A Holistic Review of Cannabis and Its Potential Risks and Benefits in Mental Health
Abstract
1. Introduction
2. Method
3. Historical Context of Cannabis
4. Basic Aspects of Cannabis
4.1. Cannabinoid Constituents of Cannabis
4.2. Routes of Consumption
5. Pharmacological Effects of Cannabis
Cannabis Pharmacokinetics
6. Negative Impacts of Cannabis on Physiological and Mental Health
6.1. Cannabis Use and Physiological Outcomes
6.2. Cannabis Use and Mental Health Outcomes
7. Cannabinoid Drugs in the Treatment of Mental Health Disorders
7.1. Epilepsy and Related Syndromes
7.2. Neurodegenerative Diseases
7.3. Neurodevelopmental Disorders
7.4. Bipolar Disorder
7.5. Anxiety Disorders and Post-Traumatic Stress Disorder
7.6. Sleep Disturbances
7.7. Chronic Pain
7.8. Fibromyalgia
7.9. Migraine
7.10. Schizophrenia
8. Gut Microbiome and Cannabinoids: The Role of Microbial Products
8.1. Cannabis Use and Gut Microbiome Dysbiosis
8.2. Intermediate Bacterial Metabolites
8.3. Signaling Molecules
8.4. Neural Pathways
9. Cannabis Use Disorder and Its Treatment
9.1. Prevalence of Cannabis Use Disorder
9.2. Common Comorbidities
9.3. Treatment
10. Social Perspectives on the Legalization of Cannabis
11. Discussion
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| eCBS | Endocannabinoid system |
| CUD | Cannabis use disorder |
| SUD | Substance use disorder |
| CNS | Central nervous system |
| BBB | Blood–brain barrier |
| HPA | Hypothalamic–pituitary–adrenal |
| GM | Gut microbiome |
| THCs | Tetrahydrocannabinols |
| Δ8-THCs | (−)-Δ8-trans-tetrahydrocannabinols |
| Δ9-THCs | (−)-Δ9-trans-tetrahydrocannabinols |
| eCBome | Endocannabinoidome |
| 2-AcGs | 2-acylglycerols |
| 2-AG | 2-arachidonoylglycerol |
| AEA | N-arachidonyl-ethanolamide |
| NAEs | N-acyl ethanolamines |
| CBCs | Cannabichromenes |
| CBLs | Cannabicyclols |
| CBDs | Cannabidiols |
| CBEs | Cannabielsoins |
| CBGs | Cannabigerols |
| CBNDs | Cannabinodiols |
| CBNs | Cannabinols |
| CBTs | Cannabitriols |
| BAs | Bile acids |
| GFA | Grifolic acid |
| KYNA | Kynurenic acid |
| DCA | Daurichromenic acid |
| SCFAs | Short-chain fatty acids |
| GABA | γ-aminobutyric acid |
| 5-HT | Serotonin |
| BDNF | Brain-derived neurotrophic factor |
| GPCR | G protein-coupled receptor |
| PPARs | Peroxisome proliferator-activated nuclear receptors |
| AN | Anorexia nervosa |
| ADHD | Attention-deficit/Hyperactivity disorder |
| ASD | Autism spectrum disorder |
| BED | Binge eating disorder |
| BD | Bipolar disorder |
| NDDs | Neurodevelopmental disorders |
| PTSD | Post-traumatic stress disorder |
| CgBT | Cognitive behavioral therapy |
| MET | Motivational enhancement therapy |
References
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef] [PubMed]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic potential of cannabis, cannabidiol, and cannabinoid-based pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.P.; Stjepanović, D.; Le Foll, B.; Hoch, E.; Budney, A.J.; Hall, W.D. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Primers 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Artamendi, S.; Fernández-Hermida, J.R.; Secades-Villa, R.; García-Portilla, M.P. Cannabis y salud mental. Actas Esp. Psiquiatr. 2011, 39, 180–190. [Google Scholar]
- Crocq, M.A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Escohotado, A. Historia General de las Drogas; Espasa Calpe: Madrid, España, 1998. [Google Scholar]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Jiang, H.E.; Li, X.; Zhao, Y.X.; Ferguson, D.K.; Hueber, F.; Bera, S.; Wang, Y.F.; Zhao, L.C.; Liu, C.J.; Li, C.S. A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J. Ethnopharmacol. 2006, 108, 414–422. [Google Scholar] [CrossRef]
- Pisanti, S.; Bifulco, M. Modern history of medical cannabis: From widespread use to prohibitionism and back. Trends Pharmacol. Sci. 2017, 38, 195–198. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse. Monitoring the Future. Secondary School Students; U.S. Department of Health and Human Services: Washington, DC, USA, 2009.
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, bioactivities, and biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef]
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A comprehensive review on Cannabis sativa ethnobotany, phytochemistry, molecular docking and biological activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef]
- Nazir, M.; Saleem, M.; Tousif, M.I.; Anwar, M.A.; Surup, F.; Ali, I.; Wang, D.; Mamadalieva, N.Z.; Alshammari, E.; Ashour, M.L.; et al. Meroterpenoids: A comprehensive update insight on structural diversity and biology. Biomolecules 2021, 11, 957. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. BioMed. Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef]
- Taura, F.; Iijima, M.; Lee, J.B.; Hashimoto, T.; Asakawa, Y.; Kurosaki, F. Daurichromenic acid-producing oxidocyclase in the young leaves of Rhododendron dauricum. Nat. Prod. Commun. 2014, 9, 1329–1332. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Asbridge, M.; Duff, C.; Marsh, D.C.; Erickson, P.G. Problems with the identification of ‘problematic’ cannabis use: Examining the issues of frequency, quantity, and drug use environment. Eur. Addict. Res. 2014, 20, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, E.; Craft, S.; Calder, R.; Hammond, D. Prevalence and use of cannabis products and routes of administration among youth and young adults in Canada and the United States: A systematic review. Addict. Behav. 2022, 129, 107258. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Streck, J.M.; Hughes, J.R.; Klemperer, E.M.; Howard, A.B.; Budney, A.J. Modes of cannabis use: A secondary analysis of an intensive longitudinal natural history study. Addict. Behav. 2019, 98, 106033. [Google Scholar] [CrossRef]
- Russell, C.; Rueda, S.; Room, R.; Tyndall, M.; Fischer, B. Routes of administration for cannabis use—Basic prevalence and related health outcomes: A scoping review and synthesis. Int. J. Drug Policy 2018, 52, 87–96. [Google Scholar] [CrossRef]
- Chaiton, M.; Kundu, A.; Rueda, S.; Di Ciano, P. Are vaporizers a lower-risk alternative to smoking cannabis? Can. J. Public Health 2022, 113, 293–296. [Google Scholar] [CrossRef]
- Spindle, T.R.; Cone, E.J.; Schlienz, N.J.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Hayes, E.; Vandrey, R. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: A crossover trial. JAMA Netw. Open 2018, 1, e184841. [Google Scholar] [CrossRef]
- Vandrey, R.; Herrmann, E.S.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; LoDico, C.; Cone, E.J. Pharmacokinetic profile of oral cannabis in humans: Blood and oral fluid disposition and relation to pharmacodynamic outcomes. J. Anal. Toxicol. 2017, 41, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Fingar, J.R.; Budney, A.J.; Naud, S.; Helzer, J.E.; Callas, P.W. Marijuana use and intoxication among daily users: An intensive longitudinal study. Addict. Behav. 2014, 39, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.D.; Haney, M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol. Depend. 2009, 103, 107–113. [Google Scholar] [CrossRef]
- Spindle, T.R.; Cone, E.J.; Schlienz, N.J.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Hayes, E.; Vandrey, R. Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J. Anal. Toxicol. 2019, 43, 233–258. [Google Scholar] [CrossRef]
- Leos-Toro, C.; Fong, G.T.; Meyer, S.B.; Hammond, D. Cannabis labelling and consumer understanding of THC levels and serving sizes. Drug Alcohol. Depend. 2020, 208, 107843. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Favrat, B.; Ménétrey, A.; Augsburger, M.; Rothuizen, L.E.; Appenzeller, M.; Buclin, T.; Pin, M.; Mangin, P.; Giroud, C. Two cases of “cannabis acute psychosis” following the administration of oral cannabis. BMC Psychiatry 2005, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Jaber-Filho, J.A.; Bento, R.M.; Damasceno, L.M.; Aquino-Neto, F.R. Delirium following ingestion of marijuana present in chocolate cookies. CNS Spectr. 2006, 11, 262–264. [Google Scholar] [CrossRef]
