Abstract
Currant pomaces were valorised using food-grade supercritical CO2 to examine how pre-drying (convective vs. freeze-drying) and species (black vs. red currant) shape extract composition and antioxidant readouts. Total phenolics (TPCs), DPPH capacity, tocopherols and fatty acids were determined; statistics employed included the Welch test with Holm adjustment and one-way ANOVA. Blackcurrant showed consistently higher TPCs than redcurrant, whereas DPPH responses were maximised in freeze-dried redcurrant. Freeze-drying increased PUFAs and concomitantly lowered SFAs within both species, with MUFAs varying within a narrower band and tending to be higher in blackcurrant. Tocopherol profiles in residues displayed homologue- and species-specific redistribution (e.g., α higher after convective drying in blackcurrant; γ/δ preferentially retained after freeze-drying), consistent with microstructure-dependent mass transfer and homologue-specific partitioning during SFE. Collectively, pre-drying emerged as the principal lever to tailor lipid class balance and antioxidant performance under fixed extraction conditions. Practically, freeze-drying is suited to PUFA-rich, antioxidant-active fractions, whereas convective drying favours more oxidation-resilient profiles. These results support process-informed ingredient design for clean-label applications and motivate yield-normalised mass balances and scale-up studies.
1. Introduction
The transition from a linear to circular economy food system has elevated the valorisation of processing by-products from a waste management afterthought to a strategic source of functional ingredients. Syntheses of agri-food side streams emphasise their richness in lipids, fibre and phenolics, and argue that well-designed recovery routes can deliver environmental and economic gains while supporting clean-label innovation [1,2,3,4].
Within berry chains, currant (Ribes spp.) pomace is compositionally attractive: seed-rich matrices provide unsaturated fatty acids, tocopherols and diverse phenolics (phenolic acids, flavonols and anthocyanins). Comparative characterisation of blackcurrant fruits, leaves and pomace show robust radical-scavenging capacity and phenolic retention after in vitro digestion, supporting the case for pomace as a precursor of antioxidant-active ingredients for novel foods [5,6]. Extending this compositional context, recent quantification of fruit- and vegetable-processing pomaces confirms chokeberry as particularly fibre-dense (notably high NDF/ADF), with apple and carrot contributing larger soluble fractions—differences that matter for techno-functionality and remain debated across varieties and processing conditions [7].
Transition into foods is feasible: proof-of-concepts with berry pomace (e.g., chokeberry) demonstrate incorporation into meat systems, although colour and texture emerge as the most sensitive attributes; low inclusion levels (~2%) appear workable provided appearance trade-offs are managed proactively [2,3,8,9].
A concurrent methodological shift has favoured green extraction over conventional reflux/Soxhlet. State-of-the-art comparisons describe how solvent choice, energy input and process intensification can be balanced to preserve bioactivity and reduce burdens; notably, integrated or hybrid sequences (stabilisation → extraction) are recommended when labile phytochemicals are targeted [10,11,12,13,14]. Among green platforms, supercritical carbon dioxide (SC-CO2) extraction is prominent: density-tuned solvating power, rapid mass transfer and low-residue products make SC-CO2 compatible with food-grade specifications and label-friendly claims. High-pressure reviews and applications outline fundamentals, scope across plant residues and alignment with scalable, modular process design [15,16,17].
Critically, SC-CO2 performance reflects synergistic control of pressure, temperature, CO2 flow, bed characteristics and co-solvent dosing; these variables govern fluid density, solubility and diffusion, and can shift selectivity among lipidic and co-extracted phenolic fractions. Recent analyses underscore such interdependencies and motivate rigorous experimental design with transparent parameter reporting [17,18]. For fruit seed and berry by-products, SC-CO2 effectively recovers oils in which PUFAs, MUFAs and SFAs collectively define nutritional profiles (e.g., linoleic and, matrix-dependently, α-linolenic acids), often accompanied by tocopherols that contribute to oxidative stability. Progress toward commercial viability highlights the importance of process intensification, eco-co-solvents and techno-economic evidence to justify scale-up [19,20].
Pre-processing is a decisive—yet debated—determinant of extract quality. Low-temperature convective drying (≤34 °C) may limit thermal damage but can enable oxidation or diffusion-related losses, whereas lyophilisation is frequently associated with improved retention of polyphenols, tocopherols and other thermolabile constituents at a higher energy input. Comparative evaluations advocate linking the pre-drying route not only to yield but also to compositional and functional endpoints, ideally within green chemistry optimisation frameworks [21,22,23,24].
Analytical strategy is integral to credibility and regulatory readiness. Recommended workflows pair GC-FID for fatty acid classes, key C18 species with HPLC for tocopherols, Folin–Ciocâlteu for total polyphenols and DPPH for radical scavenging. Robust quality control should include certified reference materials, internal standard normalisation (lipids), replicated injections with predefined RSD thresholds, matrix blanks, retention-time locking and long-term control charts for assay performance [10,11,12].
Beyond SC-CO2, the green extraction repertoire spans pressurised-liquid extraction (PLE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), enzyme-assisted extraction (EAE) and pulsed-electric-field assistance (PEF). Comparative assessments articulate method-specific performance characteristics in polyphenol-rich matrices and the implications for scale-up and sustainability metrics [13,14,21]. Biological routes extend this methodological repertoire, with enzyme-assisted and fermentation-based strategies enhancing the liberation of bound phenolics and improving downstream selectivity; integration with high-pressure separations is emergent but promising within biorefinery concepts [25].
