Abstract
Basil is susceptible to biotic or abiotic stress, negatively interfering with growth and production. Thus, the objective of this work was to evaluate the physiological effects of the application of plant regulators in basil plants that suffer from water deficit. The experiment was conducted in a randomized block design (RBD) in a 2 × 4 factorial scheme, including plants that were subjected to water stress and those that were not. In addition, plants also received five doses of Stimulate® composed of indolylbutyric acid (IBA) + gibberellic acid (GA3) + kinetin (Kt) with four repetitions each. The experiment was evaluated through the biochemical analyses of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) and lipid peroxidation performed 20, 35, and 50 days after transplanting (DAT). The mixture of plant regulators attenuateds the effects through the increasing activities of these enzymes. The plants that received the highest dosages (9 and 12 mL L−1) offered the best protetion. Parameters of growth measures such as number of leaves and leaf area also showed significant responses regarding the application of the plant growth regulators. The use of a mixture of plant regulators, despite satisfactory results, does not make basil economically viable because it presents inaccurate results regarding its use.
1. Introduction
Basil (Ocimum basilicum L.) is a species belonging to the Lamiaceae family, characterized as being an annual plant, erect, branched, and being between 30 and 50 cm of height [1]. Its main function is as a seasoning or flavoring, where green and fresh leaves are used. Basil leaves are also extracted for an essential oil which is used in cosmetics, perfumery, medicines, and food [2,3]. The main factors that interfere with its essential oil are the stage of development of the plant, seasonality, availability of light radiation, temperature, nutrients, and water [4,5].
When subjected to water stress, aromatic plants show a reduction in the growth and production of fresh and dry biomass. Altered biochemical responses include stomatal closure; an increase in the synthesis of abscisic acid and compatible solutes (such as amino acids and sugars); alterations in the content and elasticity of the cell wall; the reduction of leaf emission; and a reduction of leaf area [6,7]. In addition, it presents an increase in resistance to water flow in the vascular tissues and roots [8].
One of the ways found to mitigate the problems that occurs with water stress in the crop is the use of plant regulators, as these positively affect the expansion of the photosynthetic leaf area, the development of the root system, and the increase of antioxidative enzyme activity. Stimulate®, a product composed of a mixture of plant regulators, auxin, gibberellin, and cytokine, presents satisfactory results regarding its application in large cultures; however, for medicinal and aromatic plants, there are few studies and publications on the use of this mixture of plant regulators for the benefit of the culture.
Cavalcante et al. (2020) studied the efficiency of bio-stimulants in the treatment of water deficit in soybeans and observed that they were effective in giving these plants a greater capacity to withstand periods of draught [9]. To mitigate the effects of water deficit in sugarcane, Stimulate® was used by Torsian et al. (2020), presenting significant results when used alone but not in a mixture with other plant regulators [10]. In Oliveira et al. (2016), Stimulate® was able to maintain culture growth under water stress at doses of 10 to 15 mL L−1 [11]. In contrast, the work by Reis (2021) showed that the mixture of plant regulators did not minimize the effect of water deficit in bean cultivars [12].
During plant development, auxin is mainly produced in the apical meristems and actively transported to the roots by membrane transporters. Depending on its concentration, auxin helps to increase the rhizogenic response, which helps in the regeneration of culture [13]. Lameira et al. (1997) tested the rooting of black sage with the use of auxin, concluding that auxin had a positive influence on the formation of the root system [14]. Cytokinins are mostly synthesized in apical root meristems and transported via xylem to the shoot, where they play a fundamental role in controlling the phases of cell division in plant tissues and in breaking the dormant phase in auxiliary buds [13]. Cytokinin aided the growth and increased the production of essential oil in cultures such as Mentha piperita, M. spicata and Salvia officinalis [15]. Gibberellin is synthesized in all young developing tissues, controlling the organization of the cellulose microfibrils in such a way that the cell can expand longitudinally [13]. Mentha spicata x suaveolens, Costus spicatus (Jacq.) Sw (Zingiberaceae), and even in the culture under study (basil) demonstrate that AG increases plant height, leaf area, the number of leaves, the dry and fresh mass of the shoots, as well as the number of secondary branches [16].
Freschi and Magalhães Filho (2017) found that auxin promoted greater root development but at a significant cost to the shoot. Soy plants treated with cytokinin cracked more, but the lower development of the root system impaired water replacement during transpiration in addition to generating lower stomatal conductivity, limiting photosynthesis. In any case, gibberellin stimulated stem and petiole branching, resulting in a plant with the appearance of an unsupported vine [13]. In the same study, the use of a mixture of plant regulators displayed better responses in all evaluated aspects, thus showing that the use of plant hormones together present significant responses for cultivated plants [13].
To display the response to plant hormones when plants are under stress, antioxidant enzymes are used in order to show the plant defense system protecting them from reactive oxygen species, as these are capable of damaging cellular components.
The mixture of plant regulators such as auxin, cytokinin, and gibberellin can promote or inhibit physiological, morphological and biochemical changes, causing qualitative and quantitative changes [17]. Each of the plant hormones brings benefits to the plant, especially under stress conditions, and they have been used both individually and in combination for different purposes [18]. One of these purposes is to activate the antioxidant enzymes that are responsible for the plant’s defense. Several works already published showed the activity of antioxidant enzymes in response to stress and the use of regulators for their activation: Kang and Saltveit (2002) [19] working with cucumber seedlings; Souza et al. (2011) [20] studying rice culture; and Souza (2014) working with grapevines [18].
The enzyme superoxide dismutase controls damage caused by superoxide anions (O2−), a reactive oxygen species (ROS) that generates the oxidation of various organic molecules such as ascorbate or the reduction of metals such as ferric (Fe3+) [21]. SOD H2O2 activates molecules for plant defense in addition to producing antioxidative or cleaning enzymes [22]. Besides SOD, there are several other antioxidative enzymes capable of decomposing ROS, such as ascorbate, catalase (CAT) and peroxidase (POD) [23]. Similar to the SOD enzyme, CAT also plays a role in the defense against oxidative stress, eliminating ROS from plant tissues by converting two molecules of H2O2 into H2O and O2 [24]. Peroxidase, similar to Catalase, is an enzyme that catalyzes the oxidation–reduction between H2O2 and various reducers, which helps to control oxidative stress and, therefore, is part of several physiological processes in plants, namely lignification, suberization, auxin catabolism, and protection against attacks by pathogens and insects as well as abiotic stresses [25]. In addition, POD participates in the catabolism of IAA (indolebutyric acid), and with that they can together alter the hormonal balance in plants, causing modification of the morphogenesis. However, there are also indications that the activity of IAA oxidase is similar to that of peroxidase and that the latter may help regulate the content of IAA [26].
Lipid peroxidation or lipoperoxidation refers to the various reactions that occur as a result of the oxidative degradation of lipids. This happens when there is oxidative stress in the plant caused by an increase in ROS. Some factors are responsible for increased oxidative stress, including drought, waterlogging, temperature changes, chemical and environmental changes, and biotic and abiotic factors. This oxidative stress causes damage to plants, with proteins being the most sensitive [27]. Therefore, it is important that there is activity on the part of antioxidant enzymes so that they are able to eliminate ROS.
Nevertheless, for medicinal and aromatic plants there are few studies and publications on the use of this mixture of plant regulators and defense enzymes, and as basil is a crop capable of producing essential oil and is usually activated by some type of stress, it is of interest to investigate possible treatments so as not to impair crop productivity and increase oil production. Based on the research carried out, this work raises the hypothesis that the foliar application of Stimulate® can reduce the negative effects that water deficiency causes on the plant’s defense system, based on the activity of antioxidative enzymes. In order to verify the hypothesis, the activity of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and lipoeroxidation were evaluated, in addition to growth analyses such as number of leaves, leaf area and stem diameter in the culture of basil subjected to a water deficit and the application of different doses of Stimulate ®.
