The Potential of Parkia platycephala from Use to Cure
Abstract
1. Introduction
2. Morphological and Phytochemical Characterization of Parkia platycephala
3. Toxicity
3.1. Subacute and Systemic Toxicity
3.2. Subacute and Systemic Toxicity
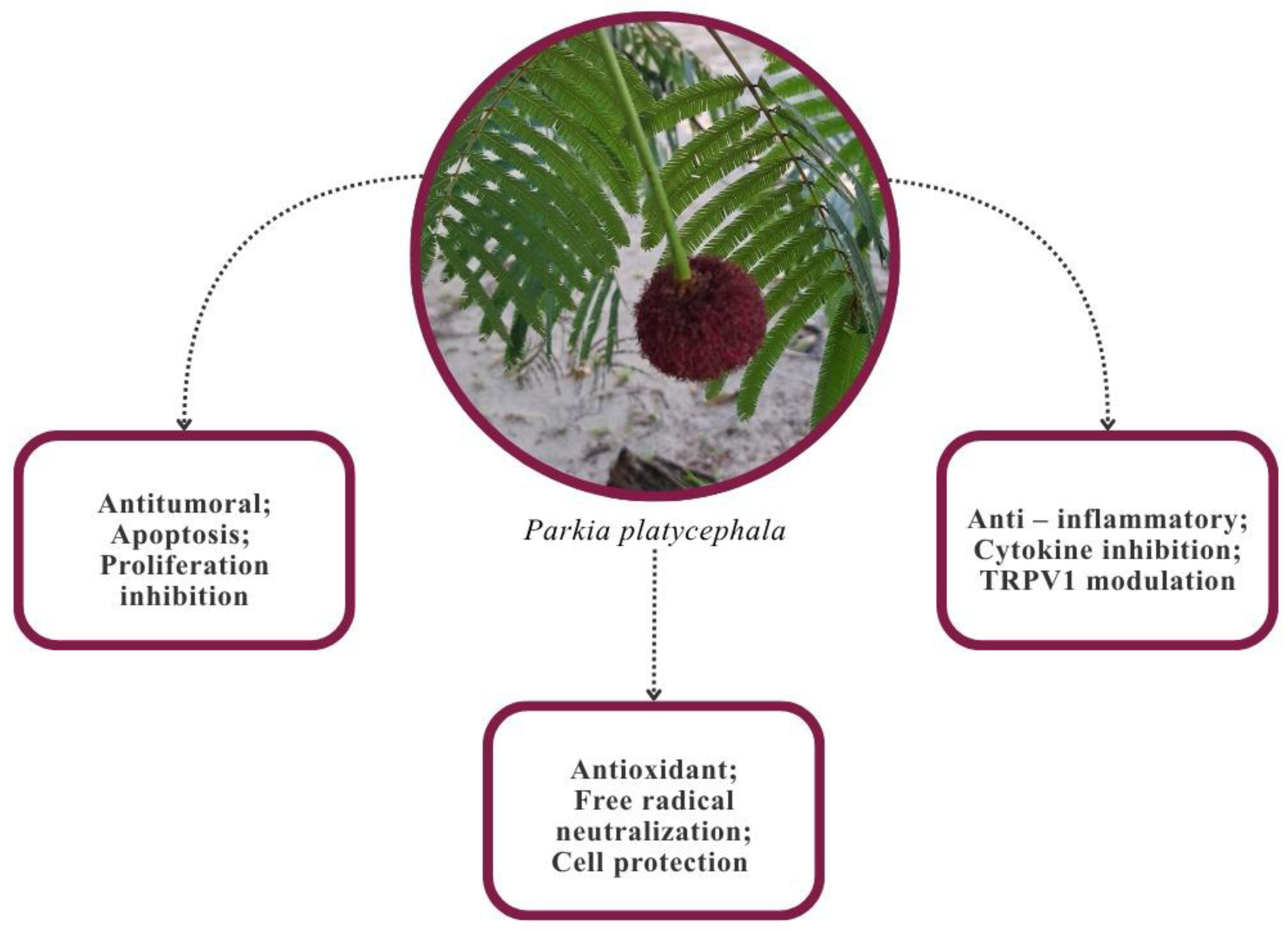
4. Biological Activities of Parkia platycephala
4.1. Antioxidant and Anti-Inflammatory Activity
4.2. Antimicrobial Activity
4.3. Antitumor Activity
5. Applications of Parkia platycephala in Animal Feed
5.1. Nutritional Potential
5.2. Application of P. platycephala Pods
5.3. Application of P. platycephala Seeds
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Saleh, M.S.M.; Jalil, J.; Zainalabidin, S.; Asmadi, A.Y.; Mustafa, N.H.; Kamisah, Y. Genus Parkia: Phytochemical, Medicinal Uses, and Pharmacological Properties. Int. J. Mol. Sci. 2021, 22, 618. [Google Scholar] [CrossRef]
- de Oliveira Silva, D.Y.B.; de Farias, S.G.G.; Dias, P.C.; e Silva, R.B.; Dias, B.A.S. Phenotypic and genotypic evaluation of Parkia platycephala families: A proposal for pre-selection. Crop Breed. Appl. Biotechnol. 2023, 23, e42792314. [Google Scholar] [CrossRef]
- Chaves, S.R.; dos Santos, R.R.; da Silva, A.L.G. Reproductive biology of Parkia platycephala Benth (Legumi-nosae, Caesalpinioideae, clado mimosoide). Braz. J. Dev. 2020, 6, 79442–79458. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Rodrigues, M.A.M.; Cardoso, C.A.L.; Alves, D.R.; Morais, S.M.; Panontin, J.F.; Scapin, E. Phytocomponents, Evaluation of Anticholinesterase Activity and Toxicity of Hydroethanolic Extracts of Parkia platy-cephala Benth. J. Braz. Chem. Soc. 2022, 33, 1414–1422. [Google Scholar]
- Fernandes, R.M.N.; Cardoso, C.A.L.; Alves, D.R.; Morais, S.M.; Scapin, E. Parkia from Cerrado: Phytochemical bioprospection, toxicity and in vitro bioactivities of bark and flower extracts. Braz. J. Biol. 2023, 83, e275733. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.C.G.; Damasceno, F.C.; Menegatti, A.C.O.; Vieira Júnior, G.M.; Chaves, M.H.; de França Ferreira, É.L. Antiradical effect and phytochemical characterisation of the leaves of Parkia platycephala Benth. Nat. Prod. Res. 2024, 39, 5403–5407. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.B.; Silva Fv BPassos, F.F.; SBezerra, R.D.; Chaves, M.H.; Oliveira, F.A.; Meneses Oliveira, R.C. Gastroprotective effect of the ethanolic extract of Parkia platycephala Benth. leaves against acute gastric lesion models in rodents. Biol. Res. 2010, 43, 451–457. [Google Scholar] [CrossRef]
- de Moura, R.; Fernandes, N. Bioprospecção Fitoquímica, Toxicidade e Atividades Antioxidante e Anticolinesterásica de Extratos da Parkia Platycephala (Benth.). Doutorado em Biodiversidade e Biotecnologia da Amazônia Legal, Programa De Pos-Graduação Em Biodiversidade E Biotecnologia-Rede Bionorte, Universidade Federal Do Tocantins, Tocantins, Brazil, 2023. [Google Scholar]
