Abstract
Drought is amongst the most important stressors affecting maize production globally. Existing strategies to offset drought impacts are centered around the rapid development of drought-tolerant cultivars through plant breeding. However, under both current conditions and projected climate changes, additional stressors such as insect pests will co-occur. To determine the impact of combined insect and drought stress on drought tolerance in maize, we assessed the effects of Dalbulus maidis, drought, and both stresses combined in drought-tolerant maize hybrids. We measured several maize morphological growth traits (i.e., plant height, stem diameter, shoot weight, root weight, root length, and root-to-shoot ratio) at the end of a 28-day period of pulse-stress and no-stress control exposure. We found that seedling growth declined when both stressors co-occurred. Nevertheless, drought-tolerant maize hybrids remained strongly tolerant to drought regardless of D. maidis infestation. While our results showed that drought tolerance is maintained in drought-tolerant maize seedlings, future studies should address any effects on maize yield. Our study highlights the importance of testing the combined effects of drought and insect stressors to better predict insect–plant interactions in the context of plant breeding for drought-tolerant traits in a changing climate.
1. Introduction
Abiotic stressors such as drought is a major threat to maize (Zea mays L. mays) production [1,2,3]. By 2050 the global demand for maize is projected to double [4,5]. Currently, an estimated 20% of annual yield loss in maize grain is attributed to drought stress [6]. Droughts harm maize market stability despite progress in breeding of drought-tolerant cultivars [7,8]. Furthermore, the United Nations Intergovernmental Panel on Climate Change (IPCC) report predicted that the Earth may reach the crucial threshold of 1.5 °C above pre-industrial levels between 2030 and 2052. The climate changes predicted by the IPCC hasten the risk of extreme droughts and likely consequences for global maize production [9].
In maize production drought is primarily addressed by breeding for traits that can improve tolerance to water deficit [6,10,11,12]. Morphological traits that are selected for under drought stress include, but are not limited to, reduced leaf area, high leaf number and short plants, thick stems, short internodes, small tassels, erect leaves, reduced root biomass, and deep root systems with little lateral root branching [13,14,15,16,17]. As water becomes scarce maize reduce transpiration and close stomata to reduce water loss, which may result in reduction in leaf area and leaf rolling [18]. A well-documented physiological response that contributes to drought tolerance in maize is redirection of growth and dry matter accumulation from shoots to roots, which enhances water uptake [13,19,20]. Thus, root-to-shoot ratio is used as a predictor of drought-tolerant response in maize [21,22,23,24]. Susceptibility to drought is related to the extent to which drought phenotypes are expressed, such as increased leaf rolling and wilting which negatively affect photosynthesis and grain yield [25,26]. Grain yield under drought stress has been shown to have strong correlation with traits such as plant height and stem thickness [27,28,29]. Therefore, plant performance parameters centered on size indices (e.g., height, stem diameter, biomass) are suitable proxies for assessing potential effects of drought stress on crop growth and yield [30,31,32,33].
Similar to drought stress, insects are important biotic stressors that threaten the production and market stability of maize [34,35,36]. Generally, insect species undergo latitudinal and altitudinal range expansions under drought conditions [37,38]. Dalbulus maidis DeLong and Wolcott (Hemiptera: Cicadellidae), the primary vector of several important maize pathogens (Spiroplasma kunkelii, Maize bushy stunt phytoplasma, Maize rayado fino virus), is an important pest to consider in the US under the projections of rising local temperatures. Dalbulus maidis feeds by removing sap from phloem tissues of maize [39]. Phloem-feeding disrupts water flow, which can lead to hydraulic stress, plant stunting, and wilting. Moreover, D. maidis excretes honeydew, which facilitates rapid growth of sooty mold fungi, which further decrease yield [40]. Dalbulus maidis is indigenous to Mexico and is most pernicious in South America, though its geographic range may likely expand to southern USA, particularly Texas, as rising temperatures improve habitat suitability in the south USA [41]. The climatic suitability of South America for maize production is expected to decline, while improving in North America [42]. Because D. maidis is a specialist herbivore on the genus Zea and has an extended co-evolutionary relationship with maize [43,44,45], it’s distribution in North America will incorporate newly suitable areas for maize production.
Interactions between drought stress and herbivory stress have not been widely explored in maize [46,47]. Under herbivory, plants either compensate between high tolerance and low resistance, vice versa, or balance between these two [48,49,50]. In some studies drought stress is hypothesized to increase susceptibility of plants to herbivory, which leads to enhanced herbivore performance [51,52,53,54], though some studies have challenged this view on the basis that insect responses vary among feeding guilds, e.g., piercing/sucking versus chewing insects [55,56,57,58]. For example, Copolovici et al. [59] showed that under combined drought/herbivory stress there was a defense priming effect due to induction of volatile organic compounds (VOCs) in the deciduous tree Alnus glutinosa, which resulted in stronger defense against green alder sawfly, Monsoma pulveratum, a chewing insect pest. In contrast, drought stress in collards (Brassica oleracea var. acephala) resulted in reduced defense against gloomy scale, Melanaspis tenebricosa, a piercing-sucking insect, evident as enhanced reproduction in the scale [52]. Meanwhile, whiteflies, Bemisia tabaci trigger a strong drought tolerance response while eliciting defense in maize [47]. The interaction between drought and insect stress is complex, and more case-by-case studies are required to elucidate the mechanisms that mediate defense expressions and trade-offs in maize as well as in other crop species.
In this study, we assessed the impacts of D. maidis on the morpho-physiological responses of drought in drought-tolerant maize hybrids by exposing maize seedlings to drought and Dalbulus maidis stress, alone and combined. The primary goal was to assess whether D. maidis may disrupt drought tolerance responses of drought-tolerant maize hybrids and provide insight to any combined effects of drought and insect stress in maize production under drought driven by climate change.
