Abstract
Aged green tea leaves, particularly from Shan Tuyet trees, represent an underutilized source of catechins—key antioxidant compounds with known health benefits. This study aims to optimize and compare three green extraction methods—Hot Water Extraction (HWE), Ultrasound-Assisted Extraction (UAE), and Ethanol–Water Extraction (EthE)—for catechin recovery from mature tea leaves. A Box–Behnken design (BBD) under Response Surface Methodology (RSM) was used to evaluate the effects of different extraction conditions. Total catechin content was quantified by HPLC, and antioxidant activities were measured using DPPH, FRAP, ORAC, and cellular antioxidant activity (CAA) assays. Results showed that while UAE and HWE produced total catechin yields of 206.0 mg/g and 202.0 mg/g, respectively, their biological efficacy was profoundly different. HWE, operating at a higher temperature (82 °C), induced significant thermal degradation, evidenced by high levels of catechin epimerization (EGCG/GCG ratio = 3.62) and hydrolysis. This loss of structural integrity resulted in the lowest cellular antioxidant activity (CAA) of 98.3 µmol QE/g. In contrast, the optimized UAE process (78 °C, 55 min, 290 W) preserved catechin stereochemistry (EGCG/GCG ratio = 9.86), yielding the highest CAA (185.2 µmol QE/g). These findings demonstrate that UAE acts as the optimal green strategy for producing high-yield, functionally superior extracts from mature tea leaves.
1. Introduction
Tea (Camellia sinensis) from ancient Shan Tuyet trees holds significant cultural and economic value in Vietnam, especially for ethnic minority communities in high-altitude regions [1,2]. Renowned for its exceptional aroma, robust taste, and health benefits, Shan Tuyet tea is both a cultural icon and an economic driver in organic and specialty markets [3,4]. For premium Shan Tuyet tea, young leaves (with high EGCG content) are selected, whereas mature leaves (with lower EGCG but still rich in bioactives) are often discarded. Mature tea leaves may have a total catechin content of ~200 mg/g, but the composition differs from young leaves. Young leaves typically contain EGCG (≈50–70% of catechins), EGC (≈12–23%), and EC (≈5–9%) [5,6,7]. As tea leaves mature, enzymatic activity (e.g., polyphenol oxidase) declines, resulting in lower EGCG and higher proportions of non-gallated catechins (e.g., EGC and EC) [5]. Consequently, although EGCG levels drop, EGC and EC levels in older leaves often remain around 50–60 mg/g [8,9]. This composition shift highlights the underappreciated potential of aged leaves as a valuable source of polyphenols and catechins, providing impetus for their efficient valorization.
Catechins are the major polyphenolic constituents in green tea and include EGCG, EGC, EC, and ECG [10]. These compounds are well-known for their potent antioxidant, anti-inflammatory, antimicrobial, and anticancer activities, making them valuable ingredients in the functional food, nutraceutical, and cosmetic industries [6]. While young tea leaves are typically richer in EGCG, previous studies have shown that mature leaves may contain relatively higher concentrations of EGC and EC [11]. This variation in chemical composition suggests that aged green tea leaves, though often overlooked, could serve as an alternative and complementary source of catechins—particularly if appropriate extraction methods are applied to recover these compounds efficiently.
Conventional catechin extraction often relies on organic solvents such as methanol, acetone, or chloroform [12]. These solvents, while effective in solubilizing a broad range of phytochemicals, pose serious concerns in terms of toxicity, flammability, environmental persistence, and regulatory constraints for use in food or pharmaceutical products [13]. In recent years, there has been a shift toward environmentally friendly processing technologies, aligning with the principles of green chemistry and the circular bioeconomy. Among the most promising approaches are Hot Water Extraction (HWE), Ultrasound-Assisted Extraction (UAE), and Ethanol–Water Extraction (EthE), which utilize non-toxic solvents and energy-efficient techniques [13,14,15]. HWE is one of the simplest and most traditional methods, using elevated temperatures to enhance mass transfer and solubilization of polar compounds. Although HWE is cost-effective and food-grade safe, prolonged heating can degrade sensitive catechins like EGCG [16]. By contrast, UAE uses acoustic cavitation to disrupt plant cell walls, increasing solvent penetration and improving extraction efficiency with lower thermal input [17]. This method has been shown to significantly increase catechin yield from various tea sources while reducing extraction time and solvent consumption [18]. Ethanol–water mixtures are another green solvent system widely used for catechin extraction due to their tunable polarity, low toxicity, and GRAS (Generally Recognized as Safe) status [19,20]. Studies have shown that ethanol concentrations of 30–70% (v/v) are particularly effective for selectively extracting catechins while preserving their stability [21]. These green technologies provide an attractive alternative for both laboratory-scale and industrial-scale applications, especially when dealing with lower-grade biomass such as mature tea leaves.
Despite the growing interest in green extraction techniques, several critical scientific gaps and methodological limitations remain in current research on catechin extraction from tea leaves. First, many investigations still use the one-factor-at-a-time (OFAT) approach, which does not account for interactions between variables and often leads to suboptimal results. In contrast, Response Surface Methodology (RSM) has been shown to be more effective in optimizing multiple factors simultaneously, as demonstrated by Vuong et al. in catechin extraction from green tea [21]. Second, most studies focus primarily on extraction yield, overlooking critical issues like catechin degradation and epimerization under heat or prolonged extraction time [22]. It is well-documented that catechins, particularly EGCG, are unstable under common experimental conditions, with degradation rates highly dependent on temperature, pH, and oxygen levels [23]. This can lead to inaccurate evaluations of extract quality and biological potential. Furthermore, the antioxidant activity of extracts is often assessed using a single chemical assay (e.g., DPPH or ABTS), which does not fully reflect biological activity in living cells [24]. Recent research suggests the need for a more comprehensive assessment, combining chemical and cell-based assays for more accurate antioxidant evaluation [25]. For example, Athirojthanakij et al. (2024) optimized catechin extraction from green tea waste using HWE, UAE, and EthE, finding that hot water yielded the highest catechin content and DPPH activity [26]. However, that study did not examine cellular antioxidant activity. This study builds on the literature by including a cell-based antioxidant assay (CAA) and focusing on aged Shan Tuyet leaves, aiming to clarify how extraction conditions and catechin structure influence bioactivity.
To address these challenges, the present study aims to develop a green and efficient strategy for extracting catechins from aged Shan Tuyet green tea leaves. The specific objectives of the research are threefold: (1) to apply multivariate optimization using RSM to three green extraction techniques (HWE, UAE, and EthE) to determine optimal catechin yield and process conditions; (2) to perform a detailed comparative analysis of the optimized extracts in terms of total catechin content, individual compound composition, and structural stability; and (3) to evaluate and compare the antioxidant activities of the extracts using both chemical assays and cell-based models, taking into account potential changes in chemical structure and the influence of extraction parameters.
2. Materials and Methods
2.1. Materials
2.1.1. Shan Tuyet Preparation
Mature Shan Tuyet green tea leaves were collected from ancient tea trees located in the high-altitude region of Tan Tien ward, Tuyen Quang province, Vietnam (22.7298° N, 104.7813° E; WGS84). Only fully developed, aged leaves not typically used for premium tea production were selected to ensure uniformity of the starting material. The leaves were thoroughly washed to remove dust and surface contaminants, then subjected to a thermal stabilization treatment via oven-drying at 60 °C for 12 h in a Memmert UF55 forced-air oven (Memmert GmbH, Schwabach, Germany). This protocol was intended to minimize enzymatic degradation by polyphenol oxidase (PPO) and peroxidase during storage and extraction. However, this step constitutes a significant thermal treatment that invariably alters the native phytochemical profile. It is well-established that oven-drying can lead to a significant loss of total phenolic content compared to non-thermal methods like freeze-drying. While the selected temperature is below the threshold for rapid catechin epimerization, prolonged exposure may still induce other forms of thermal degradation. No direct measurement of PPO activity was performed to confirm complete enzyme inactivation. Consequently, the starting material for all subsequent experiments must be understood as a thermally stabilized powder, and the results of this study reflect the impact of extraction methods on this pre-processed material, not on pristine, fresh leaves. This represents a key limitation of the study design. After drying, the leaves were ground using a mechanical grinder and sieved to obtain a uniform powder passing #18 (1.0 mm) and retained on #35 (0.5 mm). The powdered tea was stored in airtight containers at room temperature in the dark until further use.
