics in 1932 for his pioneering work in quantum mechanics, famously remarked, “The most fruitful developments frequently take place at those points where two different lines of thought meet” [303]. Looking ahead, integrative approaches will be essential. Bridging molecular redox biology with insights from neuroscience, immunology, plant physiology, and evolutionary ecology will be key to understanding how organisms maintain—or fail to maintain—homeostasis under stress.
By embracing both reductionist and holistic perspectives, we may finally approach resolution of Selye’s enduring question: what defines the tipping point between adaptation and disease? In doing so, we may also rediscover a pathway that reconnects modern science with traditional philosophical insight.
Author Contributions
Conceptualization, H.Y.; writing—original draft preparation, H.Y.; writing—review and editing, R.F.N., K.B.M. and M.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI grant number 23K05493, granted to H.Y.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Helmut Sies for generously sharing his insights into the concept of oxidative stress. We are also grateful to George Iwama for his critical reading of the manuscript. This paper is dedicated to the memory of Mitsuo Nishimura (1931–2024), whose guidance introduced H.Y. to redox biology in the context of bioenergetics.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GAS | General Adaption Syndrome |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| RSS | Reactive Sulfur Species |
| RONSS | Reactive Oxygen, Nitrogen, and Sulfur Species |
| iGluR | Ionotropic Glutamate Receptors |
| NMDAR | N-methyl-D-aspartate Receptor |
| GLR | Glutamate-Receptor-Like Channel |
| TRP | Transient Receptor Potential |
References
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, aat5314. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Dimsdale, J.E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 2008, 51, 1237–1246. [Google Scholar] [CrossRef]
- Lazarus, R. From psychological stress to the emotions: A history of changing outlooks. Annu. Rev. Psychol. 1993, 44, 1–21. [Google Scholar] [CrossRef]
- Selye, H. What is stress? Metabolism 1955, 5, 525–530. [Google Scholar]
- Selye, H. Stress and disease. Science 1955, 122, 625–631. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.N. Stress hypothesis overload: 131 hypotheses exploring the role of stress in tradeoffs, transitions, and health. Gen. Comp. Endocrinol. 2020, 288, 113355. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Wubshet, N.H.; Cai, G.; Chen, S.J.; Sullivan, M.; Reeves, M.; Mays, D.; Harrison, M.; Varnado, P.; Yang, B.; Arreguin-Martinez, E.; et al. Cellular mechanotransduction of human osteoblasts in microgravity. NPJ Microgravity 2024, 10, 35. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kang, J.H. Mechanical properties of compact bone defined by the stress-strain curve measured using uniaxial tensile test: A concise review and practical guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef]
- Rodrigo-Navarro, A.; Sankaran, S.; Dalby, M.J.; del Campo, A.; Salmeron-Sanchez, M. Engineered living biomaterials. Nat. Rev. Mater. 2021, 6, 1175–1190. [Google Scholar] [CrossRef]
- Yokoyama, M.; Gril, J.; Matsuo, M.; Yano, H.; Sugiyama, J.; Clair, B.; Kubodera, S.; Mistutani, T.; Sakamoto, M.; Ozaki, H.; et al. Mechanical characteristics of aged Hinoki wood from Japanese historical buildings. Comptes Rendus Phys. 2009, 10, 601–611. [Google Scholar] [CrossRef]
- Baloh, R.W. Biological mechanisms of psychosomatic symptoms. In Medically Unexplained Symptoms: A Brain-Centered Approach; Baloh, R.W., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 81–98. [Google Scholar]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Bracha, H.S.; Ralston, T.C.; Matsukawa, J.M.; Williams, A.E.; Bracha, A.S. Does “fight or flight” need updating? Psychosom 2004, 45, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.B. The Wisdom of the Body; W. W. Norton and Company, Inc.: New York, NY, USA, 1932. [Google Scholar]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear, and Rage: An Account of Recent Researches into the Function of Emotional Excitement; D. Appleton and Company: New York, NY, USA, 1915. [Google Scholar]
- Tan, S.Y.; Yip, A. Hans Selye (1907–1982): Founder of the stress theory. Singap. Med. J. 2018, 59, 170–171. [Google Scholar] [CrossRef]
- Szabo, S.; Tache, Y.; Somogyi, A. The legacy of Hans Selye and the origins of stress research: A retrospective 75 years after his landmark brief “letter” to the editor# of nature. Stress 2012, 15, 472–478. [Google Scholar]
- Selye, H. The evolution of the stress concept: The originator of the concept traces its development from the discovery in 1936 of the alarm reaction to modern therapeutic applications of syntoxic and catatoxic hormones. Am. Sci. 1973, 61, 692–699. [Google Scholar] [PubMed]
- Selye, H.; Szent-Györgyi, A. In Vivo: The Case for Supramolecular Biology: Presented in Six Informal, Illustrated Lectures; Liveright Publishing Corporation: New York, NY, USA, 1967. [Google Scholar]
- Mason, J.W. A re-evaluation of the concept of ‘non-specificity’in stress theory. J. Psyiatr Res. 1972, 8, 323–333. [Google Scholar]
- Hariom, S.K.; Ravi, A.; Mohan, G.R.; Pochiraju, H.D.; Chattopadhyay, S.; Nelson, E.J.R. Animal physiology across the gravity continuum. Acta Astronaut. 2021, 178, 522–535. [Google Scholar] [CrossRef]
- Mahdi, S.H.A.; Yamasaki, H.; Otaki, J.M. Heat-shock-induced color-pattern changes of the blue pansy butterfly Junonia orithya: Physiological and evolutionary implications. J. Therm. Biol. 2011, 36, 312–321. [Google Scholar] [CrossRef]
- Mahdi, S.H.; Gima, S.; Tomita, Y.; Yamasaki, H.; Otaki, J.M. Physiological characterization of the cold-shock-induced humoral factor for wing color-pattern changes in butterflies. J. Insect Physiol. 2010, 56, 1022–1031. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J. Responses of Plants to Environmental Stresses, 2nd ed.; Academic Press: New York, NY, USA, 1980; Volume 1. [Google Scholar]
- Cohen, M.F.; Mazzola, M.; Yamasaki, H. Nitric oxide research in agriculture; bridging the plant and bacterial realms. In Abiotic Stress Tolerance in Plants; Rai, A.K., Takabe, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 71–90. [Google Scholar]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Fifty-five years of research on photosynthesis, chloroplasts, and stress physiology of plants: 1958–2013. In Progress in Botany; Springer: Cham, Switzerland, 2015; pp. 3–42. [Google Scholar]
- Lichtenthaler, H.K. The stress concept in plants: An introduction. Ann. N. Y. Acad. Sci. 1998, 851, 187–198. [Google Scholar] [CrossRef]