2. Results
Drought and D. maidis treatments caused significant reduction in plant height (Drought F1,1 = 45.579 p, < 0.0001; D. maidis F1,1 = 17.910 p, < 0.0001), stem diameter (Drought F1,1 = 128.838 p, < 0.0001; D. maidis F1,1 = 137.440 p, < 0.0001), shoot weight (Drought: F1,1 = 67.837 p, < 0.0001; D. maidis F1,1 = 16.540 p, < 0.0001) and root weight (Drought F1,1 = 16.260 p, < 0.0001; D. maidis F1,1 = 20.755 p, < 0.0001). Root-to-shoot ratio was significantly increased by drought treatment (F1,1 = 16.708 p, < 0.0001) but not by D. maidis treatment (F1,1 = 0.936 p, = 0.336). Neither drought nor D. maidis treatments affected root length (Drought F1,1 = 0.018 p, = 0.895; D. maidis F1,1 = 3.747 p, = 0.056). Furthermore, the interaction term Drought × D. maidis was not significant for plant height (F1,1 = 1.0660 p, = 0.304), stem diameter (F1,1 = 0.456 p, = 0.50), shoot weight (F1,1 = 0.253 p, = 0.616), root-to-shoot ratio (F1,1 = 1.234 p, = 0.269), root weight (F1,1 = 0.424 p, = 0.517) nor root length (F1,1 = 5.283 p, = 0.024). The nested term Hybrid [Drought, D. maidis] was also not significant for plant height (F8,8 = 0.537 p, = 0.825), stem diameter (F8,8 = 0.939 p, = 0.488), shoot weight (F8,8 = 0.531 p, = 0.831), root-to-shoot ratio (F8,8 = 1.710 p, = 0.104), root weight (F8,8 = 1.127 p, = 0.351), and root length (F8,8 = 0.873 p, = 0.542). The covariate (initial stem diameter) was only significant for root weight (F1,1 = 7.788 p, = 0.006).
A 100% recovery rate was observed in the drought-tolerant maize hybrids when seedlings treated with D. maidis and drought combined stress were re-irrigated after persistent pulse-drought stress for up to 21 days (Figure 1). No seedling deaths occurred throughout the experiments for all hybrids. Water content in shoot and root was only significantly reduced in combined stress treatment (Supplemental Figure S1).
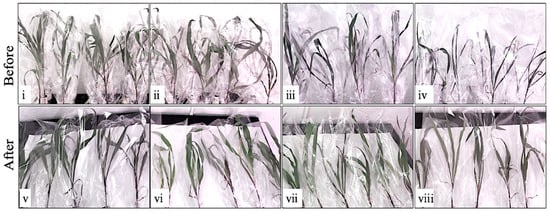
Figure 1.
Effect of Dalbulus maidis, drought, and combined stress on the above-ground performance of maize. Images of seedlings exposed to D. maidis, drought, and combined stress treatments: images (i,v) are control, images (ii,vi) are D. maidis-only, images (iii,vii) are drought-only, images (iv,viii) are combined stress (D. maidis and drought stress), images (i–iv) also show the observed drought symptoms (±wilting, leaves rolling) on the 3rd day of absolutely no watering at the 21-day point of progressive stress, while images (v–viii) show recovery 1 day after re-infiltrating the symptomatic seedlings.
Drought × D. maidis interaction effects are shown in Figure 2, while comparison between treatments and control is shown in Table 1. Results showed that plant height (Figure 2A) was reduced by 0.11- and 0.22-fold due to drought-only and combined stress treatments, respectively, and relative to control. Stem diameter (Figure 2B) was also reduced by 0.25-, 0.25-, and 0.47-fold due to D. maidis-only, drought-only, and combined stress treatment, respectively. Shoot weight (Figure 2C) was reduced by 0.14-, 0.28-, 0.41-fold due to D. maidis-only, drought-only, and combined stress treatments, respectively. Root-to-shoot ratio (Figure 2D) increased 0.13-fold by combined stress treatment compared to control. Root weight (Figure 2E) was reduced by 0.3-fold due to combined stress when compared to the control seedlings. D. maidis-only treatment caused a 0.06-fold reduction in root length (Figure 2F) compared to control seedlings.
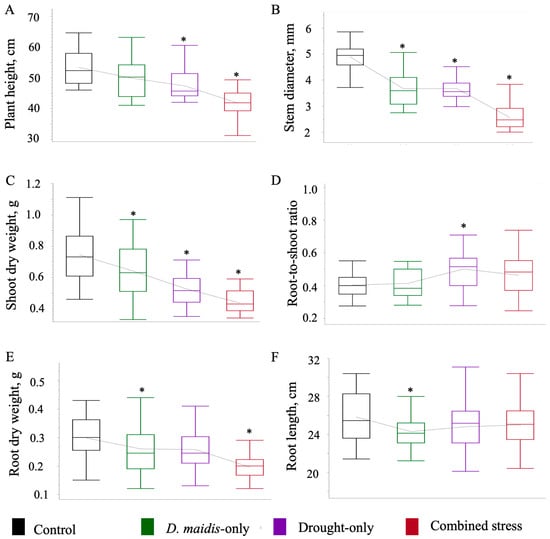
Figure 2.
Effects of drought, Dalbulus maidis, and combined stress on drought-tolerant maize hybrids. (A) plant height [Drought F1,1 = 45.579 p, < 0.0001; D. maidis F1,1 = 17.910 p, < 0.0001; drought × D. maidis F1,1 = 1.0660 p, = 0.304], (B) stem diameter [Drought F1,1 = 128.838 p, < 0.0001; D. maidis F1,1 = 137.440 p, < 0.0001; drought × D. maidis F1,1 = 0.456 p, = 0.50], (C) shoot dry weight [Drought: F1,1 = 67.837 p, < 0.0001; D. maidis F1,1 = 16.540 p, < 0.000; drought × D. maidis F1,1 = 0.253 p, = 0.616], (D) root-to-shoot ratio [Drought: F1,1 = 16.708 p, < 0.0001; D. maidis F1,1 = 0.936 p, = 0.336; drought × D. maidis F1,1 = 1.234 p, = 0.269], (E) root dry weight [Drought F1,1 = 16.260 p <0.0001; D. maidis F1,1 = 20.755 p, < 0.0001; drought × D. maidis F1,1 = 0.424 p, = 0.517], and (F) root length [Drought F1,1 = 0.018 p, = 0.895; D. maidis F1,1 = 3.747 p, = 0.056; drought × D. maidis F1,1 = 5.283 p, = 0.024]. Treatment groups are ordered from left to right by control, D. maidis-only, drought-only, and combined stress treatments. Asterisks indicate significant differences between control and treated seedlings per Dunnett’s tests (statistics are shown in Table 1). Box plots represents mean values from all three drought-tolerant hybrids (LH195 × TX772, LH195 × TX773, and TX790 × TX777).