- Sá Santos, M.M.; da Silva, F.M.P.; da Silva, J.F.M.; Pimenta, R.S. Phytochemistry and antibacterial activity of aqueous and hydroalcoholic extracts of three medicinal plants against food pathogens. Acta Sci. Biol. Sci. 2018, 40, e36674. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Junior, L.M.C.; Lima, A.S.; Silva, C.R.; Ribeiro, M.N.; Mesquista, J.W.; Rocha, C.Q.; Tangerina, M.M.; Vilegas, W. Anthel-mintic activity of plant extracts from Brazilian savanna. Vet. Parasitol. 2017, 236, 121–127. [Google Scholar] [CrossRef]
- Falya, Y.; Sumiwi, S.A.; Levita, J. Mini Review: Toxicity Study of Plant Extracts. IOSR J. Pharm. Biol. Sci. 2020, 15, 25–32. [Google Scholar]
- Costa, B.A.; Sousa, A.S.; Borges, M.S.; Damasceno, D.C.; Costa-Silva, J.H. Toxicidade sistêmica e reprodutiva induzida pelo extrato etanólico de Parkia platycephala em ratas Wistar. Rev. Bras. Farmacogn. 2013, 23, 920–926. [Google Scholar] [CrossRef]
- Fernandes, R.M.N.; Rodrigues, M.A.M.; Panontin, J.F.; Alves, D.R.; Morais, S.M.; Soares, I.M.; Scapin, E. Chemical Investigation, Toxic Potential and Acetylcholinesterase Inhibitory Effect of Parkia platycephala Leaf and Seed Extracts. J. Med. Plants Res. 2021, 15, 401–412. [Google Scholar] [CrossRef]
- Dangnon, B.; Dah-Nouvlessounon, D.; Hoteyi, S.M.I.; Sina, H.; Tomescu, J.A.; Akpo, K.J.-M.; Sangare-Oumar, M.M.; Adjanohoun, A.; Babalola, O.O.; Vamanu, E.; et al. Gastroprotective, Antioxidant, Anti-Inflammatory, and Toxicological Evaluation of Stem Bark Extracts of Vitellaria paradoxa and Parkia biglobosa. Pharmaceuticals 2025, 18, 1184. [Google Scholar] [CrossRef]
- Mann, K.; Farias, C.M.S.A.; del Sol, F.G.; Santos, C.F.; Grangeiro, T.B.; Nagano, C.S.; Cavada, B.S.; Calvete, J.J. The amino-acid sequence of the glucose/mannose-specific lectin isolated from Parkia platycephala seeds reveals three tandemly arranged jacalin-related domains. Eur. J. Biochem. 2001, 268, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.U.; Santiago, M.Q.; Osterne, V.J.S.; Pinto-Junior, V.R.; Pereira, L.P.; Silva-Filho, J.C.; Debray, H.; Rocha, B.A.M.; Delatorre, P.; Teixeira, C.S.; et al. Lectins from Parkia biglobosa and Parkia platycephala: A comparative study of structure and biological effects. Int. J. Biol. Macromol. 2016, 92, 194–201. [Google Scholar] [CrossRef]
- Souza, M.A.; Carvalho, F.C.; Ruas, L.P.; Ricci-Azevedo, R.; Roque-Barreira, M.C. The immunomodulatory effect of plant lectins: A review with emphasis on ArtinM properties. Glycoconj. J. 2013, 30, 641–657. [Google Scholar] [CrossRef]
- Cavada, B.S.; Osterne, V.J.S.; Oliveira, M.V.; Pinto-Junior, V.R.; Silva, M.T.L.; Bari, A.U.; Lima, L.D.; Lossio, C.F.; Nascimento, K.S. Reviewing Mimosoideae lectins: A group of under explored legume lectins. Int. J. Biol. Macromol. 2020, 154, 159–165. [Google Scholar] [CrossRef]
- Aladesanmi, A.J. Tetrapleura tetraptera: Molluscicidal activity and chemical constituents. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 23–36. [Google Scholar] [CrossRef]