2.1.2. Chemicals
High-purity analytical standards of eight catechins—including the four native isomers (−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epicatechin (EC), as well as their corresponding epimers (−)-gallocatechin gallate (GCG), (−)-gallocatechin (GC), (−)-catechin gallate (CG), and (+)-catechin (C)—were obtained from Sigma-Aldrich (St. Louis, MO, USA), each with ≥98% purity. Gallic acid was used as a degradation marker. HPLC-grade solvents, including acetonitrile and formic acid, were sourced from Merck (Darmstadt, Germany). Deionized water was prepared using a Milli-Q system (Merck KGaA, Darmstadt, Germany). All other reagents used in antioxidant and cell-based assays were of analytical grade.
2.2. Methods
2.2.1. Catechin Extraction
Hot Water Extraction (HWE). Water was used as solvent. For each run, 5.00 g of tea powder was combined with preheated water at the required 20:1–50:1 mL/g in a jacketed glass vessel with magnetic stirring (300 rpm) on an RCT basic magnetic stirrer (IKA-Werke GmbH & Co. KG, Staufen, Germany). A circulating water bath was set to the target 60–90 °C and verified by an in—vessel thermocouple (±1 °C). The mixture was extracted for 10–60 min, then immediately cooled on ice and filtered.
Ultrasound—Assisted Extraction (UAE). Water was used as solvent. A Qsonica Q700 probe sonicator, 20 kHz, 700 W (Qsonica LLC, Newtown, CT, USA) fitted with a 12.7—mm titanium probe was used. Slurries (5.00 g powder; 20:1–50:1 mL/g) were processed at 60–90 °C for 10–60 min while applying ultrasonic power within 100–300 W (calibrated at study start). To avoid overheating, sonication was applied in pulse mode (2 s on/2 s off) with the vessel jacketed in a thermostated bath (±1 °C) and the probe depth kept at 2 cm. Gentle magnetic stirring (300 rpm) ensured uniform cavitation. After sonication, samples were cooled and filtered.
Ethanol–Water Extraction (EthE). Ethanol (96% v/v) was diluted with deionized water to give the required 30–90% v/v. Tea powder (5.00 g) was mixed with solvent at a fixed solvent—to–solid ratio of 35:1 mL/g in a jacketed glass vessel with magnetic stirring (300 rpm). Extractions were conducted at 60–90 °C for 10–60 min. The mixtures were cooled and filtered; ethanol was recovered during rotary evaporation and recycled for subsequent runs where applicable.
After extraction, slurries were immediately cooled, filtered (Whatman No. 1, Cytiva, Marlborough, MA, USA), and the filtrates were concentrated under reduced pressure on a Büchi Rotavapor R-300 (BÜCHI Labortechnik AG, Flawil, Switzerland). Crude extracts were dried to constant mass, reconstituted/diluted as needed, and stored at −20 °C until analysis. All extractions and analytical assays were conducted in quintuplicate (n = 5) to ensure reproducibility, unless otherwise noted.
To optimize catechin extraction efficiency, Response Surface Methodology (RSM) coupled with a Box–Behnken Design (BBD) was applied for the three green extraction methods. The experimental designs were tailored to the specific variables of each method to ensure statistical robustness. For all models, the primary response variable for optimization was the total catechin yield (Y1, mg/g dry material). The antioxidant activities of the extracts produced under the final optimized conditions were subsequently evaluated in a comparative analysis to determine the most effective overall process, rather than being used as a direct response variable in the optimization models.
For Hot Water Extraction (HWE), three independent variables were investigated: temperature (X1: 60–90 °C), extraction time (X2: 10–60 min), and solvent-to-solid ratio (X3: 20:1–50:1 mL/g). A standard 15-run BBD, appropriate for three factors, was employed to model the process.
For the more complex Ultrasound-Assisted Extraction (UAE) process, four independent variables were investigated: temperature (X1: 60–90 °C), extraction time (X2: 10–60 min), solvent-to-solid ratio (X3: 20:1–50:1 mL/g), and ultrasonic power (X4: 100–300 W). To accurately model all linear, quadratic, and two-factor interaction effects, a full 27-run BBD, which is the standard requirement for a four-factor design, was conducted.
For Ethanol–Water Extraction (EthE), three independent variables were investigated: extraction temperature (X1: 60–90 °C), extraction time (X2: 10–60 min), and ethanol concentration (X3: 30–90%, v/v). A standard 15-run BBD was utilized. The solvent-to-solid ratio was held constant at 35:1 mL/g for all EthE experiments. This decision was based on preliminary single-factor experiments, which showed that catechin yield plateaued for solvent-to-solid ratios above 30:1 mL/g. This finding is consistent with literature indicating that mass transfer becomes independent of solvent volume in this range for similar plant matrices [7,11,12]. Fixing this parameter allowed the model to focus on the more sensitive and interactive effects of temperature, time, and ethanol concentration, which are the defining parameters for this selective extraction method.
For response surface modeling, coded variables x_i (level −1, 0, +1) were used, and the second-order polynomial took the form:
where Y is the predicted response (e.g., total catechins, purity, CAA), k is the number of factors, β0 the intercept, βi the linear, βii the quadratic, and βij the interaction coefficients. For HWE and EthE, k = 3 with x1 = temperature (°C), x2 = time (min), x3 = solvent-to-solid ratio (mL/g). For UAE, k = 4 with x1 = temperature (°C), x2 = time (min), x3 = solvent-to-solid ratio (mL/g), x4 = ultrasonic power (W). Coded variables were obtained as xi = (Xi − X0,i)/ΔXi. Model adequacy (ANOVA, lack-of-fit, R2, and coefficient significance were evaluated at p < 0.05.
2.2.2. High-Performance Liquid Chromatography (HPLC)
Quantitative analysis of catechins was performed using a validated HPLC method on an Agilent 1260 Infinity II system (Agilent Technologies, Santa Clara, CA, USA). Separation was achieved on a C18 reversed-phase column (250 mm × 4.6 mm, 5 µm) maintained at 30 °C. The mobile phase consisted of 0.1% formic acid in water (Solvent A) and acetonitrile (Solvent B) at a flow rate of 1.0 mL/min. The gradient elution program was as follows: 0–5 min, 10% B; 5–20 min, linear gradient from 10% to 35% B; 20–25 min, isocratic at 35% B; followed by a 5 min re-equilibration period with 10% B. The injection volume was 10 µL, and detection was performed at a wavelength of 280 nm.
The method was validated for its ability to resolve all eight target compounds. Baseline separation was achieved for the four native isomers (EGCG, EGC, ECG, and EC) and their corresponding epimers (GCG, GC, CG, and (+)-C). Compound identification was confirmed by comparing the retention times of peaks in the sample extracts with those of pure analytical standards run under identical conditions. Calibration curves for each catechin showed excellent linearity (R2 > 0.999), and the method’s precision was high (intra-day %RSD < 2%).