Table 1.
Dunnett statistics within Analysis of covariance (ANCOVA) for the independent variables (plant height, stem diameter, shoot weight, root-to-shoot ratio, root weight, and root length).
Further a priori contrast analyses showed that D. maidis caused a 0.17-fold reduction in plant height (Figure 3A) within seedlings subjected to combined stress in Midwest × South 1 (F1,53 = 10.030 p, < 0.002) hybrid, whereas it did not significantly impact the drought responses in Midwest × South 2 (F1,53 = 2.118 p, = 0.140) and South × South (F1,53 = 3.392 p, = 0.068) hybrids when compared to drought-only seedlings. Dalbulus maidis caused a 0.31-, 0.38-, and 0.22-fold significant reduction in stem diameter (Figure 3B) within seedlings subjected to combined stress in Midwest × South 1 (F1,53 = 22.837 p, < 0.0001), Midwest × South 2 (F1,53 = 29.490 p, < 0.0001) and South × South (F1,53 = 11.391 p, = 0.001) compared to drought-only seedlings. Dalbulus maidis also caused a 0.23- and 0.27-fold decrease in root weight (Figure 3D) in drought effects in Midwest × South 1 (F1,53 = 6.197 p, = 0.015), and South × South (F1,53 = 5.865 p, = 0.017), respectively, but did not affect Midwest × South 2 (F1,53 = 2.235 p, = 0.138) when compared to drought-only seedlings. Dalbulus maidis did not cause any significant differences in drought responses in shoot weight (Figure 3C) within seedlings subjected to simultaneous drought in Midwest × South 1 (F1,53 = 2.663 p, = 0.106), Midwest × South 2 (F1,53 = 3.921 p, = 0.050), or South × South (F1,53 = 0.058 p, = 0.444). Dalbulus maidis did not affect drought response for root-to-shoot ratio (Figure 3E) in Midwest × South 1 (F1,53 = 0.890 p, = 0.348), Midwest × South 2 (F1,53 = 0.367 p, = 0.546) and South × South (F1,53 = 4.949 p, = 0.028). Likewise, no comparable differences between drought-only and combined stress treatments were found in root length (Figure 3F) for Midwest × South 1 (F1,53 = 0.319 p, = 0.574), Midwest × South 2 (F1,53 = 1.295 p, = 0.258) and South × South (F1,53 = 0.020 p, = 0.887).
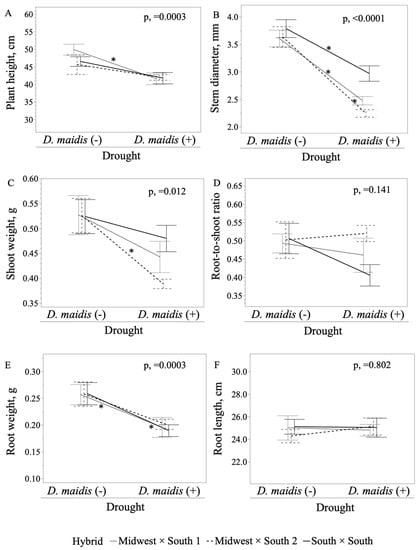
Figure 3.
Effects of Dalbulus maidis on drought responses of maize hybrids (Midwest × South 1, Midwest × South 2 and South × South). (A) plant height (F8,8 = 0.537 p, = 0.825), (B) stem diameter (F8,8 = 0.939 p, = 0.488), (C) shoot weight (F8,8 = 0.531 p, = 0.831), (D) root-to-shoot ratio (F8,8 = 1.710 p, = 0.104), (E) root weight (F8,8 = 1.127 p, = 0.351), and (F) root length (F8,8 = 0.873 p, = 0.542) for the nested term Hybrid [Drought, D. maidis]. Asterisks indicate significant differences by a priori contrast between drought-only and combined stress treatments with critical p, ≤ 0.017, per Bonferroni correction. On the x-axes, (−/+) indicates absence/presence of D. maidis during drought stress.
3. Discussion
Drought stress is a threat to maize production and is being addressed through the rapid development of drought tolerant cultivars through plant breeding [60]. Concurrent to drought, increased insect stress such as D. maidis herbivory, and pathogen transmission are potential causes of future concern. Research necessary to predict the impacts of such colliding stressors is sparse. Therefore, this study assessed the effects of drought combined with D. maidis and found that D. maidis impacted root length and stem diameter by huge effect sizes (~13.2 and ~2.3 times more than controls), as well as shoot and root weight to a lesser extent (by effect sizes of ~0.8 and ~0.7 times more than controls). Effect sizes of approximately 0.2, ~0.8, ~1.2, and ~2.2 are considered small, large, very large and huge, respectively [61,62]. Meanwhile, drought had a very large negative impact on stem diameter, shoot and root weight, plant height, and root-to-shoot ratio (effect sizes ranges from ~2.2, ~1.6, ~1.04, 0.95 and ~0.6, respectively, compared to control seedlings). Similar to the results in this study, shoot and root reductions under drought stress have been reported in maize [63,64,65]. When drought was combined with D. maidis, there were very large impacts on stem diameter, plant height, shoot weight, and root weight compared to the control seedlings (effect sizes of ~4.3, ~2.3, ~2.0, ~1.6, respectively). Prior research showed strong positive correlations between plant height and stem thickness (diameter) during vegetative growth stages and grain yield [27,28,29,32,33]. Thus, considering that plant height and stem thickness were significantly impacted by combined stresses, it is possible that maize hybrids experiencing combined stressors over extended periods of stress may lose productivity.