- Batista Gomes, P.G.; Raposo Santos, M.; de Araújo, G.G.L.; Parente, H.N.; de Oliveira Maia Parente, M.; de Moura Zanine, A.; de Jesus Ferreira, D.; Santos, E.M.; Gois, G.C.; de Sousa Santos, F.N.; et al. Parkia platycephala Replacing Ground Corn in the Diet of Confined Lambs: Intake, Digestibility, Ingestive Behaviour, Rumen Fermentation and Carcass Yield. Arch. Anim. Nutr. 2024, 78, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.H.E.; Osman MEfadil, M. Plant lectin: A promising future anti-tumor drug. Biochimie 2022, 202, 136–145. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Silva, R.R.S.; Silva, C.R.; Santos, V.F.; Barbosa, C.R.S.; Muniz, D.F.; Santos, A.L.E.; Santos, M.H.C.; Rocha, B.A.M.; Batista, K.L.R.; Costa-Júnior, L.M.; et al. Parkia platycephala lectin enhances the antibiotic activity against multi-resistant bacterial strains and inhibits the development of Haemonchus contortus. Microb. Pathog. 2019, 135, 103629. [Google Scholar] [CrossRef]
- Carvalho, A.F.U.; Farias, D.F.; da Rocha-Bezerra, L.C.B.; de Sousa, N.M.; Cavalheiro, M.G.; Fernandes, G.S.; Brasil, I.C.F.; Maia, A.A.B.; de Sousa, D.O.B.; Vasconcelos, I.M.; et al. Preliminary Assessment of the Nutritional Composition of Underexploited Wild Legumes from Semi-Arid Caatinga and Moist Forest Environments of Northeastern Brazil. J. Food Compos. Anal. 2011, 24, 487–493. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74. [Google Scholar] [CrossRef]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with Potential for Anti-Cancer Therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.L.; Zhang, S.; Tian, M.; Zhang, S.Y.; Xie, T.; Chen, D.Y.; Chen, Y.J.; He, J.; Liu, J.; Ouyang, L.; et al. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015, 48, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Bailon-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.C.; Orellana, M.I.R. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jae, I.J.; Han, J.C.; Do, Y.L.; Hyun, S.L.; Hyang, S.C.; Dae, Y.K.; Park, J.H.Y. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J. Nutr. 2005, 135, 2884–2890. [Google Scholar] [CrossRef]
- Pasban-Aliabadi, H.; Sobhani, V.; Esmaeili-Mahani, S.; Najafipour, H.; Askari, A.; Jalalian, H. Effects of Baneh (Pistacia atlantica) Gum on Human Breast Cancer Cell Line (MCF-7) and Its Interaction with Anticancer Drug Doxorubicin. Iran. J. Pharm. Res. 2019, 18, 1959–1966. [Google Scholar]
- Silva, J.H.O.; Monteiro, V.F.C.; Moura, P.H.A. Diversidade de plantas no Cerrado brasileiro: Um enfoque em Parkia platycephala. In Ciências Botânicas; Monteiro, V.F.C., Moura, P.H.A., Eds.; Atena Editora: Ponta Grossa, Brazil, 2021; pp. 79–94. [Google Scholar]
- Alves, A.A.; Sales, R.; Neiva, J.; Medeiros, A.; Braga, A.; Azevedo, A. Degradabilidade ruminal in situ de vagens de faveira (Parkia platycephala Benth.) em diferentes tamanhos de partículas. Arq. Bras. Med. Vet. Zootec. 2007, 59, 1045–1051. [Google Scholar] [CrossRef]