The High-Performance Liquid Chromatography (HPLC) method was validated according to the International Council for Harmonisation (ICH) guidelines for linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy [27]. Linearity was assessed by constructing calibration curves with at least six concentration levels for each of the eight catechin standards [28]. Precision was determined by analyzing replicate injections of a standard mixture at three concentration levels on the same day (intra-day) and on three different days (inter-day), with results expressed as relative standard deviation (%RSD). Accuracy was evaluated through recovery experiments by spiking a known number of standards into a pre-analyzed tea extract matrix [29]. The validation parameters, summarized in Table 1, confirm that the method is linear, sensitive, precise, and accurate for the quantification of all target analytes.

Table 1.
HPLC Method Validation Parameters.
2.2.3. Definition of Yield and Purity
Extraction yield (mg/g dry leaves) was calculated from the mass of the dried solid extract after solvent removal. Catechin purity (%) was defined as the mass fraction of all eight quantified catechin isomers in the dried extract, as described in Equation (2).
where Ci are HPLC-quantified masses of EGCG, EGC, ECG, EC and their epimers (GCG, GC, CG, (+)-C).
2.2.4. Antioxidant Activity Test
Antioxidant activity of the extracts was assessed using three chemical assays. The DPPH assay was used to evaluate the hydrogen/electron donating ability of the tea extracts, according to Blois (1958) with minor modifications [30]. A 0.1 mM solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) in methanol was freshly prepared. In a 96-well plate, 100 µL of DPPH solution was mixed with 100 µL of sample extract at various concentrations. The mixture was incubated in the dark at ambient temperature for 30 min, after which the absorbance was measured at 517 nm using a BioTek Synergy H1 multimode microplate reader (Agilent BioTek, Winooski, VT, USA). The antioxidant activity was expressed as % inhibition of the DPPH radical, calculated using the formula:
where Acontrol is the absorbance of the DPPH solution without extract and Asample is the absorbance of the DPPH solution containing extract. Absorbance was read at 517 nm after incubation in the dark for 30 min at ambient temperature. IC50 (µg/mL) values were obtained by fitting a four-parameter logistic model to % inhibition versus concentration.
The FRAP assay measures the ability of antioxidants to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) under acidic conditions [31]. The FRAP reagent was freshly prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) in 40 mM HCl, and 20 mM FeCl3·6H2O in a ratio of 10:1:1 (v/v/v). A 200 µL aliquot of FRAP reagent was added to 20 µL of sample or standard in a 96-well plate and incubated at 37 °C for 30 min. Absorbance was measured at 593 nm on a BioTek Synergy H1 multimode microplate reader (USA). Results were expressed as µmol Trolox equivalents (TE)/g extract using a calibration curve prepared with Trolox standard solutions.
The ORAC assay quantifies the antioxidant capacity of samples based on their ability to inhibit the oxidative degradation of fluorescein by peroxyl radicals generated from AAPH (2,2′-azobis(2-amidinopropane) dihydrochloride) [32]. In a 96-well black microplate, each well contained 25 µL of sample, 150 µL of 80 nM fluorescein, and 25 µL of 150 mM AAPH. The mixture was incubated at 37 °C, and fluorescence was recorded every minute for 60–90 min (excitation: 485 nm, emission: 535 nm) using a fluorescence microplate reader BioTek Synergy H1 (Agilent BioTek, Winooski, VT, USA). Antioxidant capacity was calculated as the area under the fluorescence decay curve (AUC) compared to the blank. Results were expressed as µmol Trolox equivalents (TE)/g extract.
2.2.5. Cellular Antioxidant Activity (CAA) Under H2O2-Induced Oxidative Stress
Cellular antioxidant activity was quantified in Caco-2 cells (ATCC, Manassas, VA, USA), a widely used human intestinal epithelial model that forms polarized monolayers, following Wolfe & Liu (2007) [33]. Cells were seeded at 1.0 × 104 cells per well in black, clear-bottom 96-well plates (Corning Incorporated, Corning, NY, USA) and maintained at 37 °C, 5% CO2 in complete medium until confluence. To evaluate dose-dependent responses, tea extracts (HWE, UAE, EthE) were freshly prepared in serum-free Hank’s balanced salt solution (HBSS) and applied at 0–200 µg/mL for 1 h; vehicle was ≤0.5% (v/v). Quercetin (0–25 µM) served as the reference antioxidant and was processed in parallel.
After pretreatment, wells were washed twice with warm HBSS, then incubated with 10 µM DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate; Invitrogen, Carlsbad, CA, USA) in HBSS for 30 min to allow cellular uptake and de-esterification to non-fluorescent DCFH. Following two gentle washes with HBSS, oxidative stress was initiated by adding H2O2 (200 µM) in HBSS. Fluorescence was recorded kinetically every 1–2 min for 30 min at Ex/Em 485/535 nm using a multimode plate reader BioTek Synergy H1 (Agilent BioTek, Winooski, VT, USA). Control conditions included untreated cells (baseline), H2O2-only (oxidative control), vehicle-only, extract-only (no H2O2), and quercetin controls.
For each well, fluorescence time courses were integrated to obtain the area under the curve (AUC). The CAA of extracts was expressed as µmol quercetin equivalents per gram of dry extract (µmol QE/g) by calibrating AUC suppression against the quercetin standard curve generated in the same plate. Unless stated otherwise, data are presented as mean ± SD from n = 5 independent experiments. Normality and homoscedasticity were verified prior to parametric testing. Dose–response data (method × concentration) were analyzed by two-way ANOVA with Tukey’s post hoc test, as specified in Section 2.2.8.
2.2.6. Cytotoxicity (MTT) Assay
MTT cytotoxicity was evaluated according to Mosmann (1983) with standard conditions [34]. Caco-2 cells were seeded at 1.0 × 104 cells/well (96-well) and incubated overnight. Extracts (HWE, UAE, EthE) were applied at 50, 200, 500 µg/mL for 24 h (vehicle ≤ 0.5% v/v). Then, 0.5 mg/mL MTT was added to the cells for 3 h at 37 °C. The resulting formazan was dissolved in 100 µL DMSO per well and absorbance was read at 570/630 nm on a BioTek Synergy H1 multimode microplate reader (USA). Viability (%) = A_sample/A_control × 100. Data are mean ± SD (n = 5); statistical significance was assessed by one-way ANOVA with Tukey’s post hoc.
2.2.7. HO-1 Expression Assay
To investigate the underlying mechanism of cytoprotection, the expression of heme oxygenase-1 (HO-1) was quantified via Western blot. Caco-2 cells were seeded and grown to confluence, then treated with the optimized extracts (200 µg/mL) for 4 h. Following treatment, cells were lysed in ice-cold RIPA buffer supplemented with a protease inhibitor cocktail. Total protein concentration in the lysates was determined using a BCA protein assay kit to ensure equal loading.
For electrophoresis, equal amounts of protein (30 µg per sample) were denatured, resolved on a 12% SDS-polyacrylamide gel (SDS-PAGE), and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was then blocked for 1 h at room temperature with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) to prevent non-specific antibody binding.
The blocked membrane was incubated overnight at 4 °C with rabbit anti-HO-1 primary antibody (HO-1 (E6Z5G) Rabbit mAb, Cell Signaling Technology, Danvers, MA, USA, #82206; WB 1:1000). After washing with TBST (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20), the membrane was incubated for 1 h at ambient temperature with HRP-conjugated goat anti-rabbit IgG secondary antibody (Cell Signaling Technology, Danvers, MA, USA, #7074; WB 1:5000). To confirm equal protein loading, membranes were stripped and re-probed with mouse anti-β-actin (clone AC-15; Sigma-Aldrich, A1978; WB 1:5000) as a loading control.