Sustainability assessments increasingly call for quantitative indicators—energy use, solvent economy, yield per unit waste and climate impacts—to accompany compositional and bioactivity data, thus aligning extraction science with circular economy decision-making and industrial feasibility [26]. At the interface of composition and efficacy, human study evidence provides a reminder that health outcomes may not follow automatically from phytochemical richness; for example, trials on New Zealand blackcurrant reported no acute or chronic effects on selected endpoints, underscoring the need to link processing choices with bioavailability and realistic use scenarios [27].
Aim and principal contribution. Against this background, we examine black and redcurrant pomace subjected to two pre-drying strategies—low-temperature convective drying (≤34 °C) and lyophilisation—followed by SC-CO2 extraction at fixed, food-relevant conditions. We characterise the resulting extracts for fatty acid profiles (GC-FID), tocopherols (HPLC), total polyphenols (Folin–Ciocâlteu) and antioxidant capacity (DPPH), and we interpret outcomes through sustainability and process-efficiency lenses. Our central conclusion is that the choice of pre-drying route materially shapes antioxidant functionality and lipid composition, informing the resource-efficient inclusion of currant-pomace extracts in edible oil carriers and emulsions, beverage fortification, clean-label antioxidant systems and encapsulated delivery formats within circular bioeconomy strategies [7,9,11,12,13,14,15,16,17,18,19,21].
2. Materials and Methods
2.1. Materials and Reagents
Fresh pomace for this study originated from two Polish suppliers—Wiatrowy Sad Juice Pressing Facility (Wiatrowy Sad Grażyna Wiatr, Dmosin, Poland), a local juice distributor, and Maspex Group (Wadowice, Poland), a large manufacturing facility. Blackcurrant pomace (Ribes nigrum) was supplied by both sources, whereas redcurrant pomace (Ribes rubrum) was obtained exclusively from Wiatrowy Sad. Reagents and standards were as follows: carbon dioxide (CO2; Air Liquide, Paris, France); HPLC-grade methanol, acetonitrile and ethanol (Honeywell, Morristown, NJ, USA); Folin–Ciocâlteu phenol reagent, gallic acid and sodium carbonate (Chempur, Piekary Śląskie, Poland); certified fatty acid methyl ester (FAME) calibration standards for GC–FID; 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid and tocopherol standards; anhydrous sodium sulphate (Sigma-Aldrich, Saint Louis, MO, USA); and type I (18.2 MΩ·cm) water produced by a Milli-Q system (Merck, Burlington, MA, USA). All chemicals were of at least analytical grade and used as received.
2.2. Process Equipment
2.2.1. Supercritical CO2 Extraction Unit (Pilot Scale)
All processing work was performed in laboratories in Wrocław, primarily at the Wrocław University of Environmental and Life Sciences. Supercritical fluid extraction (SFE) used a pilot-scale (semi-industrial) three-basket extractor (Natex Prozesstechnologie GesmbH, Ternitz, Austria) with a removable 4 dm3 extraction basket, operating in a closed-loop CO2 system. Residual air was removed via a manual vent prior to pressurisation, as shown in Figure 1. For this study, the extractor was operated isobarically at 500 bar and 30 °C for 180 min (time recorded from attainment of the set-point), and all analysed extracts were collected under these conditions. The average CO2 mass flow during extraction was ~35.6 kg·h−1; two downstream separators were held at 60 bar (S40) and 44 bar (S45), enabling continuous product collection without depressurising the basket. Temperature stability was ±0.5 °C with an external chiller. All product-contact parts were 316L stainless steel with PTFE seals.
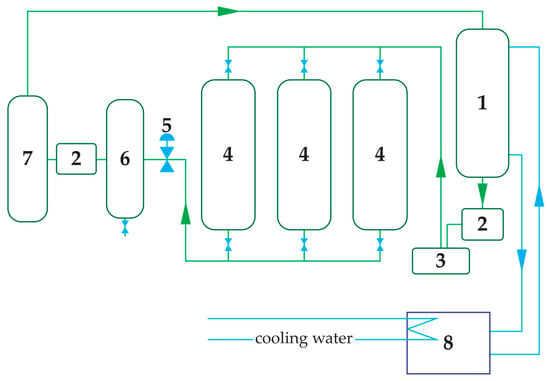
Figure 1.
Schematic of the pilot-scale supercritical CO2 extraction unit operated in a closed loop. Key components: (1) CO2 reservoir, (2) intercooler, (3) high-pressure pump, (4) extraction vessel with a 4 dm3 basket, (5) back-pressure regulator, (6) separator S40, (7) volatile compound separator S45 and (8) chiller. Green arrows indicate the closed-loop CO2 flow, whereas blue arrows indicate the circulation of the cooling medium between the intercooler and the chiller.
2.2.2. Freeze-Drying Line (Lyophilisation)
Lyophilisation employed a Delta 1–24 LSC freeze dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). Fresh blackcurrant and redcurrant pomace were spread on trays and deep-frozen at −80 °C for 48 h, then transferred to the pre-cooled dryer for primary drying (set − 40 °C, 0.120 mbar; achieved − 27 °C, 0.220 mbar) followed by secondary drying (set + 25 °C, 0.0010 mbar; achieved + 25 °C, 0.140 mbar), yielding ~3.10% for blackcurrant and subsequently ~2.86% for redcurrant moisture (R%). Pre-freezing used a low-temperature freezer (Premium U410; New Brunswick Scientific Co., Inc., Trenton, NJ, USA). The complete lyophilisation process lasted approximately 6 days for both pomace types at the stated conditions.