- Schreiber, U.; Lichtenthaler, H. Hans Kautsky’s groundbreaking discovery(ies) in 1931, its scientific environment, and the ensuing developments. Photosynthetica 2025, 63, 20–28. [Google Scholar] [CrossRef]
- Murata, N. Control of excitation transfer in photosynthesis I. Light-induced change of chlorophyll a fluoresence in Porphyridium cruentum. Biochim. Biophys. Acta 1969, 172, 242–251. [Google Scholar] [CrossRef]
- Murata, N. The discovery of state transitions in photosynthesis 40 years ago. Photosynth. Res. 2009, 99, 155–160. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Sousaraei, N.; Mashayekhi, K.; Mousavizadeh, S.J.; Akbarpour, V.; Medina, J.; Aliniaeifard, S. Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Hortic. Environ. Biotechnol. 2021, 62, 521–535. [Google Scholar] [CrossRef]
- Septiana, A.; Nakamura, S.P.; Naomasa, R.F.; Yamasaki, H. Seawater tolerance of the beach bean Vigna marina (Burm.) Merrill in comparison with mung bean (Vigna radiata) and adzuki bean (Vigna angularis). Agriculture 2025, 15, 228. [Google Scholar] [CrossRef]
- Hossain, K.K.; Nakamura, T.; Yamasaki, H. Effect of nitric oxide on leaf non-photochemical quenching of fluorescence under heat-stress conditions. Russ. J. Plant Physiol. 2011, 58, 629–633. [Google Scholar] [CrossRef]
- Buonasera, K.; Lambreva, M.; Rea, G.; Touloupakis, E.; Giardi, M.T. Technological applications of chlorophyll a fluorescence for the assessment of environmental pollutants. Anal. Bioanal. Chem. 2011, 401, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Joiner, J.; Yoshida, Y.; Vasilkov, A.P.; Yoshida, Y.; Corp, L.A.; Middleton, E.M. First observations of global and seasonal terrestrial chlorophyll fluorescence from space. Biogeosciences 2011, 8, 637–651. [Google Scholar] [CrossRef]
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M.; Berry, J.A.; Frankenberg, C.; Huete, A.R.; Zarco-Tejada, P.; Lee, J.E.; et al. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, E1327–E1333. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakamura, T.; Sakamizu, M.; van Woesik, R.; Yamasaki, H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol. 2004, 45, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Campo, K.; Baums, I.B. Fitted Fv/Fm temperature response curves: Applying lessons from plant ecophysiology to acute thermal stress experiments in coral holobionts. Coral Reefs 2024, 44, 77–84. [Google Scholar] [CrossRef]
- Hoadley, K.D.; Lockridge, G.; McQuagge, A.; Pahl, K.B.; Lowry, S.; Wong, S.; Craig, Z.; Petrik, C.; Klepac, C.; Muller, E.M. A phenomic modeling approach for using chlorophyll-a fluorescence-based measurements on coral photosymbionts. Front. Mar. Sci. 2023, 10, 1092202. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Alvarez-Noriega, M.; Alvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- van Woesik, R.; Shlesinger, T.; Grottoli, A.G.; Toonen, R.J.; Vega Thurber, R.; Warner, M.E.; Marie Hulver, A.; Chapron, L.; McLachlan, R.H.; Albright, R.; et al. Coral-bleaching responses to climate change across biological scales. Glob. Change Biol. 2022, 28, 4229–4250. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef]
- Sies, H.; Chance, B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970, 11, 172–176. [Google Scholar] [CrossRef]
- Sies, H. Findings in redox biology: From H2O2 to oxidative stress. J. Biol. Chem. 2020, 295, 13458–13473. [Google Scholar] [CrossRef] [PubMed]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and x-irradiation: A mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef]
- Sakihama, Y.; Yamasaki, H. Phytochemical antioxidants: Past, present and future. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engle 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Li, Y.R.; Cai, Z.M.; Li, S.H.; Zhu, J.F.; Zhang, F.; Liang, S.S.; Zhang, W.W.; Guan, Y.L.; Shen, D.Q.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Tas, F.; Hansel, H.; Belce, A.; Ilvan, S.; Argon, A.; Camlica, H.; Topuz, E. Oxidative stress in breast cancer. Med. Oncol. 2005, 22, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Siegrist, J.; Sies, H. Disturbed redox homeostasis in oxidative distress: A molecular link from chronic psychosocial work stress to coronary heart disease? Circ. Res. 2017, 121, 103–105. [Google Scholar] [CrossRef]
- Sanchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef]
- Hamilton, T.L. The trouble with oxygen: The ecophysiology of extant phototrophs and implications for the evolution of oxygenic photosynthesis. Free Radic. Biol. Med. 2019, 140, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Yamasaki, H.; Nishimura, M. Non-vectorial light-induced H+ release from CF1-depleted thylakoid membranes. Absence of correlation to stoichiometric H+ production and consumption coupled to electron transfer. Plant Cell Physiol. 1988, 29, 1081–1084. [Google Scholar]
- Mehler, A.H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch. Biochem. Biophys. 1951, 33, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef]
- Takahama, U.; Nishimura, M. Formation of singlet molecular oxygen in illuminated chloroplasts. Effects on photoinactivation and lipid peroxidation. Plant Cell Physiol. 1975, 16, 737–748. [Google Scholar]
- Takahama, U.; Nishimura, M. Effects of electron donor and acceptors, electron transfer mediators, and superoxide dismutase on lipid peroxidation in illuminated chloroplast fragments. Plant Cell Physiol. 1976, 17, 111–118. [Google Scholar] [CrossRef]
- Takahama, U.; Takahashi, K. Suppression of lipid peroxidation by β-carotene in illuminated chloroplast fragments: Evidence for β-carotene as a quencher of singlet molecular oxygen in chloroplasts. Plant Cell Physiol. 1978, 19, 1565–1569. [Google Scholar]
- Kaiser, W. The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim. Biophys. Acta 1976, 440, 476–482. [Google Scholar] [CrossRef]
- Kaiser, W.M. Reversible inhibition of the calvin cycle and activation of oxidative pentose phosphate cycle in isolated intact chloroplasts by hydrogen peroxide. Planta 1979, 145, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; De Gara, L.; Asard, H.; Horemans, N. Ascorbate and glutathione: Guardians of the cell cycle, partners in crime? Plant Physiol. Biochem. 2002, 40, 537–548. [Google Scholar] [CrossRef]
- Chew, O.; Whelan, J.; Millar, A.H. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003, 278, 46869–46877. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: Results from three prospective cohort studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 360203. [Google Scholar] [CrossRef]
