Abstract
Background: Exposure to early life stress significantly increases the risk of psychopathology later in life. However, the impact of early life stress on the gut microbiome and its potential role in mental health outcomes remains insufficiently understood. This narrative review examines the current knowledge on how early life stress and its associated consequences may affect the gut microbiome, with a particular focus on conditions such as anxiety, depression, and post-traumatic stress disorder. Method: A comprehensive literature search was conducted in the PubMed and Web of Science databases between January and February 2025, covering studies published between 2015 and 2025. Results: Early life stress can profoundly impact cognitive function and neurodevelopment, with maternal early-life nutrition playing a significant role in modulating the effects of prenatal and postnatal stress. Early life stress influences the gut microbiome, disrupting its composition and function by altering the synthesis of microbial metabolites, neurotransmitters, and the activation of key metabolic pathways. However, the precise role of the gut microbiome in modulating stress responses during childhood and adolescence has not yet been fully elucidated. Conclusions: Several studies have demonstrated an association between early life stress and the gut microbiome. However, causality has not yet been established due to the numerous intrinsic and extrinsic factors influencing the microbiome-gut–brain axis. In the coming years, research on key microbial regulators, such as short-chain fatty acids, amino acids, and psychobiotics, may represent a promising approach for addressing central nervous system alterations linked to early life stress. Thus, further studies will be necessary to evaluate their potential as therapeutic agents.
1. Introduction
Exposure to early life stress (ELS), including early adversities, adverse childhood experiences (ACEs), or childhood trauma, during critical developmental periods significantly increases the risk of psychopathology later in life, suggesting that ELS can disrupt normal brain development and alter both its structure and function, with potential long-term consequences for mental health [1]. ELS encompasses stressors such as abuse, neglect, or family dysfunction that occur early in life and negatively impact the health and well-being of young individuals, ultimately leading to a higher risk of morbidity and mortality in adulthood [2]. In recent years, ELS has received considerable attention due to its persistent and widespread consequences [3,4]. In fact, the relationship between ELS and adult mental health outcomes is well documented [5], with individuals exposed to ELS being more susceptible to suffer from long-lasting psychological conditions, such as anxiety, depression, and post-traumatic stress disorder (PTSD) [5,6,7,8,9].
Retrospective studies have shown a consistent association between ELS-related experiences and cognitive decline in adulthood via depressive symptoms and systemic inflammation [10,11]. These early experiences have also been related to neurological deficits in executive function, memory capacity, and processing speed [12,13,14] as they induce significant alterations in the hippocampus and prefrontal cortex (PFC) [15], thereby affecting the hypothalamic–pituitary–adrenal (HPA) axis and the neuroendocrine system, both of which are involved in the stress regulation process, mainly through the release of cortisol [16]. This hormone interacts with both cognitive and physiological processes, including immunity, inflammation, and neuroplasticity [17]. In addition, individuals exposed to ELS often exhibit psychiatric comorbidity with multiple behavioral consequences [18,19,20]. In this regard, although the relationships between ELS and mental health outcomes have been investigated from multiple perspectives, the impact of ELS on the gut microbiome (GM) and its potential role in these outcomes remains insufficiently understood.
The Human Microbiome Project was implemented as a decade-long, two-phase initiative to develop resources, methods, and knowledge to elucidate the links between human-microbiome interactions and health-related outcomes [21,22]. Although “microbiota” and “microbiome” are often used interchangeably, there are important differences between the two terms. The microbiome comprises the entire spectrum of microorganisms, including archaea, bacteria, fungi, and viruses, along with their collective genomes, structural components, metabolites, and surrounding environmental conditions. Microbiota is a more restricted term that refers to the community of commensal, symbiotic, and pathogenic microorganisms associated with a specific environment [23,24]. It is currently recognized that anaerobic bacteria make up approximately 95% of the GM, comprising about one thousand species [25]. The phyla Bacillota (formerly Firmicutes) (64%) and Bacteroidota (formerly Bacteroidetes) (23%) are the most abundant, although members of the phyla Pseudomonadota (formerly Proteobacteria) (8%), Actinomycetota (formerly Actinobacteria) (3%), Fusobacteriota (formerly Fusobacteria), and Verrucomicrobia have also been detected at lower percentages [26]. The dominant genera within the Bacillota are Bacillus, Clostridium, Enterococcus, Eubacterium, Lactobacillus, and Ruminococcus; while in the Bacteroidota, the most abundant genera are Bacteroides, Porphyromonas, and Prevotella [27,28]. In addition, archaea (Methanobrevibacter), fungi (Malassezia, Candida, and Saccharomyces), protozoa (Blastocystis), and viruses have been reported as common components of the human GM [29,30,31,32].
The GM has a measurable effect on the brain, influencing and being influenced by neuropsychiatric disorders, mood-related conditions, social behavior, and stress [33]. This microbiome-gut–brain (MGB) axis may be shaped by multiple mechanisms, such as endocrine, immune, and neural signaling pathways [34]. An essential step in correctly identifying the GM dysbiosis associated with ELS is to understand the characteristics of the microbiota in healthy children in whom no disorders are evident. Early childhood is a critical period for the establishment of the GM, with factors such as mode of delivery, initial feeding practices, and antibiotic exposure playing an important role [35,36]. Nevertheless, the composition and structure of the GM continuously evolve throughout life, influenced by factors such as physiological conditions, infectious diseases, drug use, diet, and lifestyle [37,38,39].
Given that ELS has been increasingly recognized as a critical factor influencing mental health, and considering the potential link between ELS and GM dysbiosis, this review examines the current understanding of how ELS and its associated outcomes may impact the GM, with a particular focus on conditions such as anxiety, depression, and PTSD, as these are commonly observed in individuals affected by early life adversities. By exploring this connection, this review emphasizes the significance of various biological mechanisms underlying ELS-related mental health outcomes, which may contribute to a deeper understanding of the psychological and physiological aspects of these disorders.
2. Method
This review synthesizes current findings linking GM dysbiosis to potential outcomes of ELS. Given the complexity of the subject and the heterogeneity of the available studies, a narrative approach was deemed the most appropriate over a systematic methodology or meta-analysis. Both authors independently conducted a comprehensive literature search in the PubMed and Web of Science databases between January and February 2025, covering articles published between 2015 and 2025 without search restrictions. A combination of terms related to the research topic was used to identify relevant literature. Titles and abstracts were screened for initial selection. The remaining full-text articles were thoroughly reviewed and assessed. Studies were included if they examined the involvement of the GM in traumatic experiences, ACEs, or ELS. Those that primarily focused on substance use, physiological or genetic disorders, and psychological therapies, as well as meta-analyses and reviews were excluded. Furthermore, any studies that did not align with the research objectives were excluded from further analysis.
3. ELS and Its Impact on Mental Health
ELS has lasting and pervasive effects on development [40]. Stress is the psychological response that occurs when an individual perceives being under threat or pressure. It is typically adaptive, as it triggers a variety of behavioral and physiological reactions focused on mitigating the perceived risk [41]. However, when stress becomes chronic or excessively intense, it causes prolonged activation of physiological and psychological response systems, resulting in dysregulation and contributing to adverse mental and physical outcomes [42].
The neuropsychobiological influence of ELS represents an important developmental risk factor for the onset of mental health issues in later stages of life [43]. In terms of mental health outcomes, ELS has been strongly associated with an increased and subsequent risk of depression [44,45], PTSD [46], schizophrenia [47], suicidal tendencies [48], and maladaptive behaviors such as tobacco smoking and alcohol consumption [49].
3.1. Stress System Elements
The role of the stress system functions to preserve homeostasis by reacting to both actual and perceived stressors, whether they are short-term or long-lasting [50]. The human stress system includes both central and peripheral elements that initiate adaptive responses through a variety of hormones, neuropeptides, and neurotransmitters [51]. Central nervous system (CNS) effectors of the stress system consist of (i) corticotropin-releasing hormone (CRH) that is released by CRH neurons primarily located in brain regions, such as the paraventricular nucleus (PVN) of the hypothalamus, limbic structures, bed nucleus of the stria terminalis, cerebral cortex, central nucleus of the amygdala, dorsal root neurons of the spinal cord, cerebellum, and locus coeruleus [52]—CRH has also been identified in chromaffin cells of the sympathetic ganglia of the autonomic nervous system (ANS), adrenal medulla, and in other peripheral organs and tissues, such as the skin, gastrointestinal tract, and immune system cells [52]; (ii) arginine-vasopressin, synthesized by neurons in the hypothalamic PVN [53]; (iii) the pro-opiomelanocortin-derived peptides α-melanocyte-stimulating hormone and β-endorphin, generated within the arcuate nucleus of the hypothamus [54]; and (iv) norepinephrine (NE), produced in noradrenergic neuron clusters located in specific parts of the medulla oblongata [55].
The major peripheral effectors of the stress system are the glucocorticoids (GCs) regulated by the HPA axis and the catecholamines (e.g., NE and epinephrine) modulated by the ANS, which includes (i) the sympathetic nervous system and the sympathoadrenomedullary axis, as well as (ii) the parasympathetic system. Furthermore, a diverse array of molecules such as neuropeptides, steroid hormones, and neurotransmitters engages with traditional neuroendocrine signaling pathways to preserve homeostasis [43,56].
The ANS and the HPA axis play pivotal roles in the stress response, functioning as complementary, interdependent components within a broader network of internal neural regulation (i.e., the central autonomic network). The initial reaction to a stressor is primarily driven by ANS activation, which results in elevated catecholamine levels in peripheral tissues [46]. Concurrently, CRH is released into the hypothalamic–pituitary portal vessels, triggering the synthesis and secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary into the systemic circulation. CRH has also been implicated in triggering stress-related behaviors, influenced by the rapid activation of the ANS [57]. The adrenal cortex, the main target of ACTH, responds by releasing GCs, particularly cortisol in humans [58], which are crucial for managing the intensity, duration, and attenuation of the stress response, mainly through the binding of GCs to glucocorticoid receptors (GRs) in the hippocampus [57].
Chronic or excessive activation of the stress response system can result in sustained elevations in CRH, cortisol, and catecholamine levels. This prolonged dysregulation is thought to underlie various long-term psychological and physiological disorders linked to chronic stress, such as anorexia nervosa, depression, long-term alcohol abuse, obsessive–compulsive disorder, and panic attacks, to name a few [59,60,61,62]. In contrast, several studies have shown that chronic stress conditions, such as chronic fatigue syndrome, PTSD, fibromyalgia, nicotine withdrawal, and the postpartum phase, are often associated with a reduced activity of the HPA axis [46,60].
3.2. Neurological Alterations in ELS
3.2.1. Alterations in Prefrontal–Hippocampal–Amygdala Circuits
Studies conducted in both animal models and humans indicate that ELS leads to significant alterations in the development of prefrontal–hippocampal–amygdala networks [41]. These networks are crucial for coordinating peripheral stress responses, particularly through the release of CRH and GCs, and for regulating the ANS [42]. Moreover, they are integral to emotion and memory processing, as well as to self-regulation [63,64]. In rodent models, offspring exposed to maternal neglect or separation exhibit diminished dendritic growth in the PFC and hippocampus [65]. These structural changes are linked to epigenetic modifications and to disruptions in synaptic signaling in both the amygdala and hippocampus [66,67]. Such alterations contribute to heightened sensitivity to environmental stressors, manifesting as reduced regulation of the amygdala by the PFC and hippocampus [68]. Consequently, these changes are correlated with increased anxiety and depressive-like behaviors observed in animals after ELS exposure [69,70,71]. Alterations in hippocampal synaptic plasticity have also been associated with impaired spatial memory and exaggerated threat-learning responses in rodents [72,73]. These effects are likely mediated, at least partially, by prolonged exposure to CRH and GCs due to chronic stress [74,75].