- Araújo, A.R.; Rodriguez, N.M.; Rogério, M.C.P.; Borges, I.; Saliba, E.O.S.; Santos, S.A.; Pompeu, R.C.F.F.; Fernandes, F.E.P.; Monteiro, J.P.; Muir, J.P. Nutritional Evaluation and Productivity of Supplemented Sheep Grazing in Semiarid Rangeland of Northeastern Brazil. Trop. Anim. Health Prod. 2019, 51, 957–966. [Google Scholar] [CrossRef]
- Vilela, G.; Pinheiro, G.; Castro, L.; Santos, F.; Oliveira, J.; Parente, M.; Parente, M.; Costa, J.; Barbosa, S.; Silva, S. Pontencial Nutricional Da Parkia Platycephala Na Qualidade Da Carne E Características De Carcaça De Pequenos Ruminantes. In Agropecuária e Meio Ambiente: Uma Visão Integrada; Cardoso, A., Borges, S., Silva, S., Andrade, F., Eds.; Editora Científica Digital: Guarujá, Brazil, 2025. [Google Scholar] [CrossRef]
- Batista, I.L.; Araújo, M.J.; Marques, C.A.T.; Carvalho, F.J.V.; Jácome, D.D.S.; Oliveira, R.L. Effects of Parkia platycephala on Feeding Behavior, Rumen Health, Blood Markers, and Physiological Responses of Lactating Goats. Rev. Bras. Zootec. 2020, 49, e20200096. [Google Scholar] [CrossRef]
- da Silva, L.R.F.; Alves, A.A.; Vasconcelos, V.R.; Santos do Nascimento, H.T.; Filho, M.A.M. Valor Nutritivo da Vagem de Faveira (Parkia platycephala Benth.) para Ruminantes. Rev. Bras. De Zootec. 1999, 41, 1065–1069. [Google Scholar] [CrossRef]
- Costa, L.A.; Araújo, M.J.; Edvan, R.L.; Bezerra, L.R.; Sousa, A.R.; Viana, F.J.C.; Dias-Silva, T.P. Chemical Composition, Fermentative Characteristics, and In Situ Ruminal Degradability of Elephant Grass Silage Containing Parkia platycephala Pod Meal and Urea. Trop. Anim. Health Prod. 2020, 52, 3481–3492. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Teixeira, C.S.; Pretto, A.; Costa, T.S.; de Siqueira, J.C.; Pantoja, B.T.S.; Baldisserotto, B.; Lopes, J.M. Antinutritional Effect of Lectin from Faveira (Parkia platycephala) Seeds in Tambatinga (Colossoma macropomum × Piaractus brachypomus). Bol. Inst. Pesca 2022, 48, e745. [Google Scholar] [CrossRef]
- Silva, L.R.F.; Alves, A.A.; Vasconcelos, V.R.; do Nascimento, H.T.S.; Filho, M.A.M. Nutritive Value of Diets Containing Pods of Faveira (Parkia platycephala Benth.) for Confined Finishing Sheep. Rev. Bras. Zootec. 2012, 41, 1065–1069. [Google Scholar] [CrossRef]
- Lima, J.R.L.; Rodrigues, R.C.; Sousa, G.O.C.; de Jesus, A.P.R.; da Costa, A.C.; Fonseca, A.S.R.; Cabral, L.d.S.; Costa, C.d.S.; de Oliveira, J.S.; da Silva, E.C. Performance of Grazing Sheep Kept on Tamani Grass Supplemented with Parkia platycephala Pods Replacing Corn in Multiple Supplements. N. Z. J. Agric. Res. 2025, 68, 515–531. [Google Scholar] [CrossRef]
- Sousa, G.O.C.; Rodrigues, R.C.; Lima, J.R.L.; de Jesus, A.P.R.; da Costa, A.C.; Andrade, A.C.; Rodrigues, M.M.; da Silva, E.C.; Santos, A.M.d.P.; Oliveira, P.L.R.; et al. Parkia platycephala in Multiple Supplements for Sheep Grazing on Tamani Grass Pastures: Effects on Pasture Productivity, Structural Characteristics, and Ingestive Behaviour. N. Z. J. Agric. Res. 2025, 68, 1901–1914. [Google Scholar] [CrossRef]