- Iglesias-Lepine, M.L.; Manzur-Cavalloti, I.; Epelde, F.; García-Gibert, L. Intoxicación aguda por leche de marihuana. Med. Clin. 2015, 144, 381–382. [Google Scholar] [CrossRef]
- Rezkalla, S.; Kloner, R.A. Cardiovascular effects of marijuana. Trends Cardiovasc. Med. 2019, 29, 403–407. [Google Scholar] [CrossRef]
- Chiu, R.G.; Fuentes, A.M.; Patil, S.N.; Chiu, R.; McGuire, L.S.; Mehta, A.I. Cannabis abuse and perioperative complications after treatment of intracranial aneurysms: A nationwide analysis. World Neurosurg. 2022, 158, e184–e195. [Google Scholar] [CrossRef] [PubMed]
- Hartung, B.; Kauferstein, S.; Ritz-Timme, S.; Daldrup, T. Sudden unexpected death under acute influence of cannabis. Forensic Sci. Int. 2014, 237, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Steigerwald, S.; Wong, P.O.; Khorasani, A.; Keyhani, S. The form and content of cannabis products in the United States. J. Gen. Intern. Med. 2018, 33, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Lutz, B.; Ruiz de Azua, I. The microbiome and gut endocannabinoid system in the regulation of stress responses and metabolism. Front. Cell. Neurosci. 2022, 16, 867267. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and other non-psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: Useful nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Zumbrun, E.E.; Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Epigenetic regulation of immunological alterations following prenatal exposure to marijuana cannabinoids and its long term consequences in offspring. J. Neuroimmune Pharmacol. 2015, 10, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal structure of the human cannabinoid receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef]
- Demuth, D.G.; Molleman, A. Cannabinoid signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef]
- Olah, A.; Szekanecz, Z.; Biro, T. Targeting cannabinoid signaling in the immune system: “High”-ly exciting questions, possibilities, and challenges. Front. Immunol. 2017, 8, 1487. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Aran, A.; Eylon, M.; Harel, M.; Polianski, L.; Nemirovski, A.; Tepper, S.; Schnapp, A.; Cassuto, H.; Wattad, N.; Tam, J. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol. Autism 2019, 10, 2. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Role of the endocannabinoid system in the pathophysiology of schizophrenia. Mol. Neurobiol. 2017, 54, 768–778. [Google Scholar] [CrossRef]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the endocannabinoid system in the regulation of intestinal homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V. The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 2021, 248, R83–R97. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Wiley, J.W. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology 2016, 151, 252–266. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. 2009, 60, 85–92. [Google Scholar] [CrossRef]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Role of endocannabinoid activation of peripheral CB1 receptors in the regulation of autoimmune disease. Int. Rev. Immunol. 2015, 34, 403–414. [Google Scholar] [CrossRef]
- Gyires, K.; Zádori, Z.S. Role of cannabinoids in gastrointestinal mucosal defense and inflammation. Curr. Neuropharmacol. 2016, 14, 935–951. [Google Scholar] [CrossRef]
- Sanger, G.J.; Alpers, D. Development of drugs for gastrointestinal motor disorders: Translating science to clinical need. Neurogastroenterol. Motil. 2008, 20, 177–184. [Google Scholar] [CrossRef]
- Roche, M.; Finn, D.P. Brain CB2 receptors: Implications for neuropsychiatric disorders. Pharmaceuticals 2010, 3, 2517–2553. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.L.; Duncan, M.; Sharkey, K.A. Cannabinoid CB2 receptors in the gastrointestinal tract: A regulatory system in states of inflammation. Br. J. Pharmacol. 2008, 153, 263–270. [Google Scholar] [CrossRef]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappa B-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 2004, 173, 2373–2382. [Google Scholar] [CrossRef]
- Hegde, V.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur. J. Immunol. 2010, 40, 3358–3371. [Google Scholar] [CrossRef]
- Klegeris, A.; Bissonnette, C.J.; McGeer, P.L. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br. J. Pharmacol. 2003, 139, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.; Nagarkatti, M.; Nagarkatti, P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clin. Immunol. 2007, 122, 259–270. [Google Scholar] [CrossRef]
- McKallip, R.J.; Lombard, C.; Fisher, M.; Martin, B.R.; Ryu, S.; Grant, S.; Nagarkatti, P.S.; Nagarkatti, M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 2002, 100, 627–634. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. The endocannabinoidome as a substrate for noneuphoric phytocannabinoid action and gut microbiome dysfunction in neuropsychiatric disorders. Dialogues Clin. Neurosci. 2020, 22, 259–269. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Chiarotti, M.; Costamagna, L. Analysis of 11-nor-9-carboxy-delta(9)-tetrahydrocannabinol in biological samples by gas chromatography tandem mass spectrometry (GC/MS-MS). Forensic Sci. Int. 2000, 114, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Madea, B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther. Drug Monit. 2006, 28, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chayasirisobhon, S. Mechanisms of action and pharmacokinetics of cannabis. Perm. J. 2020, 25, 1–3. [Google Scholar] [CrossRef]
- Sharma, P.; Murthy, P.; Bharath, M.M. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149–156. [Google Scholar]
- Zendulka, O.; Dovrtelova, G.; Noskova, K.; Turjap, M.; Sulcova, A.; Hanus, L.; Juřica, J. Cannabinoids and cytochrome P450 interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef]
- Almogi-Hazan, O.; Or, R. Cannabis, the endocannabinoid system and immunity—The journey from the bedside to the bench and back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Bayazit, H.; Selek, S.; Karababa, I.F.; Cicek, E.; Aksoy, N. Evaluation of oxidant/antioxidant status and cytokine levels in patients with cannabis use disorder. Clin. Psychopharmacol. Neurosci. 2017, 15, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Jelliffe-Pawlowski, L.; Schumacher, B.; Baer, R.J.; Felder, J.N.; Fuchs, J.D.; Oltman, S.P.; Steurer, M.A.; Marienfeld, C. Cannabis-related diagnosis in pregnancy and adverse maternal and infant outcomes. Drug Alcohol Depend. 2021, 225, 108757. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Jaques, S.C.; Kingsbury, A.; Henshcke, P.; Chomchai, C.; Clews, S.; Falconer, J.; Abdel-Latif, M.E.; Feller, J.M.; Oei, J.L. Cannabis, the pregnant woman and her child: Weeding out the myths. J. Perinatol. 2014, 34, 417–424. [Google Scholar] [CrossRef]
- Goldschmidt, L.; Richardson, G.A.; Willford, J.; Day, N.L. Prenatal marijuana exposure and intelligence test performance at age 6. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 254–263. [Google Scholar] [CrossRef]
- Goldschmidt, L.; Richardson, G.A.; Willford, J.A.; Severtson, S.G.; Day, N.L. School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol. Teratol. 2012, 34, 161–167. [Google Scholar] [CrossRef]
- Gray, T.R.; Eiden, R.D.; Leonard, K.E.; Connors, G.J.; Shisler, S.; Huestis, M.A. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin. Chem. 2010, 56, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, A.C.; Flanagan, R.; Kuo, B.; Staller, K. Relationship between recreational marijuana use and bowel function in a nationwide cohort study. Am. J. Gastroenterol. 2019, 114, 1894–1903. [Google Scholar] [CrossRef]