Similar alterations in brain structure and function following childhood stress exposure have been reported in the amygdala, PFC, and hippocampus in humans [41], including reduced hippocampal volume [76,77], which has been linked to psychopathological symptoms and to deficits in learning processes [78,79,80,81]. In addition, changes in amygdala and PFC responsiveness to emotional stimuli, along with disrupted connectivity between these regions, contribute to increased sensitivity to emotionally salient cues [82,83,84]. This atypical connectivity between these brain regions has been reported to occur in children with a history of emotional and physical maltreatment, sexual abuse, or in children growing up in poverty [85,86,87]. The functional connectivity between the PFC and amygdala seems to play a role in linking experiences of maltreatment with the development of anxiety and depressive symptoms [88]. Similar to animal models, evidence suggests that alterations in CRH and GC function, including epigenetic modifications, may in part mediate hippocampal alterations, psychopathological symptoms, and altered learning processes [87,89,90]. In addition, abnormal HPA responsivity has been reported to exist following a variety of ELS-related adversities, such as intrafamilial violence, low economic status, institutional neglect, and childhood abuse [87]. Prolonged exposure to GCs has been linked to hippocampal atrophy and HPA axis dysfunction [91], supporting the hypothesis that chronic HPA axis activation induces neural alterations in the PFC, hippocampus, and amygdala. These changes may contribute to impaired regulation of neural circuits involved in threat detection and adaptive responses to environmental challenges [92].
3.2.2. Alterations in Prefrontal-Striatal Dopaminergic Circuits
Emerging research indicates that ELS in animal models detrimentally impacts dopaminergic pathways implicated in motivation and reward processing [93,94], particularly within the ventral striatum, PFC, and amygdala [95]. Chronic and repeated maternal separation in rodents has been shown to disrupt dopaminergic glial cell populations, alter cell proliferation and apoptosis rates, and induce atypical dopaminergic signaling in the ventral tegmental area and substantia nigra during adulthood [96]. Other studies have linked variations in maternal care to diminished connectivity between the PFC and caudate–putamen [97], as well as structural and functional modifications in the nucleus accumbens [65,98]. These neurobiological changes have been associated with increased anhedonia-like behaviors [95] and dysregulated reward sensitivity [99].
In humans, ELS has been linked to disruptions in reward processing within the nucleus accumbens, ventral tegmental area, ventral striatum, and PFC [100,101]. These alterations have been associated with heightened vulnerability to anxiety and depressive symptoms, as well as with impairments in reward-based learning [102,103,104]. Notably, these neural circuits maintain strong connections with both the amygdala and PFC, which collectively regulate behavioral and psychological responses to stress, emotional and social learning, and self-regulatory mechanisms [105]. Consequently, disruptions in these pathways may contribute to an increased risk of maladaptive behaviors and adverse mental and physical health outcomes in later developmental stages [106].
4. Gut Microbiome in Early Life
Early childhood constitutes a critical period for the establishment of GM, with key factors such as early nutritional intake, the mode of birth, and antibiotic exposure influencing the development of the GM [35,107]. Thus, the composition and structure of GM changes constantly across the lifespan, influenced by both intrinsic and extrinsic factors, including physiological alterations, infectious diseases, dietary patterns, and lifestyle choices, among others [37,38,107]. Following birth, new microbial communities populate the neonatal gut, mainly determined by the gestational period and early-life factors, including birth circumstances and exposures during infancy [107,108,109,110,111]. The microbiome in preterm infants is often characterized by reduced diversity, a deficit in short-chain fatty acids (SCFAs), and the presence of potentially pathogenic microorganisms [112,113,114].
In preterm infants, the prevalent bacterial species are primarily from the Pseudomonadota and Bacillota phyla [113], with a notable presence of the Enterobacteriaceae and Enterococcaceae families [112], and of the genera Clostridium, Escherichia, Klebsiella, Lactobacillus, Staphylococcus, Streptococcus, and Veillonella [115,116]. The GM of infants born vaginally closely mirrors that of the maternal vaginal microbiota [117], while those delivered via cesarean section (C-section) tend to acquire a microbial profile more aligned with that of maternal skin [110,118]. For vaginally delivered infants, the most common bacteria include Bifidobacterium (phylum Actinomycetota), Enterococcus and Lactobacillus (phylum Bacillota), Bacteroides, Parabacteroides and Prevotella (phylum Bacteroidota), Sneathia (phylum Fusobacteriota), and Escherichia (phylum Pseudomonadota). Conversely, the microbiome of C-section infants is predominantly composed of Corynebacterium and Propionibacterium (phylum Actinomycetota), Staphylococcus, Streptococcus and Veillonella (phylum Bacillota), as well as Enterobacter and Haemophilus (phylum Pseudomonadota) [108,119,120,121]. Martin et al. [122] highlighted substantial differences between the meconium microbiota of vaginal and C-section births, with meconium from C-section deliveries showing lower bacterial diversity. Specifically, the C-section group had fewer Bacteroides, Bifidobacterium, and Lactobacillus species, but higher amounts of Clostridium [122,123].
Breastfeeding supplies a mixture of nutrients that contribute to several key processes, including immune system regulation and the promotion of the growth and activity of beneficial microorganisms [107,124]. The microbial composition of breast milk is diverse, typically consisting of Staphylococcus and Streptococcus species, along with other genera like Bifidobacterium spp., Propionibacterium, Veillonella, and lactic acid bacteria [125]. During the first three months of life, breastfeeding influences the composition of the GM, primarily by increasing the abundance of Staphylococcus and Bifidobacterium, while decreasing the abundance of Clostridium. Formula-fed infants, however, have a different GM composition, marked by the abundance of the bacterial genera Atopobium, Bacteroides, Bifidobacterium, Clostridium, and Enterococcus [121,122,126].
The administration of antibiotics during the first three years of life induces a reduction in the diversity and composition of the GM [127], resulting in a lower abundance of members of the order Clostridiales and in the genera Bifidobacterium, Ruminococcus, and Staphylococcus [122,128], and in an increase in members of the phyla Pseudomonadota, Actinomycetota, members of the family Enterobacteriaceae and the genera Enterococcus and Lactobacillus [129,130]. These alterations may have adverse effects on children’s health, increasing their susceptibility to a range of microbial pathogens [131], and leading to negative mental health outcomes, such as cognitive, emotional, and behavioral difficulties, including mood-related and depressive symptoms [132]. Importantly, several CNS processes, including synaptic pruning, myelination, and synaptogenesis, which occur alongside GM maturation, may be impacted by metabolites associated with specific microorganisms [133,134].
5. Microbiological Aspects of ELS
Children who experience ELS exhibit distinct neurodevelopmental trajectories [135] and face a heightened risk of developing mental health disorders throughout their lives [136]. Studies in both animal models and human populations have shed light on the mechanisms underlying ELS-related alterations and their impact on the maturation of neural circuits and psychological processes essential for cognitive function and emotional well-being [137].
5.1. ELS in Animal Models
A few studies have examined the association between ELS and GM, and most of these are based on animal models. These preclinical studies suggest that ELS may affect the GM dysbiosis in offspring and increase their risk of developing psychiatric and physiological diseases across generations [138,139,140].
In animal models, ELS induces alterations in the GM that persist into adulthood [141]. An early study reported that adult mice exposed to ELS exhibited changes in the GM, with elevated levels of Bifidobacterium bifidum, Clostridium leptum, C. coccoides, and Lactobacillus spp. [142]. Another study in adult rats found that ELS decreased the Bacillota/Bacteroidota ratio, while increased taxa linked to inflammation, such as Prevotella, Flexibacter, and Akkermansia [143]. Mice exposed to ELS and then subjected to chronic stress as adults increased members of the Bacteroidota and Pseudomonadota phyla, and Clostridium species in the GM [144].
Recent research indicates that ELS may have sex-specific effects on the GM, behavior, and gene expression in the PFC of mice [145]. In males, ELS influenced the abundance of bacterial taxa from the families Lachnospiraceae and Porphyromonadaceae, as well as the genera Alloprevotella, Bacteroides, and Lactobacillus. In contrast, females exhibited changes primarily in the genera Lactobacillus and Mucispirillum [145]. Another study similarly reported sex-dependent differences in ELS-induced microbiota alterations, with both male and female rats showing increased Bacteroides and reduced Lachnospiraceae abundance [146]. However, males displayed an increase in Streptococcus and a decrease in Staphylococcus, whereas females exhibited a rise in Sporobacter and a reduction in Mucispirillum. These microbial shifts were linked to elevated colonic IFN-γ and IL-6, as well as higher serum IL-1β levels in males, which corresponded to heightened anxiety-like behaviors. Furthermore, in rats, ELS was found to reduce Bacteroidota and increase Bacillota, an effect that was further modulated by serotonin transporter (5-HTT) genotype [147]. Specifically, rats with diminished 5-HTT expression exposed to ELS exhibited a microbiota profile oriented toward an inflammatory state, marked by an increased presence of taxa such as Fusobacterium, Mucispirillum, and Desulfovibrio.
More recently, Ke et al. [148] reviewed how the GM may contribute to PTSD by influencing inflammation (via the gut genera Roseburia and Odoribacter), neurotransmitter signaling, and stress responses, while the bidirectional gut–brain communication implies diverse pathways, mechanisms, and processes like the vagus nerve, immune system activation, and microbial metabolites. Moreover, in a preclinical study using a mouse model, Yadav et al. [149] found that recurrent exposure to social defeat stress, a well-stablished preclinical model for PTSD, induced GM dysbiosis that is characterized by a change in the Bacteroidota/Bacillota abundance ratio, an important factor in maintaining gut homeostasis and an increased abundance of members of the Lachnospiraceae family which regulate gut inflammation and gut barrier integrity. However, the decreased abundance of the members of the Muribaculaceae family suggests a dysregulation of dietary fiber fermentation and cholesterol metabolism.
5.2. ELS in Humans
Prenatal stress encompasses not only cases where the expectant mother experiences psychological distress, such as depression or anxiety [150,151], but also situations where maternal lifestyle or physical conditions may induce stress in the fetus, including exposure to adversity [152] or severe illness [153].
Postnatal research has examined children and adolescents who have experienced ELS, including studies on young populations within institutional settings [136,154,155,156], studies on adults with PTSD who have been assessed for the effects of childhood stress [157,158], and studies across several ELS domains such as life episodes, parental factors, direct victimization, contextual risks, and interpersonal stressors [159]. Table 1 summarizes recent prenatal and postnatal studies (2019–2023) on the relationship between ELS and GM.

Table 1.
Recent prenatal and postnatal studies (2019–2023) on the relationship between ELS and GM.
5.2.1. Prenatal Stress Studies
A longitudinal study tracked Dutch children from the third trimester of pregnancy through to 110 days after birth [151]. It was found that prenatal stress, which was measured via surveys and basal salivary cortisol, was associated with offspring GM composition for at least three months. Participants experiencing high levels of cumulative prenatal stress showed an increased relative abundance of genera belonging to the phylum Pseudomonadota (Enterobacter, Escherichia, and Serratia), and a lower relative abundance of genera from the phyla Bacillota (Aerococcus, Lactococcus, and Lactobacillus) and Actinomycetota (Bifidobacterium, Collinsella, and Eggerthella), suggesting an association with the GM dysbiosis.
Aatsinki et al. [150] conducted a longitudinal study in Finland. The authors found that both maternal prenatal psychological distress (PPD) and hair cortisol levels were linked to alterations in the GM of infants at 2.5 months of age (N = 446 mother–infant pairs). Chronic PPD, as assessed by various questionnaires, was negatively associated with Actinobaculum, Anaerotruncus, Akkermansia, Epulopiscium, Eubacterium, Megamonas, Megasphera, Odoribacter, Parabacteroides, Paraprevotella, Phascolarctobacterium, Propionibacterium, Pseudoramibacter, and Slackia abundance, and positively with Actinomyces, Coprococcus, Dialister, Dorea, Finegoldia, Rothia, and Veillonella abundance. Maternal depression indicators were positively correlated with the abundance of Butyricimonas and Prevotella. On the other hand, maternal hair cortisol levels were associated with changes in the infant’s GM, showing negative correlations with Actinobaculum, Anaerotruncus, Butyricimonas, Citrobacter, Enterococcus, Lactobacillus, Paraprevotella, Phascolarctobacter, Ruminococcus, and Slackia.
Another longitudinal study of 25 mother–infant dyads in the Galapagos Islands (Ecuador) found correlations between exposure to precariousness and peripartum changes in the HPA axis and in the offspring GM [152]. Maternal precariousness was assessed both during and after pregnancy using validated scales, while HPA axis activity was measured through maternal salivary cortisol levels collected during and after pregnancy. In addition, saliva, and stool samples were obtained from newborns at three days and two months of age. The authors found that both maternal precarious exposure and HPA dysregulation were consistently linked to alterations in the infants’ GM. These changes were characterized by reduced species diversity, a decrease in Bifidobacterium abundance at the genus level, and an increased presence of potential pathogens within the Enterobacteriaceae family. Figure 1 shows changes in the GM at the genus level in several prenatal stress studies.