- Yamasaki, H.; Uefuji, H.; Sakihama, Y. Bleaching of the red anthocyanin induced by superoxide radical. Arch. Biochem. Biophys. 1996, 332, 183–186. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Yamasaki, H. A function of colour. Trends Plant Sci. 1997, 2, 7–8. [Google Scholar] [CrossRef]
- Takahashi, S.; Tamashiro, A.; Sakihama, Y.; Yamamoto, Y.; Kawamitsu, Y.; Yamasaki, H. High-susceptibility of photosynthesis to photoinhibition in the tropical plant Ficus microcarpa L. f. cv. Golden Leaves. BMC Plant Biol. 2002, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Ferris, B.G., Jr. Health effects of exposure to low levels of regulated air pollutants: A critical review. J. Air Pollut. Control Assoc. 1978, 28, 482–497. [Google Scholar] [CrossRef]
- Yamasaki, H. Nitrite–dependent nitric oxide production pathway: Implications for involvement of active nitrogen species in photoinhibition in vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1477–1488. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J. Physiol. Pharmacol. 2002, 53, 503–514. [Google Scholar]
- Furchgott, R.F. Endothelium-derived relaxing factor: Discovery, early studies, and identifcation as nitric oxide (nobel lecture). Angew. Chem. Int. Ed. 1999, 38, 1870–1880. [Google Scholar] [CrossRef]
- Davis, K.L.; Martin, E.; Turko, I.V.; Murad, F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 203–236. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Konstadt, S. Nitric oxide: Has it progressed from molecule of the year to wonder drug of the decade. J. Cardiothorac. Vasc. Anesth. 1995, 9, 625–626. [Google Scholar] [CrossRef]
- Jackson, G.; Gillies, H.; Osterloh, I. Past, present, and future: A 7-year update of Viagra (sildenafil citrate). Int. J. Clin. Pract. 2005, 59, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.E.; Ohlsson, A.; Shah, P.S. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst. Rev. 2017, 8, CD005494. [Google Scholar] [CrossRef]
- Safaee Fakhr, B.; Wiegand, S.B.; Pinciroli, R.; Gianni, S.; Morais, C.C.A.; Ikeda, T.; Miyazaki, Y.; Marutani, E.; Di Fenza, R.; Larson, G.M.; et al. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 1109–1113. [Google Scholar] [CrossRef]
- Oliynyk, O.V.; Rorat, M.; Strepetova, O.V.; Dubrov, S.O.; Guryanov, V.G.; Oliynyk, Y.V.; Kulivets, O.S.; Slifirczyk, A.; Barg, W. Efficacy of sildenafil in patients with severe COVID-19 and pulmonary arterial hypertension. Viruses 2023, 15, 1157. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333. [Google Scholar] [CrossRef]
- Travis, J. NO-making enzyme no more: Cell, PNAS papers retracted. Science 2004, 306, 960. [Google Scholar] [PubMed]
- Zemojtel, T.; Fröhlich, A.; Palmieri, M.C.; Kolanczyk, M.; Mikula, I.; Wyrwicz, L.S.; Wanker, E.E.; Mundlos, S.; Vingron, M.; Martasek, P. Plant nitric oxide synthase: A never-ending story? Trends Plant Sci. 2006, 11, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.; Galli, M.; Tischner, R.; Heimer, Y.; Okamoto, M.; Mack, A. Response to Zemojtel et al.: Plant nitric oxide synthase: Back to square one. Trends Plant Sci. 2006, 11, 526–527. [Google Scholar] [CrossRef]
- Moreau, M.; Lee, G.I.; Wang, Y.; Crane, B.R.; Klessig, D.F. AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J. Biol. Chem. 2008, 283, 32957–32967. [Google Scholar] [CrossRef]
- Frohlich, A.; Durner, J. The hunt for plant nitric oxide synthase (NOS): Is one really needed? Plant Sci. 2011, 181, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Cohen, M.F. NO signal at the crossroads: Polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci. 2006, 11, 522–524. [Google Scholar] [CrossRef]
- Yamasaki, H.; Itoh, R.D.; Bouchard, J.N.; Dghim, A.A.; Hossain, K.K.; Gurung, S.; Cohen, M.F. Nitric oxide synthase-like activities in plants. In Annual Plant Reviews; Foyer, C., Zhang, H., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; Volume 42, pp. 103–125. [Google Scholar]
- Yamasaki, H.; Sakihama, Y.; Takahashi, S. An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci. 1999, 4, 128–129. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000, 468, 89–92. [Google Scholar]
- Sakihama, Y.; Nakamura, S.; Yamasaki, H. Nitric oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii: An alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol. 2002, 43, 290–297. [Google Scholar]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Desikan, R.; Griffiths, R.; Hancock, J.; Neill, S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 16314–16318. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Hancock, J.T. Nitric oxide signalling in plants. New Phytol. 2003, 159, 11–35. [Google Scholar] [CrossRef]
- Stoimenova, M.; Libourel, I.G.L.; Ratcliffe, R.G.; Kaiser, W.M. The role of nitrate reduction in the anoxic metabolism of roots II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant Soil 2003, 253, 155–167. [Google Scholar] [CrossRef]
- Stöhr, C.; Stremlau, S. Formation and possible roles of nitric oxide in plant roots. J. Exp. Bot. 2006, 57, 463–470. [Google Scholar] [CrossRef]
- Cohen, M.F.; Lamattina, L.; Yamasaki, H. Nitric oxide signaling by plant-associated bacteria. In Nitric Oxide in Plant Physiology; Hayat, M., Mori, J., Pichtel, J., Ahamad, A., Eds.; Wiley-VCH: Weinheim, Germany, 2010; pp. 161–172. [Google Scholar]
- Gladwin, M.T.; Crawford, J.H.; Patel, R.P. The biochemistry of nitric oxide, nitrite, and hemoglobin: Role in blood flow regulation. Free Radic. Biol. Med. 2004, 36, 707–717. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Millar, T.M.; Stevens, C.R.; Benjamin, N.; Eisenthal, R.; Harrison, R.; Blake, D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998, 427, 225–228. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Bender, D.; Schwarz, G. Nitrite-dependent nitric oxide synthesis by molybdenum enzymes. FEBS Lett. 2018, 592, 2126–2139. [Google Scholar] [CrossRef]
- Webb, A.J.; Milsom, A.B.; Rathod, K.S.; Chu, W.L.; Qureshi, S.; Lovell, M.J.; Lecomte, F.M.; Perrett, D.; Raimondo, C.; Khoshbin, E.; et al. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: Role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008, 103, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, A.; Jensen, B.S.; Jensen, F.B.; Fago, A. Exploring pathways of NO and H2S signaling in metabolic depression: The case of anoxic turtles. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2021, 253, 110857. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Shimoji, H.; Ohshiro, Y.; Sakihama, Y. Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide 2001, 5, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Metzen, E.; Zhou, J.; Jelkmann, W.; Fandrey, J.; Brüne, B. Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases. Mol. Biol. Cell 2003, 14, 3470–3481. [Google Scholar] [CrossRef]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef]