2. Results
2.1. Analysis of Antioxidative Enzyme Activity
The activity of SOD was greater in plants under water stress, boosting enzyme activity with the increase in Stimulate ® doses (Table 1). Meanwhile, in plants without water deficit, the influence of the regulator was negative; hence, SOD activity was reduced with increasing doses (Figure 1).

Table 1.
Analysis of variance of superoxide dismutase (SOD, U.ug.pt−1) activity of basil cv. Basil submitted to water deficit and different plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 20, 35, and 50 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
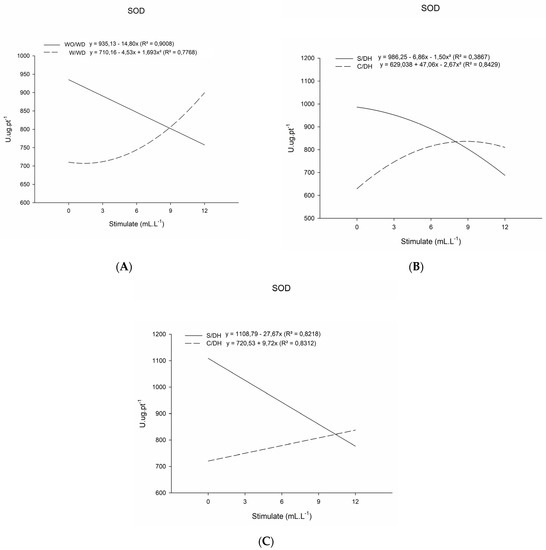
Figure 1.
Superoxide dismutase (SOD, U.ug.pt−1) enzyme activity of basil plants grown under water deficit (W/WD) and without water deficit (WO/WD) as a function plant regulator dose (0, 3, 6, 9, and 12 mL L−1), for 20 (A), 35 (B), and 50 (C) days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
There was an influence on the activity of the catalase enzyme (CAT) with water deficiency and application of the mixture of plant regulators in practically all evaluations. At 20 DAT, the results of CAT activity showed a significant interaction between the two factors (Table 2), and for plants with a water deficit, the activity of the catalase enzyme increased as a function of increasing doses.

Table 2.
Analysis of variance of catalase enzyme activity (CAT, mkat.ug.pt−1) of basil cv. Basil submitted to water deficit and different plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 20, 35, and 50 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
For the last evaluation, as previously presented, plants without a water deficit showed an increase in the activity of the CAT enzyme that was proportional to the increase in the plant regulator mixture doses (Figure 2C). Meanwhile, in plants with a water deficit, there was no significant difference for the applied doses of the plant regulator mixture, in which the action of CAT was similar between all applied doses.
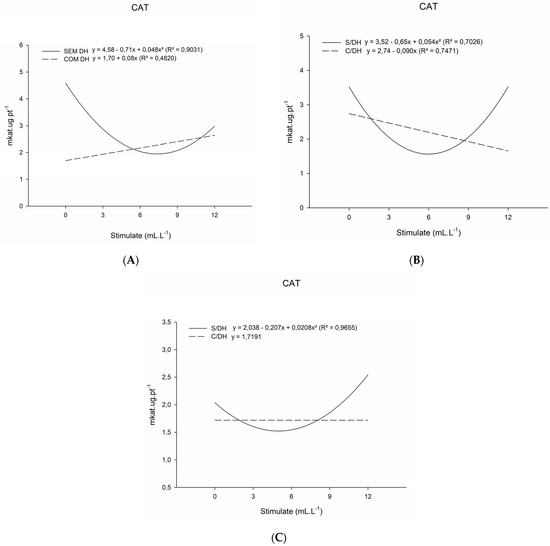
Figure 2.
Catalase enzyme activity (CAT, mkat.ug.pt−1) of basil plants grown under water deficit (W/WD) and without water deficit (WO/WD) as a function of plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 20 (A), 35 (B) and 50 (C) days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
In the results obtained for the activity of the enzyme Peroxidase (POD) (Table 3), for the first analysis, at 20 days after planting, the activity of POD was inversely proportional to the application of the plant regulator mixture in both analyzed plants with and without a water deficit (Figure 3A). As observed in the first analysis for the CAT enzyme, there was an increase in the first evaluation. This means that there may have been a greater action of the catalase enzyme that degraded H2O2 into O2 and H2O, meaning that it did not require the activity of the peroxidase enzyme for such action.

Table 3.
Analysis of variance of peroxidase enzyme activity (POD, umol.min.mg−1) of basil cv. Basil submitted to water deficit and different plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 20, 35, and 50 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
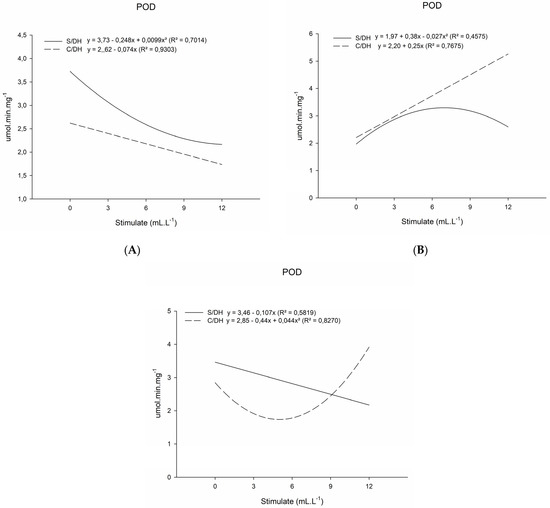
Figure 3.
Peroxidase enzyme activity (POD, umol.min.mg−1) of basil plants grown under water deficit (W/WD) and without water deficit (WO/WD) as a function of plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 20 (A), 35 (B) and 50 (C) days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
For the second evaluation, carried out at 35 DAT, the result for POD activity was the opposite of the first (Figure 3B). The application of the mixture of plant regulators together with water deficiency influenced both groups of plants so that the activity of the peroxidase enzyme increased according to the increase in the plant regulator mixture doses. In addition, the plants subjected to the deficiency presented higher POD activities when compared to the plants without a water deficiency.
In the last evaluation, the plants with a water deficiency were influenced by the plant regulator mixture doses and showed an increase in the activity of the peroxidase enzyme (Figure 3C). This result did not occur in the plants without a water deficiency. In their case, there was a decline in the activity from POD.
2.2. Lipid Peroxidation
Evaluations of lipid peroxidation were carried out in basil plants throughout their development. In the first evaluation, carried out 20 days after transplanting (Figure 4A), there was a significant interaction between the deficiency and the application of plant growth regulators (Table 4). It was observed in this first evaluation that the plants that were subjected to a water deficiency had an increase in lipid peroxidation (Figure 4A); that is, the application of the plant regulator mixture was shown to not be efficient in protecting the plants that presented a deficiency. Yet, it can also be said that in this assessment, the plant regulators may have not yet acted. This result is in line with that which was presented in the first analysis of the peroxidase enzyme activity, which showed a decline in its activity with the application of the plant regulator. For plants that did not suffer a water deficiency, there was also an increase in lipoperoxidation as well as in the evaluation of the peroxidase enzyme activity.
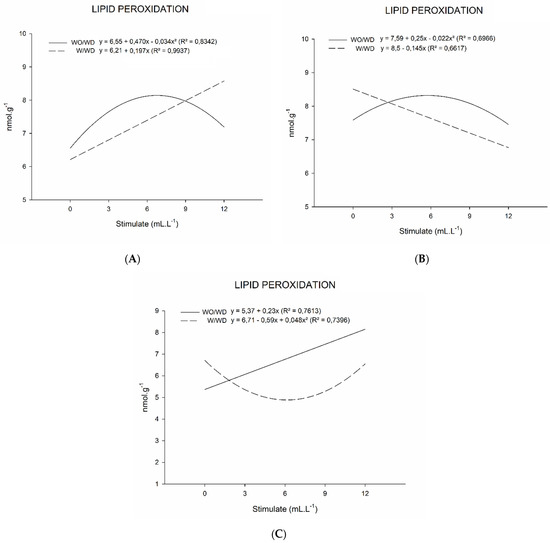
Figure 4.