2.2.3. Conventional Forced-Air Drying
Conventional low-temperature drying was conducted in a forced-air chamber dryer with PID control (EL-TECH, Wieluń, Poland) at a constant +34 °C for approximately 24 h in both cases, yielding final moisture contents of 15.22% (R%) for blackcurrant and 6.82% (R%) for redcurrant in the respective pomaces.
2.2.4. Post-Drying Comminution and Handling
Following either lyophilisation or conventional drying, samples were ground in a knife mill (IKA M20; IKA, Staufen im Breisgau, Germany) to a homogenous powder. No vibratory sieving or particle-size fractionation was performed, and samples were not vacuum-packed. The ground material was subjected to supercritical extraction on the same day as drying/lyophilisation.
2.3. Pre-Processing and Extraction Procedures
Pomace batches were assigned to one of two pre-drying routes—lyophilisation or low-temperature convective drying—implemented exactly as specified in Section 2.2.2 and Section 2.2.3. Immediately after drying, material was ground in a knife mill (IKA M20); no vibratory sieving or particle-size fractionation was applied, and samples were not vacuum-packed. The ground material was subjected to supercritical CO2 extraction on the same day. All extractions were performed under a single set of operating conditions, i.e., 500 bar, 30 °C, 180 min (time recorded from attainment of the set point), with continuous product collection and separator pressures maintained at 60 bar (S40) and 44 bar (S45); equipment configuration and ancillary settings are described in Section 2.2.1. Thus, all analysed extracts originate from these fixed SFE conditions. Mass yields were not quantified; accordingly, comparisons focus on extract composition (tocopherols, fatty acid profile, total polyphenols and antioxidant capacity), reported per gram of dry extract and—where stated—normalised to dry pomace. Analytical procedures and statistical treatment are detailed in Section 2.4 and Section 2.6, respectively.
2.4. Analytical Methods
2.4.1. Total Polyphenols (Folin–Ciocâlteu, UV-Vis)
Total polyphenols were quantified by the Folin–Ciocâlteu assay on a PerkinElmer Lambda 35 UV–vis spectrophotometer with UV WinLab (ver 6.0.4.0738) software (Perkin Elmer, Waltham, MA, USA) using 1.0 cm quartz cuvettes. The procedure followed Singleton et al. [28]: dilution of sample/standard in water, addition of Folin–Ciocâlteu reagent, a 1–8 min delay, addition of sodium carbonate, volume adjustment, colour development (~2 h at room temperature) and measurement at 760 nm; results were expressed as gallic acid equivalents (mg GAE·g−1 d.w.) from a multi-point calibration with gallic acid.
2.4.2. Antioxidant Capacity (DPPH, UV-Vis)
Radical–scavenging capacity was determined by the DPPH decolourisation assay using UV–visible spectrophotometry at 517 nm according to Laczkó-Zöld et al. [29] A freshly prepared methanolic DPPH solution (~0.10 mM) was adjusted to an initial absorbance of 0.650 ± 0.020 at 517 nm, mixed with sample aliquots and incubated in the dark at ambient temperature; the decrease in absorbance at 517 nm after the set incubation time (30 min) was used to calculate inhibition as (1):
Calibration was performed with L-ascorbic acid, and results were expressed as µmol ascorbic acid equivalents (AAE) per gram of dry matter; all measurements were conducted at least in triplicate.
2.4.3. Tocopherols (HPLC-FLD)
Quantification of α-, γ- and δ-tocopherols was performed on the solid residues retained in the extraction baskets (i.e., the spent pomace matrix), rather than on the CO2 extracts; measurements served comparative purposes across fractions. Chromatography employed a reverse-phase C18 column (5 µm, 4.6 × 150 mm) maintained at 23 °C with isocratic elution in neat methanol (100% v/v) at 1.5 mL min−1. Fluorescence was monitored at λex = 295 nm and λem = 330 nm using a Waters 600E system coupled to a 2475 Multi-λ FLD (Waters Associates, Framingham, MA, USA) under Empower™2 software control. For sample preparation, 100 mg of dried material was extracted with 10 mL ethanol, vortex-mixed, passed through a 0.45 µm nylon membrane and injected at 10 µL. External calibration with authentic tocopherol standards was applied, and results are reported on a dry-mass basis (mg g−1 d.w.).
2.4.4. Fatty Acids (GC-FID)
To characterise the lipid fraction, methyl esters of fatty acids (FAMEs) were determined in accordance with ISO 12966:2-2017 [30], a GC-FID system (TRACE GC Ultra, Thermo Fisher Scientific, Waltham, MA, USA) fitted with the 100 m capillary column specified by the standard. Peak identities were assigned by retention time matching to a certified 37-component FAME reference mixture, with data acquired and processed in Chromeleon 7.3 (v7.3.0.60919). Results are presented both as class sums (SFAs, MUFAs and PUFAs) and, separately, for the principal individual fatty acids.
2.5. Method Validation and Quality Control
All measurements followed a common QA/QC framework covering calibration, precision, detection capability and matrix/instrumental bias control. Results are reported as mean ± SD (n = 3, dry-mass basis); data were screened for outliers (Grubbs, α = 0.05) and statistically processed in STATISTICA (13.3.721.1) software with α = 0.05 for significance testing. Each analytical batch included reagent blanks, star/end calibration verification and a pooled in-house QC (spent residue extract) to monitor day-to-day drift. Linearity was verified over the working range for every assay; system suitability was checked before injections with pre-set limits (retention-time RSD ≤ 1% and peak-area RSD ≤ 2% for HPLC/GC; UV-vis replicate RSD ≤ 5%). LOD/LOQ were estimated as 3.3σ/s and 10σ/s, where σ was the SD of a low-level calibrant or procedural blank and s the calibration slope. Calibration residuals and QC chart trends were inspected for drift. Any batch failing acceptance criteria was re-analysed.