- Tartakover Matalon, S.; Azar, S.; Meiri, D.; Hadar, R.; Nemirovski, A.; Abu Jabal, N.; Konikoff, F.M.; Drucker, L.; Tam, J.; Naftali, T. Endocannabinoid levels in ulcerative colitis patients correlate with clinical parameters and are affected by cannabis consumption. Front. Endocrinol. 2021, 12, 685289. [Google Scholar] [CrossRef]
- Ghasemiesfe, M.; Barrow, B.; Leonard, S.; Keyhani, S.; Korenstein, D. Association between marijuana use and risk of cancer: A systematic review and meta-analysis. JAMA Netw. Open. 2019, 2, e1916318. [Google Scholar] [CrossRef]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology 2018, 43, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Maslov, L.N.; Singh, N.; Jaggi, A.S. Dual role of T-type calcium channels in anxiety-related behavior. J. Basic. Clin. Physiol. Pharmacol. 2020, 31, 20190067. [Google Scholar] [CrossRef]
- Baral, A.; Hanna, F.; Chimoriya, R.; Rana, K. Cannabis use and its impact on mental health in youth in Australia and the United States: A scoping review. Epidemiologia 2024, 5, 106–121. [Google Scholar] [CrossRef]
- Monteleone, P.; Matias, I.; Martiadis, V.; De Petrocellis, L.; Maj, M.; Di Marzo, V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 2005, 30, 1216–1221. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Di Marzo, V.; Aveta, T.; Piscitelli, F.; Dalle Grave, R.; Scognamiglio, P.; El Ghoch, M.; Calugi, S.; Monteleone, P.; Maj, M. Deranged endocannabinoid responses to hedonic eating in underweight and recently weight-restored patients with anorexia nervosa. Am. J. Clin. Nutr. 2015, 101, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, A.M.; Piscitelli, F.; Dalle Grave, R.; El Ghoch, M.; Di Marzo, V.; Maj, M.; Monteleone, P. Peripheral endocannabinoid responses to hedonic eating in binge-eating disorder. Nutrients 2017, 9, 1377. [Google Scholar] [CrossRef]
- Murphy, T.; Le Foll, B. Targeting the endocannabinoid CB1 receptor to treat body weight disorders: A preclinical and clinical review of the therapeutic potential of past and present CB1 drugs. Biomolecules 2020, 10, 855. [Google Scholar] [CrossRef]
- McDonald, A.J.; Kurdyak, P.; Rehm, J.; Roerecke, M.; Bondy, S.J. Age-dependent association of cannabis use with risk of psychotic disorder. Psychol. Med. 2024, 54, 2926–2936. [Google Scholar] [CrossRef] [PubMed]
- Borgan, F.; Kokkinou, M.; Howes, O. The cannabinoid CB1 receptor in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 646–659. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Parolaro, D. The endocannabinoid system and autism spectrum disorders: Insights from animal models. Int. J. Mol. Sci. 2017, 18, 1916. [Google Scholar] [CrossRef]
- Karhson, D.S.; Krasinska, K.M.; Dallaire, J.A.; Libove, R.A.; Phillips, J.M.; Chien, A.S.; Garner, J.P.; Hardan, A.Y.; Parker, K.J. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol. Autism 2018, 9, 18. [Google Scholar] [CrossRef]
- Wu, H.F.; Lu, T.Y.; Chu, M.C.; Chen, P.S.; Lee, C.W.; Lin, H.C. Targeting the inhibition of fatty acid amide hydrolase ameliorate the endocannabinoid-mediated synaptic dysfunction in a valproic acid-induced rat model of autism. Neuropharmacology 2020, 162, 107736. [Google Scholar] [CrossRef]
- Centonze, D.; Bari, M.; Di Michele, B.; Rossi, S.; Gasperi, V.; Pasini, A.; Battista, N.; Bernardi, G.; Curatolo, P.; Maccarrone, M. Altered anandamide degradation in attention-deficit/hyperactivity disorder. Neurology 2009, 72, 1526–1527. [Google Scholar] [CrossRef]
- Navarro, D.; Gasparyan, A.; Navarrete, F.; Torregrosa, A.B.; Rubio, G.; Marín-Mayor, M.; Acosta, G.B.; Garcia-Gutiérrez, M.S.; Manzanares, J. Molecular alterations of the endocannabinoid system in psychiatric disorders. Int. J. Mol. Sci. 2022, 23, 4764. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Carra, A.; Macias, M.A. Cannabis and multiple sclerosis. Expert Rev. Neurother. 2020, 20, 849–854. [Google Scholar] [CrossRef]
- Grotenhermen, F.; Müller-Vahl, K. Medicinal uses of marijuana and cannabinoids. Crit. Rev. Plant Sci. 2016, 35, 378–405. [Google Scholar] [CrossRef]
- Mandelbaum, D.E.; de la Monte, S.M. Adverse structural and functional effects of marijuana on the brain: Evidence reviewed. Pediatr. Neurol. 2017, 66, 12–20. [Google Scholar] [CrossRef]
- Albertella, L.; Le Pelley, M.E.; Copeland, J. Cannabis use in early adolescence is associated with higher negative schizotypy in females. Eur. Psychiatry 2017, 45, 235–241. [Google Scholar] [CrossRef]
- Bahorik, A.L.; Leibowitz, A.; Sterling, S.A.; Travis, A.; Weisner, C.; Satre, D.D. Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J. Affect. Disord. 2017, 213, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, J.; Simpson, T.; White, H.R.; Pardini, D. Chronic adolescent marijuana use as a risk factor for physical and mental health problems in young adult men. Psychol. Addict. Behav. 2015, 29, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Chadi, N.; Li, G.; Cerda, N.; Weitzman, E.R. Depressive symptoms and suicidality in adolescents using e-cigarettes and marijuana: A secondary data analysis from the youth risk behavior survey. J. Addict. Med. 2019, 13, 362–365. [Google Scholar] [CrossRef]
- Danielsson, A.K.; Lundin, A.; Agardh, E.; Allebeck, P.; Forsell, Y. Cannabis use, depression and anxiety: A 3-year prospective population-based study. J. Affect. Disord. 2016, 193, 103–108. [Google Scholar] [CrossRef]
- Feingold, D.; Weiser, M.; Rehm, J.; Lev-Ran, S. The association between cannabis use and anxiety disorders: Results from a population-based representative sample. Eur. Neuropsychopharmacol. 2016, 26, 493–505. [Google Scholar] [CrossRef]
- Floyd Campbell, L. Depression and marijuana use among a sample of urban females: Is stage of development important? Subst. Use Misuse 2018, 53, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Compton, W.M.; Einstein, E.B.; Volkow, N.D. Associations of suicidality trends with cannabis use as a function of sex and depression status. JAMA Netw. Open 2021, 4, e2113025. [Google Scholar] [CrossRef]
- Horwood, L.J.; Fergusson, D.M.; Coffey, C.; Patton, G.C.; Tait, R.; Smart, D.; Letcher, P.; Silins, E.; Hutchinson, D.M. Cannabis and depression: An integrative data analysis of four Australasian cohorts. Drug Alcohol Depend. 2012, 126, 369–378. [Google Scholar] [CrossRef]
- Kim, S.W.; Dodd, S.; Berk, L.; Kulkarni, J.; de Castella, A.; Fitzgerald, P.B.; Kim, J.M.; Yoon, J.S.; Berk, M. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015, 12, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, B.J.; Ames, M.E.; Linden-Carmichael, A.N. Age-varying effects of cannabis use frequency and disorder on symptoms of psychosis, depression and anxiety in adolescents and adults. Addiction 2019, 114, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Weitzman, E.R. Acute mental health symptoms in adolescent marijuana users. JAMA Pediatr. 2019, 173, 185–186. [Google Scholar] [CrossRef]
- London-Nadeau, K.; Rioux, C.; Parent, S.; Vitaro, F.; Côté, S.M.; Boivin, M.; Tremblay, R.E.; Séguin, J.R.; Castellanos-Ryan, N. Longitudinal associations of cannabis, depression, and anxiety in heterosexual and LGB adolescents. J. Abnorm. Psychol. 2021, 130, 333–345. [Google Scholar] [CrossRef]
- Meier, M.H.; Beardslee, J.; Pardini, D. Associations between recent and cumulative cannabis use and internalizing problems in boys from adolescence to young adulthood. J. Abnorm. Child Psychol. 2020, 48, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Moitra, E.; Anderson, B.J.; Stein, M.D. Reductions in cannabis use are associated with mood improvement in female emerging adults. Depress. Anxiety 2016, 33, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Galán, R.; Lana-Lander, I.; Coronado, M.; Segura, L.; Colom, J. Association between cannabis use disorder and mental health disorders in the adolescent population: A cohort study. Eur. Addict. Res. 2023, 29, 344–352. [Google Scholar] [CrossRef]