The impacts of drought in drought intolerant maize were not evaluated in this study. However, in a study in which drought intolerant maize was infested with D. maidis under drought and control conditions, Virla, Araoz and Albarracin [36] found that maize biomass loss and mortality were less severe in control (non-drought) compared to drought when exposed to both low (10 D. maidis adults/seedling) and high insect density (50 D. maidis adults/seedling). Specifically, seedling mortality was 16% greater than control under drought at high D. maidis density. This study by Virla, Araoz and Albarracin [36] provided insight for understanding the effects of D. maidis on maize in the absence of drought-tolerant traits, and its results suggested that D. maidis may worsen the effects of drought on maize production. It is well-known that drought-intolerant plants perform poorly in drought conditions compared to tolerant varieties [17,64,66]. Thus, our study focused on drought-tolerant seedlings and the effects of insect and drought stresses.
Dalbulus maidis worsened the effects of drought on stem diameter in all three drought-tolerant maize hybrids (Midwest × South 1, Midwest × South 2, and South × South). Whereas D. maidis only worsened drought effects on root weight in Midwest × South 1 and South × South hybrids and plant height in Midwest × South 1, but not Midwest × South 2 nor South × South. This suggested that the hybrid with southern parents (South × South 1) was less sensitive to D. maidis during drought relative to the hybrids with one mid-western parent (Midwest × South 1, Midwest × South 2). Interestingly, when seedlings infested with D. maidis were also subjected to drought their shoot weight, root-to-shoot ratio, and root length responses did not differ from those treated with only drought for all three drought-tolerant hybrids tested. These results suggested that D. maidis did not drastically implicate the expected responses to drought in the drought-tolerant hybrids tested. Furthermore, a 100% recovery rate was observed in the drought-tolerant maize hybrids after seedlings treated with D. maidis were re-irrigated after periods of drought. This high recovery rate observed in combined stress treatment and similarly in drought-only treated drought-tolerant seedlings suggests that D. maidis did not severely affect the drought-tolerant maize hybrids that were tested. A plausible explanation for the variations in morphological responses observed among the drought-tolerant hybrids could be that they deploy divergent drought responses [67,68]. For example, drought response mechanisms such as osmotic adjustment, stomata regulation, adjustment of their life cycle, and re-growth speed determine the morphological traits expressed during stress and can vary among hybrids [69]. Moreover, reduction in the growth traits recorded in this study are consistent with the expected expression of drought-tolerance as reported by several studies [13,14,15,16,17,19,20,21,22,23,24,70]. Though the effects of the combined stress treatment were overall not detrimental to the drought-tolerant hybrids, there was a pattern that showed larger effect sizes on maize morphological traits compared to drought-only seedlings. Piercing-sucking insects, such as D. maidis, have been shown to trigger strong drought responses. For example, Park, Bae and Ryu [47] used molecular methods to show that whiteflies trigger strong drought-tolerance responses in maize through higher expression of drought-associated genes ZmbZIP72, ZmSNAC1, and ZmABA1. Therefore, it may also be that the larger effects of D. maidis and drought stress combined (i.e., combined stress treatment) is potentially due to the triggering of a strong drought response.
Drought and D. maidis responses displayed similar patterns in several morphological traits measured (stem diameter, shoot weight, root weight, root-to-shoot ratio) when compared to the control seedlings. Piercing-sucking insects affect water transport in their host plants as they feed on plant sap and reduce water availability, which is a form of hydraulic stress. Likewise, drought is due to limited water acquisition by plants. It follows that sap-sucking insects, such as D. maidis, and drought stress could potentially trigger the activation of similar pathways to those triggered by hydraulic stress in maize. Previous studies showed that responses to some abiotic stresses, such as drought, rely on deployment of plant hormones, including abscisic acid (ABA), jasmonic acid (JA) and ethylene (ET) [71,72,73,74]. Notably, ABA, which is a dominant drought response phytohormone, is also known to promote biotic stress tolerance by suppressing salicylic acid (SA) signals [75,76,77]. Maize may also defend against the impacts of D. maidis by utilizing mechanisms of tolerance [50]. Therefore, maize cultivars that are tolerant to drought and can maintain D. maidis infestation under each single stress exposure could potentially be triggering shared components for chemical compounds being deployed for plant defense. Nevertheless, to determine the impacts of drought and D. maidis as combined stressors more comprehensive studies should explore biochemical responses in drought-tolerant maize cultivars with insects and water stress.
Currently, there is little information on the interaction between drought and insect damage in maize [46,47]. Our study highlighted the importance of testing the combined effects of a phloem feeding insect, D. maidis, and drought in the context of plant performance. By the same token, our study showed that combined drought and D. maidis stress may negatively impact the productivity of drought-tolerant maize. However, the overall results of this study indicated that the expected drought response mechanisms (reduced growth and optimized recovery) were maintained by the drought-tolerant hybrids regardless of D. maidis infestations, as evident by the high recovery rate of the seedlings after re-irrigation. Climate change will affect pest severity and incidence and severity of drought, therefore further studies that assess the interactions between insect and drought in maize are recommended.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
Three drought-tolerant maize hybrids were obtained from the Texas A&M University Crop and Soil Science Breeding and Genetics Program: pedigrees LH195 × TX772, LH195 × TX773, and TX790 × TX777. The parent line TX777 is derived from a cross between two lines that were both derived from Agricomseeds populations (Santa Cruz de la Sierra, Bolivia) and is adapted to relatively long growing seasons and multiple stress conditions common in Southern United States (US) ecosystems [78]. TX772 is derived from South American maize line with high-yielding capacity and is adapted to Southern US environments [79]. LH195 is a US elite inbred developed from the single cross LH117 × LH132 and is adapted to Midwestern US environments [80]. TX773 and TX790 are both adapted to Texas/Southern growing seasons [78]. The Hybrids were selected to meet a criterion of relatively wide representation in terms of genetic diversity and adaptation by choosing varieties whose geographic distribution ranges from Southern to Midwestern US. Subsequently, we verified the drought tolerance of these lines by halting the watering regime of seedlings for 12 consecutive days and observing whether seedlings recovered after watering at full saturation after the 12th day (unpublished). Throughout this study, hybrids LH195 × TX772, LH195 × TX773, and TX790 × TX777 are referred to as Midwest × South 1, Midwest × South 2, and South × South, respectively.