- Modin, A.; Bjorne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J.O. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Yamasaki, H.; Watanabe, N.S.; Fukuto, J.; Cohen, M.F. Nitrite-dependent nitric oxide production pathway: Diversity of NO production systems. In Studies on Pediatric Disorders; Tsukahara, H., Kaneko, K., Eds.; Springer: New York, NY, USA, 2014; pp. 35–54. [Google Scholar]
- Watanabe, N.S.; Yamasaki, H. Dynamics of nitrite content in fresh spinach leaves: Evidence for nitrite formation caused by microbial nitrate reductase activity. J. Nutr. Food Sci. 2017, 7, 1000572. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef]
- Yamasaki, H.; Imai, H.; Tanaka, A.; Otaki, J.M. Pleiotropic functions of nitric oxide produced by ascorbate for the prevention and mitigation of COVID-19: A revaluation of Pauling’s vitamin C therapy. Microorganisms 2023, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.D.; Wu, L.Y.; Jiang, B.; Yang, W.; Qi, J.S.; Cao, K.; Meng, Q.H.; Mustafa, A.K.; Mu, W.T.; Zhang, S.M.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Yamasaki, H.; Cohen, M.F. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide 2016, 55–56, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide (H2S)/Polysulfides (H2Sn) signalling and TRPA1 channels modification on sulfur metabolism. Biomolecules 2024, 14, 129. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Wozniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Morroni, F.; Tarozzi, A.; Sita, G.; Bolondi, C.; Moraga, J.M.Z.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 2013, 36, 63–71. [Google Scholar] [CrossRef]
- Yamasaki, H.; Itoh, R.D.; Mizumoto, K.B.; Yoshida, Y.S.; Otaki, J.M.; Cohen, M.F. Spatiotemporal characteristics determining the multifaceted nature of reactive oxygen, nitrogen, and sulfur species in relation to proton homeostasis. Antioxid. Redox Signal 2025, 42, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Ayadhi, L.Y.; Attia, S.M.; Al-Harbi, N.O.; Alzahrani, K.S.; Bakheet, S.A. Differential regulation of Nrf2 is linked to elevated inflammation and nitrative stress in monocytes of children with autism. Psychoneuroendocrinology 2020, 113, 104554. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Gonzalez-Gordo, S.; Munoz-Vargas, M.A.; Rodriguez-Ruiz, M.; Palma, J.M. The Modus Operandi of hydrogen sulfide (H2S)-dependent protein persulfidation in higher plants. Antioxid 2021, 10, 1686. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation: The prototypic redox-based signaling mechanism. Cell 2001, 106, 675–683. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Merchant, K.; Nudelman, R.; Beyer, W.F.; Keng, T.; DeAngelo, J.; Hausladen, A.; Stamler, J.S. OxyR: A molecular code for redox-related signaling. Cell 2002, 109, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Houk, K.N.; Foote, C.S.; Fukuto, J.M.; Ignarro, L.J.; Squadrito, G.L.; Davies, K.J. Free radical biology and medicine: It’s a gas, man! Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R491–R511. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Butler, A.R.; Woollins, J.D.; Feelisch, M. Inorganic sulfur-nitrogen compounds: From gunpowder chemistry to the forefront of biological signaling. Dalton Trans. 2016, 45, 5908–5919. [Google Scholar] [CrossRef]
- Fra, A.; Yoboue, E.D.; Sitia, R. Cysteines as redox molecular switches and targets of disease. Front. Mol. Neurosci. 2017, 10, 167. [Google Scholar] [CrossRef]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.T.; Gazi, S.K.; Barrow, R.K.; Yang, G.D.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Manta, B.; Botti, H.; Radi, R.; Trujillo, M.; Denicola, A. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 2011, 24, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Martinov, M.; Vitvitsky, V.; Seravalli, J.; Wedmann, R.; Filipovic, M.R.; Banerjee, R. Biosynthesis and reactivity of cysteine persulfides in signaling. J. Am. Chem. Soc. 2016, 138, 289–299. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 2015, 46, 14–24. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Giuffre, A.; Vicente, J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid. Med. Cell Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef]
- Musielak, Z.E.; Quarles, B. The three-body problem. Rep. Prog. Phys. 2014, 77, 065901. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.Y.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Nat. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Schulz, R.; Kelm, M.; Heusch, G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 61, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, D.; Glavnik, N.; Marinsek, M.; Zagozen, P.; Rovan, K.; Goslar, T.; Mars, T.; Podbregar, M. Total plasma sulfide in congestive heart failure. J. Card. Fail. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Edelstein, C.; Gengaro, P.; Meng, X.H.; Lucia, S.; Knotek, M.; Wangsiripaisan, A.; Shi, Y.X.; Schrier, R. Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am. J. Physiol. Ren. Physiol. 1999, 277, F383–F390. [Google Scholar] [CrossRef]
- Ahmad, F.U.D.; Sattar, M.A.; Rathore, H.A.; Abdullah, M.H.; Tan, S.; Abdullah, N.A.; Johns, E.J. Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Ren. Fail. 2012, 34, 203–210. [Google Scholar] [CrossRef]
- Ueda, J.; Tsuji, A.; Aoki, T.; Asano, R.; Kiko, T.; Hayashi, H.; Endo, H.; Nishi, N.; Takano, R.; Fujisaki, S.; et al. Phase 2 study to evaluate the efficacy and safety of inhaled nitric oxide therapy in patients with severe right heart failure associated with pulmonary hypertension. Circ. Rep. 2025, 7, 308–312. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, S.; Pan, Z.; Jiang, S.; Fan, J.; Yu, S.; Xue, L.; Yang, J.; Ma, S.; Liu, T.; et al. Hydrogen sulfide alleviates particulate matter-induced emphysema and airway inflammation by suppressing ferroptosis. Free Radic. Biol. Med. 2022, 186, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, K.; Li, M.X.; He, W.; Chang, J.R.; Liao, C.C.; Lin, F.; Qi, Y.F.; Wang, R.; Chen, Y.H. Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Sci. Rep. 2015, 5, 14971. [Google Scholar] [CrossRef]
- Pae, H.O.; Lee, Y.C.; Jo, E.K.; Chung, H.T. Subtle interplay of endogenous bioactive gases (NO, CO and H2S) in inflammation. Arch. Pharm. Res. 2009, 32, 1155–1162. [Google Scholar] [CrossRef]
- Whiteman, M.; Haigh, R.; Tarr, J.M.; Gooding, K.M.; Shore, A.C.; Winyard, P.G. Detection of hydrogen sulfide in plasma and knee-joint synovial fluid from rheumatoid arthritis patients: Relation to clinical and laboratory measures of inflammation. Ann. N. Y. Acad. Sci. 2010, 1203, 146–150. [Google Scholar] [CrossRef]