- Otten, R.; Huizink, A.C.; Monshouwer, K.; Creemers, H.E.; Onrust, S. Cannabis use and symptoms of anxiety in adolescence and the moderating effect of the serotonin transporter gene. Addict. Biol. 2017, 22, 1081–1089. [Google Scholar] [CrossRef]
- Patel, R.; Wilson, R.; Jackson, R.; Ball, M.; Shetty, H.; Broadbent, M.; Stewart, R.; McGuire, P.; Bhattacharyya, S. Cannabis use and treatment resistance in first episode psychosis: A natural language processing study. Lancet 2015, 385, S79. [Google Scholar] [CrossRef]
- Phillips, K.T.; Phillips, M.M.; Duck, K.D. Factors associated with marijuana use and problems among college students in Colorado. Subst. Use Misuse 2018, 53, 477–483. [Google Scholar] [CrossRef]
- Rabin, R.A.; Barr, M.S.; Goodman, M.S.; Herman, Y.; Zakzanis, K.K.; Kish, S.J.; Kiang, M.; Remington, G.; George, T.P. Effects of extended cannabis abstinence on cognitive outcomes in cannabis dependent patients with schizophrenia vs non-psychiatric controls. Neuropsychopharmacology 2017, 42, 2259–2271. [Google Scholar] [CrossRef]
- Rabin, R.A.; Kozak, K.; Zakzanis, K.K.; Remington, G.; George, T.P. Effects of extended cannabis abstinence on clinical symptoms in cannabis dependent schizophrenia patients versus non-psychiatric controls. Schizophr. Res. 2018, 194, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rasic, D.; Weerasinghe, S.; Asbridge, M.; Langille, D.B. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug Alcohol Depend. 2013, 129, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Pugh, B.S.; Ball, S.A. Assessing the risk of marijuana use disorder among adolescents and adults who use marijuana. Am. J. Drug Alcohol Abus. 2017, 43, 247–260. [Google Scholar] [CrossRef]
- Sagar, K.A.; Dahlgren, M.K.; Racine, M.T.; Dreman, M.W.; Olson, D.P.; Gruber, S.A. Joint effects: A pilot investigation of the impact of bipolar disorder and marijuana use on cognitive function and mood. PLoS ONE 2016, 11, e0157060. [Google Scholar] [CrossRef]
- Schoeler, T.; Theobald, D.; Pingault, J.B.; Farrington, D.P.; Coid, J.W.; Bhattacharyya, S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: Findings from 40 years of follow-up. Psychol. Med. 2018, 48, 2169–2176. [Google Scholar] [CrossRef]
- Scholes-Balog, K.E.; Hemphill, S.A.; Patton, G.C.; Toumbourou, J.W. Cannabis use and related harms in the transition to young adulthood: A longitudinal study of Australian secondary school students. J. Adolesc. 2013, 36, 519–527. [Google Scholar] [CrossRef]
- Tull, M.T.; McDermott, M.J.; Gratz, K.L. Marijuana dependence moderates the effect of posttraumatic stress disorder on trauma cue reactivity in substance dependent patients. Drug Alcohol Depend. 2016, 159, 219–226. [Google Scholar] [CrossRef]
- Weinberger, A.H.; Zhu, J.; Lee, J.; Anastasiou, E.; Copeland, J.; Goodwin, R.D. Cannabis use among youth in the United States, 2004–2016: Faster rate of increase among youth with depression. Drug Alcohol Depend. 2020, 209, 107894. [Google Scholar] [CrossRef]
- Welsh, J.W.; Knight, J.R.; Hou, S.S.; Malowney, M.; Schram, P.; Sherritt, L.; Boyd, J.W. Association between substance use diagnoses and psychiatric disorders in an adolescent and young adult clinic-based population. J. Adolesc. Health 2017, 60, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Stefanovics, E.; Rosenheck, R.A. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J. Clin. Psychiatry 2015, 76, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.L.; Halpern, C.T.; Herring, A.H.; Shanahan, M.; Ennett, S.T.; Hussey, J.M.; Harris, K.M. Testing longitudinal relationships between binge drinking, marijuana use, and depressive symptoms and moderation by sex. J. Adolesc. Health 2016, 59, 681–687. [Google Scholar] [CrossRef]
- Wong, S.S.; Zhou, B.; Goebert, D.; Hishinuma, E.S. The risk of adolescent suicide across patterns of drug use: A nationally representative study of high school students in the United States from 1999 to 2009. Soc. Psychiatry Psychiatr. Epidemiol. 2013, 48, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Zaman, T.; Malowney, M.; Knight, J.; Boyd, J.W. Co-occurrence of substance-related and other mental health disorders among adolescent cannabis users. J. Addict. Med. 2015, 9, 317–321. [Google Scholar] [CrossRef]
- Zorrilla, I.; Aguado, J.; Haro, J.M.; Barbeito, S.; López Zurbano, S.; Ortiz, A.; López, P.; Gonzalez-Pinto, A. Cannabis and bipolar disorder: Does quitting cannabis use during manic/mixed episode improve clinical/functional outcomes? Acta Psychiatr. Scand. 2015, 131, 100–110. [Google Scholar] [CrossRef]
- Knopf, A. Teen cannabis use increases risk of suicidality and depression during young adulthood. Brown Univ. Child Adolesc. Behav. Lett. 2019, 35, 3–4. [Google Scholar] [CrossRef]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Ware, M.; Marmorstein, N.; Cipriani, A.; Dendukuri, N.; et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: A systematic review and meta-analysis. JAMA Psychiatry 2019, 76, 426–434. [Google Scholar] [CrossRef]
- Power, E.; Sabherwal, S.; Healy, C.; O’Neill, A.; Cotter, D.; Cannon, M. Intelligence quotient decline following frequent or dependent cannabis use in youth: A systematic review and meta-analysis of longitudinal studies. Psychol. Med. 2021, 51, 194–200. [Google Scholar] [CrossRef]
- Sarris, J.; Sinclair, J.; Karamacoska, D.; Davidson, M.; Firth, J. Medicinal cannabis for psychiatric disorders: A clinically-focused systematic review. BMC Psychiatry 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Sexton, M.; Cuttler, C.; Finnell, J.S.; Mischley, L.K. A cross-sectional survey of medical cannabis users: Patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016, 1, 131–138. [Google Scholar] [CrossRef]
- Abizaid, A.; Merali, Z.; Anisman, H. Cannabis: A potential efficacious intervention for PTSD or simply snake oil? J. Psychiatry Neurosci. 2019, 44, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Dokmak, G.; Karaman, R. The efficacy of cannabis on multiple sclerosis-related symptoms. Life 2022, 12, 682. [Google Scholar] [CrossRef]
- Mosley, P.E.; Webb, L.; Suraev, A.; Hingston, L.; Turnbull, T.; Foster, K.; Ballard, E.; Gomes, L.; Mohan, A.; Sachdev, P.S.; et al. Tetrahydrocannabinol and cannabidiol in Tourette syndrome. NEJM Evid. 2023, 2, EVIDoa2300012. [Google Scholar] [CrossRef]
- Beletsky, A.; Liu, C.; Lochte, B.; Samuel, N.; Grant, I. Cannabis and anxiety: A critical review. Med. Cannabis Cannabinoids 2024, 7, 19–30. [Google Scholar] [CrossRef]
- Cassano, T.; Villani, R.; Pace, L.; Carbone, A.; Bukke, V.N.; Orkisz, S.; Avolio, C.; Serviddio, G. From cannabis sativa to cannabidiol: Promising therapeutic candidate for the treatment of neurodegenerative diseases. Front. Pharmacol. 2020, 11, 124. [Google Scholar] [CrossRef]
- Kim, S.H.; Yang, J.W.; Kim, K.H.; Kim, J.U.; Yook, T.H. A review on studies of marijuana for Alzheimer’s disease—Focusing on CBD, THC. J. Pharmacopunct. 2019, 22, 225–230. [Google Scholar] [CrossRef]
- Food and Drug Administration. MarinolR. New Drug Application; FDA: Silver Spring, MD, USA, 2004. [Google Scholar]
- Food and Drug Administration. CesametTM. New Drug Application; FDA: Silver Spring, MD, USA, 2006. [Google Scholar]
- European Medicines Agency. Acomplia; EMA: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Electronic Medicines Compendium. Sativex Oromucosal Spray; EMA: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Food and Drug Administration. Epidiolex. New Drug Application; FDA: Silver Spring, MD, USA, 2018. [Google Scholar]