- Porto, D.L.; de Santana Arauco, A.M.; Boechat, C.L.; de Oliveira Silva, A.; Moitinho, M.R.; de Farias, S.G.G. Arbuscular Mycorrhizal Fungi on the Initial Growth and Nutrition of Parkia platycephala Benth. under Water Stress. Cerne 2020, 26, 66–74. [Google Scholar] [CrossRef]
| Plant Part | Compounds | Biological Activity | Mechanism of Action | References |
|---|---|---|---|---|
| Leaf | Flavonoids, steroids, phenolic acids (gallic, ellagic, ferulic) | Antioxidant, anti-inflammatory anthelmintic | Significant antioxidant proprieties; ROS inhibition | [11,13,17] |
| Bark | Gallic acid, steroids ferulic acid, flavonoids | Antioxidant, antimicrobial | Lipid peroxide degradation; cell wall disintegration | [11,12] |
| Fruit (pod) | Phenolic compounds (tannins) | Antioxidant | Degradation of free radicals; preservation of cellular integrity | [8] |
| Seed | Lectin (PPL, PPL-2) | Anti-inflammatory, antimicrobial, antitumoral | TRPV1 signaling; cell cycle interruption; enhanced antibiotic action | [11,17] |
| Flower | Alkaloids, ellagic acid, phenolics | Antioxidant, antimicrobial | ROS scavenging; existence of endophytic fungi that actively combatting pathogens; significant antioxidant properties | [11,12,15] |
| Plant Part | Extract Type | Observed Toxicity | References |
|---|---|---|---|
| Leaves | Ethanolic | Moderate subacute and systemic toxicity in rats (250–1000 mg/kg, 30 days) | [19] |
| Leaves | Ethanolic | No acute toxicity (2000 mg/kg, oral); no cytotoxicity in erythrocytes | [19] |
| Leaves | Hydroethanolic (70%) | Low toxicity (IC50 between 500 and 1000 µg/mL in Artemia salina) | [15] |
| Flowers | Hydroethanolic (70%) | Low toxicity (IC50 between 500 and 1000 µg/mL in Artemia salina) | [15] |
| Bark | Hydroethanolic (70%) | Moderate toxicity (IC50 between 100 and 500 µg/mL in Artemia salina) | [15] |
| Seeds | Hydroethanolic (70%) | Non-toxic (IC50 > 1000 µg/mL in Artemia salina) | [15] |
| Seeds | Hexane | Non-toxic (IC50 > 4000 µg/mL in Artemia salina) | [20] |
| Leaves | Hexane | Non-toxic (IC50 > 4000 µg/mL in Artemia salina) | [20] |
| Leaves | Ethanolic | No apparent toxicity (2 g/kg, oral in mice, 72 h) | [11] |
| Plant Part | Isolated Compound | Activity (Cellular/Enzymatic Model) | Mechanism of Action | Reference |
|---|---|---|---|---|
| Leaf | Phenolic acids (gallic, ellagic, ferulic); flavonoids | Antioxidant in vitro (immune cells: macrophages) | Suppression of ROS; neutralization of free radicals; reduction in oxidative stress | [15] |
| Bark | Flavonoids; ferulic acid; gallic acid; steroids | Antioxidant (enzymatic model: lipid peroxide reduction) | Suppression of ROS; radical scavenging; delay of biomolecule oxidation | [12] |