Protein bands were visualized using an enhanced chemiluminescence (ECL) detection kit and imaged. The band intensities were quantified using ImageJ v1.54p software (National Institutes of Health, USA). The expression level of HO-1 was normalized to the corresponding β-actin band intensity for each sample. The results, presented as fold change relative to the untreated control, were derived from three independent biological replicates.
2.2.8. Biostatistics Analysis
All experiments were performed in five independent replicates (n = 5) unless otherwise stated. Data are reported as mean ± SD. Normality (Shapiro–Wilk) and homoscedasticity (Levene’s test) were verified prior to parametric testing. For single-factor comparisons among extraction methods, one-way ANOVA with Tukey’s HSD was applied. For dose–response CAA (0–200 µg/mL across methods), a two-way ANOVA (factors: method, concentration) with Tukey’s post hoc was used. We additionally report partial η2 as effect size. Significance thresholds were p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***). Statistical analyses were conducted in GraphPad Prism v10.3.1 (GraphPad Software, San Diego, CA, USA) with α = 0.05.
3. Results
3.1. Analytical Method Validation and Baseline Catechin Profiling
The initial step involved a detailed characterization of the mature Shan Tuyet tea leaves to provide a baseline for subsequent extraction comparisons. As detailed in Table 2, the raw material was found to be a substantial source of polyphenols, with an average total catechin content of 279.8 ± 1.3 mg/g of dry weight. The profile was dominated by the native epi-form catechins, with (−)-epigallocatechin (EGC) being the most abundant (104.5 ± 0.7 mg/g), followed by (−)-epigallocatechin gallate (EGCG, 85.1 ± 1.2 mg/g). This composition is consistent with literature reports on mature tea leaves, which tend to accumulate higher levels of non-gallated catechins. Crucially, the initial concentrations of all four non-epi form epimers—GCG, GC, CG, and C—were found to be low, collectively constituting less than 8% of the total catechin content. This low level of epimerization confirms the structural integrity of the starting material, providing a reliable baseline for evaluating extraction-induced transformations.

Table 2.
Chemical composition of mature Shan Tuyet tea leaves (raw material), including the concentration of each catechin (mg/g dry weight) measured in three independent experiments (values are presented as mean ± SD).
The accurate quantification of this complex catechin profile was enabled by a rigorously validated HPLC method. As shown in Figure 1, which overlays the chromatograms of an analytical standard mixture (black line) and a representative tea extract (red line), the method successfully achieved complete baseline separation of all eight target compounds. The elution profile shows the most prominent peak corresponding to EGC at approximately 10.5 min, followed by the epimer pair EGCG and GCG, which were clearly resolved around 16.5 min, and ECG at approximately 21 min. The precise matching of these and other retention times between the standards and the sample peaks confirms the identity of the analytes and the robustness of the method in a complex plant matrix, thereby validating its suitability for the comparative analyses performed throughout this study.
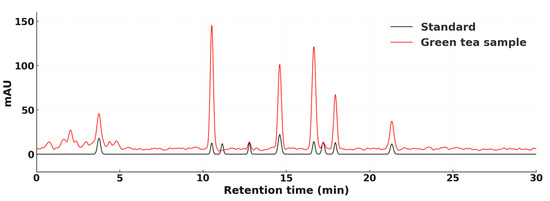
Figure 1.
HPLC chromatograms of catechin standards (black) and a representative Shan Tuyet tea extract (red) at 280 nm. The standard mixture shows baseline separation of all eight catechins (EGC, EC, C, EGCG, ECG, CG, GCG, and GC), and the extract chromatogram matches the retention times. Method validation details (linearity, LOD/LOQ, system suitability) are provided in Methods.
3.2. RSM-Based Optimization of Green Extraction Parameters and Model Validation
The optimization of catechin extraction for the three selected green methods—HWE, UAE, and EthE—was successfully conducted using Response Surface Methodology (RSM). As summarized in Table 2, the second-order polynomial models developed for each method demonstrated high statistical significance, with coefficients of determination (R2) exceeding 0.95 and non-significant lack-of-fit tests (p > 0.05). This indicates a strong correlation between the experimental data and the predicted values, confirming the models’ adequacy for navigating the experimental space. The resulting 3D response surfaces and corresponding contour plots, presented in Figure 2, visually illustrate the influence of the process variables and their interactions on catechin yield.
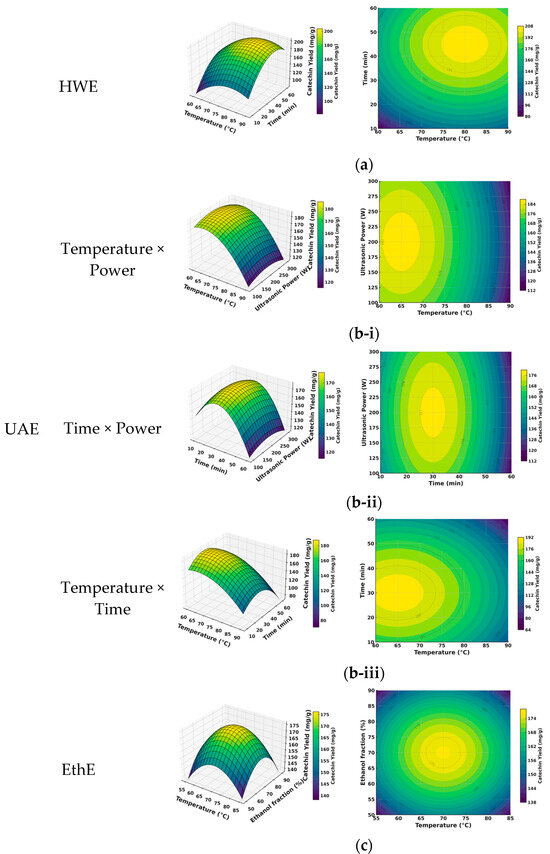
Figure 2.
Response surface (3D; left) and contour (2D; right) plots for catechin yield (mg/g). (a) HWE: temperature (60–90 °C) × time (10–60 min); ratio fixed at 45:1 mL/g. (b) UAE (27-run BBD, four factors): (i) temperature × power with time = 55 min and ratio = 48 mL/g; (ii) time × power with temperature = 78 °C and ratio = 48 mL/g; (iii) temperature × time with power = 290 W and ratio = 48 mL/g. (c) EthE: temperature (55–90 °C) × ethanol fraction (30–90% v/v); time fixed at 60 min (ratio 35:1 mL/g). Color bars indicate predicted yield (mg/g).
The specific optimal conditions predicted by the models were determined as follows. For Hot Water Extraction (HWE), the model predicted a maximum yield at a temperature of 82 °C, an extraction time of 28 min, and a solvent-to-solid ratio of 45:1 mL/g, as depicted in Figure 2a. For the more complex Ultrasound-Assisted Extraction (UAE) process, the full 27-run BBD allowed for a comprehensive analysis of four variables. The key two-factor interactions are visualized in Figure 2b(i–iii). These plots illustrate, for instance, the synergistic effect between temperature and ultrasonic power (Figure 2b-i) and between time and power (Figure 2b-ii), where higher power levels consistently enhance extraction efficiency across different temperatures and durations. The model identified a comprehensive set of optimal conditions at a temperature of 78 °C, an extraction time of 55 min, a solvent-to-solid ratio of 48:1 mL/g, and an ultrasonic power of 290 W. For Ethanol–Water Extraction (EthE) (Figure 2c), the optimal conditions were found to be a temperature of 68 °C, an extraction time of 60 min, and an ethanol concentration of 70% (v/v).