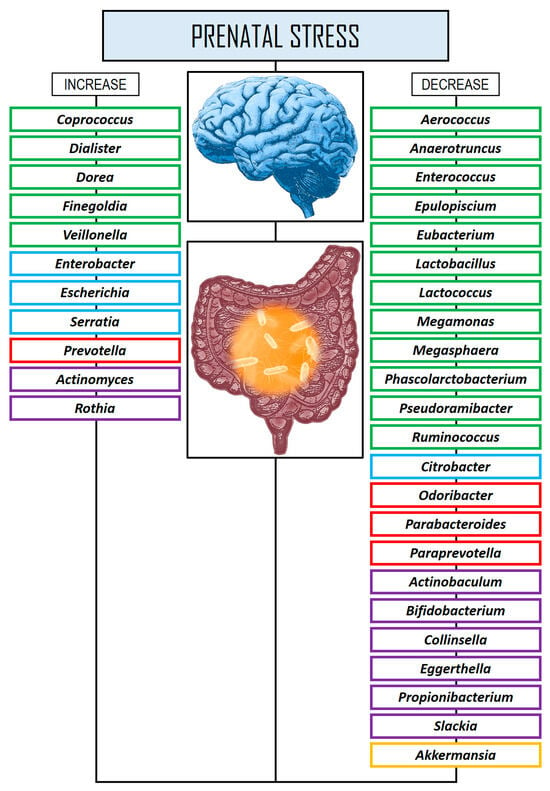
Figure 1.
Changes in the GM at the genus level in several prenatal stress studies. Rectangles in green color: phylum Bacillota. Rectangles in blue color: phylum Pseudomonadota. Rectangles in red color: phylum Bacteroidota. Rectangles in violet color: Actinomycetota. Rectangles in yellow color: phylum Verrucomicrobiota.
5.2.2. Postnatal Stress Studies
An exploratory study involving adults (N = 18) with a history of trauma and PTSD revealed a reduced overall gut microbiota abundance of taxa from the phyla Actinomycetota, Lentisphaerota, and Verrucomicrobiota in individuals who developed PTSD, compared to those who were exposed to trauma but did not develop the disorder [157]. However, no significant differences were observed between the PTSD and control groups in terms of alpha- or beta-diversity measures.
A longitudinal study examined the first six weeks of life of 82 infants stay in a neonatal intensive care unit, where stay can be stressful due to the separation of parents and infants as well as to medical procedures [154]. The authors found that higher stress scores on the Neonatal Infant Stressor Scale were associated with higher relative abundances of Proteus and Veillonella.
A study involving psychiatrically healthy women (N = 48) between 20 and 26 weeks of gestation compared a low ACEs control group with a high ACEs group, where participants had experienced multiple childhood adversities. Women in the high ACEs group exhibited a higher differential abundance of Prevotella and a lower abundance of Erysipelotrichaceae and Phascolarctobacterium compared to those in the low ACEs group [160]. Importantly, these women were psychiatrically healthy, suggesting that ACEs might affect the GM independently of current stress or psychiatric status. This study also indicates an association between GM composition and the acute glucocorticoid–immune response to stress and suggests that women with high ACEs who consume higher amounts of ω-3 polyunsaturated fatty acids may experience a reduced inflammatory response to acute stress.
A cross-sectional study conducted by Michels et al. [161] in young participants (N = 93, 8–16 years old) explored the relationship between the GM in feces and psychosocial stress, measured via questionnaires and cortisol levels. The findings revealed that elevated levels of stress were linked to a decrease in alpha-diversity, to a higher abundance of Bacteroides, Methanobrevibacter, Parabacteroides, Roseburia, and Rhodococcus, and to a lower abundance of the genera Phascolarctobacterium and the phylum Bacillota. Several genera exhibited conflicting results across different stress measures, such as Ruminococcaceae UCG014, members of the phylum Mycoplasmatota (formerly Tenericutes), Christensenellaceae R7, Prevotella 9, and Eubacterium coprostanoligenes.
Another longitudinal study focused on a shorter time frame and measured the impact of childhood adversity, specifically institutional or foster care, on the GM composition during adolescence (N = 344 participants, ages 3–18 years) [136]. Early childhood adversity was related to shifts in alpha- and beta-diversity, and decreases in Lachnospiraceae, whether related to adversity or not, were associated with brain reactivity, particularly in the PFC, during emotional processing.
A cross-sectional study involving healthy children aged 5 to 7 years (N = 40) explored the relationships between behavioral dysregulation, socioeconomic risk, diet, caregiver behaviors, and GM [162]. The findings indicated that higher levels of Bacteroides fragilis and Bifidobacterium spp. were linked to lower instances of aggression, emotional reactivity, externalizing behaviors, sadness, and impulsivity, as well as fewer family conflicts. On the other hand, two butyrate-producing bacteria, Coprococcus comes and Eubacterium rectale, were associated with greater anxiety and depression, alongside reduced inhibitory control. Interestingly, Roseburia inulinivorans, another butyrate producer, showed an inverse correlation, being linked to fewer depressive symptoms.
Keskitalo et al. [163] investigated the association between the GM composition and the cortisol response to stress in children. The study used data from the FinnBrain Birth Cohort, a large transgenerational prospective observational study that initially included 3808 mothers, 2623 fathers, and 3837 children. The nested case-control analysis focused on children who had both a fecal microbiome profile and salivary cortisol measurements at 2.5 months of age. Following exposure to a mild acute stressor, cortisol levels were assessed at four intervals: 0, 15, 25, and 35 min. The findings indicated that a reduced cortisol response to the stressor was weakly linked to GM diversity, while stronger associations were observed between cortisol levels and the specific taxonomic composition of the children’s fecal microbiome.
Reid et al. [156] examined the GM of adolescents (N = 35, aged 13–21) who had been internationally adopted from orphanages into the United States, comparing them with non-institutionalized adolescents. The study found that the stressed group had significantly higher abundances of several bacterial taxa, including the genera Bacteroides, Coprococcus, Escherichia, Prevotella, and Streptococcus, compared to the non-institutionalized group. Furthermore, notable associations were observed between the relative abundance of Escherichia, specific T-cell subsets in circulation, and cytomegalovirus seropositivity. These findings suggest a potential connection between the GM and immune system alterations resulting from early life adversity.
Laue et al. [164] carried out a 3-year prospective longitudinal study to investigate sex-specific relationships between the GM and behavioral outcomes. The study involved stool samples from 260 children taken at 6 weeks, 1 year, and 2 years of age. When the children reached approximately 3 years old, their parents completed a behavioral assessment survey to evaluate various phenotypes. The findings indicated that while most outcomes were not linked to changes in beta-diversity, a higher diversity in the microbiome at 6 weeks was associated with lower depression levels in the entire sample, as well as reduced anxiety and improved internalizing behaviors, particularly in boys. Specifically, in boys, better adaptive functioning scores correlated positively with Bifidobacterium abundance and negatively with Klebsiella abundance. In girls, Granulicatella was associated with higher anxiety scores at 6 weeks, while Streptococcus peroris was linked to better internalizing behavior at 1 year. Furthermore, certain Blautia species were connected to higher hyperactivity scores, with a stronger association observed in girls.
In a case-control study by Malan-Muller et al. [158], the relationship between the GM and mental health outcomes was examined in adults with PTSD, comparing them to trauma-exposed individuals without PTSD. The study also involved several questionnaires to assess anxiety disorders, past traumatic events, and major depressive disorder (MDD). Fecal samples were collected within the same week as the clinical assessments. The results identified a specific group of four genera (i.e., Catenibacterium, Mitsuokella, Odoribacter, and Olsenella) that were positively correlated with PTSD. Moreover, MDD was associated with a higher relative abundance of bacteria from the Bacteroidota phylum. These findings suggest that early life trauma could set the stage for microbiome alterations, which may contribute to a pro-inflammatory response, potentially increasing susceptibility to PTSD later in life.
More recently, Kraaij et al. [165] conducted a cross-sectional study of 1784 ten-year-old children from the Netherlands to characterize associations of the GM with child mental health problems. Although lower gut microbial diversity and richness was related to internalizing problems and anxious/depressed conduct problems, these associations were not significant. These authors did not find definitive evidence linking GM diversity, taxonomic traits or functions, and mental health problems in the pediatric population. However, they noted suggestive findings indicating a reduction in the genera that have previously been related to psychiatric disorders, including Anaerotruncus, Hungatella, and Oscillospiraceae. Figure 2 shows changes in the GM at the genus level in several postnatal stress studies.
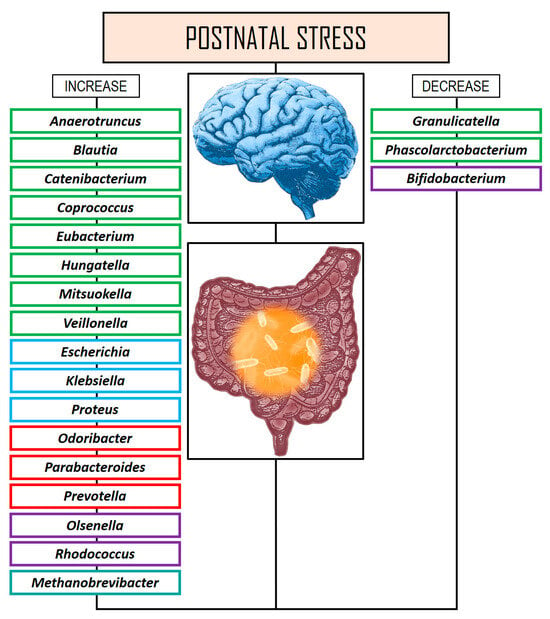
Figure 2.
Changes in the GM at the genus level in several postnatal stress studies. Rectangles in green color: phylum Bacillota. Rectangles in blue color: phylum Pseudomonadota. Rectangles in red color: phylum Bacteroidota. Rectangles in violet color: Actinomycetota. Rectangles in turquoise color: phylum Euryarchaeota.
6. Microbial Key Regulators of ELS
6.1. The Role of SCFAs in ELS
SCFAs, derived from microbial fermentation of dietary fibers, are considered a pivotal mechanism by which the GM influences the effects of maternal stress on offspring neurodevelopment [166]. These SCFAs, primarily acetate, propionate, and butyrate, are efficiently absorbed into the bloodstream and can reach the brain parenchyma [167]. They have the potential to impact brain function both prenatally and postnatally, as maternal microbiota-derived SCFAs can enter the maternal bloodstream and subsequently reach the fetal brain during pregnancy [168]. In rodent models, chronic restraint stress has been shown to reduce fecal SCFA levels [169]. Furthermore, supplementation with SCFAs has been found to mitigate social defeat stress-induced deficits in reward-seeking behavior, highlighting their potential therapeutic benefits [170].
SCFAs also influence the developing fetus by impacting the microglia, which play a key role in neuromodulation throughout development and into adulthood [171]. During embryonic development, microglia gain the ability to respond to environmental cues, and disruptions in the presence of maternal microbial metabolites can hinder their proper function and maturation [172]. While microglia do not directly express receptors for SCFAs, these molecules can diffuse into the cells, influencing gene expression by inhibiting histone deacetylase activity and preventing NF-κB nuclear translocation in response to LPS treatment in vitro [173]. Notably, acetate, which is a key SCFA derived from the microbiome, has been shown to be essential for microglial maturation. In germ-free mice, acetate regulates the microglial metabolic state and has also been found to modulate microglial phagocytosis and influence disease progression in neurodegenerative conditions [174].