- Haller, J. Anxiety modulation by cannabinoids—The role of stress responses and coping. Int. J. Mol. Sci. 2023, 24, 15777. [Google Scholar] [CrossRef]
- Hill, M.N.; McEwen, B.S. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 791–797. [Google Scholar] [CrossRef]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Aliczki, M.; Haller, J. Interactions Between Cannabinoid Signaling and Anxiety: A Comparative Analysis of Intervention Tools and Behavioral Effects; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Stasiłowicz-Krzemień, A.; Nogalska, W.; Maszewska, Z.; Maleszka, M.; Dobroń, M.; Szary, A.; Kępa, A.; Żarowski, M.; Hojan, K.; Lukowicz, M.; et al. The use of compounds derived from Cannabis sativa in the treatment of epilepsy, painful conditions, and neuropsychiatric and neurodegenerative disorders. Int. J. Mol. Sci. 2024, 25, 5749. [Google Scholar] [CrossRef]
- Porcari, G.S.; Fu, C.; Doll, E.D.; Carter, E.G.; Carson, R.P. Efficacy of artisanal preparations of cannabidiol for the treatment of epilepsy: Practical experiences in a tertiary medical center. Epilepsy Behav. 2018, 80, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Uliel-Siboni, S.; Linder, I.; Kramer, U.; Epstein, O.; Menascu, S.; Nissenkorn, A.; Yosef, O.B.; Hyman, E.; Granot, D.; et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure 2016, 35, 41–44. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S.; Cannabidiol in Dravet Syndrome Study Group. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V.; et al. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: A randomized clinical trial. JAMA Neurol. 2020, 77, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; Van Landingham, K.E.; et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef]
- Thiele, E.; Marsh, E.; Mazurkiewicz-Beldzinska, M.; Halford, J.J.; Gunning, B.; Devinsky, O.; Checketts, D.; Roberts, C. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia 2019, 60, 419–428. [Google Scholar] [CrossRef]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.P.L.G.; Kiss, A.; Black, S.E.; Lanctôt, K.L. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am. J. Geriatr. Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef]
- van den Elsen, G.A.; Tobben, L.; Ahmed, A.I.; Verkes, R.J.; Kramers, C.; Marijnissen, R.M.; Olde Rikkert, M.G.; van der Marck, M.A. Effects of tetrahydrocannabinol on balance and gait in patients with dementia: A randomised controlled crossover trial. J. Psychopharmacol. 2017, 31, 184–191. [Google Scholar] [CrossRef]
- Sousa, A.; DiFrancisco-Donoghue, J. Cannabidiol and tetrahydrocannabinol use in Parkinson’s disease: An observational pilot study. Cureus 2023, 15, e42391. [Google Scholar] [CrossRef]
- Zajicek, J.P.; Hobart, J.C.; Slade, A.; Barnes, D.; Mattison, P.G.; MUSEC Research Group. Multiple sclerosis and extract of cannabis: Results of the MUSEC trial. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1125–1132. [Google Scholar] [CrossRef]
- Aran, A.; Cassuto, H.; Lubotzky, A.; Wattad, N.; Hazan, E. Brief report: Cannabidiol-rich cannabis in children with Autism Spectrum Disorder and severe behavioral problems—A retrospective feasibility study. J. Autism Dev. Disord. 2019, 49, 1284–1288. [Google Scholar] [CrossRef]
- Barchel, D.; Stolar, O.; De-Haan, T.; Ziv-Baran, T.; Saban, N.; Fuchs, D.O.; Koren, G.; Berkovitch, M. Oral cannabidiol use in children with Autism Spectrum Disorder to treat related symptoms and co-morbidities. Front. Pharmacol. 2019, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Bar-Lev Schleider, L.; Mechoulam, R.; Saban, N.; Meiri, G.; Novack, V. Real life experience of medical cannabis treatment in autism: Analysis of safety and efficacy. Sci. Rep. 2019, 9, 200. [Google Scholar] [CrossRef]
- Fleury-Teixeira, P.; Caixeta, F.V.; Ramires da Silva, L.C.; Brasil-Neto, J.P.; Malcher-Lopes, R. Effects of CBD-enriched Cannabis sativa extract on Autism Spectrum Disorder symptoms: An observational study of 18 participants undergoing compassionate use. Front. Neurol. 2019, 10, 1145. [Google Scholar] [CrossRef]
- Desnous, B.; Beretti, T.; Muller, N.; Neveu, J.; Villeneuve, N.; Lépine, A.; Daquin, G.; Milh, M. Efficacy and tolerance of cannabidiol in the treatment of epilepsy in patients with Rett syndrome. Epilepsia Open 2024, 9, 397–403. [Google Scholar] [CrossRef]
- Abi-Jaoude, E.; Bhikram, T.; Parveen, F.; Levenbach, J.; Lafreniere-Roula, M.; Sandor, P. A double-blind, randomized, controlled crossover trial of cannabis in adults with Tourette syndrome. Cannabis Cannabinoid Res. 2023, 8, 835–845. [Google Scholar] [CrossRef]
- Anis, S.; Zalomek, C.; Korczyn, A.D.; Rosenberg, A.; Giladi, N.; Gurevich, T. Medical cannabis for Gilles de la Tourette syndrome: An open-label prospective study. Behav. Neurol. 2022, 2022, 5141773. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.E.; Williams, E.; Seegobin, S.; Tye, C.; Kuntsi, J.; Asherson, P. Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial. Eur. Neuropsychopharmacol. 2017, 27, 795–808. [Google Scholar] [CrossRef]
- Rasmussen, J.; Casey, B.J.; van Erp, T.G.M.; Tamm, L.; Epstein, J.N.; Buss, C.; Bjork, J.M.; Molina, B.S.G.; Velanova, K.; Mathalon, D.H.; et al. ADHD and cannabis use in young adults examined using fMRI of a Go/NoGo task. Brain Imaging Behav. 2016, 10, 761–771. [Google Scholar] [CrossRef]
- Zuardi, A.; Crippa, J.; Dursun, S.; Morais, S.; Vilela, J.; Sanches, R.; Hallak, J. Cannabidiol was ineffective for manic episode of bipolar affective disorder. J. Psychopharmacol. 2010, 24, 135–137. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.; Chagas, M.H.; de Oliveira, D.C.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.; Lister, R.; Evans, N.; Antley, A.; Englund, A.; Murray, R.M.; Freeman, D.; Morrison, P.D. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. J. Psychopharmacol. 2018, 32, 276–282. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in anxiety and sleep: A large case series. Perm. J. 2019, 23, 18–041. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Sisley, S.; Riggs, P.; Yazar-Klosinski, B.; Wang, J.B.; Loflin, M.J.E.; Shechet, B.; Hennigan, C.; Matthews, R.; Emerson, A.; et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS ONE 2021, 16, e0246990. [Google Scholar] [CrossRef]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the treatment of post-traumatic stress disorder: A case series. J. Altern. Complement. Med. 2019, 25, 392–397. [Google Scholar] [CrossRef]
- Greer, G.R.; Grob, C.S.; Halberstadt, A.L. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. J. Psychoact. Drugs 2014, 46, 73–77. [Google Scholar] [CrossRef]
- Jetly, R.; Heber, A.; Fraser, G.; Boisvert, D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015, 51, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Moltke, J.; Hindocha, C. Reasons for cannabidiol use: A cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. J. Cannabis Res. 2021, 3, 5. [Google Scholar] [CrossRef]
- Shannon, S.; Opila-Lehman, J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: A case report. Perm. J. 2016, 20, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: A randomized, placebo-controlled, graded-dose trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur. Neurol. 2017, 78, 320–329. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Gouaux, B.; Sakai, S.; Donaghe, H. Low-dose vaporized cannabis significantly improves neuropathic pain. J. Pain 2013, 14, 136–148. [Google Scholar] [CrossRef]
- Chaves, C.; Bittencourt, P.C.T.; Pelegrini, A. Ingestion of a THC-rich cannabis oil in people with fibromyalgia: A randomized, double-blind, placebo-controlled clinical trial. Pain Med. 2020, 21, 2212–2218. [Google Scholar] [CrossRef]
- van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, C.; Spradlin, A.; Cleveland, M.J.; Craft, R.M. Short- and long-term effects of cannabis on headache and migraine. J. Pain 2020, 21, 722–730. [Google Scholar] [CrossRef]