To confirm the reliability of the RSM models, validation experiments were performed in quintuplicate at these identified optimal conditions. As shown in Table 3, the experimental catechin yields were in close agreement with the predicted values for all three methods, with deviations of less than 5%, thereby validating the robustness of the models. The validated yield for HWE was 202.0 ± 5.0 mg/g, and for EthE was 175.0 ± 3.5 mg/g. Notably, the fully optimized UAE process yielded a validated total catechin content of 206.0 ± 4.5 mg/g. This yield was statistically comparable (p > 0.05, Tukey’s HSD) to that achieved by HWE (202.0 ± 5.0 mg/g), indicating that both methods are highly efficient in terms of gross catechin recovery. The EthE process yielded a significantly lower total catechin content of 175.0 ± 3.5 mg/g. These optimized extracts, produced under validated conditions, were subsequently used for all further comparative chemical and biological analyses.

Table 3.
Optimal conditions predicted by the Box–Behnken Design models and corresponding experimental validation for catechin extraction.
3.3. Comparative Chemical Profiling of Optimized Extracts
Following the optimization, the chemical characteristics of the extracts produced under the validated optimal conditions were comprehensively compared to evaluate the trade-offs between yield, purity, and structural integrity. The analysis revealed significant and distinct profiles for each green extraction method, as detailed in Figure 3.
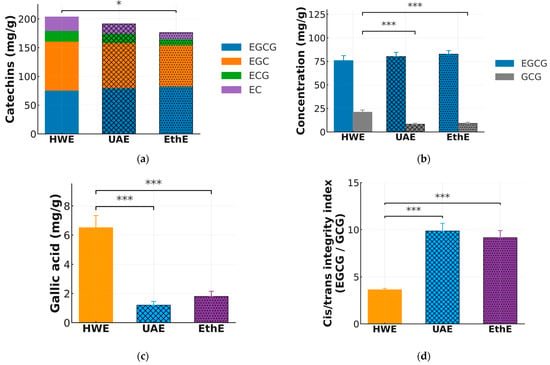
Figure 3.
Comparative analysis of catechin composition, purity, and structural transformation in extracts from optimized HWE, UAE, and EthE. (a) Stacked bars showing absolute contents of EGCG, EGC, ECG, and EC (mg/g; left y-axis) with an overlaid line for catechin purity (%; right y-axis). (b) Grouped columns comparing EGCG (cis) and its epimer GCG (trans) (mg/g). (c) Gallic acid (mg/g) as a marker of gallate hydrolysis. (d) Cis/trans integrity index expressed as the EGCG/GCG ratio (unitless). Data are mean ± SD (n = 5). One-way ANOVA with Tukey’s HSD; *, *** denote p < 0.05, p < 0.001, respectively.
Contrary to conventional expectations where harsher conditions might yield more product, the fully optimized UAE process delivered the highest total catechin yield. As shown in Figure 3a and consistent with the validation data in Table 3, UAE produced 206.0 ± 4.5 mg/g of total catechins, a yield that was statistically comparable to but slightly higher than that of HWE (202.0 ± 5.0 mg/g). However, a clear trade-off emerged concerning extract purity. EthE, while yielding the lowest total catechins (175.0 ± 3.5 mg/g), produced the purest extract, with catechins constituting 59.0 ± 2.9% of the total mass. In stark contrast, the high-yield HWE extract was the least pure (41.2 ± 2.8%), likely due to the extensive co-extraction of non-phenolic polar compounds such as polysaccharides in hot water. UAE provided a strong balance of high yield and moderate purity (50.5 ± 3.1%).
Beyond quantitative yield and purity, the extraction methods profoundly impacted the structural integrity of the thermolabile catechins, particularly through epimerization. Figure 3b provides a direct comparison of the native, biologically active (−)-EGCG and its heat-induced trans-epimer, GCG. The HWE process, operating at the highest temperature (82 °C), induced substantial epimerization, resulting in a GCG concentration of 21.0 ± 2.5 mg/g. This was significantly higher (p < 0.001) than the GCG levels in the extracts from the milder UAE (8.2 ± 1.1 mg/g) and EthE (9.1 ± 1.3 mg/g) processes, demonstrating the detrimental effect of prolonged high-temperature aqueous extraction on catechin stereochemistry.
Further evidence of thermal degradation was observed by quantifying gallic acid, a product of the hydrolysis of gallated catechins like EGCG and ECG. Consistent with the epimerization data, the HWE extract contained the highest concentration of gallic acid (6.5 ± 0.8 mg/g), significantly greater than that in the UAE and EthE extracts (Figure 3c). To synthesize these findings into a single metric of structural preservation, a cis/trans integrity index was calculated as the EGCG/GCG ratio (Figure 3d). This index was markedly higher for UAE (9.86 ± 0.79) and EthE (9.15 ± 0.76) than for HWE (3.62 ± 0.14), quantitatively demonstrating the superior ability of these methods to preserve the native, biologically active cis-conformation of EGCG.
Collectively, these results demonstrate that the three optimized green extraction methods yield chemically distinct products. While HWE and UAE provide comparable high yields, the HWE extract is characterized by significant structural degradation. In contrast, the UAE extract maintains high structural integrity, positioning it as a potentially superior source of bioactive catechins for functional applications.
3.4. Comparative Antioxidant Capacity, Cellular Bioactivity, and Mechanistic Insights
To evaluate the functional potential of the optimized extracts, a multi-tiered approach was employed, starting with chemical antioxidant assays and progressing to cell-based models. This comprehensive evaluation revealed a complex and seemingly contradictory profile of activities, as depicted in Figure 4.
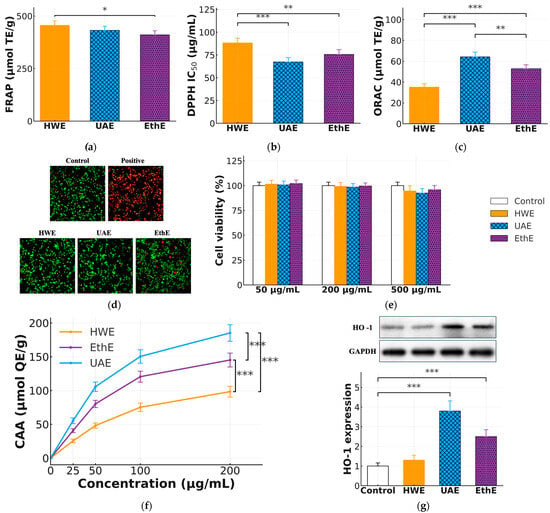
Figure 4.
Comparative evaluation of in vitro antioxidant capacity, cellular antioxidant activity, and biocompatibility of optimized extracts. (a) FRAP (µmol Trolox equivalents (TE)/g extract). (b) DPPH assay expressed as IC50 (µg/mL) (lower values indicate stronger radical scavenging). (c) ORAC (µmol TE/g extract). (d) Representative live/dead fluorescence micrographs after treatment at 200 µg/mL. (e) MTT viability (%) at 50, 200, and 500 µg/mL. (f) Cellular Antioxidant Activity (CAA) dose–response in Caco-2 cells (0–200 µg/mL; µmol quercetin equivalents (QE)/g). (g) Representative Western blot (left) and densitometric analysis (right) of relative HO-1 protein expression (fold change vs. control) in Caco-2 cells treated with extracts at 200 µg/mL for 4 h. β-actin was used as a loading control. Data are mean ± SD (n = 5). Panels (a–c,f,g): one-way ANOVA with Tukey’s HSD. Panel (d): two-way ANOVA (factors: method and concentration) with Tukey’s post hoc. *, **, *** denote p < 0.05, p < 0.01, p < 0.001, respectively.