Peroxidase enzyme activity (MDA, nmol.g−1) of basil plants grown under water deficit (W/WD) and without water deficit (WO/WD) as a function of plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 20 (A), 35 (B), and 50 (C) days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.

Table 4.
Analysis of variance of lipid peroxidation activity (MDA, nmol.g−1) of basil cv. Basil submitted to water deficit and different plant regulators doses (0, 3, 6, 9, and 12 mL L−1), for 20, 35, and 50 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
For the second evaluation at 35 DAT, it is noted that lipoperoxidation reduced in the plants with a water deficiency and with increasing doses of the plant regulator mixture (Figure 4B). The results, once again, are in line with those presented for the activity of the peroxidase enzyme, which, for this evaluation, showed an increase in enzyme activity. In the plants that did not suffer from a water deficit, the same response shown in the previous evaluation was maintained and was also equal to what was displayed in the POD enzyme evaluation.
In the last evaluation, at 50 DAT, lipid peroxidation in the water-deficient basil plants was lower at lower doses of plant regulator mixture (Figure 4C), while for higher doses, lipid peroxidation increased. Once again, the results presented were in agreement with those shown with the activity of the POD enzyme. In the plants without water deficiency, lipid peroxidation increased with increasing plant regulator doses, which goes against what occurred for the activity of the POD enzyme but which was equal to the CAT enzyme result. With this, it is worth noting that the lowest dosages may be more efficient when the plant has been subjected to a water deficiency for a longer time.
2.3. Growth Measurements
Data on plant height did not present a significant interaction for any of the isolated factors (water deficit and treatments with the mixture of plant regulators), thus showing that basil has good tolerance regarding the reduction in irrigation and that the use of the mixture of plant regulators did not influence stem growth. For the evaluations of plant dry mass, there was also no significant response from the plant regarding water deficit and application of plant growth regulators in any of the evaluated factors.
For the leaf number and leaf area data (Figure 5 and Figure 6), there was a significant interaction between the factors (Table 5). For the number of leaves (Figure 5), it was noted that plants under water stress had a greater number of leaves (65 leaves) when compared to plants without a water deficit (58 leaves). The plants of the two evaluated water regimes were influenced by the mixture of plant regulators in their highest dosage (12 mL L−1).
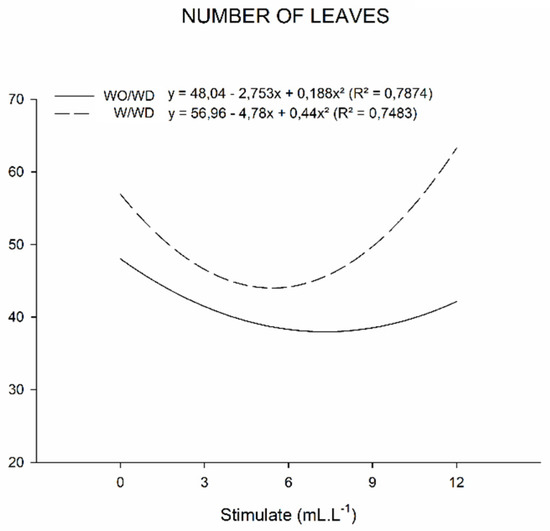
Figure 5.
Number of leaves (NL) of basil plants grown under water deficit (W/WD) and without water deficit (WO/WD) as a function of plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 60 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
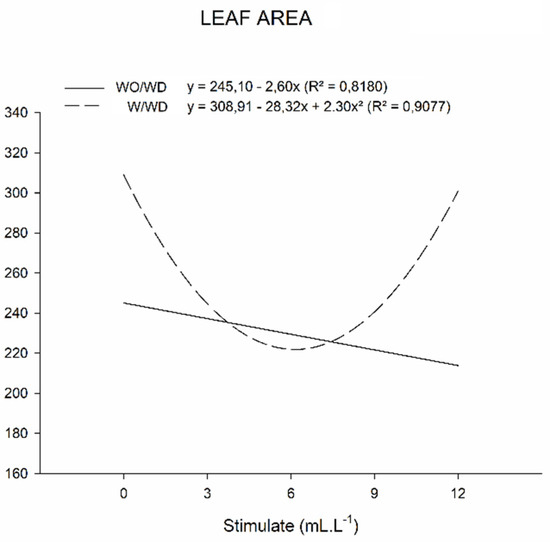
Figure 6.
Leaf area (cm²) of basil plants grown under water deficit (W/WD) and without water deficit (WO/WD) as a function of plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 60 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.

Table 5.
Analysis of variance of number of leaves (NL) and leaf area (cm²) of basil cv. Basil submitted to water deficit and different plant regulators doses (0, 3, 6, 9, and 12 mL L−1), for 60 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
It was also verified that in the plants without water deficiency the use of the mixture of plant regulators negatively affected the leaf area (Figure 6) as the dose of the product increased. The same was observed with the number of leaves for this group of plants, contradicting the possibility that a high number of leaves can reduce the leaf area due to shading.
For stem diameter (Table 6), basil plants grown under water deficit responded positively to the application of plant growth regulators (Figure 7), since the diameter increased proportionally to the product doses. It can also be observed that this behavior in the growth of the stem diameter was repeated in the three evaluations carried out (Figure 7A–C), where the plant responded to the application of plant growth regulators during the evaluated period.

Table 6.
Analysis of variance of diameter (mm) of basil cv. Basil submitted to water deficit and different plant regulators doses (0, 3, 6, 9, and 12 mL L−1), for 20, 35, and 50 days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
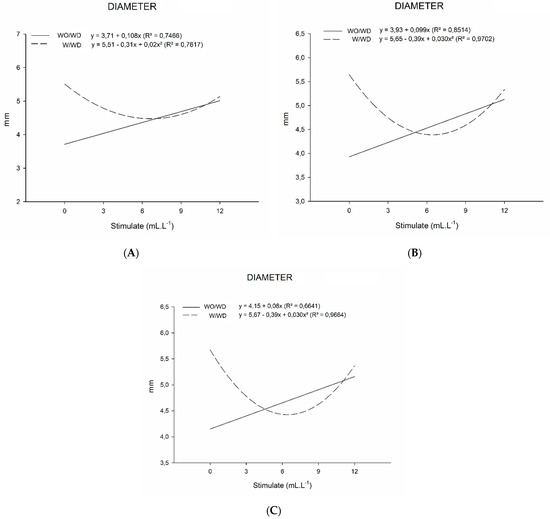
Figure 7.
Diameter (mm) of basil plants grown under water deficit (W/WD) and without water deficit (WO/WD) as a function of plant regulator doses (0, 3, 6, 9, and 12 mL L−1), for 20 (A), 35 (B), and 50 (C) days after transplanting (DAT). Botucatu city, São Paulo State, Brazil, 2020.
3. Discussion
The use of the plant regulator mixture, especially in the highest dosages (9 and 12 mL L−¹), increased the antioxidative enzymes that serve as protection for the plant when it is in conditions of water deficiency. The increase in enzymes suggests that they are protecting the basil plant from possible damage caused by the dehydration of the crop.
3.1. Analysis of Antioxidative Enzyme Activity
Data on increased SOD activity in basil plants show that the plant regulator mixture present in the commercial product Stimulate© was able to help defend the plant when it was subjected to some type of deficiency. In the case of this work, this deficiency was water, since SOD activity increased significantly in plants that were under water deficit, which may be a defense mechanism of plants when they are subjected to oxidative problems resulting from stress [28]. Results such as this were also found by [29], in which corn plants under water stress were subjected to the application of a plant regulator mixture and had an increase in SOD activity. In the work carried out by [30], rice plants under water stress showed higher SOD activity than in plants that did not suffer stress—results that were also found in the present work. On the other hand, in a study carried out by [31], gerbera plants under normal conditions and to which were applied plant regulators, showed an increase in SOD activity differing from the present work, in which the plants under normal water conditions and with the application of the product had a reduction in SOD activity.