- Kloesch, B.; Liszt, M.; Krehan, D.; Broell, J.; Kiener, H.; Steiner, G. High concentrations of hydrogen sulphide elevate the expression of a series of pro-inflammatory genes in fibroblast-like synoviocytes derived from rheumatoid and osteoarthritis patients. Immunol. Lett. 2012, 141, 197–203. [Google Scholar] [CrossRef]
- Li, L.; Hsu, A.; Moore, P.K. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation—A tale of three gases! Pharmacol. Ther. 2009, 123, 386–400. [Google Scholar] [CrossRef]
- Kamalian, A.; Asl, M.S.; Dolatshahi, M.; Afshari, K.; Shamshiri, S.; Roudsari, N.M.; Momtaz, S.; Rahimi, R.; Abdollahi, M.; Abdolghaffari, A.H. Interventions of natural and synthetic agents in inflammatory bowel disease, modulation of nitric oxide pathways. World J. Gastroenterol. 2020, 26, 3365–3400. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lu, H.Y.; Zhou, Q.; Chen, D.F.; Zeng, Y.Y.; Shi, J.Y.; Zhang, Y.M.; Wang, X.W.; Shen, X.K.; Cai, X.J. H2S-releasing versatile montmorillonite nanoformulation trilogically renovates the gut microenvironment for inflammatory bowel disease modulation. Adv. Sci. 2024, 11, e2308092. [Google Scholar] [CrossRef]
- Vieira, N.F.; Barbosa, T.P.; Cárnio, E.C. Performance of nitric oxide in sepsis: A scoping review. Acta Paul. Enferm. 2024, 37, eAPE00512. [Google Scholar] [CrossRef]
- Cao, G.D.; Zeng, Y.C.; Zhao, Y.H.; Liang, L.; Luo, X.Q.; Guo, L.C.; Zhang, Y.X.; Cheng, Q.H. H2S regulation of ferroptosis attenuates sepsis-induced cardiomyopathy. Mol. Med. Rep. 2022, 26, 335. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Dawoud, A.; Youness, R.A.; Nafea, H.; Manie, T.; Bourquin, C.; Szabo, C.; Abdel-Kader, R.M.; Gad, M.Z. Pan-inhibition of the three H2S synthesizing enzymes restrains tumor progression and immunosuppression in breast cancer. Cancer Cell Int. 2024, 24, 136. [Google Scholar] [CrossRef]
- Malicka, I.; Martynowicz, H.; Dziegiel, P.; Podhorska-Okolow, M.; Wozniewski, M.; Szuba, A. Asymmetric dimethylarginine in an NMU-induced rat mammary tumor model. Anticancer Res. 2023, 43, 97–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, C.; Deng, Y.; Ye, C.; Peng, H. HIF-1alpha signaling: Essential roles in tumorigenesis and implications in targeted therapies. Genes Dis. 2024, 11, 234–251. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Modis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-ß-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S. Anti-obesity and anti-diabetic effects of nitrate and nitrite. Nitric Oxide 2017, 70, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Katsouda, A.; Szabo, C.; Papapetropoulos, A. Reduced adipose tissue H2S in obesity. Pharmacol. Res. 2018, 128, 190–199. [Google Scholar] [CrossRef]
- Pokorski, M.; Pozdzik, M.; Mazzatenta, A. Antioxidant treatment for impaired hypoxic ventilatory responses in experimental diabetes in the rat. Respir. Physiol. Neurobiol. 2018, 255, 30–38. [Google Scholar] [CrossRef]
- Beltowski, J.; Wojcicka, G.; Jamroz-Wisniewska, A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: Implications for the pathogenesis and treatment of diabetes mellitus. Biochem. Pharmacol. 2018, 149, 60–76. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- Austin, S.A.; Santhanam, A.V.; Hinton, D.J.; Choi, D.S.; Katusic, Z.S. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 2013, 127, 691–700. [Google Scholar] [CrossRef]
- Vandini, E.; Ottani, A.; Zaffe, D.; Calevro, A.; Canalini, F.; Cavallini, G.M.; Rossi, R.; Guarini, S.; Giuliani, D. Mechanisms of hydrogen sulfide against the progression of severe Alzheimer’s disease in transgenic mice at different ages. Pharmacology 2019, 103, 50–60. [Google Scholar] [CrossRef]
- Kim, J.; Han, J.Y.; Lee, Y.; Kim, K.; Choi, Y.P.; Chae, S.; Hoe, H.S. Genetic deletion of nitric oxide synthase 2 ameliorates Parkinson’s disease pathology and neuroinflammation in a transgenic mouse model of synucleinopathy. Mol. Brain 2023, 16, 7. [Google Scholar] [CrossRef]
- Cao, X.; Cao, L.; Ding, L.; Bian, J.S. A new hope for a devastating disease: Hydrogen sulfide in Parkinson’s disease. Mol. Neurobiol. 2018, 55, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 273283. [Google Scholar] [CrossRef]
- Solomon, A.; Mangialasche, F.; Richard, E.; Andrieu, S.; Bennett, D.A.; Breteler, M.; Fratiglioni, L.; Hooshmand, B.; Khachaturian, A.S.; Schneider, L.S. Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 2014, 275, 229–250. [Google Scholar] [CrossRef]
- Ince, P.G.; Codd, G.A. Return of the cycad hypothesis—Does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol. Appl. Neurobiol. 2005, 31, 345–353. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Heng, B.; Lane, D.J.R.; Bush, A.I.; Guillemin, G.J.; Tan, V.X. Neuropathological mechanisms of β-N-methylamino-L-alanine (BMAA) with a focus on iron overload and ferroptosis. Neurotox. Res. 2022, 40, 614–635. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, A.R.; Mortimer, J.A.; Schellenberg, G.D.; Galasko, D. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology 2009, 72, 473. [Google Scholar] [CrossRef]
- Borenstein, A.; Mortimer, J.; Schofield, E.; Wu, Y.; Salmon, D.; Gamst, A.; Olichney, J.; Thal, L.; Silbert, L.; Kaye, J. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology 2007, 68, 1764–1771. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef]
- Oyanagi, K. The nature of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam and magnesium deficiency. Park. Relat. Disord. 2005, 11 (Suppl. 1), S17–S23. [Google Scholar] [CrossRef]
- Gehringer, M.M.; Pengelly, J.J.; Cuddy, W.S.; Fieker, C.; Forster, P.I.; Neilan, B.A. Host selection of symbiotic cyanobacteria in 31 species of the Australian cycad genus: Macrozamia (Zamiaceae). Mol. Plant Microbe Interact. 2010, 23, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.F.; Sakihama, Y.; Takagi, Y.C.; Ichiba, T.; Yamasaki, H. Synergistic effect of deoxyanthocyanins from symbiotic fern Azolla spp. on hrmA gene induction in the cyanobacterium Nostoc punctiforme. Mol. Plant Microbe Interact. 2002, 15, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.P.; Pereira, A.L.; Azevedo, J.; Bujak, A.A.; Leshyk, V.; Pham Gia, M.; Stadtlander, T.; Vasconcelos, V.; Winstead, D.J. Azolla as a safe food: Suppression of cyanotoxin-related genes and cyanotoxin production in Its symbiont, Nostoc azollae. Plants 2024, 13, 2707. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Peters, S.J.; Mitrovic, S.M.; Rodgers, K.J.; Bishop, D.P. Bioaccumulation of β-methylamino-L-alanine (BMAA) by mussels exposed to the cyanobacteria Microcystis aeruginosa. Environ. Pollut. 2024, 363, 125081. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A.; Elnour, R.O.; Alamri, S.; Hashem, M.; Alshehri, A.M.; Campos, A.; Vasconcelos, V.; Badawye, H. Occurrence of β-N-methylamino-L-alanine (BMAA) toxin in irrigation water and field vegetable plants and assessing its potential risk to human health. Water Air Soil Pollut. 2024, 235, 72. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, W.R.; Liu, Z.Q.; Lin, T.F. Reaction pathways and kinetics of a cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) during chlorination. Environ. Sci. Technol. 2017, 51, 1303–1311. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Popova, A.A.; Rasmussen, U.; Semashko, T.A.; Govorun, V.M.; Koksharova, O.A. Stress effects of cyanotoxin β-methylamino-L-alanine (BMAA) on cyanobacterial heterocyst formation and functionality. Environ. Microbiol. Rep. 2018, 10, 369–377. [Google Scholar] [CrossRef]