Seeds were germinated between two sheets of moist paper towels incubated in Petri dishes for approximately 3 days. Newly germinated seeds, with emerged radicle and cotyledon, were transplanted into cone-trainers (SC10R, Greenhouse megastore, IL, USA) filled with modified soil mixture consisting of sand, peat, and maize soil (organic soil taken from a field located at Foggy Valley Rd. Moody, Texas) in a 1:1:2 proportion. Seedlings were irrigated every 2 days with 30 mL of water. The soil was fertilized (N-P-K/20-20-20) once every two weeks to remediate the reduced nutrient content of sandy soil. Maize seedlings were maintained in a growth room with an average lighting condition of up to 1030 µmoL/m2/s photosynthetic photon flux density (PPFD) over a 4′ × 4′ canopy using SPYDRx PLUS led lights (Fluence Bioengineering, TX, USA) and a photoperiod of 12:12 (L:D). Growth condition was also maintained at an average humidity of 33%, and a temperature of 24–26 °C.
4.2. Dalbulus maidis Rearing
Insects used in this study came from a colony established in 2009 at the Biological Control Facility, Texas A&M University, College Station Texas. Insects were kept in a plastic-frame mesh cage (BugDorm-44545F) on seedlings of a Mexican maize landrace (Elotes Occidentales) in their 4th–6th vegetative growth stage (V4–V6) in a growth room with a photoperiod of 12:12 (L:D) and a temperature of 24–26 °C, as previously described by Bellota, Dávila-Flores and Bernal [45]. Mature adult females assumed to be mated were taken from the colony and used for oviposition in this study.
4.3. Drought Induction and Water Regime
Water stress status of maize hybrids was quantified by saturating each pot to 100% soil water holding capacity and then weighing on a balance (Metler Toledo, Greifensee Switzerland) daily for 1 week, to determine evapotranspiration rate (ET water loss). Cumulative ET water loss was calculated by subtracting the weight of the maize pot each day from the weight of the same pot at saturation (day 0) (Figure 4). In preliminary steps, soil water status was verified by quantifying gravimetric and volumetric water content (GWC, Ɵ). Soil dry weight was determined by drying soil in a precision gravity convection oven (Acme Revival, Memphis, TN, USA) at 70 °C for 3 days and used to determine gravimetric water content (ƟG = [saturated soil-dry soil]/dry soil) where ƟG was calculated to be 0.273. Additionally, soil core volume was calculated by volume of container, V = 1/3πr2h, and used to determine the volumetric water content (ƟV = ([dry soil/soil core volume] × GWC), where ƟV was calculated to be 0.613.
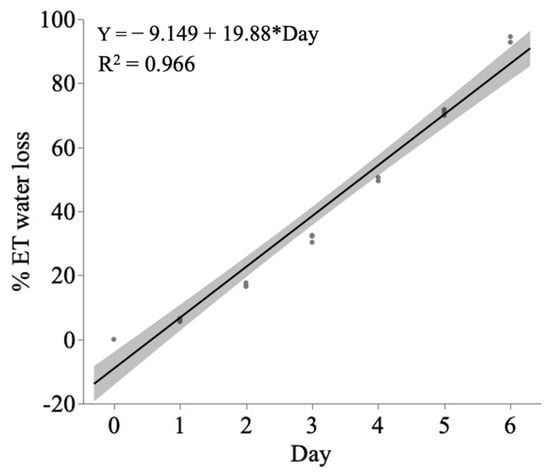
Figure 4.
Establishing water infiltration regime for inducing drought stress. Drought induction water regime was determined by assessing the relationship between evapotranspiration rate against day in maize hybrids using linear regression analysis. Days taken to reach 50% ET water loss was determined by the equation of the line (Y = −9.149 + 15.88 × Day) and calculated to be ≅4 days (=3.7 days). Additionally, watering at 2-day intervals would keep ET water loss within the desired ranges 0–30% (=23% ET water loss). The shaded region shows the 95% confidence of the fitted line, which is the daily ET water loss for the mean of three hybrids (Midwest × South 1, Midwest × South 2, and South × South). There was no significant difference in the mean lines for each hybrid (not shown, n = 21, F2,2 0.014, p = 0.986).
Drought stress was defined as 50% ET (i.e., equivalence of 50% ƟV) water loss, which was found to have occurred at day 4 post saturation with no subsequent watering (preliminary results, Figure 4). Pots with maize seedlings used as control water treatment were maintained by a water regime of 30 mL (100% infiltration, non-drought conditions) every 2 days to replenish an ET water loss such that soil water was maintained within a range of 70–100% (i.e., 0–30% total ET). Simultaneously, maize pots in which drought stress was induced were maintained by a water regime of watering with 15 mL (50% infiltration, drought conditions) every 2 days, such that the soil water was maintained at a range of 50–30%, thus maintaining a persistently pulsed-stressed scenario.
4.4. Treatments
Three maize hybrids (Midwest × South 1, Midwest × South 2 and South × South) were evaluated under two drought levels (+, −), and two D. maidis levels (+, −) in this study. Thus, the hybrids were subjected to one of four treatments: (i) drought (−)/D. maidis (−) (hereafter “control”); (ii) drought (−)/D. maidis (+) (“D. maidis-only”); (iii) drought (+)/D. maidis (−) (“drought-only”); and (iv) drought (+)/D. maidis (+) (“combined stress”). A total of 10 experimental replicates were established. Each replicate consisted of seedlings of the three maize hybrids subjected to one of the four 4 treatments, i.e., 12 seedlings per replicate, and a total of 120 seedlings for the entire experiment.