- Stith, S.S.; Diviant, J.P.; Brockelman, F.; Keeling, K.; Hall, B.; Lucern, S.; Vigil, J.M. Alleviative effects of cannabis flower on migraine and headache. J. Integr. Med. 2020, 18, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Boggs, D.L.; Surti, T.; Gupta, A.; Gupta, S.; Niciu, M.; Pittman, B.; Schnakenberg Martin, A.M.; Thurnauer, H.; Davies, A.; D’Souza, D.C.; et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 2018, 235, 1923–1932. [Google Scholar] [CrossRef]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef]
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and epilepsy. Neurotherapeutics 2015, 12, 747–768. [Google Scholar] [CrossRef]
- Arachchige, A.S.P.M. Marijuana’s potential in neurodegenerative diseases: An editorial. AIMS Neurosci. 2023, 10, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef] [PubMed]
- Thanabalasingam, S.J.; Ranjith, B.; Jackson, R.; Wijeratne, D.T. Cannabis and its derivatives for the use of motor symptoms in Parkinson’s disease: A systematic review and metaanalysis. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211018561. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Neurodevelopmental disorders associated with gut microbiome dysbiosis in children. Children 2024, 11, 796. [Google Scholar] [CrossRef]
- Milosev, L.M.; Psathakis, N.; Szejko, N.; Jakubovski, E.; Muller-Vahl, K.R. Treatment of Gilles de la Tourette syndrome with cannabis-based medicine: Results from a retrospective analysis and online survey. Cannabis Cannabinoid Res. 2019, 4, 265–274. [Google Scholar] [CrossRef]
- Koppel, B.S. Cannabis in the treatment of dystonia, dyskinesias, and tics. Neurotherapeutics 2015, 12, 788–792. [Google Scholar] [CrossRef]
- Dias-de Freitas, F.; Pimenta, S.; Soares, S.; Gonzaga, D.; Vaz-Matos, I.; Prior, C. The role of cannabinoids in neurodevelopmental disorders of children and adolescents. Rev. Neurol. 2022, 75, 189–197. [Google Scholar] [CrossRef]
- Parrella, N.F.; Hill, A.T.; Enticott, P.G.; Barhoun, P.; Bower, I.S.; Ford, T.C. A systematic review of cannabidiol trials in neurodevelopmental disorders. Pharmacol. Biochem. Behav. 2023, 230, 173607. [Google Scholar] [CrossRef]
- Kosiba, J.D.; Maisto, S.A.; Ditre, J.W. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: Systematic review and meta-analysis. Soc. Sci. Med. 2019, 233, 181–192. [Google Scholar] [CrossRef]
- Schlag, A.K.; O’Sullivan, S.E.; Zafar, R.R.; Nutt, D.J. Current controversies in medical cannabis: Recent developments in human clinical applications and potential therapeutics. Neuropharmacology 2021, 191, 108586. [Google Scholar] [CrossRef]
- Graczyk, M.; Łukowicz, M.; Dzierzanowski, T. Prospects for the use of cannabinoids in psychiatric disorders. Front. Psychiatry 2021, 12, 620073. [Google Scholar] [CrossRef]
- Botsford, S.L.; Yang, S.; George, T.P. Cannabis and cannabinoids in mood and anxiety disorders: Impact on illness onset and course, and assessment of therapeutic potential. Am. J. Addict. 2020, 29, 9–26. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Egerton, A.; Kim, E.; Rosso, L.; Riano Barros, D.; Hammers, A.; Brammer, M.; Turkheimer, F.E.; Howes, O.D.; McGuire, P. Acute induction of anxiety in humans by delta-9-tetrahydrocannabinol related to amygdalar cannabinoid-1 (CB1) receptors. Sci. Rep. 2017, 7, 15025. [Google Scholar] [CrossRef]
- Bisson, J.I.; Cosgrove, S.; Lewis, C.; Robert, N.P. Post-traumatic stress disorder. BMJ 2015, 351, h6161. [Google Scholar] [CrossRef] [PubMed]
- Betthauser, K.; Pilz, J.; Vollmer, L.E. Use and effects of cannabinoids in military veterans with posttraumatic stress disorder. Am. J. Health Syst. Pharm. 2015, 72, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.; Phillips, J.A. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018, 3, 152–161. [Google Scholar] [CrossRef]
- Kaul, M.; Zee, P.C.; Sahni, A.S. Effects of cannabinoids on sleep and their therapeutic potential for sleep disorders. Neurotherapeutics 2021, 18, 217–227. [Google Scholar] [CrossRef]
- Meng, H.; Johnston, B.; Englesakis, M.; Moulin, D.E.; Bhatia, A. Selective cannabinoids for chronic neuropathic pain: A systematic review and meta-analysis. Anesth. Analg. 2017, 125, 1638. [Google Scholar] [CrossRef]
- Strand, N.H.; Maloney, J.; Kraus, M.; Wie, C.; Turkiewicz, M.; Gomez, D.A.; Adeleye, O.; Harbell, M.W. Cannabis for the treatment of fibromyalgia: A systematic review. Biomedicines 2023, 11, 1621. [Google Scholar] [CrossRef]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and pain: New insights from old molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Horz, H.P. Archaeal lineages within the human microbiome: Absent, rare or elusive? Life 2015, 5, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, L.W.; Walters, W.A.; Lauber, C.L.; Clemente, J.C.; Berg-Lyons, D.; Teiling, C.; Kodira, C.; Mohiuddin, M.; Brunelle, J.; Driscoll, M.; et al. Communities of microbial eukaryotes in the mammalian gut within the context of environmental eukaryotic diversity. Front. Microbiol. 2014, 5, 298. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Noverr, M.C. The emerging world of the fungal microbiome. Trends Microbiol. 2013, 21, 334–341. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Stensvold, C.R.; Rajilić-Stojanović, M.; Heilig, H.G.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef]
- Zárate, S.; Taboada, B.; Yocupicio-Monroy, M.; Arias, C.F. Human virome. Arch. Med. Res. 2017, 48, 701–716. [Google Scholar] [CrossRef]
- Mills, S.; Shanahan, F.; Stanton, C.; Hill, C.; Coffey, A.; Ross, R.P. Movers and shakers: Influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes 2013, 4, 4–16. [Google Scholar] [CrossRef]
- Reynoso-García, J.; Miranda-Santiago, A.E.; Meléndez-Vázquez, N.M.; Acosta-Pagán, K.; Sánchez-Rosado, M.; Díaz-Rivera, J.; Rosado-Quiñones, A.M.; Acevedo-Márquez, L.; Cruz-Roldán, L.; Tosado-Rodríguez, E.L.; et al. A complete guide to human microbiomes: Body niches, transmission, development, dysbiosis, and restoration. Front. Syst. Biol. 2022, 2, 951403. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy human gastrointestinal microbiome: Composition and function after a decade of exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Nalage, D.; Kale, R.; Sontakke, T.; Pradhan, V.; Biradar, A.; Senevirathna, J.D.M.; Jaweria, R.; Dighe, T.; Dixit, P.; Patil, R.; et al. Bacterial phyla: Microbiota of kingdom Animalia. Acad. Biol. 2024, 2, 1–19. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Influence of human gut microbiome on the healthy and the neurodegenerative aging. Exp. Gerontol. 2024, 194, 112497. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int. Microbiol. 2025, 128, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Iizumi, T.; Battaglia, T.; Ruiz, V.; Perez Perez, G.I. Gut microbiome and antibiotics. Arch. Med. Res. 2017, 48, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019, 44, 117. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and gut dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef]
- Hrncir, T. Gut microbiota dysbiosis: Triggers, consequences, diagnostic and therapeutic options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Cluny, N.L.; Keenan, C.M.; Reimer, R.A.; Le Foll, B.; Sharkey, K.A. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PLoS ONE 2015, 10, e0144270. [Google Scholar] [CrossRef] [PubMed]
