Enhancing Salinity Tolerance of Fig Transplants Cv. Conadria via Exogenous Application of Sodium Nitroprusside
Abstract
1. Introduction
2. Results
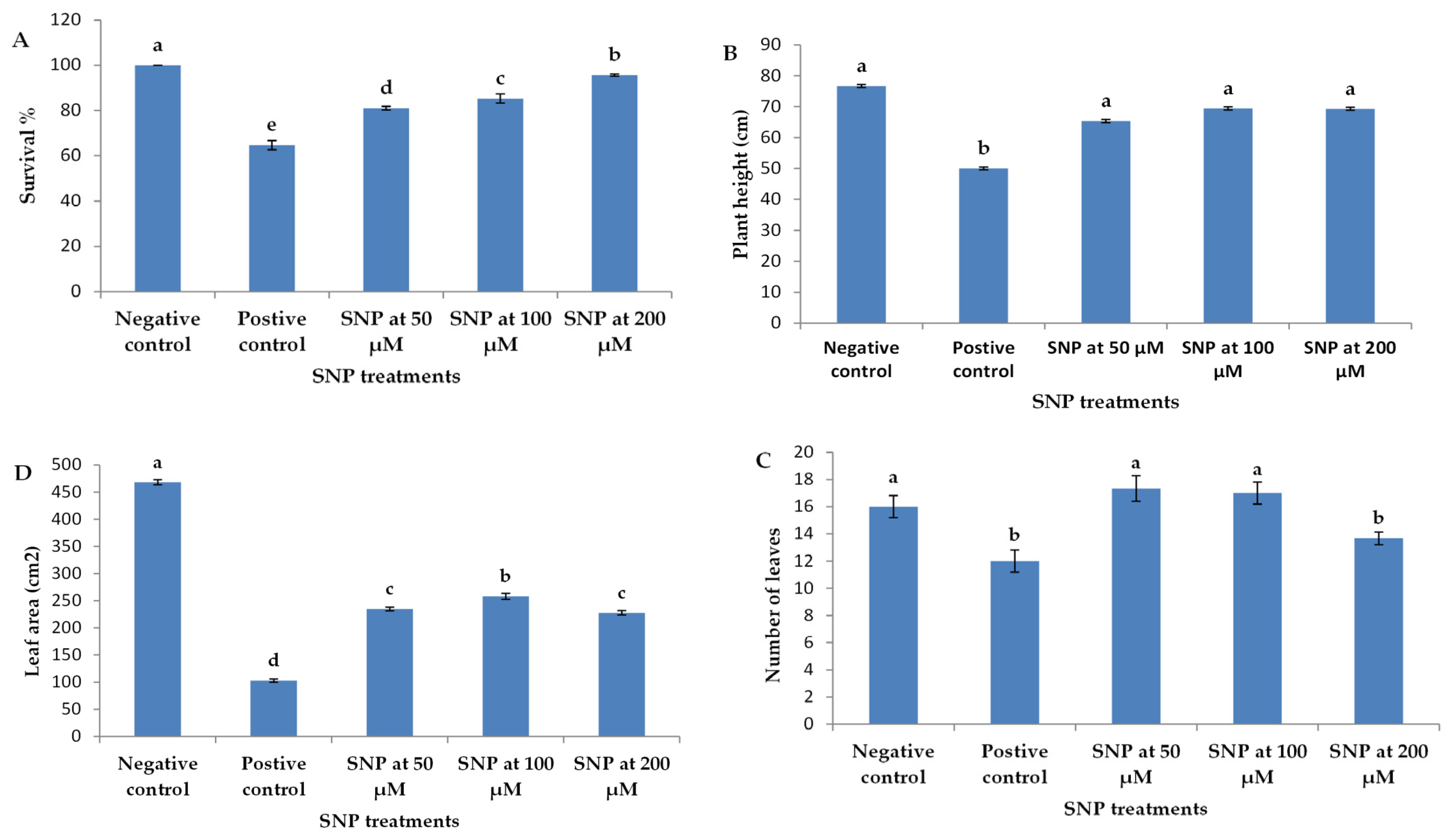
2.1. Biomass and Plant Growth
2.2. Analysis of Mineral Elements
2.3. Chlorophyll Content
2.4. Proline Content
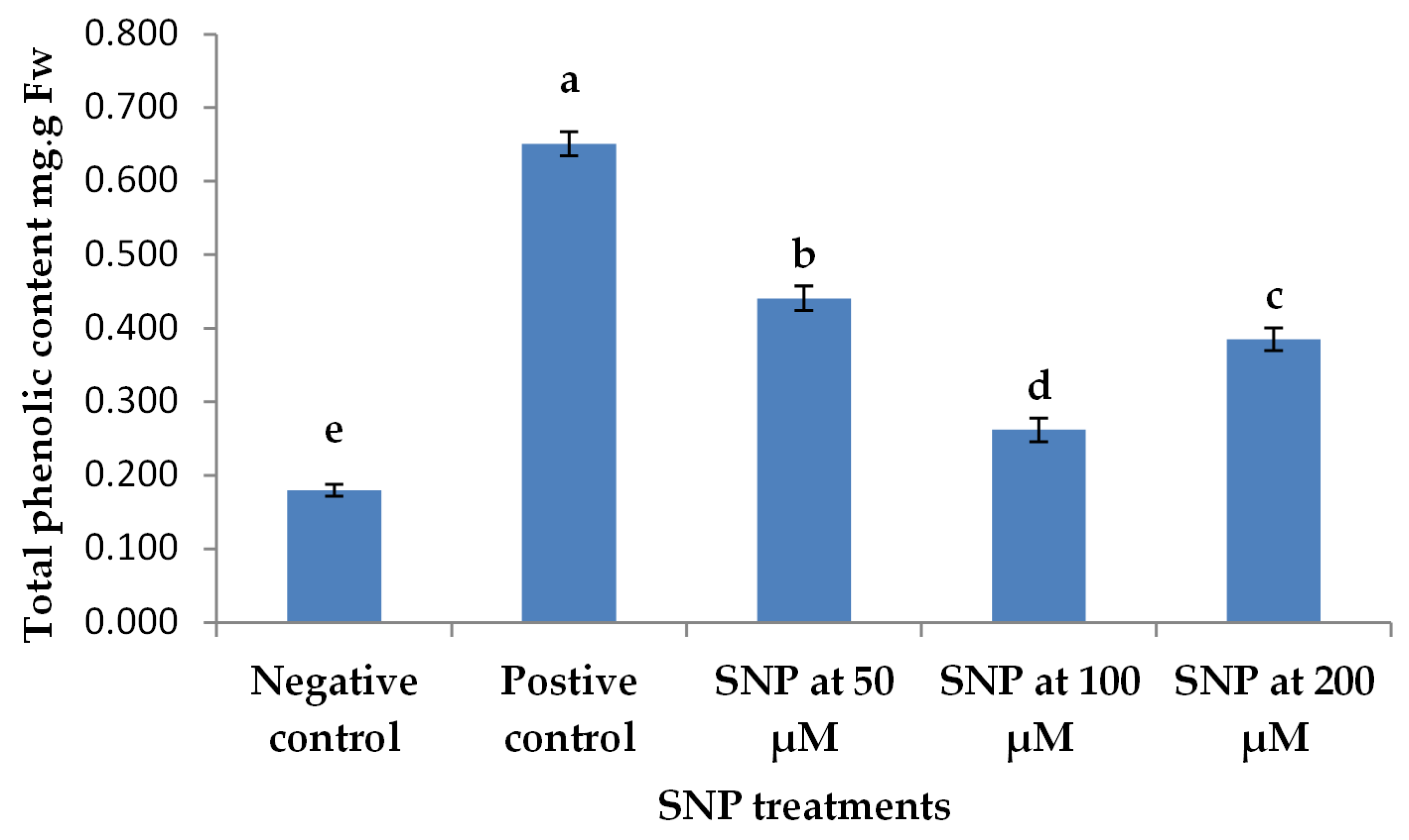
2.5. Total Phenol Content
2.6. Lipid Peroxidation
2.7. Antioxidant Enzymes Activity
2.8. The Hierarchical Clustering Heatmap
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Salinity Stress Treatments
4.2. Biomass and Plant Growth
4.3. Analysis of Mineral Elements
4.4. Chlorophyll Content
4.5. Proline
4.6. Total Phenol Content
4.7. Lipid Peroxidation
4.8. Extraction and Determination of Antioxidant Enzymes Activity
4.8.1. Catalase (CAT) Activity
4.8.2. Peroxidase (POX) Activity
4.8.3. Superoxide Dismutase (SOD) Activity
4.9. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Morales-González, J.A.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Valadez-Vega, C.; Álvarez-González, I.; Chamorro-Cevallos, G.; Morales-González, Á.; Garcia-Melo, L.F. A review of Ficus L. genus (Moraceae): A source of bioactive compounds for health and disease. Part 1. Am. J. Transl. Res. 2024, 16, 6236–6273. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Vaya, J. Fig, Carob, Pistachio, and Health. In Bioactive Foods in Promoting Health; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 245–263. [Google Scholar]
- Ercisli, S.; Tosun, M.; Karlidag, H.; Dzubur, A.; Hadziabulic, S.; Aliman, Y. Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from Northeastern Turkey. Plant Foods Hum. Nutr. 2012, 67, 271–276. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 February 2025).