| Fruit | Phenolic compounds (tannins); | Antioxidant (chemical and cellular assays) | Free radical scavenging; inhibition of lipid and protein oxidation; protection of cellular and tissue integrity | [12] |
| Seed | PPL; | Anti-inflammatory and antinociceptive (in vivo/in vitro models) | Modulation of pro-inflammatory cytokines; COX-2 inhibition; inhibition of TRPV1 channel activation | [15] |
| Flowers | Octadecenamide; phenolic compounds; steroids; alkaloids; ellagic acid | Antioxidant (endothelial cells) | Electron donation; free radical scavenging; stabilization of ROS | [12] |
| Plant Part | Extract/Fraction/ Isolated Compound Class | Activity (Cellular/Enzymatic Model) | Mechanism of Action | Reference |
|---|---|---|---|---|
| Leaf | Aqueous extract: tannins, saponins, and sesquiterpenes | Inhibitory activity against resistant S. aureus, B. subtilis, and S. choleraesuis | Membrane disruption; inhibition of cell wall transpeptidases | [23] |
| Bark | Phenolic acids (tannins, saponins); flavonoids | Activity against microbial development | Modulation of microbial metabolism; metal ion binding; inhibition of metabolic processes | [16] |
| Flower | ND | Inhibition of bacterial growth | Interference with cell wall; disruption of protein synthesis and cytoplasmic membrane integrity | [31] |
| Seed | PPL | Synergism with gentamicin (S. aureus, E. coli) | Specific binding to membrane/cell wall monosaccharides; disruption of membrane integrity; inhibition of bacterial adhesion processes; inhibition of bacterial cell proliferation | [31] |
| Fruit | Bioactive extracts; metabolites | ND | ND | ND |
| Plant Part | Isolated Compound | Activity (Cellular/Enzymatic Model) | Mechanism of Action | Reference |
|---|---|---|---|---|
| Leaf | Lectins (glycoproteins, proteins) | Autophagy/apoptosis in lung cancer (A549), liver cancer, and colorectal cancer | Recognition of altered glycan structures on cancer cell surfaces; modulation of cell proliferation and death; synergistic interaction with therapeutic agents | [20] |
| Seed | PPL | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, J.; Medeiros, R.; Melonio, M.C.; da Rocha, C.Q.; Cerqueira, F. The Potential of Parkia platycephala from Use to Cure. Compounds 2025, 5, 47. https://doi.org/10.3390/compounds5040047
Nunes J, Medeiros R, Melonio MC, da Rocha CQ, Cerqueira F. The Potential of Parkia platycephala from Use to Cure. Compounds. 2025; 5(4):47. https://doi.org/10.3390/compounds5040047
Chicago/Turabian StyleNunes, Joana, Rui Medeiros, Matheus Chagas Melonio, Cláudia Quintino da Rocha, and Fátima Cerqueira. 2025. "The Potential of Parkia platycephala from Use to Cure" Compounds 5, no. 4: 47. https://doi.org/10.3390/compounds5040047
APA StyleNunes, J., Medeiros, R., Melonio, M. C., da Rocha, C. Q., & Cerqueira, F. (2025). The Potential of Parkia platycephala from Use to Cure. Compounds, 5(4), 47. https://doi.org/10.3390/compounds5040047