- Downing, S.; Banack, S.; Metcalf, J.; Cox, P.; Downing, T. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-L-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef]
- Koksharova, O.A.; Butenko, I.O.; Pobeguts, O.V.; Safronova, N.A.; Govorun, V.M. ß-N-methylamino-L-alanine (BMAA) causes severe stress in Nostoc sp. PCC 7120 cells under diazotrophic conditions: A proteomic study. Toxins 2021, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Steele, J.R.; Phillips, C.R.; Violi, J.P.; Rodgers, K.J. The cyanobacterial toxins BMAA and 2,4-DAB perturb the L-serine biosynthesis pathway and induce systemic changes in energy metabolism in human neuroblastoma cells: A proteomic study. Toxicol. Vitr. 2025, 106, 106058. [Google Scholar] [CrossRef] [PubMed]
- Lobner, D.; Piana, P.M.T.; Salous, A.K.; Peoples, R.W. β-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 2007, 25, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Pierozan, P.; Piras, E.; Brittebo, E.; Karlsson, O. The cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) targets the olfactory bulb region. Arch. Toxicol. 2020, 94, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Kamiya, H.; Tsuzuki, K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 1998, 54, 581–618. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutarnatergic system—Too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Liu, L.D.; Wong, T.P.; Pozza, M.F.; Lingenhoehl, K.; Wang, Y.S.; Sheng, M.; Auberson, Y.P.; Wang, Y.T. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 2004, 304, 1021–1024. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry 2004, 9, 979+984–997. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Hartley, M.; Heinemann, S.F. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 1995, 268, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Kotermanski, S.E.; Johnson, J.W. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J. Neurosci. 2009, 29, 2774–2779. [Google Scholar] [CrossRef]
- Stoll, L.; Hall, J.; Van Buren, N.; Hall, A.; Knight, L.; Morgan, A.; Zuger, S.; Van Deusen, H.; Gentile, L. Differential regulation of ionotropic glutamate receptors. Biophys. J. 2007, 92, 1343–1349. [Google Scholar] [CrossRef]
- Mothet, J.P.; Parent, A.T.; Wolosker, H.; Brady, R.O.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4926–4931. [Google Scholar] [CrossRef]
- Butler, T.R.; Self, R.L.; Smith, K.J.; Sharrett-Field, L.J.; Berry, J.N.; Littleton, J.M.; Pauly, J.R.; Mulholland, P.J.; Prendergast, M.A. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neuroscience 2010, 165, 525–534. [Google Scholar] [CrossRef]
- Chen, H.S.V.; Lipton, S.A. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006, 97, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014, 41, 4–10. [Google Scholar] [CrossRef]
- Avshalumov, M.V.; Rice, M.E. NMDA receptor activation mediates hydrogen peroxide-induced pathophysiology in rat hippocampal slices. J. Neurophysiol. 2002, 87, 2896–2903. [Google Scholar] [CrossRef]
- Rauk, A. β-N-Methylamino-L-alanine (BMAA) not involved in Alzheimer’s disease. J. Phys. Chem. B 2018, 122, 4472–7780. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.N.; Omelchenko, I.; Ross, S.M.; Spencer, P. The neurotoxin, ß-N-methylamino-L-alanine (BMAA) interacts with the strychnine-insensitive glycine modulatory site of the N-methyl D-aspartate receptor. Neuropharmacology 1995, 34, 651–658. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Braidy, N.; Guillemin, G.J.; Welch, J.H.; Neilan, B.A. Excitotoxic potential of the cyanotoxin β-methyl-amino-L-alanine (BMAA) in primary human neurons. Toxicon 2012, 60, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gao, Q.F.; Yang, M.; Shao, Q.L.; Xu, X.P.; Zhang, Y.B.; Luan, S. Ca2+/calmodulin-mediated desensitization of glutamate receptors shapes plant systemic wound signalling and anti-herbivore defence. Nat. Plants 2024, 10, 145–160. [Google Scholar] [CrossRef]
- Price, M.B.; Jelesko, J.; Okumoto, S. Glutamate receptor homologs in plants: Functions and evolutionary origins. Front. Plant Sci. 2012, 3, 235. [Google Scholar] [CrossRef]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijó, J.A. Glutamate Receptor-Like genes Form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.L.; Gao, Q.F.; Lhamo, D.; Zhang, H.S.; Luan, S. Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci. Signal. 2020, 13, eaba1453. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 mediate cold acclimation-induced chilling tolerance by regulating apoplastic H2O2 production and redox homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef]
- Gokce, A.; Sekmen Cetinel, A.H.; Turkan, I. Involvement of GLR-mediated nitric oxide effects on ROS metabolism in Arabidopsis plants under salt stress. J. Plant Res. 2024, 137, 485–503. [Google Scholar] [CrossRef]
- Brenner, E.D.; Martinez-Barboza, N.; Clark, A.P.; Liang, Q.S.; Stevenson, D.W.; Coruzzi, G.M. Arabidopsis mutants resistant to S (+)-β-methyl-α, β-diaminopropionic acid, a cycad-derived glutamate receptor agonist. Plant Physiol. 2000, 124, 1615–1624. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B-Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Inoue, R.; Morii, T.; Takahashi, N.; Yamamoto, S.; Hara, Y.; Tominaga, M.; Shimizu, S.; Sato, Y.; Mori, Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006, 2, 596–607. [Google Scholar] [CrossRef]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S.; Kiyonaka, S.; Takahashi, N.; Wajima, T.; Hara, Y.; Negoro, T.; Hiroi, T.; Kiuchi, Y.; Okada, T.; et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008, 14, 738–747. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Neuberger, A.; Sobolevsky, A.I. Molecular pharmacology of the onco-TRP channel TRPV6. Channels 2023, 17, 2266669. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Nadezhdin, K.D.; Zakharian, E.; Sobolevsky, A.I. Structural mechanism of TRPV3 channel inhibition by the plant-derived coumarin osthole. EMBO Rep. 2021, 22, e53233. [Google Scholar] [CrossRef]