DPPH (517 nm) and TPCs (Folin–Ciocâlteu, 765 nm). External calibration used ascorbic acid (DPPH; results as µmol AAE·g−1 d.w.) and gallic acid (TPCs; mg GAE·g−1 d.w.). The DPPH reagent blank served as A0 for % inhibition. Incubation was fixed at 30 min, ~21 °C and verified across runs. Reagent blanks were prepared daily; dilution linearity (2×–10×) and spike recovery checks assessed matrix effects. Calibration residuals were inspected for randomness/lack of curvature; batches failing criteria were repeated.
Tocopherols (HPLC-FLD) and comparative assessment in post-SFE residues). Quantification of γ- and δ-tocopherol in solid residues recovered from extraction baskets (used for comparative purposes across fractions) employed external calibration with authentic standards. System suitability required adequate homologue resolution (Rs ≥ 1.5), retention time stability (RSD ≤ 1% or ±0.1 min), acceptable peak symmetry (0.8–1.5) and S/N ≥ 10 at the LOQ. Solvent blanks bracketed injections to check carry-over; continuing calibration verification was run approximately every 10 samples. Precision was judged against a priori targets (repeatability RSDr ≤ 2–3% for standard addition in a representative residue); spike recovery evidenced accuracy in the presence of co-extractives.
Fatty acid (GC-FID as FAME) identity was assigned by retention time matching to a certified 37-component FAME mixture on the 100 m capillary specified by the standard. System suitability followed ISO 12966:2-2017 [30], including checks of column efficiency, critical pair separations (cis/trans C18:2; C18:3 isomers), retention time locking via reference peaks and verification of baseline resolution for adjacent PUFA isomers; oxidation artefacts were monitored via blanks and inspection of late-eluting/ghost peaks. Concentration-dependent repeatability limits were applied: RSD ≤ 2% for major constituents (≥10% m/m), ≤5% for 1–10%, ≤10% for 0.1–1% and ≤20% for trace levels; exceedances triggered re-extraction/reinjection. Reporting prioritised mass-based results over area normalisation.
2.6. Data Processing and Statistical Analysis
All results were normalised to dry mass. Where replicate measurements were available, values are reported as mean ± SD with homogeneous groups; where replication was not feasible, means only are presented. Data were screened for outliers (Grubbs, α = 0.05) and where applicable, for distributional assumptions (normality and variance homogeneity). All analyses were conducted in STATISTICA 13.3.721.1 (StatSoft, Kraków, Poland) using two-tailed tests with α = 0.05 and Holm adjustment for multiple comparisons.
Comparisons were performed both within species (contrasting pre-drying variants) and across fractions. Pairwise contrasts used Welch’s test (with non-parametric back-up where assumptions were untenable). For fatty acid classes (PUFAs, MUFAs and SFAs), differences were evaluated by one-way ANOVA, and effect sizes were reported as R2 (eta-squared) and ω2 alongside F and p. Homogeneous group lettering in tables derives from these procedures.
3. Results
Results are reported for blackcurrant and redcurrant fractions obtained under fixed SFE conditions (500 bar, 30 °C, 180 min) following convective or freeze-drying pretreatments. Where available, quantitative data are presented as mean ± SD with homogeneous groups indicated by differing superscripts; in the remaining instances, means only are provided without dispersion estimates or group lettering. The presentation proceeds from phenolic metrics (TPCs and DPPH) to lipid-associated markers (tocopherols in post-SFE residues) and fatty acid composition at class and constituent levels.
3.1. Total Polyphenols
Folin–Ciocâlteu determinations (Table 1) evidenced a statistically robust interspecific main effect, with blackcurrant extracts (B-5, B-6) exhibiting higher TPCs (mg GAE·g−1 d.w.) than redcurrant (R-5, R-6). Within species, the pre-drying factor (convective vs. freeze-drying) did not yield distinguishable means under the adopted decision threshold. Given the fixed supercritical carbon dioxide regime, the predominantly lipophilic solvating environment characteristic of SC-CO2 is expected to restrict the co-extraction of polar phenolics; consequently, pre-drying-induced microstructural disparities exerted only a secondary influence on the phenolic content of the extracts. Residual within-species variability is plausibly attributable to opposing contributions from modified cell-wall porosity and reduced water activity, on the one hand, and thermo-oxidative alteration during convective drying, on the other.

Table 1.
Total phenolic content of currant fractions, determined by the Folin–Ciocâlteu assay and expressed as mg GAE·g−1 d.w. Values are mean ± SD. Different letters denote homogenous groups at p < 0.05 tested within species and where indicated, across all fractions.
3.2. Antioxidant Capacity
DPPH outcomes (Table 2) are reported as µmol AAE·g−1 d.w. alongside IC50. The ordering of activity was R-6 > B-6 > (B-5 × R-5), with R-6 exhibiting the highest AAE and lowest IC50; Welch tests with Holm adjustment supported these contrasts at p < 0.05. This hierarchy only partially corresponds to TPCs (Section 3.1), which is consistent with the expectation that, in lipid-rich SC-CO2 extracts, the DPPH read-out reflects not solely residual phenolics that are assay-soluble, but also the contribution of co-extracted lipophilic antioxidants and matrix-dependent optical/viscosity effects. Compared with the convectively dried counterparts, the freeze-dried variants (R-6 and B-6) showed a modestly enhanced in vitro radical-scavenging capacity, consistent with greater recovery of lipophilic antioxidants under identical extraction conditions; however, DPPH is a single-endpoint, solvent-dependent chemical assay that is sensitive to matrix effects and should not be over-interpreted as a predictor of in vivo efficacy or oxidative shelf-life.