In the chemical assays, the extracts exhibited distinct behaviors depending on the underlying reaction mechanism. The HWE extract, rich in total reducing compounds, displayed the highest activity in the Ferric Reducing Antioxidant Power (FRAP) assay (Figure 4a), with a value of 455.4 ± 21.5 µmol Trolox equivalents (TE)/g. In contrast, in assays based on hydrogen atom transfer and radical scavenging mechanisms, the UAE extract proved to be significantly more potent. It exhibited the lowest DPPH IC50 value (67.3 ± 4.8 µg/mL), indicating the strongest radical-scavenging ability, and the highest ORAC value (64.2 ± 4.5 µmol TE/g) (Figure 4b,c). These divergent results underscore the limitation of relying on a single chemical assay and suggest that the structural quality of the catechins, not just their total quantity, is a critical determinant of specific antioxidant functions.
To assess true biological efficacy in a physiological context, the Cellular Antioxidant Activity (CAA) assay was conducted using Caco-2 cells. The results revealed a striking divergence from both the chemical assay patterns and the extraction yields. As shown in the dose–response curves (Figure 4f), the UAE extract demonstrated the most potent cytoprotective activity, reaching 185.2 ± 12.5 µmol QE/g at a concentration of 200 µg/mL. The EthE extract showed moderate activity (145.1 ± 10.5 µmol QE/g), while the HWE extract, despite its high catechin yield, exhibited the lowest cellular protective effect (98.3 ± 8.1 µmol QE/g). This finding highlights a critical disconnect between total catechin yield and cellular bio-efficacy, suggesting that the harsh conditions of HWE compromised the biological functionality of the extracted compounds.
To ensure that the observed differences in cellular activity were not confounded by cytotoxicity, the biocompatibility of the extracts was evaluated. The MTT assay (Figure 4e) revealed no significant reduction in cell viability at concentrations up to 500 µg/mL, with viability remaining above 90% for all treatments. The biocompatibility at the effective concentration of 200 µg/mL was further confirmed visually by live/dead fluorescence microscopy (Figure 4d), which showed a high population of viable cells (green fluorescence) across all treatments, consistent with the quantitative MTT results.
To investigate the mechanistic basis for the superior cellular performance of the UAE extract, we hypothesized that its bioactivity might be linked to the activation of endogenous cellular defense pathways. We therefore measured the expression of the cytoprotective enzyme heme oxygenase-1 (HO-1), a key downstream target of the Nrf2 antioxidant response pathway. As shown by the Western blot analysis (Figure 4g), the extracts induced markedly different levels of HO-1 protein. The UAE extract was the most potent inducer, causing a nearly four-fold increase in HO-1 expression relative to the control. The EthE extract showed moderate induction, while the HWE extract failed to elicit a significant response. This induction pattern (UAE > EthE > HWE) closely mirrored the CAA results. The strong correlation provides evidence that the superior biological performance of the UAE extract is attributable not only to direct radical scavenging but also to its enhanced capacity to activate endogenous cellular defense mechanisms.
3.5. Correlating Extraction Yield with Biological Efficacy
To synthesize the comprehensive findings of this study, a direct comparison was made between the quantitative extraction efficiency (validated catechin yield) and the ultimate biological efficacy (cellular antioxidant activity). This final analysis, visualized in Figure 5, serves to integrate the chemical and biological data, providing a clear verdict on the most effective green extraction strategy.
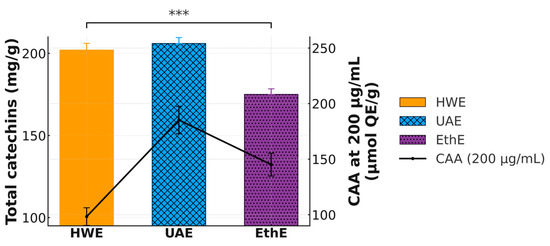
Figure 5.
The Comprehensive Superiority of UAE: Leading in Both Catechin Yield and Cellular Bio-efficacy. Combined plot showing total validated catechin yield (mg/g; left y-axis) and Cellular Antioxidant Activity (CAA) at 200 µg/mL (µmol QE/g; right y-axis). The results demonstrate that the optimized UAE process is superior in both extraction quantity and biological quality compared to HWE and EthE, *** denote p < 0.001.
The results unequivocally challenge the simple paradigm that higher extraction yield is the sole metric of a successful process. While HWE produced a high catechin yield (202.0 ± 5.0 mg/g), its biological efficacy was severely compromised, as evidenced by the lowest CAA value (98.3 ± 8.1 µmol QE/g). These results indicate that the structural degradation observed in Figure 3 directly translates to a loss of functional activity at the cellular level. In contrast, the optimized UAE process stands out as the superior method, achieving not only the highest validated catechin yield (206.0 ± 4.5 mg/g) but also the highest cellular bioactivity (185.2 ± 12.5 µmol QE/g). This powerful combination of high yield and high efficacy underscores the critical importance of process conditions that preserve the structural integrity of bioactive compounds. These findings collectively establish that the quality of the extracted catechins, defined by their structural preservation, is a more critical determinant of biological function than the gross quantity alone.
4. Discussion
This study demonstrates that mature Shan Tuyet tea leaves remain a valuable source of catechins despite their lower market value compared to young leaves. Our findings revealed a total catechin content of ≈280 mg/g (Table 2), with high levels of epigallocatechin (EGC) and epigallocatechin gallate (EGCG), consistent with earlier studies on the influence of leaf maturity on catechin profiles [5,6,7,10]. Such compositional shifts highlight the potential of utilizing aged tea leaves as a sustainable polyphenol source, supporting both local agricultural valorization and green processing initiatives [1,2,3,4]. The observed differences in catechin distribution further emphasize the importance of adapting extraction techniques to preserve the bioactivity of these compounds, especially when considering functional food and nutraceutical applications. For clarity of scope, the interpretations below pertain to material stabilized by oven-drying at 60 °C for 12 h prior to extraction.
Comparative evaluation of Hot Water Extraction (HWE), Ultrasound-Assisted Extraction (UAE), and Ethanol–Water Extraction (EthE) revealed trade-offs between yield, purity, and structural stability. HWE achieved the highest catechin yield (~202 mg/g) but also induced substantial epimerization (EGCG → GCG) and degradation into gallic acid, in agreement with prior findings on the thermal instability of catechins [18,19]. These structural transformations explain why HWE extracts showed the lowest antioxidant activity in both DPPH and CAA assays, despite strong reducing capacity in FRAP. By contrast, UAE maintained catechin integrity (epimers < 10 mg/g), resulting in superior antioxidant efficacy (CAA~185 µmol QE/g). EthE offered the highest extract purity (59%) by reducing co-extraction of proteins and polysaccharides [11,13], yielding moderately high bioactivity and making it attractive for applications prioritizing selectivity over maximum yield. The strong correlation between the EGCG/GCG ratio and cellular outcomes points towards a deeper mechanistic implication related to molecular stereochemistry. The superior performance of the UAE extract in inducing HO-1, a key enzyme in the Nrf2-mediated antioxidant response pathway [26,27,28], suggests that the native cis-conformation of EGCG is a more effective activator of this pathway than its trans-epimer, GCG. While GCG is not biologically inert, its altered three-dimensional structure may result in a lower binding affinity for the upstream cellular sensors that trigger Nrf2 activation, such as Keap1, or other protein targets [35]. This stereoselective interaction would explain why the HWE extract, despite containing a high total amount of catechins, fails to elicit a significant HO-1 response due to its high proportion of the less active GCG epimer. This finding elevates the discussion beyond simple “degradation” and proposes a specific structure-function mechanism: the preservation of native catechin stereochemistry by milder extraction techniques like UAE is critical for engaging and activating endogenous cellular defense pathways, an effect that is lost during harsher thermal processing. We note that this mechanistic inference is correlative; direct head-to-head testing of purified EGCG vs. GCG in the same cellular model will be performed in future work.