- Neuberger, A.; Trofimov, Y.A.; Yelshanskaya, M.V.; Khau, J.; Nadezhdin, K.D.; Khosrof, L.S.; Krylov, N.A.; Efremov, R.G.; Sobolevsky, A.I. Molecular pathway and structural mechanism of human oncochannel TRPV6 inhibition by the phytocannabinoid tetrahydrocannabivarin. Nat. Commun. 2023, 14, 4630. [Google Scholar] [CrossRef]
- Neuberger, A.; Trofimov, Y.A.; Yelshanskaya, M.V.; Nadezhdin, K.D.; Krylov, N.A.; Efremov, R.G.; Sobolevsky, A.I. Structural mechanism of human oncochannel TRPV6 inhibition by the natural phytoestrogen genistein. Nat. Commun. 2023, 14, 2659. [Google Scholar] [CrossRef]
- Agam, K.; von Campenhausen, M.; Levy, S.; Ben-Ami, H.C.; Cook, B.; Kirschfeld, K.; Minke, B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J. Neurosci. 2000, 20, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Persson, A.; Cheung, B.; de Bono, M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr. Biol. 2006, 16, 649–659. [Google Scholar] [CrossRef]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013, 27, 2451–2457. [Google Scholar] [CrossRef]
- Kozai, D.; Ogawa, N.; Mori, Y. Redox regulation of transient receptor potential channels. Antioxid. Redox Signal 2014, 21, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Wo, Z.G.; Oswald, R.E. Unraveling the modular design of glutamate-gated ion channels. Trends Neurosci. 1995, 18, 161–168. [Google Scholar]
- Sies, H. Dynamics of intracellular and intercellular redox communication. Free Radic. Biol. Med. 2024, 225, 933–939. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef]
- Dzhalilova, D.; Makarova, O. Differences in tolerance to hypoxia: Physiological, biochemical, and molecular-biological characteristics. Biomedicines 2020, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Shams, I.; Avivi, A.; Nevo, E. Hypoxic stress tolerance of the blind subterranean mole rat: Expression of erythropoietin and hypoxia-inducible factor 1 alpha. Proc. Natl. Acad. Sci. USA 2004, 101, 9698–9703. [Google Scholar] [CrossRef]
- Horsman, J.W.; Heinis, F.I.; Miller, D.L. A novel mechanism to prevent H2S toxicity in Caenorhabditis elegans. Genetics 2019, 213, 481–490. [Google Scholar] [CrossRef]
- Mustroph, A.; Lee, S.C.; Oosumi, T.; Zanetti, M.E.; Yang, H.; Ma, K.; Yaghoubi-Masihi, A.; Fukao, T.; Bailey-Serres, J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010, 152, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Perata, P. ERFVII transcription factors and their role in the adaptation to hypoxia in Arabidopsis and crops. Front. Genet. 2023, 14, 1213839. [Google Scholar] [CrossRef]
- Yamasaki, H.; Ogura, M.P.; Kingjoe, K.A.; Cohen, M.F. D-Cysteine-induced rapid root abscission in the water fern Azolla pinnata: Implications for the linkage between D-amino acid and reactive sulfur species (RSS) in plant environmental responses. Antioxidants 2019, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H. Blood nitrate and nitrite modulating nitric oxide bioavailability: Potential therapeutic functions in COVID-19. Nitric Oxide 2020, 103, 29–30. [Google Scholar] [CrossRef]
- Weitzberg, E.; Hezel, M.; Lundberg, J.O. Nitrate-nitrite-nitric oxide pathway lmplications for anesthesiology and Intensive care. Anesthesiology 2010, 113, 1460–1475. [Google Scholar] [CrossRef]
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate-nitrite-ntric oxide pathway: A mechanism of hypoxia and anoxia tolerance in plants. Int. J. Mol. Sci. 2022, 23, 11522. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. The therapeutic potential of hydrogen sulfide: Separating hype from hope. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R297–R312. [Google Scholar] [CrossRef]
- Dombkowski, R.A.; Russell, M.J.; Olson, K.R. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R678–R685. [Google Scholar] [CrossRef]
- Szabo, C.; Ransy, C.; Modis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef]
- Dey, A.; Prabhudesai, S.; Zhang, Y.S.; Rao, G.; Thirugnanam, K.; Hossen, M.N.; Dwivedi, S.K.D.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathione β-synthase regulates HIF-1α stability through persulfidation of PHD2. Sci. Adv. 2020, 6, eaaz8534. [Google Scholar] [CrossRef]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006, 209, 4011–4023. [Google Scholar] [CrossRef] [PubMed]
- Capra, F. The Tao of Physics: An Exploration of the Parallels Between Modern Physics and Eastern Mysticism; Shambhala Publications: Boston, MA, USA, 1975. [Google Scholar]
- Edinger, E.F.; Wesley, D.A. The Aion Lectures: Exploring the Self in CG Jung’s Aion Studie; Inner City Books: Toronto, ON, Canada, 1996. [Google Scholar]
- Osmond, C. Quintessential inefficiencies of plant bioenergetics: Tales of two cultures. Funct. Plant Biol. 1995, 22, 123–129. [Google Scholar] [CrossRef]
- Society for Experimental Biology. Journal of Experimental Botany. Available online: https://academic.oup.com/jxb?login=false (accessed on 27 May 2025).
- Yamasaki, H. Nitric oxide research in plant biology: Its past and future. In Nitric Oxide SIgnaling in Higher Plants; Magakhaes, J.R., Singh, R.P., Passos, L.P., Eds.; Studium Press: Houston, TX, USA, 2004; pp. 1–23. [Google Scholar]
- Santambrogio, L.; Franco, A. The yin/yang balance of the MHC-self-immunopeptidome. Front. Immunol. 2022, 13, 1035363. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K.; Gross, M.M.; Calleja-Agius, J.; Turner, J.D. The yin and yang of the oxytocin and stress systems: Opposites, yet interdependent and intertwined determinants of lifelong health trajectories. Front. Endocrinol. 2024, 15, 1272270. [Google Scholar] [CrossRef]
- Yamasaki, H. The NO world for plants: Achieving balance in an open system. Plant Cell Environ. 2005, 28, 78–84. [Google Scholar] [CrossRef]
- Reston, J. Now, About My Operation in Peking. The New York Times, 26 July 1971. [Google Scholar]
- Harrison, T.M.; Churgin, S.M. Acupuncture and traditional Chinese veterinary medicine in zoological and exotic animal medicine: A review and introduction of methods. Vet. Sci. 2022, 9, 74. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Sato, E.F.; Inoue, M.; Asada, A. Acupuncture enhances generation of nitric oxide and increases local circulation. Anesth. Analg. 2007, 104, 301–307. [Google Scholar] [CrossRef]
- Su, X.T.; Wang, L.; Ma, S.M.; Cao, Y.; Yang, N.N.; Lin, L.L.; Fisher, M.; Yang, J.W.; Liu, C.Z. Mechanisms of acupuncture in the regulation of oxidative stress in treating ischemic stroke. Oxid. Med. Cell Longev. 2020, 2020, 7875396. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.X.; Li, X.Y.; Sakurai, T.; Pandjaitan, M. Evidence of enhanced non-enzymatic generation of nitric oxide on the skin surface of acupuncture points: An innovative approach in humans. Nitric Oxide 2007, 17, 60–68. [Google Scholar] [CrossRef]