4.5. Effects of Dalbulus maidis and Drought on Drought-Tolerant Maize Hybrids
Dalbulus maidis and drought treatments were initiated two weeks after planting. Twelve 2-week-old maize seedlings (4 from each of 3 plant types) of approximately the same size were used as a single experimental replicate. Each of the 4 seedlings from each hybrid were subjected to one of the 4 treatments. Dalbulus maidis stress was applied by confining two D. maidis females on the midpoint area of the 2nd true leaf of maize seedlings for 24 h. Cages holding D. maidis females were made of two foam sheets (external dimension 5.5 × 5.5 × 1 cm each) enclosed with anti-insect netting mesh on the outer-sides, which sandwiched the leaf (internal cage dimensions 3.5 × 3.5 × 2 cm) to allow oviposition to occur over a 24 h period, after which females were removed from each seedling. After this, each seedling (infested and non-infested) was covered with a crystal clear micro-perforated plastic bag (dimension 11″ × 20″/60 holes per square inch) (ClearBags, CA, USA). Drought treatment was induced on the onset of hatching (~10 days after infestation), by separating seedlings (i.e., both newly infested by oviposition and non-infested) in two groups and halting watering of the set designated as drought (containing both infested and non-infested seedlings) for four consecutive days, then re-infiltrating with half the volume of the non-drought labeled seedlings, i.e., 15 mL and 30 mL of water, respectively, following a scheduled, 2 days routine. The water treatment regime was maintained consistently until seedlings were harvested 28 days after oviposition took place (sufficient for the onset of D. maidis maturing into adults).
Several maize seedling traits were measured, including plant height (taken from crown root base of the seedlings to the tip of the longest leaf), stem diameter (average of two measurements taken at shorter and longer axes at a position between 2nd and 3rd true leaf), dry shoot weight (stem and leaves), dry root weight, root length (measurement taken from crown base of crown root to the tip of the longest root), and root-to-shoot ratio (root weight/shoot weight). The dry weights of shoots and roots were taken after oven drying in a precision gravity convection oven (Acme Revival, United States) for ≥3 days at 80 °C.
4.6. Statistical Analyses
Statistical analyses relied on a nested analysis of covariance (ANCOVA) with the independent variables drought and D. maidis, the interaction term drought × D. maidis, the nested term hybrid [drought × D. maidis], and the response variables plant height, stem diameter, shoot weight, root-to-shoot ratio, root weight and root length. Additionally, the model included the independent covariate initial stem diameter, which was measured immediately prior to applying treatments to seedlings. The covariate was included to account for variability in seedling sizes at the time that treatments were initiated. Hybrids were nested in the model to allow for comparisons among hybrids under drought × D. maidis interactions, if warranted. Dunnett’s tests were used to compare LS means between the control and the remaining three treatments. Additionally, a priori contrasts within the nested term hybrid [drought × D. maidis] were used to compare between the drought-only and combined stress treatments within each of the hybrids. Effect sizes (Cohen’s d) for comparisons were determined to compare the magnitude of the differences between treatments. The critical P value for three contrast comparisons was adjusted to 0.017 per Bonferroni correction [81]. All statistical analyses were conducted using JMP PRO 15.0.0 (SAS Institute Inc., Cary, NC, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/stresses2030023/s1.
Author Contributions
Conceptualization, T.-K.L.J., R.F.M. and J.S.B.; methodology, T.-K.L.J.; validation, R.F.M. and J.S.B.; formal analysis, T.-K.L.J. and J.S.B.; investigation, T.-K.L.J.; resources, R.F.M. and J.S.B.; data curation, T.-K.L.J.; writing—original draft preparation, T.-K.L.J.; writing—review and editing, R.F.M. and J.S.B.; visualization, T.-K.L.J.; supervision, R.F.M. and J.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Seth Murray for supplying the drought-tolerant maize hybrids used in this study. We also thank Keyan Zhu-salzman, Mariana Mateos and Astri Wayadande for providing reviews on this manuscript. Finally, we acknowledge the Texas A&M AgriLife facility in Weslaco Texas and the Department of Entomology, College Station Texas, for support through graduate research assistantship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rafique, S. Drought responses on physiological attributes of Zea mays in relation to nitrogen and source-sink relationships. In Abiotic Stress in Plants; IntechOpen: London, UK, 2020. [Google Scholar]
- Bhusal, B.; Poudel, M.R.; Rishav, P.; Regmi, R.; Neupane, P.; Bhattarai, K.; Maharjan, B.; Bigyan, K.C.; Acharya, S. A Review on Abiotic Stress Resistance in Maize (Zea mays L.): Effects, Resistance Mechanisms and Management. J. Biol. Today’s World 2021, 10, 1–3. [Google Scholar]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Maazou, A.-R.S.; Tu, J.; Qiu, J.; Liu, Z. Breeding for drought tolerance in maize (Zea mays L.). Am. J. Plant Sci. 2016, 7, 1858. [Google Scholar] [CrossRef]
- Tigchelaar, M.; Battisti, D.S.; Naylor, R.L.; Ray, D.K. Future warming increases probability of globally synchronized maize production shocks. Proc. Natl. Acad. Sci. USA 2018, 115, 6644. [Google Scholar] [CrossRef]
- Gurian-Sherman, D. High and Dry: Why Genetic Engineering Is Not Solving Agriculture’s Drought Problem in a Thirsty World; Union of Concerned Scientists: Cambridge, MA, USA, 2012. [Google Scholar]
- IPCC. Global Warming of 1.5 °C: Summary of Policy Makers; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018.