Table 2.
DPPH radical-scavenging capacity of currant fractions. Antioxidant capacity reported as mg GAE·g−1 d.w. together with IC50 (sample concentration affording 50% inhibition). Values are mean ± SD. Higher AAE and lower IC50 indicate stronger activity. Different letters indicate significant differences (p < 0.05) evaluated globally.
3.3. Tocopherols
Tocopherol homologues (α, γ, δ) were quantified in the post-extraction solid residues retained in the extraction basket (Table 3) for explicitly comparative assessment. In blackcurrant α-tocopherol was greater following convective drying (B-3 > B-4), whereas γ- and δ-tocopherol were greater after freeze-drying (B-4 > B-3). In redcurrant α and δ were higher in R-3 than R-4, whilst γ was higher in R-4 (all within-species differences were significant at p < 0.05). These opposing shifts are most plausibly explained by homologue-specific partitioning between the supercritical CO2 phase and the residual matrix under the fixed extraction conditions: homologues preferentially transferred to the supercritical phase will appear detected in the residual matrix, and the balance is modulated by pre-drying-dependent microstructures together with differential thermal lability (α-tocopherol typically being more labile than γ/δ under warm air). Because measurements were undertaken on residues rather than extracts, direct concordance with extract-based DPPH/TPCs is neither anticipated nor required; instead, these data provide orthogonal evidence concerning tocopherol retention/partitioning under fixed SC-CO2 conditions.

Table 3.
Tocopherol homologues in post-SFE solid residues, which were quantified by HPLC-FLD as mg g−1 d.w. without SD (replicate variability was negligible, 0.000–0.003), whereas compositional shares are given as % of total tocopherols (mean ± SD). Different superscript letters mark homogenous groups with species.
3.4. Fatty Acids
Class-level fatty acid composition (Table 4). Class-wise fatty acid profiles differed substantially among fractions. PUFAs were greatest in B-6, followed by R-6, whereas B-5 and R-5 formed a lower, homogeneous pair. MUFAs were elevated in blackcurrant relative to redcurrant, while SFAs exhibited the inverse gradient. The ANOVA class effects were significant for all three lipid classes; detailed outcomes are summarised in Table 5a. To delineate species-specific contributions, within-species contrasts for PUFAs are provided in Table 5b.

Table 4.
Fatty acid composition of extracts by class, expressed as % of total FAME. Values are mean ± SD. Different superscript letters indicate p < 0.05 and denote global homogeneous groups determined across all fractions.

Table 5.
(a). One-way ANOVA for fatty acid class composition across fractions. (b). One-way ANOVA with species contrasts for PUFAs: B-5 vs. B-6 and R-5 vs. R-6.
Taken together, the ANOVA effects in Table 5a and the within-species effects in Table 5b indicate that higher PUFAs and lower SFAs are primarily associated with freeze-drying within each species, consistent with enhanced recovery of seed-derived unsaturated fatty acids under the fixed SFE regime.
Key individual fatty acids (Table 4). Trends at the class level are driven predominantly by linoleic (LA) and α-linoleic (ALA) acids on the PUFA axis, and palmitic (PA) and stearic (SA) acids on the SFA axis, with oleic acid (OA) varying within a comparatively narrow MUFA band. The freeze-dried blackcurrant extract (B-6) shows the strongest shift towards LA/ALA-rich profiles-consistent with improved accessibility of seed lipids under the adopted SC-CO2 regime-whereas higher SFAs in convectively dried variants are compatible with a greater relative contribution of cuticular/waxy constituents in less open matrices. Functionally, as PUFAs increase and SFAs decrease, susceptibility to oxidation tends to rise (despite potential gains in DPPH performance), whereas SFA-richer profiles would be expected to display enhanced oxidative robustness.
In the present dataset, fractions with higher LA/ALA proportions are predisposed to higher instantaneous DPPH indices yet lower oxidative shelf-life, necessitating formulation safeguards (e.g., tocopherol/phenolic co-antioxidants, oxygen- and light-barrier packaging and microencapsulation). Conversely, SFA-enriched profiles exhibit greater oxidative stability but typically do not achieve the same AAE values. The MUFA fraction (predominantly OA) modulates fluidity and processing behaviour and may temper extremes in stability without strongly determining DPPH outcomes. Note that DPPH is a chemical, single-timepoint assay and should not be over-interpreted as a predictor of long-term storage performance; alignment with accelerated oxidation tests is recommended for product development.
4. Discussion
The data demonstrate that pre-drying treatment and botanical origin jointly shaped the compositional and functional profiles of currant pomace subjected to supercritical CO2 extraction. Freeze-drying was associated with higher PUFA recovery and, in some fractions, enhanced antioxidant capacity, whereas convective drying resulted in relatively higher SFA levels and selective depletion of labile tocopherol homologues. These findings are consistent with the premise that microstructural integrity and water activity strongly affect mass transfer and solute partitioning during extraction [31].