- Milano, M.; Ruelland, D.; Fernandez, S.; Dezetter, A.; Fabre, J.; Servat, E.; Fritsch, J.-M.; Ardoin-Bardin, S.; Thivet, G. Current state of Mediterranean water resources and future trends under climatic and anthropogenic changes. Hydrol. Sci. J. 2013, 58, 498–518. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L.; Flores, F.; Moneo, M. Challenges to manage the risk of water scarcity and climate change in the Mediterranean. Water Resour. Manag. 2007, 21, 775–788. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Khan, M.M.; Siddiqi, S.A.; Farooque, A.A.; Iqbal, Q.; Shahid, S.A.; Akram, M.T.; Rahman, S.; Al-Busaidi, W.; Khan, I. Towards sustainable application of wastewater in agriculture: A review on reusability and risk assessment. Agronomy 2022, 12, 1397. [Google Scholar] [CrossRef]
- FAO. Global network on integrated soil management for sustainable use of salt-affected soils. In FAO Land and Plant Nutrition Management Service Rome; FAO: Rome, Italy, 2005. [Google Scholar]
- Gupta, R.K.; Abrol, I.P.; Finkl, C.W.; Kirkham, M.B.; Arbestain, M.C.; Macías, F.; Chesworth, W.; Germida, J.J.; Loeppert, R.H.; Cook, M.G.; et al. Soil salinity and salinization. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2011. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Loupassaki, M.; Bertaki, M.; Androulakis, I. Effects of NaCl salinity on growth, ion content and CO2 assimilation rate of six olive cultivars. Sci. Hortic. 2002, 96, 235–247. [Google Scholar] [CrossRef]
- Arora, N.; Bhardwaj, R.; Sharma, P.; Arora, H.K. Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. Acta Physiol. Plant 2008, 30, 833–839. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Hu, L.; Hu, T.; Zhang, X.; Pang, H.; Fu, J. Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J. Am. Soc. Hortic. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E.; Turan, M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci. Agric. 2009, 66, 180–187. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, J.; Anwar-ul-Haq, M.; Yang, A.; Akhtar, S.S.; Jacobsen, S.E. Integrating role of ethylene and ABA in tomato plants adaptation to salt stress. Sci. Hortic. 2014, 172, 109–116. [Google Scholar] [CrossRef]
- Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Plants use calcium to resolve salt stress. Trends Plant Sci. 1998, 3, 411–412. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crop Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, X.; Wu, Y.; Zhang, L. Enhanced sensitivity to oxidative stress in an Arabidopsis nitric oxide synthase mutant. J. Plant Physiol. 2007, 164, 737–745. [Google Scholar] [CrossRef]
- Lamotte, O.; Courtois, C.; Dobrowolska, G.; Besson, A.; Pugin, A.; Wendehenne, D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic. Biol. Med. 2006, 40, 1369–1376. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Moro, C.F.; Gaspar, M.; da Silva, F.R.; Pattathil, S.; Hahn, M.G.; Salgado, I.; Braga, M.R. S-nitrosoglutathione promotes cell wall remodelling, alters the transcriptional profile and induces root hair formation in the hairless root hair defective 6 (rhd6) mutant of Arabidopsis thaliana. New Phytol. 2017, 213, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Ali, H.M.; Khan, M.N. Sodium nitroprusside and indole acetic acid improve the tolerance of tomato plants to heat stress by protecting against DNA damage. J. Plant Interact. 2017, 12, 177–186. [Google Scholar] [CrossRef]
- Lindberg, S.; Premkumar, A. Ion changes and signaling under salt stress in wheat and other important crops. Plants 2023, 13, 46. [Google Scholar] [CrossRef]
- Karami, A.; Sepehri, A. Beneficial role of MWCNTs and SNP on growth, physiological and photosynthesis performance of barley under NaCl stress. J. Soil Sci. Plant Nutr. 2018, 18, 752–771. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Abd Elhamid, E.M.; Sadak, M.S. Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull. Natl. Res. Cen. 2019, 43, 118. [Google Scholar] [CrossRef]
- Al-Jarah, T.M.; Jerry, A.N.; Jasim, A.M. Effect of Sodium Nitroprusside (SNP) on minerals content of cabbage Brassica oleracea var. capitata L. grown under salt stress. Basrah J. Agric. Sci. 2019, 32, 44–59. [Google Scholar] [CrossRef]