The mixture of plant regulators is interesting for increasing the SOD enzyme, as the results presented by Moreira et al. (2018) [32] show that the use of auxin applied alone in the sugarcane crop intensified the stress in the plant instead of decreasing it. When auxin was applied together with other plant hormones, the mixture helped to control stress in addition to increasing the production quality of sugarcane juice, which is of great interest to the industry.
Cytokinin assists in the synthesis of compounds that are capable of reducing stress in addition to being able to produce a compound that is efficient in removing the superoxide radical, thus acting similarly to the enzyme superoxide dismutase (SOD). Cytokinin can also help combat oxidative stress and cause an increased activity of antioxidant enzymes [33]. With this important function, cytokine becomes interesting in being combined with other hormones, and Sousa (2016) [33] presented results in which the combination of plant regulators, in which cytonin was a part, increased the activity of SOD when compared to its use alone. In addition, the mixture of regulators was also able to decrease the production of free radicals.
The results obtained for the first two CAT evaluations contradict the results found in the work carried out by [34] in which rice plants subjected to water stress showed an increase in CAT activity over several days; that is, CAT activity was higher in the last day’s evaluation and in plants that had been subjected to deficiency for a longer time. The obtained results indicates that the CAT was not enough to eliminate hydrogen peroxide (H2O2), since what is indicated for the removal of H2O2 is the increase of catalase enzyme activity. This is because CAT is the main enzyme involved in the elimination of hydrogen peroxide [35], and in the present work the opposite occurred—the activity of the enzyme decreased.
These results show that, for the first days of deficiency, the plant regulator mixture was able to protect the plant against possible damage caused by water stress. In plants without water deficit, the opposite result was obtained (Figure 2A), in which CAT activity was higher in the control plants in relation to the plants that received the application of the plant regulator mixture. The increase in CAT enzyme activity in the basil plants with a water deficit and that were subjected to the application of the mixture of plant regulators shows that these help in the elimination of H2O2, which consequently becomes effective for water stress resistance [36]. CAT then acts in conjunction with other antioxidant enzymes to prevent the oxidative stress produced at the site of infection [37]. In the second evaluation, carried out at 35 DAT (Figure 2B), CAT activity in the plants with a water deficit was inverse, showing lower enzymatic activity when plant regulator doses were applied. This showed that, for this evaluation, the plant regulator mixture was unable to protect the plant from the damage caused by water deficiency. That happened because CAT, which helps in this defense, showed decreasing results, while the plants that did not suffer from a water deficiency showed an increase in enzymatic activity at the highest dose of the plant regulator mixture.
With this as a possible explanation for the last result, it can be said that the plant regulator mixture Stimulate© was not able to help protect basil plants against damage from oxidative stress caused by a lack of water since, in plants that did not experience a water deficiency, the activity of the CAT enzyme increased as the days went by. Results in which CAT activity was higher in non-stressed plants were also found in tomato plants treated with Putrescine [28]. Furthermore, as already mentioned in related works, the CAT enzyme is active under conditions of high stress in the plant. The fact that basil is a crop that apparently tolerates drought well may explain why it does not show so much damage when subjected to a water deficit [38].
Gibberellin, which is present in the composition of Stimulate, plays an important role in the resistance and adaptation of plants when they are subjected to a water deficiency. For this, the studies carried out by Garcia (2014) [39] showed that the use of gibberellin in tomato plants subjected to water deficiency increased the activity of antioxidant enzymes—especially the CAT enzyme, which presented the highest activity among all.
The POD results were also presented in the first hours of evaluation in castor bean plants subjected to stress, which subsequently displayed an increase in the activity of this enzyme [40]. Another study described how pineapple plants under saline stress displayed an increase in POD activity in the first days of evaluation—the possible cause of which being the adaptation of the crop to stressors [41]. These results were contrary to the ones obtained in the present work, in which it can be said that, for the first evaluation, the water deficiency was not severe to the point of harming the plant in relation to oxidative stress.
A study previously carried out evaluated brassica species under water stress and observed similar results to the present work, in which there was an increase in the POD enzyme, especially in plants that were subjected to a water deficit [42]. This result also occurred in the work carried out with Umbu plants, which showed greater POD enzyme activity in plants subjected to water stress [43].
The increase in the activity of the POD enzyme in plants subjected to a water deficit and treated with a plant regulator mixture was also reported in corn and soybean plants under water stress. The application of the plant regulator mixture presented growth in the activity of the peroxidase enzyme [29], showing that Stimulate© helped to protect basil plants when they were subjected to some type of stress.
The hormones present in the Stimulate formulation (Ak–Ck–GA) were responsible for reducing the stress levels of the plants, and therefore the mixture between them becomes interesting. However, the use of one of these hormones combined with others that are also available on the market can help protect the plant. In the work carried out by Camargo (2016) [44], there was an increase in POD activity in grapevines treated with cytokinin alone and together with putrescine.
On the other hand, in the work presented by Carvalho (2016) [45], auxin was used to reverse the stress caused by viruses, and thus the POD activity increased with the foliar application of isolated auxin; however, when used in combination with gibberellin, the POD enzyme activity decreased, thus showing the importance of regular studies with the hormones used at work.
3.2. Lipid Peroxidation
In a previous study carried out [46], the increase in the activity of antioxidant enzymes and the reduction of lipid peroxidation resulted in the control of the possible effects caused by ROS. Another study [46] presented results showing an increase in lipid peroxidation in the first evaluation carried out in corn crop under stress, with a subsequent reduction. Pea plants subjected to drought also showed increased lipid peroxidation [27].
Yet another study [47] showed how bean leaves treated with herbicide dosages displayed an increase in lipid peroxidation for the first evaluation, followed by a reduction for the next evaluation, as occurred in the present work. A study carried out on tomato crops with a water deficiency showed a reduction in lipid peroxidation in all evaluations carried out on tomato leaves and also showed an increase in the activity of antioxidative enzymes [28].
Literature suggests that, over time, the plant can minimize the damage caused by oxidative stress, and therefore, high doses of plant regulators are not necessary to attenuate this damage [44]. Other results also indicate differences between the evaluations carried out and suggest that the plants adapt to the possible stress to which they are submitted [48].
To reduce the damage caused by oxidative stress, Soares et al. (2017) [49] studied the application of auxin alone and together with catasterone in apple culture. The mixture of auxin with the other organic compound showed less lipid peroxidation activity compared to its isolated use.
The increase in lipid peroxidation indicates that water stress in the plant caused damage to the cell membrane due to the increase in free radicals. In work carried out by Garcia (2014) [39], water stress without application of any plant regulator showed high activity of lipid peroxidation. Subsequently, when gibberellin was applied, the activity of this enzyme decreased, showing a positive response to the application of gibberellin, which showed less lipid peroxidation in the tomato culture.
3.3. Growth Measurements
A study carried out by Santos (2017) shows that the basil crop has a low sensitivity to water deficiency in terms of dry mass production [50]. Likewise, studies carried out by Rossi (2011) presented a positive response for the plant height and plant dry mass of the common bean crop with the application of plant regulators [51]. The increase in the number of leaves with the use of plant growth regulators was also observed by Gonçalves et al. (2017) in passion fruit seedlings at a dosage of 150 mL L−1 [52].
Although the highest dose (12 mL L−1) presents the highest stem diameter value, doses greater than this can cause a reduction in these values in passion fruit plants, as displayed by Gonçalves et al. (2017), whose study show that doses ranging from 30 to 150 mL L−1 inhibited the growth of the passion fruit stem diameter [51]. For plants subjected to a water deficit, the diameter was greater at the lowest dose, which is consistent with the results already shown (high doses can negatively interfere with the diameter of plants).