The TPC data (Table 1) confirmed a robust species effect, with blackcurrant fractions exceeding redcurrant, though pre-drying did not produce within-species differences. By contrast, DPPH responses (Table 2) showed that freeze-dried fractions (particularly R-6) expressed higher µmol AAE·g−1 d.w. and a lower IC50, indicating stronger radical scavenging. This divergence from TPCs highlights the contribution of lipophilic antioxidants such as tocopherols, which modulate chemical assay outcomes beyond polyphenol abundance [32].
Tocopherol analysis (Table 3) revealed species- and pre-drying-specific patterns that are in accord with known chemotaxonomic differences in Ribes seeds: blackcurrant typically features a higher share of α-tocopherol, whereas redcurrant is comparatively enriched in γ/δ homologues [33]. From a physicochemical standpoint, the observed profiles are consistent with the protective role of tocopherols towards polyunsaturated lipids and with reported associations between tocopherol class proportions, total tocopherols and oil content in Ribes oils [33]. Partitioning effects under identical SC-CO2 conditions-sensitive to temperature/pressure history and matrix microstructure-further explain the homologue shifts between oil and spent solids [34]. In parallel, optimisation studies in related berry systems show that adjusting extraction variables by design (e.g., response-surface optimisation) can materially influence antioxidant readouts and cross-assay correlations (TPCs and DPPH), underlining the assay and solvent dependence of such metrics [35].
Fatty acid profiling reinforced the influence of pre-drying. Class-level ANOVA (Table 5a,b) showed highly significant differences (p < 0.001), with PUFAs increasing as SFAs decreased within species. Table 4 reports class sums (PUFAs, MUFAs and SFAs), whereas Table 6 details the individual acids underpinning these shifts-linoleic (LA, C18:2n−6) and α-linolenic (ALA, C18:3n−3) as principal PUFA drivers, and palmitic (PA, C16:0) and stearic (SA, C18:0) for SFAs. These tendencies accord with established patterns in Ribes seed oils-LA/ALA-rich profiles with measurable GLA in blackcurrant-and help to explain the species effect observed here [33]. Moreover, raw material heterogeneity, especially seed load, plausibly amplifies these differences: typical seed oil contents reported for blackcurrant versus redcurrant (~26 vs. ~9 g oil/100 g seed) imply that batches richer in seeds will exhibit proportionally higher PUFAs and associated lipid-soluble antioxidants, thereby modulating the effective composition of dried pomace subjected to SFE [36].

Table 6.
Composition in the most relevant fatty acids of currant extracts. Results are mean % of total FAME. Different superscript letters indicate p < 0.05 and denote global homogenous groups computed for each fatty acid across all fractions.
At the methodological level, our use of fixed extraction conditions (500 bar, 30 °C, 180 min) offers a controlled baseline, yet the literature emphasises that factorial optimisation of pressure, temperature and co-solvent is essential for maximising yields and preserving bioactivity [37]. Comparisons with scalable anthocyanin recovery show that SC-CO2 and hybrid systems can match or exceed solvent-based approaches in activity while reducing environmental burden [35]. These benefits position SC-CO2 within the broader context of circular bioeconomy valorisation strategies, where fruit by-products are increasingly viewed as sources of natural antioxidants and preservatives [38].
Mass yield data were not quantified, and extraction yield (gravimetric recovery) was not reported in this study. The pilot-scale, closed-loop SC-CO2 system was configured for compositional endpoints under continuous collection in two separators, and runs were not instrumented for accurate per-separator or per-batch gravimetry. Consequently, reliable mass-normalised metrics could not be derived. This limitation does not affect the comparative conclusions on composition and antioxidant readouts, but it precludes yield-normalised reporting; future work will incorporate calibrated collection vessels and full mass balances to complement the compositional data.
Additional insights come from emerging processing technologies. Thermosonication of blackcurrant juices enhanced antioxidant retention and colour quality while reducing microbial load [39]. Such evidence complements our finding that freeze-drying preserved antioxidant capacity more effectively than convective drying, underscoring the importance of non-thermal or gentle pre-treatments in preserving functional quality. Moreover, process modelling of plant bioactives under SC-CO2 confirms that extraction behaviour is governed by interdependencies between solute polarity, fluid density and matrix porosity, further justifying the observed species- and drying-specific outcomes [40].
In sum, the study demonstrates that currant pomaces can be strategically valorised into PUFA-rich, tocopherol-containing fractions with measurable antioxidant potential. The combined interpretation of Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 and Figure 1, together with current literature, supports the conclusion that judicious selection of pre-drying and SC-CO2 parameters allows tailoring of extract functionality. Future work should integrate yield-normalised mass balances, explore scale-up and address bioavailability in vivo to bridge compositional metrics with nutritional efficacy.
5. Conclusions
Under a single, food-grade SC-CO2 regime (500 bar, 30 °C, 180 min), pre-drying was the primary determinant of composition and activity. Freeze-dried extracts (B-6 and R-6) showed higher PUFAs and lower SFAs than convectively dried (B-5 and R-5) counterparts, while MUFAs varied within a narrower band and tended to be higher in blackcurrant. Antioxidant capacity (DPPH) was modestly enhanced in vitro after freeze-drying (lowest IC50 and highest AAE, notably in R-6). TPCs were consistently higher in blackcurrant than redcurrant, with no within-species effect of pre-drying.