- Tang, L.; Li, Y.; Xie, H.; Shu, Q.; Yang, F.; Liu, Y.L.; Liang, F.; Wang, H.; Huang, W.; Zhang, G.J. A sensitive acupuncture needle microsensor for real-time monitoring of nitric oxide in acupoints of rats. Sci. Rep. 2017, 7, 6446. [Google Scholar] [CrossRef]
- Guo, J.; Wei, T.; Huang, Q.; Li, M.; Yang, C.; Mou, J.; Shi, L.; Gao, T.; Li, G. Direct acupuncture of nitric oxide by an electrochemical microsensor with high time-space resolution. Biosens. Bioelectron. 2022, 195, 113667. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K. Cholesterol: Evolution of structure and function. In Biochemistry of Lipids, Lipoproteins and Membranes; Vance, D.E., Vance, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 20, pp. 363–381. [Google Scholar]
- Lefebvre, B.; Furt, F.; Hartmann, M.A.; Michaelson, L.V.; Carde, J.P.; Sargueil-Boiron, F.; Rossignol, M.; Napier, J.A.; Cullimore, J.; Bessoule, J.J.; et al. Characterization of lipid rafts from Medicago truncatula root plasma membranes: A proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007, 144, 402–418. [Google Scholar] [CrossRef]
- Miserocchi, G. Early endothelial signaling transduction in developing lung edema. Life 2023, 13, 1240. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 2014, 1838, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Caveolae: Structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef]
- Navarro, G.; Borroto-Escuela, D.O.; Fuxe, K.; Franco, R. Potential of caveolae in the therapy of cardiovascular and neurological diseases. Front. Physiol. 2014, 5, 370. [Google Scholar] [CrossRef]
- Anagnostopoulou, A.; Camargo, L.L.; Rodrigues, D.; Montezano, A.C.; Touyz, R.M. Importance of cholesterol-rich microdomains in the regulation of Nox isoforms and redox signaling in human vascular smooth muscle cells. Sci. Rep. 2020, 10, 17818. [Google Scholar] [CrossRef]
- Noirot, E.; Der, C.; Lherminier, J.; Robert, F.; Moricova, P.; Kieu, K.; Leborgne-Castel, N.; Simon-Plas, F.; Bouhidel, K. Dynamic changes in the subcellular distribution of the tobacco ROS-producing enzyme RBOHD in response to the oomycete elicitor cryptogein. J. Exp. Bot. 2014, 65, 5011–5022. [Google Scholar] [CrossRef]
- Shaul, P.W.; Smart, E.J.; Robinson, L.J.; German, Z.; Yuhanna, I.S.; Ying, Y.; Anderson, R.G.; Michel, T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996, 271, 6518–6522. [Google Scholar] [CrossRef]
- GarciaCardena, G.; Oh, P.; Liu, J.W.; Schnitzer, J.E.; Sessa, W.C. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling. Proc. Natl. Acad. Sci. USA 1996, 93, 6448–6453. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, M.A.; Lolo, F.N.; Echarri, A. Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.M.; Martens, J.R.; Tamkun, M.M. Localization of ion channels to lipid raft domains within the cardiovascular system. Trends Cardiovasc. Med. 2004, 14, 37–42. [Google Scholar] [CrossRef]
- Angelini, C.; Morellato, A.; Alfieri, A.; Pavinato, L.; Cravero, T.; Bianciotto, O.T.; Salemme, V.; Natalini, D.; Centonze, G.; Raspanti, A.; et al. p140Cap regulates the composition and localization of the NMDAR complex in synaptic lipid rafts. J. Neurosci. 2022, 42, 7183–7200. [Google Scholar] [CrossRef]
- Payrits, M.; Zsidó, B.Z.; Nehr-Majoros, A.K.; Börzsei, R.; Helyes, Z.; Hetényi, C.; Szoke, É. Lipid raft disruption inhibits the activation of transient receptor potential vanilloid 1, but not TRP melastatin 3 and the voltage-gated L-type calcium channels in sensory neurons. Front. Cell Dev. Biol. 2024, 12, 1452306. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Patra, S.K. Lipid raft facilitated receptor organization and signaling: A functional rheostat in embryonic development, stem cell biology and cancer. Stem Cell Rev. Rep. 2023, 19, 2–25. [Google Scholar] [CrossRef]
- Levental, I.; Lyman, E. Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 2023, 24, 107–122. [Google Scholar] [CrossRef]
- Cohen, M.F.; Yamasaki, H.; Mazzola, M. Brassica napus seed meal soil amendment modifies microbial community structure, nitric oxide production and incidence of Rhizoctonia root rot. Soil. Biol. Biochem. 2005, 37, 1215–1227. [Google Scholar] [CrossRef]
- Esteban, D.J.; Hysa, B.; Bartow-McKenney, C. Temporal and spatial distribution of the microbial community of Winogradsky columns. PLoS ONE 2015, 10, e0134588. [Google Scholar] [CrossRef]
- Tokuda, G.; Yamada, A.; Nakano, K.; Arita, N.; Yamasaki, H. Occurrence and recent long-distance dispersal of deep-sea hydrothermal vent shrimps. Biol. Lett. 2006, 2, 257–260. [Google Scholar] [CrossRef]
- Tokuda, G.; Yamada, A.; Nakano, K.; Arita, N.O.; Yamasaki, H. Colonization of Sulfurovum sp. on the gill surfaces of Alvinocaris longirostris, a deep-sea hydrothermal vent shrimp. Mar. Ecol. 2007, 29, 106–114. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef] [PubMed]
- Kentgens, A.C.; Pusterla, O.; Bauman, G.; Santini, F.; Wyler, F.; Curdy, M.S.; Willers, C.C.; Bieri, O.; Latzin, P.; Ramsey, K.A. Simultaneous multiple breath washout and oxygen-enhanced magnetic resonance imaging in healthy adults. Respir. Med. Res. 2023, 83, 100993. [Google Scholar] [CrossRef] [PubMed]
- Fago, A. New insights into survival strategies to oxygen deprivation in anoxia-tolerant vertebrates. Acta Physiol. 2022, 235, e13841. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Gomez, C.R.; Richards, J.G.; Milsom, W.K. Mitochondrial responses to prolonged anoxia in brain of red-eared slider turtles. Biol. Lett. 2016, 12, 20150797. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Hogg, D.W.; Buck, L.T. Endogenous reductions in N-methyl-D-aspartate receptor activity inhibit nitric oxide production in the anoxic freshwater turtle cortex. FEBS Lett. 2008, 582, 1738–1742. [Google Scholar] [CrossRef]
- Chiu, J.; DeSalle, R.; Lam, H.M.; Meisel, L.; Coruzzi, G. Molecular evolution of glutamate receptors: A primitive signaling mechanism that existed before plants and animals diverged. Mol. Biol. Evol. 1999, 16, 826–838. [Google Scholar] [CrossRef]
- Burnstock, G.; Verkhratsky, A. Evolutionary origins of the purinergic signalling system. Acta Physiol. 2009, 195, 415–447. [Google Scholar] [CrossRef]
- Bright, M.; Gollner, S.; de Oliveira, A.L.; Espada-Hinojosa, S.; Fulford, A.; Hughes, I.V.; Hourdez, S.; Karthauser, C.; Kolar, I.; Krause, N.; et al. Animal life in the shallow subseafloor crust at deep-sea hydrothermal vents. Nat. Commun. 2024, 15, 8466. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Damer, B. Can life begin on Enceladus? A perspective from hydrothermal chemistry. Astrobiology 2017, 17, 834–839. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).