The same effect of reducing leaf area was observed in studies carried out by Oliveira et al. (2016), who analyzed the leaf area in Zea mays Everta corn, obtaining a higher number of leaves and a smaller leaf area [51]. In contrast, Souza et al. (2013) observed an increase in leaf area in yellow passion fruit seedlings using a dose of 12 mL L−1 of the plant regulator mixture [52]. When compared to the plants with a water deficit, the control plants showed a greater number of leaves and leaf area, as did the ones with the highest dosage of the plant regulator mixture. This result shows that water deficiency did not directly affect the leaf area of the basil plants, differing from the studies carried out by Oliveira et al. (2016) with Zea mays Everta corn under saline stress that showed a decrease in leaf area, relating this response to the defense mechanisms of the plant [11].
The increase in leaf numbers with Stimulate dosages and the varying amount of cytokinin was observed by Neiva and Souza (2012) [53] in cassava plants. The number of leaves increased (both in live and dead leaves) with the application of the plant regulator, especially at dosages in which the cytokinin concentration increased. Concerning the results of isolated regulators, in the work conducted by Torres and Borges (2012) [54], the application of isolated gibberellin presented an increase in the number of leaves when compared to control plants.
In the leaf area analysis, the lowest dosages showed negative results, and according to a study by Gonçalves et al. (2015) [51], despite the increase in the number of leaves, the formed leaves were smaller. Tecchio et al. (2015) [55] observed an increase in the number of leaves and leaf area with the application of Stimulate in citrus at high dosages.
The increase in diameter can be explained by the composition of the mixture of plant regulators used, which contained auxin, gibberellin, and cytokinin, acting on the secondary growth and development of the plant, as highlighted by Oliveira et al. (2005) [56]. In the work carried out by Santos et al. (2020), the application of compound plant regulator in soybean seeds showed a superior result in stem diameter when compared to the use of a nutrient complex or even when compared to the use of a plant regulator mixture added to the nutrient complex [57]. A possible explanation for this fact, according to Scalon et al. (2009), is that the combined presence of plant regulators promoted greater absorption and use of nutrients by the roots, reflecting on the plant stem, promoting growth in stem thickness, and helping in the resistance to lodging [58].
The results obtained in tomato culture by Cerezer and Cin (2020) [59] show the positive effects of the application of auxin and gibberellin for plant diameter, wherein they showed an increase in the stem diameter when compared to the control plants. Both auxin and gibberellin can help in the vegetative growth of plants and in the increase of expansive enzymes and xyloglucanases in the culture stem, which explains the increase in diameter.
Martinez et al. (2013) [60] observed an increase in stem diameter and number of leaves in tomato plants that were treated with gibberellin. Gibberellin plays an important role in cell expansion and stem growth, especially in plants that are damaged by biotic and abiotic stresses [61]. This information regarding gibberellin shows the importance of the plant regulator for the development of the crop as a whole.
The use of gibberellin and cytokinin was studied by Leite et al. (2003) [62] in soybean plants, in which the mixture of both plant hormones decreased several aspects of growth, such as stem diameter, leaf area and biomass production. The possible explanation for these results was presented by Almeida and Rodrigues (2016) [63], who put forth the possibility that cytokinin action had reduced the effects of gibberellin. However, in the same work studied, the isolated application of cytokinin did not have a significant effect on the culture.
4. Materials and Methods
4.1. Experiment Setup and Conduction
The experiment was conducted in a greenhouse at the Department of Biostatistics, Plant Biology, Parasitology and Zoology (BBVPZ) in the Institute of Biosciences from São Paulo State University (UNESP), Botucatu Campus, in the city of Botucatu—geographical coordinates 22°53′13″ S latitude and 48°29′50″ W longitude. The climate in the region is classified as mesothermal type Cwa, humid subtropical, with rainy summers and dry winters according to Köppen-Geiger (2009). The internal temperature of the greenhouse varied between 15 and 25 °C (average temperature 18.1 °C) and had an air humidity of 73% during the days of the experiment. The sowing of basil seeds was carried out in trays using the cultivar Alfavaca Basilicão. The seedlings were kept in a greenhouse in a controlled environment at the Department of Forest Science, Soils and Environment at the School of Agricultural Sciences for 30 days. Irrigation of the site was carried out by sprinkling, with a flow rate of 3 m³/hour. To carry out the transplant, the soil used was taken from the Lageado Farm of the School of Agricultural Sciences. The soil was of the Red Latosol type and was submitted to chemical analysis for subsequent correction, fertilization, and appropriate liming. Soil analysis was performed at the Agricultural and Environmental Analysis Laboratory (AGRILAB), while fertilization and liming were performed according to the results obtained (Table 7). Based on the recommendation for mint crops using Bulletin 100, the fertilization for basil was adapted, as there are no recommendations specifically for basil crops.

Table 7.
Results of soil chemical analysis. UNESP/FCA. Botucatu city, São Paulo State, Brazil, 2020.
After the chemical analysis of the soil, calculations were made for the application of lime, which was 210 g per pot, while for the application of nitrogen (N), phosphorus (P), and potassium (K), 0.2 g, 2.7 g, and 0.07 g were respectively applied per pot.
The seedlings were transplanted into pots with a capacity of 8.5 L under a protected environment thirty days after sowing. The experiment was carried out in a randomized block design (RBD) in a factorial scheme with two factors, with water regimes of 50 and 100% of stomatal conductance, and with five treatments with a mixture of plant regulators: T1—0 mL L−1 (control); T2—3 mL L−1; T3—6 mL L−1; T4—9 mL L−1, and T5—12 mL L−1. Each treatment had 12 pots per repetition, each with 2 plants. Only the 4 central ones were evaluated, while the others were considered borders, totaling 120 pots in the whole experiment.
For the mixture of plant regulators, the commercial product Stimulate® was used (manufactured by Stoller do Brasil Ltd.), consisting of 90 mg L−1 of kinetin (Kt, cytokinin), 50 mg L−1 of indolylbutyric acid (IBA, auxin), and 50 mg L−1 of gibberellic acid (GA3, gibberellin). Stimulate® was applied foliarly 20 days after transplanting (DAT) the seedlings and when the water deficit was initiated, using a pressurized CO2 manual sprayer with a pressure of 4 kgf cm−2, flow rate of 0.2 L min−1, and open conical nozzle. Other applications were made every 15 days until reaching 50 DAT. Applications were made on 8 May 2020; 23 May 2020; and 12 June 2020.
Water deficit was defined by measuring the stomatal conductance rate in fully expanded leaves, using an InfraRed Gas Analyzer (IRGA), brand LI-COR, model 6400. The stomatal conductance rate was measured in irrigated plants, presenting values from 250 to 300 mmol m−2 s−1. After that, irrigation was interrupted, and daily evaluations of stomatal conductance were performed. When the stomatal conductance values reached 50% (between 100 and 150 mmol m−2 s−1) of the initially measured value, the plants were considered to be under water deficit and as such maintained for 7 days before being rehydrated on the 8th day. Seven water-deficit cycles were performed until reaching sixty days. After exposing the plants to water deficit, the plant regulator mixture was applied every 15 days, followed by evaluations. Evaluations were carried out on basil plants, looking for the activity of antioxidative enzymes and lipid peroxidation.
Analyses were carried out at the Laboratory of Plant Physiology at the Department of BBVPZ, Institute of Biosciences, São Paulo State University (UNESP), Botucatu city campus.
4.2. Analysis of Antioxidative Enzyme Activity
The activities of the antioxidative enzymes superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) were evaluated. For these analyses, the leaves were collected in the morning at 11:00 am every 15 days. These leaves were stored in plastic bags wrapped in aluminum foil and immediately kept in Styrofoam containing liquid nitrogen for subsequent storing in a freezer. Thus, the leaves were ground and macerated until they turned into a dry powder, and the resulting dry matter was weighed for each enzyme. To determine SOD activity, the enzyme’s ability to inhibit Nitrotetrazolium Blue chloride (NBT) photoreduction was considered. To determine this activity, 50 µL of crude extract were added to a solution containing 13 mM of methionine, 75 µL of nitrotetrazolium, 100 nM of EDTA, and 2 µM of riboflavin in 3 mL of 50 mM potassium phosphate buffer pH 7.8.