Tocopherols measured in post-SFE residues displayed homologue- and species-specific redistribution: in blackcurrant, α-tocopherol was higher after convective drying, whereas γ/δ increased after freeze-drying; in redcurrant the converse pattern was observed. Taken together, the data indicate a trade-off between PUFA enrichment and oxidative robustness (higher PUFAs accompany lower SFAs and higher DPPH), and they highlight complementarity between extracts and residue: the oil phase concentrate unsaturates while the spent matrix retains distinct tocopherol profiles. These findings provide direct composition-based guidance for tailoring currant pomace fractions to application needs (activity versus stability) and support whole-steam utilisation rather than single-product optimisation.
Author Contributions
Conceptualisation, F.H., M.K. and T.K.; methodology, F.H. and T.K.; formal analysis, F.H.; data curation, F.H.; writing—original draft preparation, F.H., M.K. and T.K.; writing—review and editing, F.H.; visualisation, F.H.; supervision, M.K. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to confidentiality and commercialisation provisions arising from a tri-party collaboration/industrial PhD agreement.
Acknowledgments
The fifth edition of the implementation doctorate programme-Ministry of Science and Higher Education.
Conflicts of Interest
Author Filip Herzyk and Tomasz Krusiński were employed by Wroclaw Technology Park. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAE | Ascorbic Acid Equivalent |
| ALA | α-Linoleic acid |
| d.w. | Dry Weight |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FAME | Fatty Acids Methyl Esters |
| GAE | Gallic Acid Equivalent |
| GLA | γ-Linoleic acid |
| LA | Linoleic acid |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
| OA | Oleic acid |
| PA | Palmitic acid |
| QC | Quality Control |
| SA | Stearic acid |
| SC-CO2 | Supercritical-Carbon Dioxide |
| SFE | Supercritical Fluid Extraction |
References
- Darko, H.S.O.; Ismaiel, L.; Fanesi, B.; Pacetti, D.; Lucci, P. Current Trends in Food Processing By-Products as Sources of High Value-Added Compounds in Food Fortification. Foods 2024, 13, 2658. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Pascoalino, L.; Barros, L.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Reis, F.S. Closing the Loop: Exploring Apple Pomace as a Source of Bioactive Compounds in the Framework of Circular Economy. Sustain. Food Technol. 2025, 3, 81–95. [Google Scholar] [CrossRef]
- Peña-Portillo, G.-C.; Acuña-Nelson, S.-M.; Bastías-Montes, J.-M. From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products—A Review. Antioxidants 2024, 13, 992. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Akram, M.U.; Rybak, K.; Witrowa-Rajchert, D.; Nowacka, M. Bioactive Compounds from Plants and By-Products: Novel Extraction Methods, Applications, and Limitations. AIMS Mol. Sci. 2024, 11, 150–188. [Google Scholar] [CrossRef]
- Untea, A.E.; Oancea, A.-G.; Vlaicu, P.A.; Varzaru, I.; Saracila, M. Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization before and after In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation. Foods 2024, 13, 1514. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Oancea, A.G.; Varzaru, I.; Vlaicu, P.A. Comparative Analysis of Black Chokeberry (Aronia melanocarpa L.) Fruit, Leaves, and Pomace for Their Phytochemical Composition, Antioxidant Potential, and Polyphenol Bioaccessibility. Foods 2024, 13, 1856. [Google Scholar] [CrossRef]
- Cegiełka, A. An Attempt to Use Black Chokeberry Pomace in the Production of Hamburgers. Food Biotechnol. Agric. Sci. 2024, 78, 68–73. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. [Google Scholar] [CrossRef]
- Nawirska–Olszańska, A.; Oziembłowski, M. Fruit and Vegetable Processing Waste as Potential Raw Material for Food Enrichment with Dietary Fiber. Food Sci. Nutr. 2025, 13, e70766. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food By-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Cao, S.; Liang, J.; Chen, M.; Xu, C.; Wang, X.; Qiu, L.; Zhao, X.; Hu, W. Comparative Analysis of Extraction Technologies for Plant Extracts and Absolutes. Front. Chem. 2025, 13, 1536590. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Peron, G. Green Extraction and Valorisation of Bioactive Compounds from Food and Food Waste. Appl. Sci. 2024, 14, 11619. [Google Scholar] [CrossRef]
- da Silva Bastos, K.V.L.; de Souza, A.B.; Tomé, A.C.; Souza, F. de M. New Strategies for the Extraction of Antioxidants from Fruits and Their By-Products: A Systematic Review. Plants 2025, 14, 755. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Process 2021, 9, 357. [Google Scholar] [CrossRef]
- Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. [Google Scholar] [CrossRef]
- Oro, C.E.D.; Wancura, J.H.C.; dos Santos, M.S.N.; Venquiaruto, L.D.; Dallago, R.M.; Tres, M.V. High-Pressure Extraction Techniques for Efficient Recovery of Flavonoids and Coumarins from Flower Seeds. Processes 2025, 13, 300. [Google Scholar] [CrossRef]
- Fahrudin, F.I.; Phongthai, S.; Wirjantoro, T.I.; Intipunya, P. Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts. Foods 2025, 14, 2189. [Google Scholar] [CrossRef]