- Moghaddam, A.; Larijani, H.R.; Oveysi, M.; Moghaddam, H.R.T.; Nasri, M. Alleviating the adverse effects of salinity stress on Salicornia persica using sodium nitroprusside and potassium nitrate. BMC Plant Biol. 2023, 23, 166. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Guo, J.; Yang, Y.; Li, B.; Zhang, L. Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 2004, 134, 849–857. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Q.; Wu, F.; Dong, X.; He, J.; Pei, Z.; Zheng, H. Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H+-ATPase and Na+/H+ antiporter under high salinity. Tree Physiol. 2010, 30, 1570–1585. [Google Scholar] [CrossRef]
- Sabater, B.; Rodrguez, M.T. Control of chlorophyll degradation in detached leaves of barley and oat through effect of kinetin on chlorophyllase levels. Physiol. Plant. 1978, 43, 274–276. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Wu, Y.X.; Hu, Y.; Jia, X.M.; Zhao, T.; Cheng, L.; Wang, Y.X. Tolerance of two apple rootstocks to short-term salt stress: Focus on chlorophyll degradation, photosynthesis, hormone and leaf ultrastructures. Acta Physiol. Plant. 2019, 41, 87. [Google Scholar] [CrossRef]
- Aras, S.; Keles, H.; Eşitken, A. SNP Mitigates Malignant Salt Effects on Apple Plants. Erwerbs-Obstbau 2020, 62, 107–115. [Google Scholar] [CrossRef]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil. Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Yasir, T.A.; Khan, A.; Skalicky, M.; Wasaya, A.; Rehmani, M.I.A.; Sarwar, N.; Mubeen, K.; Aziz, M.; Hassan, M.M.; Hassan, F.A.S.; et al. Exogenous sodium nitroprusside mitigates salt stress in lentil (Lens culinaris Medik.) by affecting the growth, yield, and biochemical properties. Molecules 2021, 26, 2576. [Google Scholar] [CrossRef]
- Heuer, B. Role of proline in plant response to drought and salinity. In Handbook of Plant and Crop Stress, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 213–238. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, X.; Yang, F. Adaptive responses to progressive drought stress in two Populus cathayana populations. Silva Fenn. 2008, 42, 705–719. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Kong, J.; Liu, S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Sakhabutdinova, A.R.; Fatkhutdinova, D.R.; Bezrukova, M.V.; Shakirova, F.M. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant Physiol. 2003, 21, 314–319. [Google Scholar]
- Namdjoyan, S.; Kermanian, H.; Soorki, A.A.; Tabatabaei, S.M.; Elyasi, N. Effects of exogenous salicylic acid and sodium nitroprusside on α-tocopherol and phytochelatin biosynthesis in zinc-stressed safflower plants. Turk. J. Bot. 2018, 42, 271–279. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants Under Salt Stress; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1, 217037. [Google Scholar] [CrossRef]
- Hmmam, I.; Raza, A.; Đalović, I.; Khedr, N.; Abdellatif, A. An in vitro approach to investigate the role of abscisic acid in alleviating the negative effects of chilling stress on banana shoots. Phyton-Int. J. Exp. Bot. 2023, 92, 1695–1711. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I. Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Notulae. Bot. Horti Agrobotanici 2013, 41, 44–57. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef]
- Farouk, S.; Arafa, S.A. Mitigation of salinity stress in canola plants by sodium nitroprusside application. Span. J. Agric. Res. 2018, 16, e0802. [Google Scholar] [CrossRef]
- Christou, A.; Manganaris, G.A.; Fotopoulos, V. Systemic mitigation of salt stress by hydrogen peroxide and sodium nitroprusside in strawberry plants via transcriptional regulation of enzymatic and non-enzymatic antioxidants. Environ. Exp. Bot. 2014, 107, 46–54. [Google Scholar] [CrossRef]
- Sheokand, S.; Kumari, A.; Sawhney, V. Effect of nitric oxide and putrescine on antioxidative responses under NaCl stress in chickpea plants. Physiol. Mol. Biol. Plants. 2008, 14, 355–362. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. Available online: https://search.informit.org/doi/10.3316/informit.282079847301776 (accessed on 1 March 2025).