The results also highlight the limitations of relying on single antioxidant assays. While HWE extracts appeared potent in FRAP, they underperformed in DPPH and CAA, underscoring that biological relevance requires multi-assay evaluation [24,25]. Overall, each method demonstrated unique advantages: HWE for cost-effective large-scale extraction, UAE for maximizing functional efficacy, and EthE for producing high-purity extracts. Future work should include direct comparisons of purified catechin isomers to better clarify structure–activity relationships and explore advanced green technologies such as deep eutectic solvents or microwave-assisted extraction [12,13]. Within the conditions investigated (with a full four-factor BBD for UAE), UAE delivered the highest CAA and strongest HO-1 induction despite a slightly lower yield than HWE, indicating that preserving specific catechin structures under milder thermal–mechanical regimes may be more critical for cellular efficacy than maximizing total yield. In summary, maintaining the cis-conformation of EGCG and related catechins is critical for biological activity, positioning UAE as the most effective method when biofunctionality is prioritized.
The three extraction routes rely on low-toxicity solvents (water, ethanol–water) and moderate temperatures. Compared with HWE, UAE reduced the process time while delivering the highest biofunctional efficacy (CAA), thus improving energy-per-unit-benefit. EthE achieved the highest catechin purity (59%), enabling downstream savings in purification. We estimate that solvent consumption per gram of catechins is lowest for UAE/EthE under optimized conditions; ethanol can be recovered and reused by rotary evaporation, further reducing the E-factor [36,37]. From a circularity perspective, converting under-valued mature leaves into high-value catechin concentrates reduces agricultural residues while creating local value capture. Relative to HWE, the UAE optimum achieves comparable yield at shorter residence times and with less epimerization, implying lower energy-per-kg-product and a reduced purification burden [38,39]. To substantiate these advantages, future analyses will quantify ethanol recovery (%), specific energy input (kWh per g extract), E-factor/solid waste, greenhouse-gas footprint (kg CO2e per kg extract), and cost per functional unit within a streamlined TEA/LCA framework [40].
Alongside these strengths, a few considerations set the proper context for interpreting our findings. First and foremost, the use of oven-drying at 60 °C for 12 h to prepare the starting material introduces a significant confounding variable. This thermal pre-treatment likely altered the native catechin profile before the extractions began, meaning our findings strictly apply to the valorization of thermally stabilized tea leaves rather than fresh ones [41]. Moreover, polyphenol oxidase (PPO) activity was not measured post-drying; therefore, complete enzyme inactivation cannot be confirmed. Future studies should employ non-thermal methods such as freeze-drying to establish a more accurate baseline for evaluating extraction-induced degradation. Second, while our data strongly suggest a differential bioactivity between EGCG and its epimer GCG, this conclusion is inferential. The study did not involve direct comparative testing of the purified isomers. Future work should isolate EGCG and GCG to definitively characterize their respective potencies in inducing the Nrf2/HO-1 pathway. Finally, this study was limited to a single source of Shan Tuyet tea; geographical and seasonal variations could influence the initial catechin composition and thus the optimal extraction parameters. Future multi-site, multi-season sampling will be pursued to generalize the optimization more broadly.
5. Conclusions
In conclusion, this study optimized and comprehensively compared three green extraction methods for valorizing catechins from underutilized mature Shan Tuyet tea leaves under thermally stabilized starting material (oven-dried, 60 °C/12 h). These findings challenge the conventional notion that harsher extraction conditions necessarily yield more catechins. Instead, they highlight the importance of preserving catechin integrity to maximize biological function. Among three green extraction routes—hot water extraction (HWE), ultrasound-assisted extraction (UAE), and ethanol–water extraction (EthE)—UAE achieved the most favorable balance between quantity and biological function, delivering a validated total catechin yield of 206.0 ± 4.5 mg/g together with the highest cellular antioxidant activity (185.2 ± 12.5 µmol QE/g at 200 µg/mL). Mechanistically, the UAE extract preserved catechin stereochemistry (higher EGCG/GCG), accompanied by the strongest induction of heme oxygenase-1 (HO-1), consistent with the enhanced engagement of endogenous antioxidant defenses. These mechanistic inferences are correlative and will be confirmed in future head-to-head assays using purified EGCG and GCG in the same cellular model.
By comparison, HWE produced a similar total catechin yield (202.0 ± 5.0 mg/g) but showed pronounced epimerization and the lowest cellular antioxidant activity, indicating that harsher thermal conditions compromise functional quality despite high gross yield. EthE afforded the highest catechin purity (59.0 ± 2.9%) at a lower total yield (175.0 ± 3.5 mg/g), which can reduce downstream purification burdens when selectivity is prioritized.
Ultimately, the choice of extraction technology must be tailored to the intended application. For producing high-efficacy functional ingredients where both yield and bioactivity are critical, UAE is the optimal strategy. Although caffeine was not quantified in this study, its contribution to the selected antioxidant/cellular endpoints is expected to be minor and relatively uniform across extracts; therefore, its omission does not alter the method ranking. Quantification was performed by HPLC-UV/DAD with authentic standards and validated per ICH Q2(R2); targeted LC–MS will be incorporated in follow-up to profile minor degradation products. Future research should focus on: (1) elucidating the specific molecular interactions within the Nrf2/HO-1 signaling pathway using purified catechin epimers to confirm the proposed stereoselective mechanism; (2) investigating the impact of the initial leaf drying method (e.g., freeze-drying vs. oven-drying) on the efficacy of subsequent UAE; and (3) conducting a comprehensive techno-economic and life-cycle assessment to validate the industrial scalability and sustainability of the optimized UAE process for producing high-value functional ingredients from tea by-products.
Author Contributions
Conceptualization, D.T.N. and D.V.N.; methodology, D.T.N. and T.Q.N.; validation, P.T.T.P. and D.T.N.; formal analysis, D.T.N.; investigation, X.T.N. and P.T.T.P.; resources, U.T.P.; data curation, D.T.N.; writing—original draft, D.T.N.; writing—review & editing, X.T.N. and U.T.P.; visualization, P.T.T.P.; supervision, D.V.N. and T.Q.N.; project administration, P.T.T.P.; funding acquisition, P.T.T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vietnam Academy of Science and Technology, grant number THTEXS.02/23-25.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
During the preparation of this manuscript/study, the authors used ChatGPT 5 for the purposes of drawing graphs. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
HWE (Hot Water Extraction); UAE (Ultrasound-Assisted Extraction); EthE (Ethanol–Water Extraction); HPLC (High-Performance Liquid Chromatography); DAD (Diode-Array Detector); LC–MS (Liquid Chromatography–Mass Spectrometry); ICH Q2(R2) (International Council for Harmonisation guideline Q2(R2)); DPPH (2,2-Diphenyl-1-picrylhydrazyl); FRAP (Ferric Reducing Antioxidant Power); ORAC (Oxygen Radical Absorbance Capacity); CAA (Cellular Antioxidant Activity); HO-1 (Heme Oxygenase-1); Nrf2 (Nuclear Factor Erythroid 2–Related Factor 2); BBD (Box–Behnken Design); RSM (Response Surface Methodology); PRESS (Predicted Residual Error Sum of Squares); TEA (Techno-Economic Assessment); LCA (Life-Cycle Assessment); PPO (Polyphenol Oxidase); RT (Retention Time); S/S (Solvent-to-Solid ratio); SD (Standard Deviation); UV (Ultraviolet); WGS84 (World Geodetic System 1984); EGCG ((–)-Epigallocatechin Gallate); GCG ((–)-Gallocatechin Gallate); EGC ((–)-Epigallocatechin); EC ((–)-Epicatechin); ECG ((–)-Epicatechin Gallate); w/w (weight/weight); v/v (volume/volume).