6.2. The Role of Amino Acids in ELS
In a study using maternally separated (MS) mice, Zhu et al. [175] reported that decreased levels of amino acid transporters in the gut of MS mice led to lower blood glutamine (Gln) levels and synaptic dysfunction in the medial PFC. Reduced blood Gln availability limits its use in the brain for the reuptake of presynaptic neurotransmitters like glutamate (Glu) and γ-aminobutyric acid (GABA). In addition, MS mice exhibited GM dysbiosis, specifically a decrease in the relative abundance of Limosilactobacillus (formerly Lactobacillus) reuteri. Supplementation with L. reuteri alleviated neurobehavioral deficits in MS mice by enhancing intestinal amino acid transport and restoring synaptic transmission in the medial prefrontal cortex. Moreover, supplementation with other amino acids like tryptophan (Trp) altered the GM, with increased levels of Intestinimonas and decreased levels of Turicibacter in adult offspring at 12 weeks of age in Sprague–Dawley rats [176]. These authors also observed that Trp supplementation provided protection against hypertension induced by maternal chronic kidney disease. Stress during pregnancy in mice disrupted placental Trp metabolism and reduced the availability of Trp during fetal neurogenesis, partly by diminishing the maternal Trp-metabolizing microbiota [177]. Key gut microbes involved in Trp metabolism, such as Parasutterella and Bifidobacterium, were reduced in both the dams and their offspring following prenatal stress [177]. This suggests that maternal malnutrition or drug exposure during pregnancy may influence placental Trp availability, alter the kynurenine (Kyn) metabolic pathway, and/or decrease the abundance of Trp-metabolizing microbes in the gut, potentially contributing to neuropsychiatric disorders in offspring.
In humans, Trp is primarily derived from diet and serves as a precursor of serotonin (5-HT), which is associated with metabolic disorders [178], and is also directly and indirectly implicated in regulating brain functions [179], playing a central role in the development of social and emotional behaviors in offspring [180]. Alterations in Trp metabolism are a fundamental biological marker of prenatal maternal stress induced by intrauterine inflammation [181], which increases the potential risk for neurodevelopmental disorders [36,182,183] and is associated with peer victimization in adolescents [184,185,186]. Children with autism spectrum disorder (ASD) have been found to have impaired Trp metabolism in the gut, CNS, and blood, which may underlie the pathophysiology of ASD [187,188].
The fetal-placental unit controls Trp metabolism, primarily via two pathways: 5-HT and Kyn. The Kyn pathway, which is active in the brain, liver, intestine, and placenta, degrades over 95% of Trp and plays a role in neurotransmission and inflammation, as well as in immune modulation and responses during pregnancy [189]. Key enzymes in the Kyn pathway include indoleamine-2,3-dioxygenase type 1 and type 2, and Trp-2,3-dioxygenase [190]. The 5-HT pathway uses up to 5% of Trp, primarily in pancreatic cells, adipose tissue, the gut, and the CNS, regulating responses to environmental stimuli such as feeding behavior, cognition, and sleep. Key enzymes in this pathway include tryptophan hydroxylase type 1 in the enterochromaffin cells of the gastrointestinal tract, adipose tissue, and β-cell pancreatic cells, whereas tryptophan hydroxylase type 2 hydrolyzes Trp in central neurons [191].
The GM plays a critical role in the Trp metabolism, directly converting it to various molecules such as indole and its derivatives, indole acrylic acid, indole-3-acetic acid, indole-3-propionic acid, indole-3-acetaldehyde, and tryptamine [192]. These products help maintain intestinal balance by regulating the expression of pro- and anti-inflammatory cytokines [193,194]. The specific gut bacteria involved in Trp metabolism include the genera Anaerostipes, Bacteroides, Bifidobacterium, Clostridium, Lactobacillus, and Ruminococcus. Other bacteria, like Providencia, Pseudomonas, and Staphylococcus, mediates the conversion of Trp to Kyn [195]. Indole and its derivatives play important roles in gastrointestinal function, inflammation control, antioxidation, and immune system regulation. Indole also promotes the release of glucagon-like peptide-1, which slows down gastric emptying and reduces appetite. Moreover, tryptamine encourages the secretion of 5-HT from enterochromaffin cells, stimulating gastrointestinal movement by influencing intestinal neurons [193,195,196].
Interestingly, the excess of Trp supply during pregnancy may reduce the aggressiveness in the male offspring via modification of the MGB axis function [197]. In addition, probiotics may prevent aggressive behavior by modifying the central serotogenic system, and thus the GM may act as a complementary endocrine organ [198]. Trp exposure may also increase catecholamine concentrations in the thalamus and in the hypothalamus, affecting energy homeostasis and social interaction, which may be related to changes in the GM and in the GB axis’ function [199]. Stress-induced HPA axis overactivity is related to reactive aggression [200], and the HPA axis has been involved in several adverse mental conditions, such as attention deficit hyperactivity disorder (ADHD), anxiety, and depression [201,202].
Striatal dopamine (DA) synthesis is elevated in ASD and has been linked to social defeat [203]. DA serves as the precursor for NE and EP, both of which play key roles in the reward system by contributing to feelings of satisfaction [204]. Functionally, EP and NE possess strong similarities as neurotransmitters or hormones, both acting to regulate the response to stress [205]. An NE reuptake inhibitor, viloxazine, by blocking the actions of the DA receptors and transporters, enhances the presence of DA and NE [206], thereby alleviating symptoms of impulsivity and inattention in ADHD, which is directly linked to bullying behavior in males [207,208]. The 5-HT and DA systems interact at a neurophysiological level in the regulation of impulsive aggression, which is central to several mental health disorders, such as borderline personality disorder and antisocial personality disorder [209,210].
6.3. The Role of Psychobiotics in ELS
The modulation of the GM and its metabolites via psychobiotic administration appears to be a highly promising strategy for addressing CNS alterations resulting from ELS [211]. Psychobiotics consist of probiotics, prebiotics, synbiotics, and postbiotics that provide mental benefits by acting on the GM and reducing symptoms of stress, anxiety, and depression [212]. With respect to ELS, few studies have been performed using probiotics to minimize the effects on mental health and CNS function, through the ability of probiotics to synthesize neuroactive compounds, such as GABA, 5-HT, DA, NE, and acetylcholine [33,213].
Male Sprague–Dawley rats subjected to MS were exposed to probiotics (i.e., Lactobacillus helveticus and Lacticaseibacillus rhamnosus) through the maternal drinking water during the stress period [214]. The study revealed that the stressed rat pups exhibited premature activation of the medial PFC during fear regulation, a phenomenon that could be mitigated by probiotic treatment.
Stressful social experiences in early life were investigated by Karen et al. [215] by examining how they can induce changes in the MGB axis. These authors found that the supplementation with the probiotic Lactocaseibacillus paracasei strain HT6 had a significant beneficial effect on anxiety-like behavior in stressed rats, along with normalized levels of ACTH, corticosterone, GRs, 5-HT, DA, and NE.
De Santa et al. [216] explored the effects of prolonged consumption of a multi-strain probiotic mixture on behavioral alterations in a MS mouse model, focusing on adult male mice. The study found that probiotic treatment, which included strains such as Bifidobacterium animalis subsp. lactis BL03, B. animalis subsp. lactis BI04, B. breve BB02, Lactobacillus acidophilus BA05, L. helveticus BD08, L. paracasei BP07, Lactiplantibacillus plantarum BP06, and Streptococcus thermophilus BT01, successfully reversed anxiety- and depressive-like behaviors. Moreover, it normalized the neuroinflammatory state by restoring the quiescent state of microglia and induced a proneurogenic effect. Additionally, the probiotic treatment significantly influenced the production of SCFAs, particularly increasing butyrate levels.
7. Discussion
This review examined ELS-related outcomes and their association with the GM. Traumatic experiences during childhood or adolescence constitute major negative life events that profoundly affect the cognitive function and development, and can lead to adverse mental conditions such as anxiety, depression, and PTSD. In turn, there are various ways through which the GM can impact human psychological processes, including the nervous system (i.e., autonomic nervous system, enteric nervous system, and vagus nerve), the immune system, and the HPA axis [217]. The effect of GM on mental disorders is mainly mediated via the synthesis of neurotransmitters and microbial metabolites, as well as through the activation of certain metabolic pathways such as Trp metabolism. Nevertheless, the precise role of the GM in modulating stress responses and influencing social behavior in children and adolescents has not yet been fully elucidated and remains as a widely debated topic. For this reason, research should aim at determining to which extent ELS is associated with alterations in the GM and whether dysbiosis early in life may induce the onset and development of various mental disorders later in life. This will highlight the GM as a novel biological factor influencing vulnerability versus resilience to stress and potentially identify new treatment targets for several mental disorders.
The influence of prenatal stress on the infant GM is supported by longitudinal evidence showing that both psychological and physiological markers of maternal distress are associated with specific microbial alterations in early life. Aatsinki et al. [150] and Jahnke et al. [152] independently demonstrated that maternal stress, HPA axis dysregulation, and hair cortisol concentrations were linked to changes in the composition of the infant GM. Notably, Veillonella abundance was consistently elevated in association with maternal stress markers in both cohorts, suggesting a potential role for this bacterial genus as a microbial correlate of prenatal stress exposure. Nevertheless, there are divergences between both studies. Aatsinki et al. [150] identified broad shifts, including reductions in beneficial genera such as Anaerotruncus, Phascolarctobacterium, and Ruminococcus, as well as elevations in Coprococcus and Prevotella, with associations varying depending on whether distress was assessed psychologically or via cortisol biomarkers. In contrast, Jahnke et al. [152] reported a reduction in alpha-diversity and increased relative abundance of pro-inflammatory taxa such as members of the families Enterobacteriaceae and Streptococcaceae, emphasizing compositional changes potentially linked to immune modulation. These differences may reflect the distinct stress metrics used, as well as methodological variability or statistical power given the much smaller sample in Jahnke et al. [152]. Postnatal studies, in turn, present significant variability in the results, but also reveal common patterns. In the study by D’Agata et al. [154], neonatal stress, measured by the Neonatal Infant Stressor Scale, was associated with higher relative abundances of the genera Proteus and Veillonella. Reid et al. [156] found that adolescents who experienced stress during childhood, as a result of being internationally adopted from orphanages, exhibited higher abundances of the genera Bacteroides and Prevotella. Flannery et al. [162] and Michels et al. [161] identified higher abundances of Bacteroides, Rhodococcus, and Roseburia. Interestingly, Laue et al. [164], found that greater diversity in the infant microbiome was associated with lower levels of depression and anxiety, particularly in boys, suggesting that GM diversity may play a protective role against psychosocial stress. In contrast, the study by Kraaij et al. [165], in a larger sample of children, reported that lower GM diversity and richness were related to internalizing problems and anxious/depressed conduct problems. These findings highlight the complexity of the relationship between GM and mental health, with results varying depending on the context, age, and nature of the stress experienced. However, a trend is observed, particularly a reduction in Phascolarctobacterium and an increase in the Bacteroides genus being associated with greater emotional and behavioral difficulties. Therefore, while prenatal exposures appear to more consistently influence taxa associated with immune maturation and colonization resistance, potentially reflecting early disruptions in vertical transmission and gut-immune crosstalk, postnatal stress exposures, particularly during sensitive periods of neurodevelopment, seem to associate more with genera linked to emotional regulation and cognition, such as Roseburia, Bacteroides, and Coprococcus, as well as functional pathways involved in monoamine metabolism [162]. Furthermore, while prenatal stress studies identified a potential reduction in microbial diversity [152], postnatal studies present a more variable pattern, with some of them reporting reduced richness [165] and others highlighting taxonomic shifts without diversity loss [164].
In essence, across the reviewed literature, there is growing evidence linking psychosocial stress, both prenatal and postnatal, with alterations in the GM composition across developmental stages. Despite methodological heterogeneity among studies (e.g., variations in sample sizes, age ranges, stress assessments, and sequencing techniques), the genera Veillonella, Prevotella, and Coprococcus were repeatedly associated with stress-related exposures in both infant and adolescent populations, suggesting a potential shared microbial signature of psychosocial stress. Specifically, Veillonella was positively associated with maternal HPA axis dysregulation [152] and neonatal stress exposure [154], while Prevotella was consistently elevated in individuals with high adversity exposure across different age groups [150,156,160]. Conversely, taxa such as Phascolarctobacterium and Anaerotruncus tended to show decreased abundance in relation to stress or psychopathology [150,161,165], possibly reflecting a stress-induced depletion of beneficial commensals. On the other hand, while Coprococcus was enriched in adolescents exposed to early institutionalization [156]; it was also associated with increased anxiety symptoms in younger children [162], pointing to potential developmental or contextual moderators. In addition, while some studies [163,164] reported associations between GM diversity and psychological outcomes, others found no clear links, highlighting ongoing debate regarding the role of diversity per se as a biomarker of stress resilience or vulnerability.