- Castonguay-Paradis, S.; Lacroix, S.; Rochefort, G.; Parent, L.; Perron, J.; Martin, C.; Lamarche, B.; Raymond, F.; Flamand, N.; Di Marzo, V.; et al. Dietary fatty acid intake and gut microbiota determine circulating endocannabinoidome signaling beyond the effect of body fat. Sci. Rep. 2020, 10, 15975. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, J.A.; Hussain, S.K.; Cook, R.; Li, F.; Tobin, N.H.; Ragsdale, A.; Shoptaw, S.; Gorbach, P.M.; Aldrovandi, G.M. Effects of substance use and sex practices on the intestinal microbiome during HIV-1 infection. J. Infect. Dis. 2018, 218, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Panee, J.; Gerschenson, M.; Chang, L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J. Neuroimmune Pharm. 2018, 13, 113–122. [Google Scholar] [CrossRef]
- Vijay, A.; Kouraki, A.; Gohir, S.; Turnbull, J.; Kelly, A.; Chapman, V.; Barrett, D.A.; Bulsiewicz, W.J.; Valdes, A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes 2021, 13, 1997559. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiong, L.; Li, L.; Li, M.; Chen, M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2017, 32, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of cannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigates experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. Brain Behav. Immun. 2019, 82, 25–35. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Alghetaa, H.K.; Zhou, J.; Chatterjee, S.; Nagarkatti, P.; Nagarkatti, M. Protective effects of Δ(9)-tetrahydrocannabinol against enterotoxin-induced acute respiratory distress syndrome are mediated by modulation of microbiota. Br. J. Pharmacol. 2020, 177, 5078–5095. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Ahearn, O.C.; Watson, M.N.; Rawls, S.M. Chemokines, cytokines and substance use disorders. Drug Alcohol Depend. 2021, 220, 108511. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.; Kim, M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Taylor, J.R.; Wolf, M.E.; Shaham, Y. Circuit and synaptic plasticity mechanisms of drug relapse. J. Neurosci. 2017, 37, 10867–10876. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S.; Godino, A.; Peck, E.G.; Salery, M.; Mervosh, N.L.; Landry, J.A.; Russo, S.J.; Hurd, Y.L.; Nestler, E.J.; Kiraly, D.D. Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat. Commun. 2018, 9, 9. [Google Scholar] [CrossRef]
- de Timary, P.; Stärkel, P.; Delzenne, N.M.; Leclercq, S. A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology 2017, 122, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hofford, R.S.; Russo, S.J.; Kiraly, D.D. Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur. J. Neurosci. 2019, 50, 2562–2573. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Epigenetic mechanisms in aging: Extrinsic factors and gut microbiome. Genes 2024, 15, 1599. [Google Scholar] [CrossRef]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.I.; Alexander, D.C.; Riesche, S.L.; Heller, E.A.; Nestler, E.J.; et al. Alcohol metabolism contributes to brain histone acetylation. Nature 2019, 574, 717–721. [Google Scholar] [CrossRef]
- Thomas, S.P.; Denu, J.M. Short-chain fatty acids activate acetyltransferase p300. eLife 2021, 10, e72171. [Google Scholar] [CrossRef]
- Walker, D.M.; Nestler, E.J. Neuroepigenetics and addiction. Handb. Clin. Neurol. 2018, 148, 747–765. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Vilca, S.J.; Margetts, A.V.; Pollock, T.A.; Tuesta, L.M. Transcriptional and epigenetic regulation of microglia in substance use disorders. Mol. Cell. Neurosci. 2023, 125, 103838. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: A complex interconnected loop influencing human health status. Int. J. Tryptophan Res. 2019, 12, 1178646919852996. [Google Scholar] [CrossRef]
- Leclercq, S.; Schwarz, M.; Delzenne, N.M.; Stärkel, P.; de Timary, P. Alterations of kynurenine pathway in alcohol use disorder and abstinence: A link with gut microbiota, peripheral inflammation and psychological symptoms. Transl. Psychiatry 2021, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Morales-Puerto, N.; Giménez-Gómez, P.; Pérez-Hernández, M.; Abuin-Martínez, C.; Gil de Biedma-Elduayen, L.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacol. Therapeut. 2021, 223, 107807. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The role of the gut microbiota in bile acid metabolism. Ann. Hepatol. 2017, 16, s15–s20. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Minakata, K.; Yamagishi, I.; Nozawa, H.; Hasegawa, K.; Suzuki, M.; Gonmori, K.; Suzuki, O.; Watanabe, K. Sensitive identification and quantitation of parent forms of six synthetic cannabinoids in urine samples of human cadavers by liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2017, 35, 275–283. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Simpson, S.; Mclellan, R.; Wellmeyer, E.; Matalon, F.; George, O. Drugs and bugs: The gut-brain axis and substance use disorders. J. Neuroimmune Pharmacol. 2022, 17, 33–61. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Ciccocioppo, R. The role of serotonin in craving: From basic research to human studies. Alcohol Alcohol. 1999, 34, 244–253. [Google Scholar] [CrossRef]
- Müller, C.P.; Homberg, J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015, 277, 146–192. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Michaelides, M.; Baler, R. The neuroscience of drug reward and addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Wallace, B.D.; Roberts, A.B.; Pollet, R.M.; Ingle, J.D.; Biernat, K.A.; Pellock, S.J.; Venkatesh, M.K.; Guthrie, L.; O’Neal, S.K.; Robinson, S.J.; et al. Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chem. Biol. 2015, 22, 1238–1249. [Google Scholar] [CrossRef]
- Jasirwan, C.O.M.; Lesmana, C.R.A.; Hasan, I.; Sulaiman, A.S.; Gani, R.A. The role of gut microbiota in non-alcoholic fatty liver disease: Pathways of mechanisms. Biosci. Microbiota Food Health 2019, 38, 81–88. [Google Scholar] [CrossRef]
- Al-Khazaleh, A.K.; Jaye, K.; Chang, D.; Münch, G.W.; Bhuyan, D.J. Buds and bugs: A fascinating tale of gut microbiota and cannabis in the fight against cancer. Int. J. Mol. Sci. 2024, 25, 872. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2013, 17, 1197759. [Google Scholar] [CrossRef]
- Raybould, H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. Basic Clin. 2010, 153, 41–46. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Mawe, G.M. The enteric nervous system. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef]
- Dowling, L.R.; Strazzari, M.R.; Keely, S.; Kaiko, G.E. Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. J. Allergy Clin. Immunol. 2022, 150, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, B.; Roy, S. Gut-microbiome implications in opioid use disorder and related behaviors. Adv. Drug Alcohol Res. 2022, 2, 10311. [Google Scholar] [CrossRef]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J. The role of glial cells in drug abuse. Curr. Drug Abus. Rev. 2009, 2, 76–82. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef]
- Zhou, R.; Qian, S.; Cho, W.C.S.; Zhou, J.; Jin, C.; Zhong, Y.; Wang, J.; Zhang, X.; Xu, Z.; Tian, M.; et al. Microbiota-microglia connections in age-related cognition decline. Aging Cell 2022, 21, e13599. [Google Scholar] [CrossRef]
- Antoine, D.; Venigalla, G.; Truitt, B.; Roy, S. Linking the gut microbiome to microglial activation in opioid use disorder. Front. Neurosci. 2022, 16, 1050661. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Huang, H.; Pant, A.; Westgate, P.M.; Bada, H.S.; Bauer, J.A.; Giannone, P.J.; Sithisarn, T. Plasma brain-derived neurotrophicfactor levels in newborn infants with neonatal abstinence syndrome. Front. Pediatr. 2017, 5, 238. [Google Scholar] [CrossRef]
- Palma-Álvarez, R.F.; Ros-Cucurull, E.; Amaro-Hosey, K.; Rodriguez-Cintas, L.; Grau-López, L.; Corominas-Roso, M.; Sánchez-Mora, C.; Roncero, C. Peripheral levels of BDNF and opiate-use disorder: Literature review and update. Rev. Neurosci. 2017, 28, 499–508. [Google Scholar] [CrossRef]