References
- Vietnam+ (VietnamPlus). Shan Tuyet Tea, Valuable Timber Plants Named ‘Vietnam Heritage Trees’. Vietnam+ (VietnamPlus). Available online: https://en.vietnamplus.vn/shan-tuyet-tea-valuable-timber-plants-named-vietnam-heritage-trees-post239267.vnp (accessed on 18 July 2025).
- Tea, T.J. What Makes Vietnamese Tea Unique: Regions and Traditions. Available online: https://teajtea.com/blogs/tea-tours/vietnamese-tea-uniqueness (accessed on 18 July 2025).
- Efforts Made to Preserve Ancient Shan Tuyet Tea Trees in Ha Giang. Available online: https://en.qdnd.vn/economy/special-reports/efforts-made-to-preserve-ancient-shan-tuyet-tea-trees-in-ha-giang-509977 (accessed on 18 July 2025).
- Economic Value of Shan Tuyet Tea in Cao, Bo. Available online: https://baohagiang.vn/english/Economy/202505/economic-value-of-shan-tuyet-tea-in-cao-bo-bd80140/ (accessed on 18 July 2025).
- Zhang, L.-Q.; Wei, K.; Cheng, H.; Wang, L.-Y.; Zhang, C.-C. Accumulation of catechins and expression of catechin synthetic genes in Camellia sinensis at different developmental stages. Bot. Stud. 2016, 57, 31. [Google Scholar] [CrossRef]
- Nain, C.W.; Mignolet, E.; Herent, M.-F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The Catechins Profile of Green Tea Extracts Affects the Antioxidant Activity and Degradation of Catechins in DHA-Rich Oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Lee, L.-S.; Kim, S.-H.; Kim, Y.-B.; Kim, Y.-C. Quantitative Analysis of Major Constituents in Green Tea with Different Plucking Periods and Their Antioxidant Activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Geiss, C.; Zarse, K.; Madreiter-Sokolowski, C.T.; Ristow, M. Green tea catechins EGCG and ECG enhance the fitness and lifespan of Caenorhabditis elegans by complex I inhibition. Aging 2021, 13, 22629–22648. [Google Scholar] [CrossRef] [PubMed]
- Danial, A.M.; Koh, S.; Kahar, A.; Razak, D.A.; Long, K.; Azali, A.; Sani, N.; Agus, B. Bioactive compounds distribution in tea leaves from different maturity stagesand altitude in Malaysia. Food Res. 2024, 8 (Suppl. 3), 66–74. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; de Bruijn, W.J.C.; Vincken, J.-P. A comparison of the phenolic composition of old and young tea leaves reveals a decrease in flavanols and phenolic acids and an increase in flavonols upon tea leaf maturation. J. Food Compos. Anal. 2020, 86, 103385. [Google Scholar] [CrossRef]
- Cioanca, O.; Lungu, I.-I.; Mita-Baciu, I.; Robu, S.; Burlec, A.F.; Hancianu, M.; Crivoi, F. Extraction and Purification of Catechins from Tea Leaves: An Overview of Methods, Advantages, and Disadvantages. Separations 2024, 11, 171. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Ozsefil, I.C.; Ziylan-Yavas, A. Green approach for polyphenol extraction from waste tea biomass: Single and hybrid application of conventional and ultrasound-assisted extraction. Environ. Res. 2023, 235, 116703. [Google Scholar] [CrossRef]
- Dutta, S.; Priyadarshini, S.R.; Moses, J.A.; Anandharamakrishnan, C. Supercritical Fluid and Ultrasound-assisted Green Extraction Technologies for Catechin Recovery. ChemBioEng Rev. 2021, 8, 654–664. [Google Scholar] [CrossRef]
- Koina, I.M.; Sarigiannis, Y.; Hapeshi, E. Green Extraction Techniques for the Determination of Active Ingredients in Tea: Current State, Challenges, and Future Perspectives. Separations 2023, 10, 121. [Google Scholar] [CrossRef]
- Ahmad, R.; Aldholmi, M.; Alqathama, A.; Althomali, E.; Aljishi, F.; Mostafa, A.; Alqarni, A.M.; Shaaban, H. The effect of natural antioxidants, pH, and green solvents upon catechins stability during ultrasonic extraction from green tea leaves (Camellia sinensis). Ultrason. Sonochem. 2023, 94, 106337. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Influence of ethanol, water, and their mixtures as co-solvents of the supercritical carbon dioxide in the extraction of phenolics from purple corn cob (Zea mays L.). J. Supercrit. Fluids 2016, 118, 11–18. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Oxidation and degradation of (epi)gallocatechin gallate (EGCG/GCG) and (epi)catechin gallate (ECG/CG) in alkali solution. Food Chem. 2023, 408, 134815. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Zhu, Q.Y.; Wong, Y.F.; Zhang, Z.; Chung, H.Y. Stabilizing Effect of Ascorbic Acid on Green Tea Catechins. J. Agric. Food Chem. 1998, 46, 2512–2516. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Athirojthanakij, W.; Rashidinejad, A. Optimizing catechin extraction from green tea waste: Comparative analysis of hot water, ultrasound-assisted, and ethanol methods for enhanced antioxidant recovery. Food Sci. Nutr. 2024, 12, 5121–5130. [Google Scholar] [CrossRef]
- Abraham, J. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In Handbook of Transnational Economic Governance Regimes; Tietje, C., Brouder, A., Eds.; Brill|Nijhoff: Leiden, The Netherlands, 2010; pp. 1041–1053. [Google Scholar] [CrossRef]
- Unno, T.; Sagesaka, Y.M.; Kakuda, T. Analysis of Tea Catechins in Human Plasma by High-Performance Liquid Chromatography with Solid-Phase Extraction. J. Agric. Food Chem. 2005, 53, 9885–9889. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants in food. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xiao, M.; Hu, S.; Wang, M. Keap1-Nrf2 pathway: A key mechanism in the occurrence and development of cancer. Front. Oncol. 2024, 14, 1381467. [Google Scholar] [CrossRef]
- Bouchez, A.; Vauchel, P.; Périno, S.; Dimitrov, K. Multi-Criteria Optimization including Environmental Impacts of a Microwave-Assisted Extraction of Polyphenols and Comparison with an Ultrasound-Assisted Extraction Process. Foods 2023, 12, 1750. [Google Scholar] [CrossRef]
- Pappas, V.M.; Samanidis, I.; Stavropoulos, G.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Analysis of Five-Extraction Technologies’ Environmental Impact on the Polyphenols Production from Moringa oleifera Leaves Using the Life Cycle Assessment Tool Based on ISO 14040. Sustainability 2023, 15, 2328. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Chen, F.; Hu, X.; Xu, D.; Cao, Y. Epimerisation and hydrolysis of catechins under ultrasonic treatment. Int. J. Food Sci. Technol. 2021, 56, 312–320. [Google Scholar] [CrossRef]
- Hiep, N.T.; Duong, H.T.; Anh, D.T.; Nguyen, N.H.; Thai, D.Q.; Linh, D.T.T.; Anh, V.T.H.; Khoi, N.M. Subcritical Water Extraction of Epigallocatechin Gallate from Camellia sinensis and Optimization Study Using Response Surface Methodology. Processes 2020, 8, 1028. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Pătrăuțanu, O.-A.; Teodosiu, C.; Volf, I. Life cycle assessment of polyphenols extraction processes from waste biomass. Sci. Rep. 2020, 10, 13632. [Google Scholar] [CrossRef] [PubMed]
- Serdar, G.; Demir, E.; Sökmen, M. Sequential Green Extraction of Caffeine and Catechins from Green Tea. Int. J. Second. Metab. 2019, 6, 283–291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).