The reaction started when the tubes were illuminated in a chamber composed of fluorescent lamps at 25 °C. After 5 minutes of incubation, the end of catalysis was determined by the interruption of light. The blue compound that was formed by the photoreduction of nitretetrazolium was determined by reading it in a spectrophotometer at 560 nm. The SOD unit is defined as the enzymatic activity required to inhibit 50% of NBT photoreduction. This activity was defined by the percentage of inhibition obtained, the sample volume, and the protein concentration in the sample (µg µL−1). CAT activity was determined by spectrophotometer at a wavelength of 240 nm by monitoring the variation in hydrogen peroxide absorption, according to the method by Peixoto et al. (1999) [64].
Further, 50 µL of crude extract was added to 950 µL of 50 nm potassium phosphate buffer pH 7, which was supplemented with hydrogen peroxide to a final concentration of 12.5 mM. The change in absorption was calculated over a 60-second interval. Enzyme activity was calculated using a molar extinction coefficient of 39.4 mM cm−1. Catalase takes into account the concentration of soluble protein in the test. POD activity was determined using collected plant material, which was weighed and macerated in 5 mL of 0.2 M phosphate buffer pH 6.7. The obtained residue was filtered twice using gauze to obtain the crude extract, a procedure that was performed in a gel bath with substrate based on hydrogen peroxide and pyrogallol. POD activity determination was performed according to the spectrophotometric method of Teisseire and Guy (2000) [65]. The activity was expressed in µM of decomposed H2O2 min−1 mg−1 of protein.
4.3. Total Soluble Proteins
For the analysis of total soluble protein content, the method of Bradford (1976) [66] was used. In test tubes of 15 mL, 100 mg of lyophilized MS/5.0 mL of extraction buffer (Tris-HCl 25 mM pH 7.6) were added. After that, it was shaken with sealed tubes for 2 hours in the shaker. Then, after extraction, the tubes were centrifuged in a benchtop centrifuge (2000 rpm for 10 min) and the supernatant was collected to measure soluble proteins. Soon after, 100 µL of sample was added in test tubes with an additional 2.5 mL of Bradford reagent. The tubes were then gently shaken manually so as not to denature the proteins. Fifteen minutes after the process, the reading was performed at 595 nm against the blank with 100 µL of water + 2.5 mL of Bradford reagent. The results were expressed in mg protein/g DM [67].
4.4. Lipid Peroxidation
Lipid peroxidation was determined according to the method described by Heath and Packer (1968) [68]. For 200 mg of plant material, homogenization was performed with 0.25% thiobarbituric acid and 10% trichloroacetic acid. The material was incubated in a water bath at 90 °C for one hour. Subsequently, it was cooled and centrifuged at 10,000 rpm for ten minutes. Then, it was collected and subjected to readings at 560 to 600 nm in a spectrophotometer.
4.5. Growth Measurements
The growth analysis was performed based on measurements of leaf area, number of leaves, and diameter. The collections for leaf area and number of leaves were performed at the end of 60 days with the separation and weighing of fresh leaves. Leaf area was measured using a LICOR model 3100 benchtop leaf area meter, while the number of leaves was taken from manual leaf counts on the plants. The diameter was recorded every 15 days before and after application of the mixture of plant regulators while the plants were still in the pots, using a digital caliper (mm).
4.6. Statistical Analysis
The results obtained were submitted to tests of normality and homogeneity before the analysis of variance (ANOVA). The means were compared by the F test for a qualitative factor (water deficiency) and regression analysis for a quantitative factor (plant regulator doses) to a 5% level of probability. Statistical tests were performed using the AgroEstat and SigmaPlot software.
5. Conclusions
With the results obtained, it can be concluded that the basil plants were sensitive to water deficiency. The application of Stimulate® showed an increase in SOD enzyme activity in stressed plants in all evaluations. However, for the CAT enzyme, only the first day of application showed a positive response to the application. The POD enzyme showed an increase from the second day of application of Stimulate®, and this enzyme had a direct effect on the reduction of lipid peroxidation. The growth measures obtained better responses in the highest doses applied.
Author Contributions
Conceptualization, J.D.R.; data curation, B.L.C.; formal analysis, B.L.C.; investigation, B.L.C. and E.S.A.; methodology, B.L.C. and E.S.A.; project administration, B.L.C.; supervision, J.D.R. and E.O.O.; validation, J.D.R. and E.O.O.; visualization, J.D.R. and E.O.O.; writing—original draft, B.L.C.; writing—review and editing, E.S.A., J.D.R. and E.O.O. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc., via emails from our system or the assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES), grant number 001.
Data Availability Statement
All data included in the main text.
Acknowledgments
The authors would like to express their gratitude to the School of Agronomy Science of São Paulo State University (UNESP), Botucatu campus, and all its servers who contributed to the development of this study. The authors would also like to thank the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais no Brasil: Nativas e Exóticas, 2nd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008. (In Portuguese) [Google Scholar]
- Barbosa, D.D. Desenvolvimento Vegetativo e Parâmetros Fisiológicos em Genótipos de Amendoim sob Déficit Hídrico e Inoculados com Rizóbios 76 f. Master’s Thesis, Universidade Estadual da Paraíba/Embrapa Algodão, Campina Grande, Brazil, 2016. [Google Scholar]
- Blank, A.F.; Filho, J.L.D.C.; Neto, A.L.D.S.; Alves, P.B.; Arrigoni-Blank, M.D.F.; Silva-Mann, R.; Mendonça, M.D.C. Morphological and agronomic characterization of basil and basil accessions. Hortic. Bras. 2004, 22, 113–116. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Schenkel, E.P.; Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Óleos Voláteis. In Farmacognosia: Da planta ao Medicamento 5; Editora da UFSC: Florianópolis, Brazil, 2004; p. 475. (In Portuguese) [Google Scholar]
- De Morais, L.A.S. Influência dos fatores abióticos na composição química dos óleos essenciais. Embrapa Meio Ambiente-Artig. Em An. De Congr. (ALICE) 2009, 27, S4050–S4063. [Google Scholar]
- Joly, R.J.; Zaerr, J.B.; Dalton, D.A.; Hanus, F.J.; Russell, S.A.; Evans, H.J. Alteration of Cell-Wall Water Content and Elasticity in Douglas-Fir during Periods of Water Deficit. Plant Physiol. 1987, 83, 418–422. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S. Acclimation and adaptive responses of woody plants to environmetal stresses. Bot. Rev. 2002, 68, 279–334. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger Ian Max Møller, E.; Murphy, A. Fisiologia e Desenvolvimento Vegetal—6a Edição; Artmed Editora: São Paulo, Brasil, 2017. [Google Scholar]
- Cavalcante, W.S.D.S.; Da Silva, N.F.; Teixeira, M.B.; Filho, F.R.C.; Nascimento, P.E.R.; Corrêa, F.R. Eficiência dos Bioestimulantes no Manejo do Déficit Hídrico na Cultura da Soja. Irriga 2020, 25, 754–763. [Google Scholar] [CrossRef]
- Torsian, W.S.; Kikuti, A.L.P.; Kikuti, H.; Pereira, C.E. Bioestimulantes no desenvolvimento da cana-de-açúcar. Magistra 2011, 31, 625–634. [Google Scholar]
- De Oliveira, F.A.; De Medeiros, J.F.; Da Cunha, R.C.; De Souza, M.W.L.; Lima, L.A. Use of biostimulants in relieving salt stress in popcorn. Rev. Ciência Agronômica 2016, 47, 307–315. [Google Scholar] [CrossRef]
- Reis, M.A.M. Bioestimulante Como Estratégia ao Déficit Hídrico em Feijoeiro Comum. 69 f. Doctoral Dissertation, Universidade Estadual Paulista Júlio de Mesquita Filho, Jaboticabal, São Paulo, Brazil, 2021. [Google Scholar]
- Freschi, J.; Magalhães Filho, J.R. O poder dos Hormônios Vegetais em Modular o Desenvolvimento da Planta. Available online: https://www.stoller.com.br/o-poder-dos-hormonios-vegetais-em-modular-o-desenvolvimento-da-planta/ (accessed on 9 December 2022).