- Nastić, N.; Mazumder, J.A.; Banat, F. Supercritical CO2 Extraction of Oil from Fruit Seed By-Product: Advances, Challenges, and Pathways to Commercial Viability. Crit. Rev. Food Sci. Nutr. 2025, 146, 122–135. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative Methods of Bioactive Compounds and Oils Extraction from Berry Fruit By-Products—A Review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Ballistreri, G.; Amenta, M.; Fabroni, S.; Timpanaro, N.; Platania, G.M. Sustainable Extraction Protocols for the Recovery of Bioactive Compounds from By-Products of Pomegranate Fruit Processing. Foods 2024, 13, 1793. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Junior, T.K.; de Moura, C.; Cruz, T.M.; Marques, M.B.; do Carmo, M.A.V.; Deolindo, C.T.P.; Daguer, H.; Azevedo, L.; Granato, D. Optimization of the Green Chemistry-like Extraction of Phenolic Compounds from Grape (Vitis labrusca L.) and Blackberry (Rubus fruticosus L.) Seeds with Concomitant Biological and Antioxidant Activity Assessments. Plants 2023, 12, 2618. [Google Scholar] [CrossRef]
- Tourabi, M.; Faiz, K.; Ezzouggari, R.; Louasté, B.; Merzouki, M.; Dauelbait, M.; Bourhia, M.; Almaary, K.S.; Siddique, F.; Lyoussi, B.; et al. Optimization of Extraction Process and Solvent Polarities to Enhance the Recovery of Phytochemical Compounds, Nutritional Content, and Biofunctional Properties of Mentha longifolia L. Extracts. Bioresour. Bioprocess. 2025, 12, 24. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-Industrial By-Products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Raj, B.; Seetharam, D.S.; Patil, S.J. Green Extraction of Bioactive Compounds from Marine Constituents. Scr. Medica 2025, 56, 329–341. [Google Scholar] [CrossRef]
- Morton, L.C.; Paton, C.D.; Aberkane, R.; Braakhuis, A.J. No Effect of Acute or Chronic New Zealand Blackcurrant Extract on Cycling Performance or Physiological Responses in Trained Cyclists. Eur. J. Sport Sci. 2025, 25, e12267. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 14, 152–178. [Google Scholar] [CrossRef]
- Laczkó-Zöld, E.; Komlósi, A.; Ülkei, T.; Fogarasi, E.; Croitoru, M.; Fülöp, I.; Domokos, E.; Ştefănescu, R.; Varga, E. Extractability of Polyphenols from Black Currant, Red Currant and Gooseberry and Their Antioxidant Activity. Acta Biologica Hungarica 2018, 69, 156–169. [Google Scholar] [CrossRef] [PubMed]
- ISO 12966:2-2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters (Part 2: Preparation of Methyl Esters of Fatty Acids). British Standards Institution: London, UK, 2017. Available online: https://www.iso.org/standard/72142.html (accessed on 25 September 2025).
- Blejan, A.M.; Nour, V.; Păcularu-Burada, B.; Popescu, S.M. Wild Bilberry, Blackcurrant, and Blackberry By-Products as a Source of Nutritional and Bioactive Compounds. Int. J. Food Prop. 2023, 26, 1579–1595. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Andlid, T.; Lundin, L.; Ahrné, L.; Alminger, M. Supercritical Fluid Extraction of Berry Seeds: Chemical Composition and Antioxidant Activity. J. Food Qual. 2018, 2018, 6046074. [Google Scholar] [CrossRef]
- Goffman, F.D.; Galletti, S. Gamma-Linolenic Acid and Tocopherol Contents in the Seed Oil of 47 Accessions from Several Ribes Species. J. Agric. Food Chem. 2000, 49, 349–354. [Google Scholar] [CrossRef]
- Wójciak, M.; Mazurek, B.; Tyśkiewicz, K.; Kondracka, M.; Wójcicka, G.; Blicharski, T.; Sowa, I. Blackcurrant (Ribes nigrum L.) Seeds—A Valuable Byproduct for Further Processing. Molecules 2022, 27, 8679. [Google Scholar] [CrossRef]
- Costa, D.; Rupasinghe, H.P.V. Development of a Scalable Extraction Process for Anthocyanins of Haskap Berry (Lonicera caerulea). Molecules 2025, 30, 1071. [Google Scholar] [CrossRef]
- Bada, J.C.; León-Camacho, M.; Copovi, P.; Alonso, L. Characterization of Berry and Currant Seed Oils from Asturias, Spain. Int. J. Food Prop. 2013, 17, 77–85. [Google Scholar] [CrossRef][Green Version]
- Muhammad, D.R.A.; Ayouaz, S.; Rachmawati, A.N.; Madani, K.; Fibri, D.L.N.; Rafi, M.; Julianti, E.; Fahmy, K. Advanced and Potential Methods for Extraction of Bioactive Compounds from Avocado Peel—A Review. Appl. Sci. 2024, 14, 6018. [Google Scholar] [CrossRef]
- Sabzal, I.; Shehzadi, F.; Sabzal, N.; Hassan, S.A.; Arif, M.A.; Aslam, N.; Mousavi Khaneghah, A.; Aadil, R.M. Transforming Fruit Waste into Meat Preserving Agents: Harnessing Natural Extracts to Enhance Meat Quality and Extend Shelf Life. J. Agric. Food Res. 2025, 21, 101973. [Google Scholar] [CrossRef]
- Qiu, X.; Su, J.; Nie, J.; Zhang, Z.; Ren, J.; Wang, S.; Pei, Y.; Li, X. Effects of Thermosonication on the Antioxidant Capacity and Physicochemical, Bioactive, Microbiological, and Sensory Qualities of Blackcurrant Juice. Foods 2024, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Chammam, A.; Smirnova, I.; Fillaudeau, L.; Romdhane, M.; Zetzl, C.; Bouajila, J. Supercritical CO2 Extraction of Bioactives from P. Halepensis Petals: Process Modeling, Mass Transfer, and Bioactivity Characterization. J. Supercrit. Fluids 2025, 225, 106701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).