It is pivotal to acknowledge the limitations inherent in the studies reviewed, as these must be carefully considered when interpreting the findings of this review. First, several of these studies adopt a cross-sectional design, often resulting in small sample sizes that reduce statistical power and increase susceptibility to confounding bias. Second, most GM studies have been conducted at the genus and phylum levels, despite evidence suggesting that species-level differences are critical for inferring functional variations in the GM. Third, 16S rRNA gene sequencing, the predominant method used, has limited taxonomic resolution and does not account for other key microbial components, such as protozoa, viruses, and fungi. Fourth, the bacterial sequencing techniques employed are unable to reliably differentiate between beneficial and pathogenic taxa. Consequently, whole-genome metagenomics, such as shotgun sequencing, is expected to surpass 16S rRNA gene sequencing in future research. Finally, diet represents a major confounding factor in the association between the GM and ELS, and it is often not adequately controlled in these studies.
A recent systematic review of human studies [218] identifies emerging patterns and significant gaps in the relationship between ELS and the GM. Prenatal stress appears consistently associated with increased abundance of Proteobacteria (currently Pseudomonadota), particularly the family Enterobacteriaceae, and decreased levels of Bifidobacterium, a genus with well-documented immunomodulatory and neuroactive properties. These changes were linked to maternal cortisol levels and early infant stress markers, suggesting potential mechanistic pathways linking maternal stress physiology to microbial colonization. However, postnatal studies present more conflicting findings, partly due to variability in stress definitions, microbiome assessment methods, and population characteristics. While some studies report reductions in alpha-diversity and shifts in beta-diversity among institutionalized or traumatized children, others fail to replicate these results, highlighting the need for greater taxonomic resolution and longitudinal designs. Moreover, discrepancies in the abundance of taxa such as Bacteroides, Eubacterium, and Coprococcus, which are variably associated with both protective and detrimental outcomes, underscore the limitations of current approaches and the influence of confounding factors such as sex, medication, diet, and socioeconomic conditions. The present narrative review and the systematic review by Agusti et al. [218] identify specific microbial genera, including Veillonella, Prevotella, and Coprococcus, as being associated with psychosocial stress across developmental stages, suggesting a potential shared microbial signature. However, the implications of these findings remain uncertain due to methodological variability across studies. Both reviews acknowledge key limitations, such as the predominance of 16S rRNA sequencing with limited taxonomic resolution, the absence of species-level analysis, and insufficient control over confounding factors like diet and socioeconomic conditions. While the review by Agusti et al. [218] emphasizes the need for longitudinal studies and a deeper understanding of the role of maternal cortisol in microbial colonization, our review also underscores the biological pathways through which the GM may influence psychological processes, particularly through neurotransmitter synthesis, microbial metabolites, and metabolic pathways such as tryptophan metabolism. Furthermore, our review highlights the roles of SCFAs, amino acids, and psychobiotics as key microbial regulators in the context of ELS.
A more comprehensive understanding of the GM and its effects on human behavior is essential to clarify its implications in the field of psychosocial sciences. In this sense, Maes et al. [219] have recently introduced a novel clinimetric approach, known as “precision nomothetic psychiatry”, which facilitates the exploration of causal relationships between causome/protectome factors, recurrence of illness index (ROI), cognitive deficits, and a quantitative score of the phenome of depression. This model shows that ACEs and the increased translocation of Gram-negative bacteria are robustly related to depression, with these effects being mediated by ROI, reduced antioxidant defenses such as lowered high-density lipoprotein cholesterol and the activation of immune and oxidative stress pathways. Nevertheless, there is currently no evidence revealing whether ACEs and ROI affect the GM, nor whether compositional dysbiosis may mediate the effects of ACEs on the depression phenotype, which encompasses cognitive deficits and suicidal behavior.
ELS can contribute to the perpetuation of social disparities and cycles of mental health vulnerability, placing a considerable strain on public resources, healthcare systems, and overall societal well-being. To mitigate these undesirable effects, it is essential to implement public policies that promote safe and supportive environments for children and adolescents. This includes raising awareness about the impact of ELS and educating about the potential factors influencing its onset, which should be prioritized in health professional training and community programs. Research focused on understanding the psychological processes and emotional states involved in early adversities, such as the role of humiliation in the context of bullying victimization [220], could offer valuable insights for addressing ELS and its long-term consequences. Furthermore, interdisciplinary studies on the GM and its interaction with physiological, psychological, and environmental factors may be promising to shape and regulate human behavior from infancy.
8. Conclusions
ELS can profoundly impact cognitive function and neurodevelopment, with maternal early-life nutrition playing a significant role in modulating the effects of prenatal and postnatal stress. ELS influences the GM, disrupting its composition and function by altering the synthesis of microbial metabolites, neurotransmitters, and the activation of key metabolic pathways. However, the precise role of the GM in modulating stress responses during childhood and adolescence has not yet been fully elucidated.
Although several studies have demonstrated an association between ELS and the GM, causality has not yet been established due to the numerous intrinsic and extrinsic factors influencing the MGB axis. In the coming years, research on key microbial regulators, such as SCFAs, amino acids, and psychobiotics, may represent a promising approach for addressing CNS alterations linked to ELS. Thus, further studies will be necessary to evaluate their potential as therapeutic agents.
Author Contributions
Conceptualization, A.B.-R. and J.J.B.; investigation, J.J.B.; writing—original draft preparation, A.B.-R. and J.J.B.; writing—review and editing, A.B.-R.; supervision, J.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACTH | Adrenocorticotropin |
| ACEs | Adverse childhood experiences |
| ADHD | Attention deficit hyperactivity disorder |
| ASD | Autism spectrum disorder |
| ANS | Autonomic nervous system |
| CNS | Central nervous system |
| CRH | Corticotropin-releasing hormone |
| C-section | Cesarean section |
| DA | Dopamine |
| ELS | Early life stress |
| EP | Epinephrine |
| GABA | γ-aminobutyric acid |
| Gln | Glutamine |
| Glu | Glutamate |
| GM | Gut microbiome |
| GCs | Glucocorticoids |
| GRs | Glucocorticoid receptors |
| 5-HT | Serotonin |
| 5-HTT | Serotonin transporter |
| HPA | Hypothalamic–pituitary–adrenal |
| IFN | Interferon |
| IL | Interleukin |
| Kyn | Kynurenine |
| LPS | Lipopolysaccharide |
| MDD | Major depressive disorder |
| MS | Maternally separated |
| MGB | Microbiome-gut–brain |
| NE | Norepinephrine |
| NF-κB | Nuclear factor kappa B |
| PVN | Paraventricular nucleus |
| PTSD | Post-traumatic stress disorder |
| PFC | Prefrontal cortex |
| PPD | Prenatal psychological distress |
| ROI | Recurrence of illness index |
| SCFAs | Short-chain fatty acids |
| Trp | Tryptophan |
References
- Shin, S.H.; Kim, Y.K. Early life stress, neuroinflammation, and psychiatric illness of adulthood. Adv. Exp. Med. Biol. 2023, 1411, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Boullier, M.; Blair, M. Adverse childhood experiences. Paediatr. Child Health 2018, 28, 132–137. [Google Scholar] [CrossRef]
- Alhowaymel, F.; Kalmakis, K.; Jacelon, C. Developing the concept of adverse childhood experiences: A global perspective. J. Pediatr. Nurs. 2021, 56, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Merrick, M.T.; Ford, D.C.; Ports, K.A.; Guinn, A.S. Prevalence of adverse childhood experiences from the 2011–2014 behavioral risk factor surveillance system in 23 States. JAMA Pediatr. 2018, 172, 1038–1044. [Google Scholar] [CrossRef]
- Merrick, M.T.; Ports, K.A.; Ford, D.C.; Afifi, T.O.; Gershoff, E.T.; Grogan-Kaylor, A. Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse Negl. 2017, 69, 10–19. [Google Scholar] [CrossRef]
- Bishop, M.; Rosenstein, D.; Bakelaar, S.; Seedat, S. An analysis of early developmental trauma in social anxiety disorder and posttraumatic stress disorder. Ann. Gen. Psychiatry 2014, 13, 16. [Google Scholar] [CrossRef]
- Chi, X.; Jiang, W.; Guo, T.; Hall, D.L.; Luberto, C.M.; Zou, L. Relationship between adverse childhood experiences and anxiety symptoms among Chinese adolescents: The role of self-compassion and social support. Curr. Psychol. 2023, 142, 12822–12834. [Google Scholar] [CrossRef] [PubMed]
- Elmore, A.L.; Crouch, E. The association of adverse childhood experiences with anxiety and depression for children and youth, 8 to 17 years of age. Acad. Pediatr. 2020, 20, 600–608. [Google Scholar] [CrossRef]
- Lew, D.; Xian, H. Identifying distinct latent classes of adverse childhood experiences among US children and their relationship with childhood internalizing disorders. Child Psychiatry Hum. Dev. 2019, 50, 668–680. [Google Scholar] [CrossRef]
- Dye, H. The impact and long-term effects of childhood trauma. J. Hum. Behav. Soc. Environ. 2018, 28, 381–392. [Google Scholar] [CrossRef]
- Lowry, E.; McInerney, A.; Schmitz, N.; Deschênes, S.S. Adverse childhood experiences and cognitive function in adulthood: Examining the roles of depressive symptoms and inflammation in a prospective cohort study. Soc. Psychiatry Psychiatr. Epidemiol. 2022, 57, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, K.; Rief, W.; Euteneuer, F. Childhood adversity and cognitive functioning in patients with major depression. Child Abuse Negl. 2017, 70, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Gould, F.; Clarke, J.; Heim, C.; Harvey, P.D.; Majer, M.; Nemeroff, C.B. The effects of child abuse and neglect on cognitive functioning in adulthood. J. Psychiatr. Res. 2012, 46, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Majer, M.; Nater, U.M.; Lin, J.M.; Capuron, L.; Reeves, W.C. Association of childhood trauma with cognitive function in healthy adults: A pilot study. BMC Neurol. 2010, 10, 61. [Google Scholar] [CrossRef]
- Lee, M.; Park, K.H. Overgeneral memory in depression: Differences in with or without history of trauma, negative mood, and functional impairment. J. Korean Assn. Learn.-Cent. Curric. Instr. 2021, 21, 403–417. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry 2004, 65, 18–28. [Google Scholar]
- Ho, T.C.; King, L.S. Mechanisms of neuroplasticity linking early adversity to depression: Developmental considerations. Transl. Psychiatry 2021, 11, 517. [Google Scholar] [CrossRef]
- Enoch, M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology 2011, 214, 17–31. [Google Scholar] [CrossRef]