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Jin, J.W.; Xu, Y.F.; Huang, Y.F. Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. Afr. J. Biotechnol. 2010, 9, 1619–1627. [Google Scholar] [CrossRef]
- Sahay, S.; Gupta, M. An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 2017, 67, 39–52. [Google Scholar] [CrossRef]
- Bavita, A.; Shashi, B.; Navtej, S.B. Nitric oxide alleviates oxidative damage induced by high temperature stress in wheat. Indian J. Exp. Biol. 2012, 50, 372–378. Available online: http://nopr.niscpr.res.in/handle/123456789/14132 (accessed on 1 March 2025).
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. Exogenous nitric oxide (as sodium nitroprusside) ameliorates polyethylene glycol-induced osmotic stress in hydroponically grown maize roots. J. Plant Growth Regul. 2014, 33, 683–696. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis. Part 2, Chemical and Microbiological and Properties, 2nd ed.; Page, A.L., Miller, R.H., Kenney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Agricultural Chemists, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Temminghoff, E.E.J.M.; Houba, V.J.G. Plant Analysis Procedures, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 94–96. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bärlocher, F.; Graça, M.A. Total phenolics. In Methods to Study Litter Decomposition; Bärlocher, F., Gessner, M., Graça, M.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 157–161. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fu, Y.; Wang, N. Effects of aqueous chlorine dioxide treatment on browning of fresh-cut lotus root. LWT-Food Sci. Technol. 2009, 42, 654–659. [Google Scholar] [CrossRef]
- Kong, F.X.; Hu, W.; Chao, S.Y.; Sang, W.L.; Wang, L.S. Physiological responses of the lichen Xanthoparmelia mexicana to oxidative stress of SO2. Environ. Exp. Bot. 1999, 42, 201–209. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Iowa State University Press: Ames, IA, USA, 1967. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegazi, E.S.; Abdallatif, A.; Burshaid, R. Enhancing Salinity Tolerance of Fig Transplants Cv. Conadria via Exogenous Application of Sodium Nitroprusside. Stresses 2025, 5, 36. https://doi.org/10.3390/stresses5020036
Hegazi ES, Abdallatif A, Burshaid R. Enhancing Salinity Tolerance of Fig Transplants Cv. Conadria via Exogenous Application of Sodium Nitroprusside. Stresses. 2025; 5(2):36. https://doi.org/10.3390/stresses5020036
Chicago/Turabian StyleHegazi, El Said, Abdou Abdallatif, and Rashid Burshaid. 2025. "Enhancing Salinity Tolerance of Fig Transplants Cv. Conadria via Exogenous Application of Sodium Nitroprusside" Stresses 5, no. 2: 36. https://doi.org/10.3390/stresses5020036
APA StyleHegazi, E. S., Abdallatif, A., & Burshaid, R. (2025). Enhancing Salinity Tolerance of Fig Transplants Cv. Conadria via Exogenous Application of Sodium Nitroprusside. Stresses, 5(2), 36. https://doi.org/10.3390/stresses5020036