- Boote, K.J.; Ibrahim, A.M.H.; Lafitte, R.; McCulley, R.; Messina, C.; Murray, S.C.; Specht, J.E.; Taylor, S.; Westgate, M.E.; Glasener, K. Position statement on crop adaptation to climate change. Crop Sci. 2011, 51, 2337–2343. [Google Scholar] [CrossRef]
- Ziyomo, C.; Bernardo, R. Drought tolerance in maize: Indirect selection through secondary traits versus genomewide selection. Crop Sci. 2013, 53, 1269–1275. [Google Scholar] [CrossRef]
- Edmeades, G.O. Progress in Achieving and Delivering Drought Tolerance in Maize-an Update; ISAAA: Ithaca, NY, USA, 2013; p. 130. [Google Scholar]
- Ribaut, J.-M.; Betran, J.; Monneveux, P.; Setter, T. Drought tolerance in maize. In Handbook of Maize: Its Biology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 311–344. [Google Scholar]
- Araus, J.L.; Serret, M.D.; Edmeades, G. Phenotyping maize for adaptation to drought. Front. Physiol. 2012, 3, 305. [Google Scholar] [CrossRef]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Meeks, M.; Murray, S.C.; Hague, S.; Hays, D. Measuring Maize Seedling Drought Response in Search of Tolerant Germplasm. Agronomy 2013, 3, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Hasibuzzaman, A.S.M.; Akter, F.; Bagum, S.A.; Hossain, N.; Akter, T.; Uddin, M.S. Morpho-Physiological Mechanisms of Maize for Drought Tolerance. In Plant Stress Physiology; IntechOpen: London, UK, 2021; p. 229. [Google Scholar]
- Jenks, M.A.; Hasegawa, P.M.; Jain, S.M. Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hsiao, T.C.; Xu, L.K. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Nejad, T.S. Effect of drought stress on shoot/root ratio. World Acad. Sci. Eng. Technol. 2011, 5, 539–541. [Google Scholar]
- Khalil, A.M.; Murchie, E.H.; Mooney, S.J. Quantifying the influence of water deficit on root and shoot growth in wheat using X-ray Computed Tomography. AoB Plants 2020, 12, plaa036. [Google Scholar] [CrossRef]
- Comas, L.; Becker, S.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Shin, S.; Kim, S.G.; Lee, J.S.; Go, T.-H.; Shon, J.; Kang, S.; Lee, J.-S.; Bae, H.H.; Son, B.-Y.; Shim, K.-B.; et al. Impact of the consecutive days of visible wilting on growth and yield during tassel initiation in maize (Zea Mays L.). J. Crop Sci. Biotechnol. 2015, 18, 219–229. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Wu, X.; Wang, W. Identification of drought tolerant mechanisms in a drought-tolerant maize mutant based on physiological, biochemical and transcriptomic analyses. BMC Plant Biol. 2020, 20, 315. [Google Scholar] [CrossRef]
- Ali, F.; Ahsan, M.; Ali, Q.; Kanwal, N. Phenotypic Stability of Zea mays Grain Yield and Its Attributing Traits under Drought Stress. Front. Plant Sci. 2017, 8, 1397. [Google Scholar] [CrossRef]
- Mousavi, S.M.N.; Nagy, J. Evaluation of plant characteristics related to grain yield of FAO410 and FAO340 hybrids using regression models. Cereal Res. Commun. 2021, 49, 161–169. [Google Scholar] [CrossRef]
- Slewinski, T.L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Evci, G.; Durak, S.; Pekcan, V.; Gücer, T. Determining the relationships between yield and yield attributes in sunflower. Turk. J. Agric. For. 2007, 31, 237–244. [Google Scholar]
- Liang, X.-L.; Abu, L.-L.; Feng, G.J. Yield Performance of Maize Hybrids and Analysis of Correlation between Yield and Agronomic Characteristics. Maize Sci. 2001, 9, 1. [Google Scholar]
- Pedersen, I.F.; Christensen, J.T.; Sørensen, P.; Christensen, B.T.; Holton Rubæk, G. Early plant height: A defining factor for yields of silage maize with contrasting phosphorus supply. Soil Use Manag. 2021, 38, 537–548. [Google Scholar] [CrossRef]
- Aman, J.; Bantte, K.; Alamerew, S.; Sbhatu, D.B. Correlation and path coefficient analysis of yield and yield components of quality protein maize (Zea mays L.) hybrids at Jimma, western Ethiopia. Int. J. Agron. 2020, 2020, 6998871. [Google Scholar] [CrossRef]
- Culy, M.D. Yield loss of field corn from insects. In Biotic Stress and Yield Loss; CRC Press: Boca Raton, FL, USA, 2000; pp. 57–86. [Google Scholar]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef]
- Virla, E.G.; Araoz, M.V.C.; Albarracin, E.L. Estimation of direct damage to maize seedlings by the corn leafhopper, Dalbulus maidis (Hemiptera: Cicadellidae), under different watering regimes. Bull. Entomol. Res. 2021, 111, 438–444. [Google Scholar] [CrossRef]
- Chander, S.; Husain, M.; Pal, V. Insect Pest Management in Climate Change; Studera Press: Delhi, India, 2016. [Google Scholar]
- Konvicka, M.; Maradova, M.; Benes, J.; Fric, Z.; Kepka, P. Uphill shifts in distribution of butterflies in the Czech Republic: Effects of changing climate detected on a regional scale. Glob. Ecol. Biogeogr. 2003, 12, 403–410. [Google Scholar] [CrossRef]
- Panizzi, A.R. Suboptimal nutrition and feeding behavior of hemipterans on less preferred plant food sources. An. Soc. Entomol. Bras. 2000, 29, 1–12. [Google Scholar] [CrossRef]
- Waquil, J.M. Sampling and abundance of leafhoppers and damage by Dalbulus maidis (DeLong&Wolcott) (Homoptera: Cicadellidae) to maize seedling. An. Soc. Entomol. Bras. 1997, 26, 27–33. [Google Scholar]
- Santana Jr, P.A.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanço, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Global alterations in areas of suitability for maize production from climate change and using a mechanistic species distribution model (CLIMEX). Sci. Rep. 2017, 7, 5910. [Google Scholar] [CrossRef]
- Nault, L.R.; Delong, D.M. Evidence for co-evolution of leafhoppers in the genus Dalbulus (Cicadellidae: Homoptera) with maize and its ancestors. Ann. Entomol. Soc. Am. 1980, 73, 349–353. [Google Scholar] [CrossRef]
- Medina, R.F.; Reyna, S.M.; Bernal, J.S. Population genetic structure of a specialist leafhopper on Zea: Likely anthropogenic and ecological determinants of gene flow. Entomol. Exp. Appl. 2012, 142, 223–235. [Google Scholar] [CrossRef]