- Gilbert, R.; Widom, C.S.; Browne, K.; Fergusson, D.; Webb, E.; Janson, S. Burden and consequences of child maltreatment in high-income countries. Lancet 2009, 373, 68–81. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Greif Green, J.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatry 2012, 69, 1151–1160. [Google Scholar] [CrossRef]
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Salvucci, E. Microbiome, holobiont and the net of life. Crit. Rev. Microbiol. 2016, 42, 485–494. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Jang, H.H.; Kim, G.; Zouiouich, S.; Cho, S.Y.; Kim, H.J.; Kim, J.; Choe, J.S.; Gunter, M.J.; Ferrari, P.; et al. Taxonomic composition and diversity of the gut microbiota in relation to habitual dietary intake in Korean adults. Nutrients 2021, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morales, P.; Orellana, C.A.; Moutafis, G.; Moonen, G.; Rincon, G.; Nielsen, L.K.; Marcellin, E. Revisiting the evolution and taxonomy of Clostridia, a phylogenomic update. Genome Biol. Evol. 2019, 11, 2035–2044. [Google Scholar] [CrossRef]
- Raethong, N.; Nakphaichit, M.; Suratannon, N.; Sathitkowitchai, W.; Weerapakorn, W.; Keawsompong, S.; Vongsangnak, W. Analysis of human gut microbiome: Taxonomy and metabolic functions in Thai adults. Genes 2021, 12, 331. [Google Scholar] [CrossRef]
- Chabé, M.; Lokmer, A.; Ségurel, L. Gut protozoa: Friends or foes of the human gut microbiota? Trends Parasitol. 2017, 33, 925–934. [Google Scholar] [CrossRef]
- Kim, J.Y.; Whon, T.W.; Lim, M.Y.; Kim, Y.B.; Kim, N.; Kwon, M.S.; Kim, J.; Lee, S.H.; Choi, H.J.; Nam, I.H.; et al. The human gut archaeome: Identification of diverse haloarchaea in Korean subjects. Microbiome 2020, 8, 114. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Pargin, E.; Roach, M.J.; Skye, A.; Papudeshi, B.; Inglis, L.K.; Mallawaarachchi, V.; Grigson, S.R.; Harker, C.; Edwards, R.A.; Giles, S.K. The human gut virome: Composition, colonization, interactions, and impacts on human health. Front. Microbiol. 2023, 14, 963173. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Neurodevelopmental disorders associated with gut microbiome dysbiosis in children. Children 2024, 11, 796. [Google Scholar] [CrossRef]
- Adamek, K.; Skonieczna-Żydecka, K.; Węgrzyn, D.; Łoniewska, B. Prenatal and early childhood development of gut microbiota. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9667–9680. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int. Microbiol. 2025, 28, 1–15. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Pechtel, P.; Pizzagalli, D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 2011, 214, 55–70. [Google Scholar] [CrossRef]
- Smith, K.E.; Pollak, S.D. Early life stress and development: Potential mechanisms for adverse outcomes. J. Neurodev. Disord. 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. The resilient brain: Epigenetics, stress and the lifecourse. Psychoneuroendocrinology 2017, 83, 76. [Google Scholar] [CrossRef]
- Agorastos, A.; Pervanidou, P.; Chrousos, G.P.; Kolaitis, G. Early life stress and trauma: Developmental neuroendocrine aspects of prolonged stress system dysregulation. Hormones 2018, 17, 507–520. [Google Scholar] [CrossRef] [PubMed]
- LeMoult, J.; Humphreys, K.L.; Tracy, A.; Hoffmeister, J.A.; Ip, E.; Gotlib, I.H. Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Nanni, V.; Uher, R.; Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry 2012, 169, 141–151. [Google Scholar] [CrossRef]
- Pervanidou, P.; Makris, G.; Chrousos, G.; Agorastos, A. Early life stress and pediatric posttraumatic stress disorder. Brain Sci. 2020, 10, 169. [Google Scholar] [CrossRef]
- Senner, F.; Schneider-Axmann, T.; Kaurani, L.; Zimmermann, J.; Wiltfang, J.; von Hagen, M.; Vogl, T.; Spitzer, C.; Senner, S.; Schulte, E.C.; et al. Association of early life stress and cognitive performance in patients with schizophrenia and healthy controls. Schizophr. Res. Cogn. 2023, 32, 100280. [Google Scholar] [CrossRef]
- Zatti, C.; Rosa, V.; Barros, A.; Valdivia, L.; Calegaro, V.C.; Freitas, L.H.; Ceresér, K.M.M.; da Rocha, N.S.; Bastos, A.G.; Schuch, F.B. Childhood trauma and suicide attempt: A meta-analysis of longitudinal studies from the last decade. Psychiatry Res. 2017, 256, 353–358. [Google Scholar] [CrossRef]
- Khoury, L.; Tang, Y.L.; Bradley, B.; Cubells, J.F.; Ressler, K.J. Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress. Anxiety 2010, 27, 1077–1086. [Google Scholar] [CrossRef]
- Makris, G.; Agorastos, A.; Chrousos, G.P.; Pervanidou, P. Stress system activation in children and adolescents with autism spectrum disorder. Front. Neurosci. 2022, 15, 756628. [Google Scholar] [CrossRef]
- Haykin, H.; Rolls, A. The neuroimmune response during stress: A physiological perspective. Immunity 2021, 54, 1933–1947. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Liu, Y. The molecular physiology of CRH neurons. Front. Neuroendocrinol. 2012, 33, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.H.; Abdulai-Saiku, S.; Vyas, A. Arginine vasopressin in the medial amygdala causes greater post-stress recruitment of hypothalamic vasopressin neurons. Mol. Brain 2021, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Cawley, N.X.; Li, Z.; Loh, Y.P. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol. 2016, 56, T77–T97. [Google Scholar] [CrossRef]
- Atzori, M.; Cuevas-Olguin, R.; Esquivel-Rendon, E.; Garcia-Oscos, F.; Salgado-Delgado, R.C.; Saderi, N.; Miranda-Morales, M.; Treviño, M.; Pineda, J.C.; Salgado, H. Locus ceruleus norepinephrine release: A central regulator of CNS spatio-temporal activation? Front. Synaptic Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Bucci, M.; Marques, S.S.; Oh, D.; Harris, N.B. Toxic stress in children and adolescents. Adv. Pediatr. 2016, 63, 403–428. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Gold, P.W.; Gabry, K.E.; Yasuda, M.R.; Chrousos, G.P. Divergent endocrine abnormalities in melancholic and atypical depression: Clinical and pathophysiologic implications. Endocrinol. Metab. Clin. North Am. 2002, 31, 37–62. [Google Scholar] [CrossRef] [PubMed]
- Lähdepuro, A.; Savolainen, K.; Lahti-Pulkkinen, M.; Eriksson, J.G.; Lahti, J.; Tuovinen, S.; Kajantie, E.; Pesonen, A.K.; Heinonen, K.; Räikkönen, K. The impact of early life stress on anxiety symptoms in late adulthood. Sci. Rep. 2019, 9, 4395. [Google Scholar] [CrossRef]
- Eichenbaum, H. Prefrontal–hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 2017, 18, 547–558. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Morrison, J.H. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- Monroy, E.; Hernández-Torres, E.; Flores, G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J. Chem. Neuroanat. 2010, 40, 93–101. [Google Scholar] [CrossRef]
- Danielewicz, J.; Hess, G. Early life stress alters synaptic modification range in the rat lateral amygdala. Behav. Brain Res. 2014, 265, 32–37. [Google Scholar] [CrossRef]
- Malter Cohen, M.; Jing, D.; Yang, R.R.; Tottenham, N.; Lee, F.S.; Casey, B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. USA 2013, 110, 18274–18278. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E.; Way, B.M.; Seeman, T.E. Early adversity and adult health outcomes. Dev. Psychopathol. 2011, 23, 939–954. [Google Scholar] [CrossRef]
- Berman, A.K.; Lott, R.B.; Donaldson, S.T. Periodic maternal deprivation may modulate offspring anxiety-like behavior through mechanisms involving neuroplasticity in the amygdala. Brain Res. Bull. 2014, 101, 7–11. [Google Scholar] [CrossRef]
- Ishikawa, J.; Nishimura, R.; Ishikawa, A. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur. J. Neurosci. 2015, 41, 442–453. [Google Scholar] [CrossRef]
- Wei, L.; David, A.; Duman, R.S.; Anisman, H.; Kaffman, A. Early life stress increases anxiety-like behavior in Balbc mice despite a compensatory increase in levels of postnatal maternal care. Horm. Behav. 2010, 57, 396–404. [Google Scholar] [CrossRef]
- Callaghan, B.L.; Richardson, R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav. Neurosci. 2011, 125, 20–28. [Google Scholar] [CrossRef]
- Oomen, C.A.; Soeters, H.; Audureau, N.; Vermunt, L.; Van Hasselt, F.N.; Manders, E.M.M.; Joëls, M.; Lucassen, P.J.; Krugers, H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J. Neurosci. 2010, 30, 6635–6645. [Google Scholar] [CrossRef]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef]
- Ivy, A.S.; Rex, C.S.; Chen, Y.; Dubé, C.; Maras, P.M.; Grigoriadis, D.E.; Gall, C.M.; Lynch, G.; Baram, T.Z. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010, 30, 13005–13015. [Google Scholar] [CrossRef]
- Hanson, J.L.; Nacewicz, B.M.; Sutterer, M.J.; Cayo, A.A.; Schaefer, S.M.; Rudolph, K.D.; Shirtcliff, E.A.; Pollak, S.D.; Davidson, R.J. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol. Psychiatry 2015, 77, 314–323. [Google Scholar] [CrossRef]
- Teicher, M.H.; Anderson, C.M.; Ohashi, K.; Khan, A.; McGreenery, C.E.; Bolger, E.A.; Rohan, M.L.; Vitaliano, G.D. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage 2018, 169, 443–452. [Google Scholar] [CrossRef]
- Chen, M.C.; Hamilton, J.P.; Gotlib, I.H. Decreased hippocampal volume in healthy girls at risk of depression. Arch. Gen. Psychiatry 2010, 67, 270–276. [Google Scholar] [CrossRef]
- Gorka, A.X.; Hanson, J.L.; Radtke, S.R.; Hariri, A.R. Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biol. Mood Anxiety Disord. 2014, 4, 12. [Google Scholar] [CrossRef]
- Hanson, J.L.; van den Bos, W.; Roeber, B.J.; Rudolph, K.D.; Davidson, R.J.; Pollak, S.D. Early adversity and learning: Implications for typical and atypical behavioral development. J. Child Psychol. Psychiatry 2017, 58, 770–778. [Google Scholar] [CrossRef]
- Woon, F.L.; Hedges, D.W. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus 2008, 18, 729–736. [Google Scholar] [CrossRef]
- Gee, D.G.; Gabard-Durnam, L.J.; Flannery, J.; Goff, B.; Humphreys, K.L.; Telzer, E.H.; Hare, T.A.; Bookheimer, S.Y.; Tottenham, N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. USA 2013, 110, 15638–15643. [Google Scholar] [CrossRef]
- VanTieghem, M.R.; Tottenham, N. Neurobiological programming of early life stress: Functional development of amygdala prefrontal circuitry and vulnerability for stress related psychopathology. Curr. Top. Behav. Neurosci. 2018, 38, 117–136. [Google Scholar] [CrossRef]
- Wolf, R.C.; Herringa, R.J. Prefrontal–amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 2016, 41, 822–831. [Google Scholar] [CrossRef]
- Jedd, K.; Hunt, R.H.; Cicchetti, D.; Hunt, E.; Cowell, R.A.; Rogosch, F.A.; Toth, S.L.; Thomas, K.M. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Dev. Psychopathol. 2015, 27, 1577–1589. [Google Scholar] [CrossRef]
- Kim, P.; Evans, G.W.; Angstadt, M.; Ho, S.S.; Sripada, C.S.; Swain, J.E.; Liberzon, I.; Phan, K.L. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. USA 2013, 110, 18442–18447. [Google Scholar] [CrossRef]
- Koss, K.J.; Gunnar, M.R. Annual Research Review: Early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. J. Child Psychol. Psychiatry 2017, 59, 327–346. [Google Scholar] [CrossRef]
- Pagliaccio, D.; Luby, J.L.; Bogdan, R.; Agrawal, A.; Gaffrey, M.S.; Belden, A.C.; Botteron, K.N.; Harms, M.P.; Barch, D.M. Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J. Abnorm. Psychol. 2015, 124, 817–833. [Google Scholar] [CrossRef]
- Heim, C.M.; Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef]
- Turecki, G.; Meaney, M.J. Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biol. Psychiatry 2016, 79, 87–96. [Google Scholar] [CrossRef]
- McEwen, B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef]
- McEwen, C.A.; McEwen, B.S. Social structure, adversity, toxic stress, and intergenerational poverty: An early childhood model. Annu. Rev. Sociol. 2017, 43, 445–472. [Google Scholar] [CrossRef]
- Birn, R.M.; Roeber, B.J.; Pollak, S.D. Early childhood stress exposure, reward pathways, and adult decision making. Proc. Natl. Acad. Sci. USA 2017, 114, 13549–13554. [Google Scholar] [CrossRef]