- Lameira, O.A.; Pinto, J.E.B.P.; Arrigoni, M.F.B. Enraizamento de miniestacas de erva-baleeira. Hortic. Brasileira. 1997, 5, 114–116. [Google Scholar]
- El-Keltawi, N.E.; Croteau, R. Influence of foliar applied cytokinins on growth and essential oil content of several members of the lamiaceae. Phytochemistry 1987, 26, 891–895. [Google Scholar] [CrossRef]
- Paes, L.; Mendonça, M.S.; Casas, L. Aspectos Estruturais e Fitoquímicos de partes vegetativas de Costus spicatus (Jacq.) Sw. (Costaceae). Rev. Bras. de Plantas Med. 2012, 15, 380–390. [Google Scholar] [CrossRef]
- Spinelli, F.A.W.; Rademacher, B.E.; Sabatini, A.C.G.; Costa, A. Reduction of scabincidence (Venturia inaequalis) in apple with prohexadione-Ca and trinexapac-ethyl, two growth regulating acylcyclohexanediones. Crop Prot. 2010, 29, 691–698. [Google Scholar] [CrossRef]
- Souza, E.R. Fenologia e Mistura de Reguladores Vegetais e de Fertilizante Foliar no Metabolismo da Videira cv. Sweet Sunshine em Clima Semiárido 143 f. Doctor’s Dissertation, Universidade Estadual Paulista, São Paulo, Brazil, 2014. [Google Scholar]
- Kang, H.-M.; Saltveit, M.E. Reduced chilling tolerance in elongating cucumber seedling radicles is related to their reduced antioxidant enzyme and DPPH-radical scavenging activity. Physiol. Plant. 2002, 115, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.L.; Woyann, L.G.; Ahlert, R.J.; Almeida, A.M.; Costa, O. A Peroxidação lipídica em cultivares de arroz irrigado Submetidas ao estresse por ferro em hidroponia. In Proceedings of the Anais. CIC XX Congresso de iniciação científica. UFPEL, Pelotas, Brazil, 8–11 November 2011. [Google Scholar]
- Hu, Z.-H.; Shen, Y.-B.; Shen, F.-Y.; Su, X.-H. Effects of feeding Clostera anachoreta on hydrogen peroxide accumulation and activities of peroxidase, catalase, and ascorbate peroxidase in Populus simonii × P. pyramidalis ‘Opera 8277’ leaves. Acta Physiol. Plant. 2009, 31, 995–1002. [Google Scholar] [CrossRef]
- Nascimento, J.; Barrigossi, J.A. O Papel das Enzimas Antioxidantes na Defesa das Plantas Contra Insetos Herbívoros e Fitopatógenos. Agrar. Acad. 2014, 1, 234–250. [Google Scholar] [CrossRef]
- Rossi, V.S.; Costa, M.F. Mecanismo antioxidante em plantas. In Proceedings of the 10º mostra acadêmica UNIMEP, Piracicaba, Brazil, 10–12 November 2012; pp. 1–4. Available online: http://www.unimep.br/phpg/mostraacademica/anais/10mostra/4/31.pdf. (accessed on 5 July 2021).
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.C.G.; Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev. Nut. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Prado, D.Z. Enraizamento de Eucalyptus Grandis x Eucalyptus Urophylla Pela Ação de Peróxido de Hidrogênio, Quercetina e Ácido Indolbutírico 106 f. Master’s Thesis, Curso de Ciências Biológicas (Botânica), Fisiologia e e Bioquímica Vegetal, Universidade Estadual Paulista, Júlio de Mesquita Filho, Botucatu, Brazil, 2014. [Google Scholar]
- Lopes, M.J.C.; Souza, I.R.P.; Magalhães, P.C.; Gama, E.E.G. Oxidação proteíca e peroxidação lípidica em plantas de diferentes ciclos de seleção do milho ‘SARACURA’, sob encharcamento contínuo. Rev. Bras. De Milho E Sorgo Lavras 2005, 4, 362–373. [Google Scholar] [CrossRef]
- Torres, T.P. Putrescina no Desenvolvimento do Tomateiro cv. Justyne em Condições de Estresse Hídrico 56 f. Master’s Thesis, Curso de Agronomia, Horticultura, Universidade Estadual Paulista Júlio de Mesquita Filho, Botucatu, Brazil, 2020. [Google Scholar]
- De Vasconcelos, A.C.F.; Zhang, X.; Ervin, E.H.; Kiehl, J.D.C. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Sci. Agricola 2009, 66, 395–402. [Google Scholar] [CrossRef]
- Deus, K.E. Atividade Enzimática e Expressão Diferencial da Superóxido Dismutase (SOD) em Plantas de Arroz de Terras Altas sob Deficiência Hídrica 131 f. Master’s Thesis, Curso de Biologia, Universidade Federal de Goiás, Goiânia, Brazil, 2014. [Google Scholar]
- Nunes, R.C.A.; Viana, R.S.; Machado, N.B., Neto. Atividade enzimática da superóxido dismutase em resposta aos fitorreguladores em Gerbera jamensonii. Comunicata Scientiae 2015, 6, 83–89. [Google Scholar]
- Moreira, B.R.A.; Viana, R.S.; Manarelli, F.; Viana, C.R.A.; Nakamune, A.C.M.S. Parâmetros Tecnológicos e Avaliação de Enzimas Antioxidantes da Cana-de-açúcar (Saccharum spp.) quando Aplicados Maturadores Químicos. Revista Virtual de Química 2018, 10, 1–23. [Google Scholar]
- Sousa, M.C. Reguladores Vegetais e Nutrientes Minerais no Metabolismo de Plantas de Tomateiro 76 f. Master’s Thesis, Curso de Agronomia, Horticultura, Universidade Estadual Paulista Júlio de Mesquita Filho, Botucatu, Brazil, 2016. [Google Scholar]
- Rossatto, T. Alterações Fisiológicas, Bioquímicas e Moleculares de Arroz, cv BRS AG, em Resposta ao Estresse Salino 117 f. Master’s Thesis, Curso de Biologia, Fisiologia Vegetal, Universidade Federal de Pelotas, Pelotas, Brazil, 2016. [Google Scholar]
- Bispo, G.L. Fenologia e desempenho ecofisiológico de Vasconcellea quercifolia A.St.-Hill 88 f. Doctoral Dissertation, Curso de Agronomia, Universidade Estadual Paulista (Unesp), Botucatu, Brazil, 2020. [Google Scholar]
- Fernandes, C.F.; Vieira, J.R., Jr.; Silva, D.S.G.; Alves, R.C. Estresse Oxidativo e o Mecanismo de Defesa de Plantas Contra Patógenos; Embrapa Rondônia: Porto Velho, Rondônia, Brazil, 2013. [Google Scholar]
- Sharma, S.; Ghoshal, C.; Arora, A.; Samar, W.; Nain, L.; Paul, D. Strain Improvement of Native Saccharomyces cerevisiae LN ITCC 8246 Strain Through Protoplast Fusion to Enhance Its Xylose Uptake. Appl. Biochem. Biotechnol. 2021, 193, 2455–2469. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.P.O. Efeitos de Giberelina Para Respostas em Plantas de Tomate à Deficiência Hídrica 71 f. Master’s Thesis, Curso de Agronomia, Fisiologia Vegetal, Universidade Federal de Viçosa, Viçosa, Brazil, 2014. [Google Scholar]
- Soares, A.M.S. Análise da Resposta Antioxidativa em Plantas de Ricinus Communis Submetidas ao Estresse por Metiljasmonato 93 f. Master’s Thesis, Curso de Biologia, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos do Goytacazes, Brazil, 2006. [Google Scholar]
- Piza, I.M.T.; Lima, G.P.P.; Brasil, O.G. Peroxidase Activity and Proteins Levels on Pineapple Plants Micropropagated on Salinity Medium. Revista Brasileiro Agrociência 2003, 9, 361–366. [Google Scholar]
- Uprety, B.; Kaja, A.; Ferdoush, J.; Sen, R.; Bhaumik, S.R. Regulation of Antisense Transcription by NuA4 Histone Acetyltransferase and Other Chromatin Regulatory Factors. Mol. Cell. Biol. 2016, 36, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; de Melo, N.F.; de Araujo, F.P.; Fernandes, K.V.S.; Pinto, M.S.T. Efeito do estresse hídrico sobre a atividade de enzimas antioxidantes em xilopódio de umbuzeiro (Spondias tuberosa. Arruda). In Proceedings of the XXII Congresso Brasileiro de Fruticultura, Bento Gonçalves, Brazil, 22–26 October 2012; pp. 573–576. Available online: https://www.alice.cnptia.embrapa.br/alice/bit-stream/doc/938810/1/00000003904SWB.pdf. (accessed on 5 June 2021).