- Bellota, E.; Dávila-Flores, A.; Bernal, J.S. A Bird in the Hand Versus Two in the Bush? The Specialist Leafhopper Dalbulus maidis (Hemiptera: Cicadellidae) Does Not Discriminate Against Sub-optimal Host Plants (Zea spp.). Neotrop. Entomol. 2017, 47, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Arias, C.C.; Ligarreto-Moreno, G.A.; Ramírez-Godoy, A.; Restrepo-Díaz, H. Maize Responses Challenged by Drought, Elevated Daytime Temperature and Arthropod Herbivory Stresses: A Physiological, Biochemical and Molecular View. Front. Plant Sci. 2021, 12, 1512. [Google Scholar] [CrossRef]
- Park, Y.-S.; Bae, D.-W.; Ryu, C.-M. Aboveground whitefly infestation modulates transcriptional levels of anthocyanin biosynthesis and jasmonic acid signaling-related genes and augments the cope with drought stress of maize. PLoS ONE 2015, 10, e0143879. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Müller-Schärer, H.; Schaffner, U.; Steinger, T. Evolution in invasive plants: Implications for biological control. Trends Ecol. Evol. 2004, 19, 417–422. [Google Scholar] [CrossRef]
- Chinchilla-Ramírez, M.; Borrego, E.J.; DeWitt, T.J.; Kolomiets, M.V.; Bernal, J.S. Maize seedling morphology and defence hormone profiles, but not herbivory tolerance, were mediated by domestication and modern breeding. Ann. Appl. Biol. 2017, 170, 315–332. [Google Scholar] [CrossRef]
- White, T.C.R. A hypothesis to explain outbreaks of looper caterpillars, with special reference to populations of Selidosema suavis in a plantation of Pinus radiata in New Zealand. Oecologia 1974, 16, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.G.; Frank, S.D. Warming and drought combine to increase pest insect fitness on urban trees. PLoS ONE 2017, 12, e0173844. [Google Scholar] [CrossRef] [PubMed]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Koricheva, J.; Larsson, S.; Haukioja, E. Insect performance on experimentally stressed woody plants: A meta-analysis. Annu. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef] [Green Version]
- Huberty, A.; Denno, R. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Beetge, L.; Krüger, K. Drought and heat waves associated with climate change affect performance of the potato aphid Macrosiphum euphorbiae. Sci. Rep. 2019, 9, 3645. [Google Scholar] [CrossRef]
- Xie, H.; Shi, J.; Shi, F.; Wang, X.; Xu, H.; He, K.; Wang, Z. Aphid fecundity and defenses in wheat exposed to a combination of heat and drought stress. J. Exp. Bot. 2020, 71, 2713–2722. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef]
- Cooper, M.; Gho, C.; Leafgren, R.; Tang, T.; Messina, C. Breeding drought-tolerant maize hybrids for the US corn-belt: Discovery to product. J. Exp. Bot. 2014, 65, 6191–6204. [Google Scholar] [CrossRef]
- Sawilowsky, S.S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods 2009, 8, 26. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Ashraf, M.; Nawazish, S.; Athar, H.-U.-R. Are chlorophyll fluorescence and photosynthetic capacity potential physiological determinants of drought tolerance in maize (Zea mays L.). Pak. J. Bot. 2007, 39, 1123–1131. [Google Scholar]
- Rahbarian, R.; Khavari-Nejad, R.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietinum L.) genotypes. Acta Biol. Crac. Ser. Bot. 2011, 53, 47–56. [Google Scholar] [CrossRef]
- Jin, Z.; Xue, Q.-W.; Jessup, K.E.; Hou, X.-B.; Hao, B.-Z.; Marek, T.H.; Xu, W.-W.; Evett, S.R.; O’Shaughnessy, S.A.; Brauer, D.K. Shoot and root traits in drought tolerant maize (Zea mays L.) hybrids. J. Integr. Agric. 2018, 17, 1093–1105. [Google Scholar]
- Aslam, M.; Maqbool, M.A.; Cengiz, R. Effects of drought on maize. In Drought Stress in Maize (Zea mays L.); Springer: Berlin/Heidelberg, Germany, 2015; pp. 5–17. [Google Scholar]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Medina, V. Drought Adaptation Mechanisms Should Guide Experimental Design. Trends Plant Sci. 2016, 21, 639–647. [Google Scholar] [CrossRef]
- Kränzlein, M.; Geilfus, C.-M.; Franzisky, B.L.; Zhang, X.; Wimmer, M.A.; Zörb, C. Physiological Responses of Contrasting Maize (Zea mays L.) Hybrids to Repeated Drought. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Avramova, V.; Nagel, K.A.; AbdElgawad, H.; Bustos, D.; DuPlessis, M.; Fiorani, F.; Beemster, G.T.S. Screening for drought tolerance of maize hybrids by multi-scale analysis of root and shoot traits at the seedling stage. J. Exp. Bot. 2016, 67, 2453–2466. [Google Scholar] [CrossRef]
- Li, X.-J.; Yang, M.-F.; Chen, H.; Qu, L.-Q.; Chen, F.; Shen, S.-H. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: Proteomic evidence. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 929–940. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Bostock, R.M.; Pye, M.F.; Roubtsova, T.V. Predisposition in plant disease: Exploiting the nexus in abiotic and biotic stress perception and response. Annu. Rev. Phytopathol. 2014, 52, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dommel, M.; Mou, Z. Abscisic acid promotes proteasome-mediated degradation of the transcription coactivator NPR 1 in Arabidopsis thaliana. Plant J. 2016, 86, 20–34. [Google Scholar] [CrossRef]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A.; et al. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Ung, H.; Mosher, S.; Yoshioka, K. SA-ABA antagonism in defense responses. Plant Signal. Behav. 2010, 5, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.C.; Mayfield, K.; Pekar, J.; Brown, P.; Lorenz, A.; Isakeit, T.; Odvody, G.; Xu, W.; Betran, J. Tx741, Tx777, Tx779, Tx780, and Tx782 Inbred Maize Lines for Yield and Southern United States Stress Adaptation. J. Plant Regist. 2019, 13, 258–269. [Google Scholar] [CrossRef]
- Llorente, C.F.; Betrán, F.J.; Bockholt, A.; Fojt Iii, F. Registration of Tx772 Maize. Crop Sci. 2004, 44, 1036–1037. [Google Scholar] [CrossRef]
- Foley, T.J. Inbred Corn Line LH195. U.S. Patant 5059745A, 22 October 1991. [Google Scholar]
- Abdi, H. Bonferroni and Šidák corrections for multiple comparisons. Encycl. Meas. Stat. 2007, 3, 103–107. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).