- Novick, A.M.; Levandowski, M.L.; Laumann, L.E.; Philip, N.S.; Price, L.H.; Tyrka, A.R. The effects of early life stress on reward processing. J. Psychiatr. Res. 2018, 101, 80–103. [Google Scholar] [CrossRef]
- Risbrough, V.B.; Glynn, L.M.; Davis, E.P.; Sandman, C.A.; Obenaus, A.; Stern, H.S.; Keator, D.B.; Yassa, M.A.; Baram, T.Z.; Baker, D.G. Does anhedonia presage increased risk of posttraumatic stress disorder?: Adolescent anhedonia and posttraumatic disorders. In Behavioral Neurobiology of PTSD; Current Topics in Behavioral Neurosciences; Springer: Cham, Switzerland, 2018; Volume 38, pp. 249–265. [Google Scholar] [CrossRef]
- Chocyk, A.; Dudys, D.; Przyborowska, A.; Majcher, I.; Maćkowiak, M.; Wędzony, K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience 2011, 173, 1–18. [Google Scholar] [CrossRef]
- Yan, C.G.; Rincón-Cortés, M.; Raineki, C.; Sarro, E.; Colcombe, S.; Guilfoyle, D.N.; Yang, Z.; Gerum, S.; Biswal, B.B.; Milham, M.P.; et al. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl. Psychiatry 2017, 7, e1005. [Google Scholar] [CrossRef]
- Peña, C.J.; Neugut, Y.D.; Calarco, C.A.; Champagne, F.A. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur. J. Neurosci. 2014, 39, 946–956. [Google Scholar] [CrossRef]
- Rodrigues, A.J.; Leão, P.; Carvalho, M.; Almeida, O.F.X.; Sousa, N. Potential programming of dopaminergic circuits by early life stress. Psychopharmacology 2011, 214, 107–120. [Google Scholar] [CrossRef]
- Dennison, M.J.; Rosen, M.L.; Sambrook, K.A.; Jenness, J.L.; Sheridan, M.A.; McLaughlin, K.A. Differential associations of distinct forms of childhood adversity with neurobehavioral measures of reward processing: A developmental pathway to depression. Child Dev. 2017, 90, e96–e113. [Google Scholar] [CrossRef]
- Marusak, H.A.; Hatfield, J.R.; Thomason, M.E.; Rabinak, C.A. Reduced ventral tegmental area-hippocampal connectivity in children and adolescents exposed to early threat. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 130–137. [Google Scholar] [CrossRef]
- Corral-Frías, N.S.; Nikolova, Y.S.; Michalski, L.J.; Baranger, D.A.A.; Hariri, A.R.; Bogdan, R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol. Med. 2015, 45, 2605–2617. [Google Scholar] [CrossRef]
- Goff, B.; Gee, D.G.; Telzer, E.H.; Humphreys, K.L.; Gabard-Durnam, L.; Flannery, J.; Tottenham, N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience 2013, 249, 129–138. [Google Scholar] [CrossRef]
- Kamkar, N.H.; Lewis, D.J.; van den Bos, W.; Morton, J.B. Ventral striatal activity links adversity and reward processing in children. Dev. Cogn. Neurosci. 2017, 26, 20–27. [Google Scholar] [CrossRef]
- Ledoux, J.E.; Daw, N.D. Surviving threats: Neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat. Rev. Neurosci. 2018, 19, 269–282. [Google Scholar] [CrossRef]
- Nelson, C.A.; Scott, R.D.; Bhutta, Z.A.; Harris, N.B.; Danese, A.; Samara, M. Adversity in childhood is linked to mental and physical health throughout life. BMJ 2020, 371, m3048. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Cong, X.; Xu, W.; Janton, S.; Henderson, W.A.; Matson, A.; McGrath, J.M.; Maas, K.; Graf, J. Gut microbiome developmental patterns in early life of preterm infants: Impacts of feeding and gender. PLoS ONE 2016, 11, e0152751. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Morreale, C.; Giaroni, C.; Baj, A.; Folgori, L.; Barcellini, L.; Dhami, A.; Agosti, M.; Bresesti, I. Effects of perinatal antibiotic exposure and neonatal gut microbiota. Antibiotics 2023, 12, 258. [Google Scholar] [CrossRef]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernandez-Barranco, A.; Margolles, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef]
- Moles, L.; Gómez, M.; Heilig, H.; Bustos, G.; Fuentes, S.; de Vos, W.; Fernández, L.; Rodríguez, J.M.; Jiménez, E. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS ONE 2013, 8, e66986. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of gut microbiota in the first 1000 days after birth and potential interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef]
- Madan, J.C.; Hoen, A.G.; Lundgren, S.N.; Farzan, S.F.; Cottingham, K.L.; Morrison, H.G.; Sogin, M.L.; Li, H.; Moore, J.H.; Karagas, M.R. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old infants. JAMA Pediatr. 2016, 170, 212–219. [Google Scholar] [CrossRef]
- Hansen, R.; Scott, K.P.; Khan, S.; Martin, J.C.; Berry, S.H.; Stevenson, M.; Okpapi, A.; Munro, M.J.; Hold, G.L. First-pass meconium samples from healthy term vaginally-delivered neonates: An analysis of the microbiota. PLoS ONE 2015, 10, e0133320. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in Caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a neonate is born, so is a microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef]
- Karlsson, C.L.; Molin, G.; Cilio, C.M.; Ahrné, S. The pioneer gut microbiota in human neonates vaginally born at term—A pilot study. Pediatr. Res. 2011, 70, 282–286. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The role of microbiota in infant health: From early life to adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef]
- Martin, R.; Makino, H.; Cetinyurek Yavuz, A.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef] [PubMed]
- Hesla, H.M.; Stenius, F.; Jäderlund, L.; Nelson, R.; Engstrand, L.; Alm, J.; Dicksved, J. Impact of lifestyle on the gut microbiota of healthy infants and their mothers—The ALADDIN birth cohort. FEMS Microbiol. Ecol. 2014, 90, 791–801. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Farver, M.; Smilowitz, J.T. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr. Metab. Insights 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef]
- Fouhy, F.; Guinane, C.M.; Hussey, S.; Wall, R.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 2012, 56, 5811–5820. [Google Scholar] [CrossRef]
- Tanaka, S.; Kobayashi, T.; Songjinda, P.; Tateyama, A.; Tsubouchi, M.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K.; Nakayama, J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009, 56, 80–87. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Thompson, J.; Waldie, K.E.; Murphy, R.; Wall, C.; Mitchell, E.A. Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatr. 2017, 106, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Damiani, F.; Cornuti, S.; Tognini, P. The gut-brain connection: Exploring the influence of the gut microbiota on neuroplasticity and neurodevelopmental disorders. Neuropharmacology 2023, 231, 109491. [Google Scholar] [CrossRef]
- Troller-Renfree, S.V.; Brito, N.H.; Desai, P.M.; Leon-Santos, A.G.; Wiltshire, C.A.; Motton, S.N.; Meyer, J.S.; Isler, J.; Fifer, W.P.; Noble, K.G. Infants of mothers with higher physiological stress show alterations in brain function. Dev. Sci. 2020, 23, e12976. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.L.; Fields, A.; Gee, D.G.; Gabard-Durnam, L.; Caldera, C.; Humphreys, K.L.; Goff, B.; Flannery, J.; Telzer, E.H.; Shapiro, M.; et al. Mind and gut: Associations between mood and gastrointestinal distress in children exposed to adversity. Dev. Psychopathol. 2020, 32, 309–328. [Google Scholar] [CrossRef]
- Vogel, S.C.; Brito, N.H.; Callaghan, B.L. Early life stress and the development of the infant gut microbiota: Implications for mental health and neurocognitive development. Curr. Psychiatry Rep. 2020, 22, 61. [Google Scholar] [CrossRef]
- Kemp, K.M.; Colson, J.; Lorenz, R.G.; Maynard, C.L.; Pollock, J.S. Early life stress in mice alters gut microbiota independent of maternal microbiota inheritance. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 320, R663–R674. [Google Scholar] [CrossRef]
- Otaru, N.; Kourouma, L.; Pugin, B.; Constancias, F.; Braegger, C.; Mansuy, I.M.; Lacroix, C. Transgenerational effects of early life stress on the fecal microbiota in mice. Commun. Biol. 2024, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.; Wu, Y.; Hu, C.; Zhu, Z.; Ye, D.; Long, G.; Chen, B.; Guo, R.; Shu, X.; Zheng, W.; et al. Early life stress induces irritable bowel syndrome from childhood to adulthood in mice. Front. Microbiol. 2023, 14, 1255525. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef]
- Amini-Khoei, H.; Haghani-Samani, E.; Beigi, M.; Soltani, A.; Mobini, G.R.; Balali-Dehkordi, S.; Haj-Mirzaian, A.; Rafieian-Kopaei, M.; Alizadeh, A.; Hojjati, M.R.; et al. On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. Int. Immunopharmacol. 2019, 66, 242–250. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Kuti, D.; Winkler, Z.; Horváth, K.; Juhász, B.; Paholcsek, M.; Stágel, A.; Gulyás, G.; Czeglédi, L.; Ferenczi, S.; Kovács, K.J. Gastrointestinal (non-systemic) antibiotic rifaximin differentially affects chronic stress-induced changes in colon microbiome and gut permeability without effect on behavior. Brain Behav. Immun. 2020, 84, 218–228. [Google Scholar] [CrossRef]
- Rincel, M.; Aubert, P.; Chevalier, J.; Grohard, P.A.; Basso, L.; de Oliveira, C.M.; Helbling, J.C.; Lévy, É.; Chevalier, G.; Leboyer, M.; et al. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav. Immun. 2019, 80, 179–192. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.A.; Kang, W.S.; Kim, J.W. Early-life stress modulates gut microbiota and peripheral and central inflammation in a sex-dependent manner. Int. J. Mol. Sci. 2021, 22, 1899. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Ramsteijn, A.S.; Dini-Andreote, F.; van Eijk, R.; Houwing, D.J.; Salles, J.F.; Olivier, J.D.A. Serotonin transporter genotype modulates the gut microbiota composition in young rats, an effect augmented by early life stress. Front. Cell. Neurosci. 2017, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Hartmann, J.; Ressler, K.J.; Liu, Y.Y.; Koenen, K.C. The emerging role of the gut microbiome in posttraumatic stress disorder. Brain Behav. Immun. 2023, 114, 360–370. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ahmad, R.; Moshfegh, C.M.; Sankarasubramanian, J.; Joshi, V.; Elkhatib, S.K.; Chhonker, Y.S.; Murry, D.J.; Talmon, G.A.; Guda, C.; et al. Repeated social defeat stress induces an inflammatory gut milieu by altering the mucosal barrier integrity and gut microbiota homeostasis. Biol. Psychiatry Glob. Open Sci. 2023, 3, 824–836. [Google Scholar] [CrossRef]
- Aatsinki, A.K.; Keskitalo, A.; Laitinen, V.; Munukka, E.; Uusitupa, H.M.; Lahti, L.; Kortesluoma, S.; Mustonen, P.; Rodrigues, A.J.; Coimbra, B.; et al. Maternal prenatal psychological distress and hair cortisol levels associate with infant fecal microbiota composition at 2.5 months of age. Psychoneuroendocrinology 2020, 119, 104754. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Jahnke, J.R.; Roach, J.; Azcarate-Peril, M.A.; Thompson, A.L. Maternal precarity and HPA axis functioning shape infant gut microbiota and HPA axis development in humans. PLoS ONE 2021, 16, e0251782. [Google Scholar] [CrossRef] [PubMed]
- Grant-Beurmann, S.; Jumare, J.; Ndembi, N.; Matthew, O.; Shutt, A.; Omoigberale, A.; Martin, O.A.; Fraser, C.M.; Charurat, M. Dynamics of the infant gut microbiota in the first 18 months of life: The impact of maternal HIV infection and breastfeeding. Microbiome 2022, 12, 61. [Google Scholar] [CrossRef]
- D’Agata, A.L.; Wu, J.; Welandawe, M.K.V.; Dutra, S.V.O.; Kane, B.; Groer, M.W. Effects of early life NICU stress on the developing gut microbiome. Dev. Psychobiol. 2019, 61, 650–660. [Google Scholar] [CrossRef]
- Hermes, G.D.A.; Eckermann, H.A.; de Vos, W.M.; de Weerth, C. Does entry to center-based childcare affect gut microbial colonization in young infants? Sci. Rep. 2020, 24, 10235. [Google Scholar] [CrossRef]
- Reid, B.M.; Horne, R.; Donzella, B.; Szamosi, J.C.; Coe, C.L.; Foster, J.A.; Gunnar, M.R. Microbiota-immune alterations in adolescents following early life adversity: A proof of concept study. Dev. Psychobiol. 2021, 63, 851–863. [Google Scholar] [CrossRef]