- Camargo, R.B. Citocinina, piraclostrobina e putrescina: Influência no desenvolvimento de mudas em três cultivares de videiras em diferentes ambientes 80 f. Doctoral Dissertation, Curso de Agronomia, Horticultura, Universidade Estadual Paulista Júlio de Mesquita Filho, Botucatu, Brazil, 2016. [Google Scholar]
- Yamamoto, Y.; Kobayashi, Y.; Matsumoto, H. Lipid Peroxidation Is an Early Symptom Triggered by Aluminum, But Not the Primary Cause of Elongation Inhibition in Pea Roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Junqua, M.; Biolley, J.-P.; Pie, S.; Kanoun-Boulé, M.; Duran, R.; Goulas, P. In vivo occurrence of carbonyl residues in Phaseolus vulgaris proteins as a direct consequence of a chronic ozone stress. Plant Physiol. Biochem. 2000, 38, 853–861. [Google Scholar] [CrossRef]
- Souza, J.B. Caracterização Fisiológica e Bioquímica de Linhagens de Milho Visando Tolerância ao Alumínio 70 f. Master’s Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2003. [Google Scholar]
- Giannopolotis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Soares, C.S.M.; Ramos, A.P.; Cruz, N.F. Junior; Steffens, C.A.; Amarante, C.V.T. Ação de brassinosteroide e auxina sobre o estresse oxidativo em maçãs ’gala’. Available online: https://www.udesc.br/arquivos/udesc/id_cpmenu/3168/31_A__o_de_brassinosteroide_e_auxina_sobre_o_estresse_oxidativo_em_ma__s_Gala_1503494829714_3168.pdf (accessed on 11 January 2023).
- Santos, J.F. dos. Cultivo Hidropônico de Manjericão sob Estresse Salino: Crescimento, Produção e Aspectos Bioquímicos 44 f. Doctoral Dissertation, Curso de Engenharia Agrícola, Universidade Federal do Recôncavo da Bahia, Cruz das Almas, Brazil, 2017. [Google Scholar]
- Rossi, R. Nitrogênio em Cobertura e Bioestimulante Aplicado via Foliar em Feijoeiro de Inverno no Sistema Plantio Direto 62 f. Master’s Thesis, Curso de Agronomia, Universidade Estadual Paulista Júlio de Mesquita Filho, Ilha Solteira, Brazil, 2011. [Google Scholar]
- Gonçalves, B.H.L.; Souza, J.M.A.; Ferraz, R.A.; Tecchio, M.A.; Leonel, S. Efeito do bioestimulante Stimulate® no desenvolvimento de mudas de maracujazeiro cv. BRS Rubi do Cerrado. Revista de Ciências Agrárias 2017, 41, 147–155. [Google Scholar] [CrossRef]
- Souza, J.M.A.; Gonçalves, B.H.L.; Santos, A.M.F.; Ferraz, R.A.; Leonel, S. Efeito de bioestimulante no desenvolvimento inicial de plântulas do porta-enxerto cítrico tangerineira ‘Cleópatra’. Sci. Plena. 2013, 9, 1–8. [Google Scholar]
- Neiva, L.S. Filho; Souza, A.S. Stimulate® na Micropropagação da Mandioca (Manihot esculenta Crantz). In 6º Jornada Científica; Embrapa Mandioca e Fruticultura: Bahia, Brazil, 2012. [Google Scholar]
- Torres, R.C.; Borges, K.C.A.S. Effects of the action of gibberellin in the initial growth of pepper plants (Capsicum frutescens). Cadernos UniFOA (Centro Universitário De Volta Redonda) 2013, 8, 11–16. [Google Scholar]
- Tecchio, M.A.; Leonel, S.; Dos Reis, L.L.; Simonetti, L.M.; Da Silva, M.J.R. Stimulate no Desenvolvimento de Mudas de Kunquat ‘Nagami’. Irriga 2015, 1, 97–106. [Google Scholar] [CrossRef]
- De Oliveira, A.; Ferreira, G.; Rodrigues, J.D.; Ferrari, T.B.; Kunz, V.L.; Primo, M.A.; Poletti, L.D. Efeito de reguladores vegetais no desenvolvimento de mudas de Passiflora alata Curtis. Rev. Bras. de Frutic. 2005, 27, 9–13. [Google Scholar] [CrossRef]
- Dos Santos, L.P.; Barbacena, D.R.; Gonçalves, R.C.; Nascimento, C.A.C.; Carvalho, F.L.D.C.; França, L.C.; Adorian, G.C. Aplicação de Bioestimulante e Complexo de Nutrientes no Tratamento de Sementes de Soja. Agri-Environ. Sci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Scalon, S.D.P.Q.; De Lima, A.A.; Filho, H.S.; Vieira, M.D.C. Germinação de sementes e crescimento inicial de mudas de Campomanesia adamantium Camb.: Efeito da lavagem, temperatura e de bioestimulantes. Rev. Bras. Sement. 2009, 31, 96–103. [Google Scholar] [CrossRef]
- Cerezer, B.; Cin, G.D. Ação de Giberelina e Auxina no Desenvolvimento da Planta e na Qualidade de Tomates ‘Gaúcho’ 42 f. Graduation Thesis, Curso de Agronomia, Instituto Federal de Santa Catarina, São Miguel do Oeste, Brazil, 2020. [Google Scholar]
- Martínez, L.D.O.; Mendoza, O.J.; Valenzuela, M.C.; Serrano, P.A.; Olarte, S.J. Efecto de las giberelinas sobre el crecimiento y calidad de plántulas de tomate. Biotecnia 2013, 15, 56–60. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Biosynthesis, signal transduction, action, 3rd ed.; Springer: New York, NY, USA, 2010; p. 805. [Google Scholar]
- Leite, V.M.; Rosolem, C.A.; Rodrigues, J.D. Gibberellin and cytokinin effects on soybean growth. Sci. Agric. 2003, 60, 537–541. [Google Scholar] [CrossRef]
- Almeida, G.M.; Rodrigues, J.G.L. Desenvolvimento de plantas através da interferência de auxinas, citocininas, etileno e giberelinas. Braz. J. Appl. Technol. Agric. Sci. 2016, 1, 111–117. [Google Scholar] [CrossRef]
- Peixoto, P.H.P.; Cambraia, J.; Sant’Anna, R.; Mosquim, P.R.; Moreira, M.A. Aluminium effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Revista Brasileira de Fisiologia Vegetal 1999, 11, 137–143. [Google Scholar]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abade, M.; Ávila, M.T.; Lima, L.G.S.; Silva, E.G.; Oliveira, C.S.; Silva, R.T.L. Determinação dos Teores de Proteínas Solúveis Totais em Plantas de Girassol (Helianthus annuus L.). In Proceedings of the VII Workshop de Agroenergia Matérias Primas Ribeirão Preto, Anais [...], Infobibos, Ribeirão Preto, Brazil, 29–30 March 2014; pp. 1–4. Available online: http://www.infobibos.com/Agroenergia/CD_2014/Resumos/ResumoAgroenergia_201 4_010.pdf (accessed on 5 September 2019).
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).