- Hemmings, S.M.J.; Malan-Müller, S.; van den Heuvel, L.L.; Demmitt, B.A.; Stanislawski, M.A.; Smith, D.G.; Bohr, A.D.; Stamper, C.E.; Hyde, E.R.; Morton, J.T.; et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: An exploratory study. Psychosom. Med. 2017, 79, 936–946. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; van den Heuvel, L.L.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M.J. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 2022, 56, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.H.; Kraaij, R.; Schuurmans, I.K.; Frances-Cuesta, C.; Sanz, Y.; Medina-Gomez, C.; Duijts, L.; Rivadeneira, F.; Tiemeier, H.; Jaddoe, V.W.; et al. Early-life stress and the gut microbiome: A comprehensive population-based investigation. Brain Behav. Immun. 2024, 118, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Jašarević, E.; Criniti, S.; McGeehan, B.; Tanes, C.; Sammel, M.D.; Elovitz, M.A.; Compher, C.; Wu, G.; Epperson, C.N. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav. Immun. 2019, 75, 240–250. [Google Scholar] [CrossRef]
- Michels, N.; Van de Wiele, T.; Fouhy, F.; O’Mahony, S.; Clarke, G.; Keane, J. Gut microbiome patterns depending on children’s psychosocial stress: Reports versus biomarkers. Brain Behav. Immun. 2019, 80, 751–762. [Google Scholar] [CrossRef]
- Flannery, J.E.; Stagaman, K.; Burns, A.R.; Hickey, R.J.; Roos, L.E.; Giuliano, R.J.; Fisher, P.A.; Sharpton, T.J. Gut feelings begin in childhood: The gut metagenome correlates with early environment, caregiving, and behavior. mBio 2020, 11, e02780-19. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, A.; Aatsinki, A.K.; Kortesluoma, S.; Pelto, J.; Korhonen, L.; Lahti, L.; Lukkarinen, M.; Munukka, E.; Karlsson, H.; Karlsson, L. Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months. Stress 2021, 24, 551–560. [Google Scholar] [CrossRef]
- Laue, H.E.; Karagas, M.R.; Coker, M.O.; Bellinger, D.C.; Baker, E.R.; Korrick, S.A.; Madan, J.C. Sex-specific relationships of the infant microbiome and early-childhood behavioral outcomes. Pediatr. Res. 2022, 92, 580–591. [Google Scholar] [CrossRef]
- Kraaij, R.; Schuurmans, I.K.; Radjabzadeh, D.; Tiemeier, H.; Dinan, T.G.; Uitterlinden, A.G.; Hillegers, M.; Jaddoe, V.W.; Duijts, L.; Moll, H.; et al. The gut microbiome and child mental health: A population-based study. Brain Behav. Immun. 2023, 108, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, M.C.; Verosky, B.; Chen, H.J.; Davis, O.; Gur, T.L. The maternal microbiome as a map to understanding the impact of prenatal stress on offspring psychiatric health. Biol. Psychiatry 2024, 95, 300–309. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Pessa-Morikawa, T.; Husso, A.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K.; Iivanainen, A.; Niku, M. Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta. BMC Microbiol. 2022, 22, 46. [Google Scholar] [CrossRef]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.; Xie, J.; Chen, J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Thion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 2018, 172, 500–516.e16. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2276.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhong, Z.; Shi, L.; Huang, L.; Lin, C.; He, Y.; Xia, X.; Zhang, T.; Ding, W.; Yang, Y. Gut microbiota mediate early life stress-induced social dysfunction and anxiety-like behaviors by impairing amino acid transport at the gut. Gut Microbes 2024, 16, 2401939. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Tiao, M.M.; Tain, Y.L. Maternal tryptophan supplementation protects adult rat offspring against hypertension programmed by maternal chronic kidney disease: Implication of tryptophan-metabolizing microbiome and aryl hydrocarbon receptor. Int. J. Mol. Sci. 2020, 21, 4552. [Google Scholar] [CrossRef]
- Galley, J.D.; Chen, H.J.; Antonson, A.M.; Gur, T.L. Prenatal stress-induced disruptions in microbial and host tryptophan metabolism and transport. Behav. Brain Res. 2021, 414, 113471. [Google Scholar] [CrossRef]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Steenbergen, L.; Jongkees, B.J.; Sellaro, R.; Colzato, L.S. Tryptophan supplementation modulates social behavior: A review. Neurosci. Biobehav. Rev. 2016, 64, 346–358. [Google Scholar] [CrossRef]
- Keane, J.M.; Khashan, A.S.; McCarthy, F.P.; Kenny, L.C.; Collins, J.M.; O’Donovan, S.; Brown, J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; et al. Identifying a biological signature of prenatal maternal stress. JCI Insight 2021, 6, e143007. [Google Scholar] [CrossRef]
- Chen, L.W.; Wang, S.T.; Wang, L.W.; Kao, Y.C.; Chu, C.L.; Wu, C.C.; Chiang, C.H.; Huang, C.C. Early neurodevelopmental trajectories for autism spectrum disorder in children born very preterm. Pediatrics 2020, 146, e20200297. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Feng, Z.; Cheng, H.W. Perspective: Gestational tryptophan fluctuation altering neuroembryogenesis and psychosocial development. Cells 2022, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Cappadocia, M.C.; Weiss, J.A.; Pepler, D. Bullying experiences among children and youth with autism spectrum disorders. J. Autism Dev. Disord. 2012, 42, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.M.; Fedina, C. Cyber bullying in ADHD and Asperger syndrome populations. Res. Autism Spectr. Disord. 2011, 5, 1201–1208. [Google Scholar] [CrossRef]
- Zinner, S.H.; Conelea, C.A.; Glew, G.M.; Woods, D.W.; Budman, C.L. Peer victimization in youth with Tourette syndrome and other chronic tic disorders. Child Psychiatry Hum. Dev. 2012, 43, 124–136. [Google Scholar] [CrossRef]
- Higazi, A.M.; Kamel, H.M.; Abdel-Naeem, E.A.; Abdullah, N.M.; Mahrous, D.M.; Osman, A.M. Expression analysis of selected genes involved in tryptophan metabolic pathways in Egyptian children with autism spectrum disorder and learning disabilities. Sci. Rep. 2021, 11, 6931. [Google Scholar] [CrossRef]
- Roussin, L.; Prince, N.; Perez-Pardo, P.; Kraneveld, A.D.; Rabot, S.; Naudon, L. Role of the gut microbiota in the pathophysiology of autism spectrum disorder: Clinical and preclinical evidence. Microorganisms 2020, 8, 1369. [Google Scholar] [CrossRef]
- Santana-Coelho, D. Does the kynurenine pathway play a pathogenic role in autism spectrum disorder? Brain Behav. Immun.-Health 2024, 40, 100839. [Google Scholar] [CrossRef]
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G.; et al. Kynurenic acid in schizophrenia: A systematic review and meta-analysis. Schizophr. Bull. 2017, 43, 764–777. [Google Scholar] [CrossRef]
- Oh, C.M.; Park, S.; Kim, H. Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab. J. 2016, 40, 89–98. [Google Scholar] [CrossRef]
- Wei, G.Z.; Martin, K.A.; Xing, P.Y.; Agrawal, R.; Whiley, L.; Wood, T.K.; Hejndorf, S.; Ng, Y.Z.; Low, J.Z.Y.; Rossant, J.; et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2021091118. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021, 41, 1061–1088. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan metabolism by gut microbiome and gut-brain-axis: An in silico analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Young, S.N. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos. Trans. R. Soc. London B Biol. Sci. 2013, 368, 20110375. [Google Scholar] [CrossRef]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef] [PubMed]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Duran, N.L.; Olson, S.L.; Hajal, N.J.; Felt, B.T.; Vazquez, D.M. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. J. Abnorm. Child Psychol. 2009, 37, 169–182. [Google Scholar] [CrossRef]
- Buske-Kirschbaum, A.; Trikojat, K.; Tesch, F.; Schmitt, J.; Roessner, V.; Luksch, H.; Rösen-Wolff, A.; Plessow, F. Altered hypothalamus-pituitary-adrenal axis function: A relevant factor in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder? Psychoneuroendocrinology 2019, 105, 178–186. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA axis as target for depression outdated, or is there a new hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Schalbroeck, R.; van Velden, F.H.P.; de Geus-Oei, L.F.; Yaqub, M.; van Amelsvoort, T.; Booij, J.; Selten, J.P. Striatal dopamine synthesis capacity in autism spectrum disorder and its relation with social defeat: An [18F]-FDOPA PET/CT study. Transl. Psychiatry 2021, 11, 47. [Google Scholar] [CrossRef]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The brain’s reward system in health and disease. In Circadian Clock in Brain Health and Disease; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1344, pp. 57–69. [Google Scholar] [CrossRef]
- Goldstein, D.S. Adrenal responses to stress. Cell. Mol. Neurobiol. 2010, 30, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Garcia-Olivares, J.; Candler, S.; Schwabe, S.; Maletic, V. New insights into the mechanism of action of viloxazine: Serotonin and norepinephrine modulating properties. J. Exp. Pharmacol. 2020, 12, 285–300. [Google Scholar] [CrossRef]
- Bacchini, D.; Affuso, G.; Trotta, T. Temperament, ADHD and peer relations among schoolchildren: The mediating role of school bullying. Aggress. Behav. 2008, 34, 447–459. [Google Scholar] [CrossRef]
- Stenseng, F.; Skalická, V.; Skaug, S.S.; Belsky, J.; Wichstrøm, L. Attention-deficit hyperactivity disorder symptoms and bullying victimization from childhood to adolescence—A within-person cross-lagged approach. Dev. Psychopathol. 2024; 1–11, Advance online publication. [Google Scholar] [CrossRef]
- Dalley, J.W.; Roiser, J.P. Dopamine, serotonin and impulsivity. Neuroscience 2012, 215, 42–58. [Google Scholar] [CrossRef]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress. Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Lingrand, L.; Maillard, M.; Feuz, B.; Tompkins, T.A. The effects of psychobiotics on the microbiota-gut-brain axis in early-life stress and neuropsychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110142. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Psychobiotics: A new perspective on the treatment of stress, anxiety, and depression. Anxiety Stress 2024, 30, 79–93. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays 2011, 33, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.S.M.; Stylianakis, A.A.; Richardson, R. Early-life stress, microbiota, and brain development: Probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev. Cogn. Neurosci. 2019, 37, 100627. [Google Scholar] [CrossRef]
- Karen, C.; Shyu, D.J.H.; Rajan, K.E. Lactobacillus paracasei supplementation prevents early life stress-induced anxiety and depressive-like behavior in maternal separation model-possible involvement of microbiota-gut-brain axis in differential regulation of microRNA124a/132 and glutamate receptors. Front. Neurosci. 2021, 15, 719933. [Google Scholar] [CrossRef]
- De Santa, F.; Strimpakos, G.; Marchetti, N.; Gargari, G.; Torcinaro, A.; Arioli, S.; Mora, D.; Petrella, C.; Farioli-Vecchioli, S. Effect of a multi-strain probiotic mixture consumption on anxiety and depression symptoms induced in adult mice by postnatal maternal separation. Microbiome 2024, 12, 29. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef]
- Agusti, A.; Lamers, F.; Tamayo, M.; Benito-Amat, C.; Molina-Mendoza, G.V.; Penninx, B.W.J.H.; Sanz, Y. The gut microbiome in early life stress: A systematic review. Nutrients 2023, 15, 2566. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Vasupanrajit, A.; Jirakran, K.; Klomkliew, P.; Chanchaem, P.; Tunvirachaisakul, C.; Plaimas, K.; Suratanee, A.; Payungporn, S. Adverse childhood experiences and reoccurrence of illness impact the gut microbiome, which affects suicidal behaviours and the phenome of major depression: Towards enterotypic phenotypes. Acta Neuropsychiatr. 2023, 35, 328–345. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Fernández, S. Humiliation and its relationship with bullying victimization: A narrative review. Psychol. Soc. Educ. 2024, 16, 42–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).