- Hinds, J.A.; Sanchez, E.R. The role of the hypothalamus-pituitary-adrenal (HPA) axis in test-induced anxiety: Assessments, physiological responses, and molecular details. Stresses 2022, 2, 146–155. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. Motivación intrínseca y consumo de drogas: Una revisión de estudios sobre los motivos de curiosidad y de expansión [Intrinsic motivation and drug consumption: A review of studies on curiosity and expansion motives]. Health Addict. 2024, 24, 47–67. [Google Scholar] [CrossRef]
- Vandrey, R.G.; Budney, A.J.; Hughes, J.R.; Liguori, A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008, 92, 48–54. [Google Scholar] [CrossRef]
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Connor, J.P.; Stjepanović, D.; Budney, A.J.; Le Foll, B.; Hall, W.D. Clinical management of cannabis withdrawal. Addiction 2022, 117, 2075–2095. [Google Scholar] [CrossRef] [PubMed]
- Budney, A.J.; Moore, B.A.; Vandrey, R. Health consequences of marijuana use. In Handbook of the Medical Consequences of Alcohol and Drug Abuse; Brick, J., Ed.; Haworth Press: Philadelphia, PA, USA, 2004; pp. 171–217. [Google Scholar]
- Allsop, D.J.; Copeland, J.; Norberg, M.M.; Fu, S.; Molnar, A.; Lewis, J.; Budney, A.J. Quantifying the clinical significance of cannabis withdrawal. PLoS ONE 2012, 7, e44864. [Google Scholar] [CrossRef]
- Davis, J.P.; Smith, D.C.; Morphew, J.W.; Lei, X.; Zhang, S. Cannabis withdrawal, posttreatment abstinence, and days to first cannabis use among emerging adults in substance use treatment: A prospective study. J. Drug Issues 2016, 46, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Haney, M. The marijuana withdrawal syndrome: Diagnosis and treatment. Curr. Psychiatry Rep. 2005, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology 2018, 43, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Balodis, I.; MacKillop, J. Cannabis Use Disorder; IntechOpen: London, UK, 2019. [Google Scholar]
- Compton, W.M.; Han, B.; Jones, C.M.; Blanco, C.; Hughes, A. Marijuana use and use disorders in adults in the USA, 2002–2014: Analysis of annual cross-sectional surveys. Lancet Psychiatry 2016, 3, 954–964. [Google Scholar] [CrossRef]
- Rotermann, M.; Langlois, K. Prevalence and correlates of marijuana use in Canada, 2012. Health Rep. 2015, 26, 10–15. [Google Scholar]
- Rotermann, M.; Macdonald, R. Analysis of trends in the prevalence of cannabis use in Canada, 1985 to 2015. Health Rep. 2018, 29, 10–20. [Google Scholar]
- Shah, K.; Farwa, U.E.; Vanaparti, A.; Patel, S.; Kanumuri, M.; Vashishth, O.; Hossain, N.; Dahiya, R.; Banala, M.; Enamorado, F.R.P.; et al. Global epidemiology of cannabis use disorders and its trend from 1990 to 2019: Benchmarking analysis of the global burden of disease study. J. Fam. Med. Prim. Care 2024, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Farmer, R.F.; Kosty, D.B.; Seeley, J.R.; Duncan, S.C.; Lynskey, M.T.; Rohde, P.; Klein, D.N.; Lewinsohn, P.M. Natural course of cannabis use disorders. Psychol. Med. 2015, 45, 63–72. [Google Scholar] [CrossRef]
- Hasin, D.S.; Kerridge, B.T.; Saha, T.D.; Huang, B.; Pickering, R.; Smith, S.M.; Jung, J.; Zhang, H.; Grant, B.F. Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Am. J. Psychiatry 2016, 173, 588–599. [Google Scholar] [CrossRef]
- UNODC. World Drug Report 2015; United Nations Office on Drugs and Crime: Geneva, Switzerland, 2015. [Google Scholar]
- Degenhardt, L.; Ferrari, A.J.; Calabria, B.; Hall, W.D.; Norman, R.E.; McGrath, J.; Flaxman, A.D.; Engell, R.E.; Freedman, G.D.; Whiteford, H.A.; et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: Results from the GBD 2010 study. PLoS ONE 2013, 8, e76635. [Google Scholar] [CrossRef]
- Rubio, J.M.; Olfson, M.; Villegas, L.; Pérez-Fuentes, G.; Wang, S.; Blanco, C. Quality of life following remission of mental disorders: Findings from the National Epidemiologic Survey on alcohol and related conditions. J. Clin. Psychiatry 2013, 74, e445–e450. [Google Scholar] [CrossRef]
- Solmi, M.; De Toffol, M.; Kim, J.Y.; Choi, M.J.; Stubbs, B.; Thompson, T.; Firth, J.; Miola, A.; Croatto, G.; Baggio, F.; et al. Balancing risks and benefits of cannabis use: Umbrella review of meta-analyses of randomised controlled trials and observational studies. BMJ 2023, 382, e072348. [Google Scholar] [CrossRef]
- Hayley, A.C.; Stough, C.; Downey, L.A. DSM-5 cannabis use disorder, substance use and DSM-5 specific substance-use disorders: Evaluating comorbidity in a population-based sample. Eur. Neuropsychopharmacol. 2017, 27, 732–743. [Google Scholar] [CrossRef]
- Silins, E.; Horwood, L.J.; Patton, G.C.; Fergusson, D.M.; Olsson, C.A.; Hutchinson, D.M.; Spry, E.; Toumbourou, J.W.; Degenhardt, L.; Swift, W.; et al. Young adult sequelae of adolescent cannabis use: An integrative analysis. Lancet Psychiatry 2014, 1, 286–293. [Google Scholar] [CrossRef]
- Ranganathan, M.; Skosnik, P.D.; D’Souza, D.C. Marijuana and madness: Associations between cannabinoids and psychosis. Biol. Psychiatry 2016, 79, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.; Radhakrishnan, R.; D’Souza, D.C.; Ranganathan, M. Human laboratory studies on cannabinoids and psychosis. Biol. Psychiatry 2016, 79, 526–538. [Google Scholar] [CrossRef]
- Gage, S.H.; Hickman, M.; Zammit, S. Association between cannabis and psychosis: Epidemiologic evidence. Biol. Psychiatry 2016, 79, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.K.; Mills, L.; Doyle, M.; Sahid, A.; Montebello, M.; Monds, L.; Arunogiri, S.; Haber, P.; Lorenzetti, V.; Lubman, D.I.; et al. A phase III multisite randomised controlled trial to compare the efficacy of cannabidiol to placebo in the treatment of cannabis use disorder: The CBD-CUD study protocol. BMC Psychiatry 2024, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Chatters, R.; Cooper, K.; Day, E.; Knight, M.; Lagundoye, O.; Wong, R.; Kaltenthaler, E. Psychological and psychosocial interventions for cannabis cessation in adults: A systematic review. Addict. Res. Theory 2016, 24, 93–110. [Google Scholar] [CrossRef]
- Gates, P.J.; Sabioni, P.; Copeland, J.; Le Foll, B.; Gowing, L. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst. Rev. 2016, 2016, CD005336. [Google Scholar] [CrossRef]
- Davis, M.L.; Powers, M.B.; Handelsman, P.; Medina, J.L.; Zvolensky, M.; Smits, J.A. Behavioral therapies for treatment-seeking cannabis users: A meta-analysis of randomized controlled trials. Eval. Health Prof. 2015, 38, 94–114. [Google Scholar] [CrossRef]
- Budney, A.J.; Stanger, C.; Tilford, J.M.; Scherer, E.B.; Brown, P.C.; Li, Z.; Li, Z.; Walker, D.D. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol. Addict. Behav. 2015, 29, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.M.; Nich, C.; Lapaglia, D.M.; Peters, E.N.; Easton, C.J.; Petry, N.M. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: Less can be more, more or less. Addiction 2012, 107, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Rains, L.S.; Steare, T.; Mason, O.; Johnson, S. Improving substance misuse outcomes in contingency management treatment with adjunctive formal psychotherapy: A systematic review and meta-analysis. BMJ Open 2020, 10, e034735. [Google Scholar] [CrossRef] [PubMed]
- Sabioni, P.; Le Foll, B. Psychosocial and pharmacological interventions for the treatment of cannabis use disorder. F1000Research 2018, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Brezing, C.A.; Levin, F.R. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology 2018, 43, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Zhand, N.; Milin, R. What do we know about the pharmacotheraputic management of insomnia in cannabis withdrawal: A systematic review. Am. J. Addict. 2018, 27, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Meyyappan, A.C.; Hawken, E.R.; Tibbo, P.G. Pharmacotherapies for cannabis use disorder: A systematic review and network meta-analysis. Int. J. Drug Policy 2021, 97, 103295. [Google Scholar] [CrossRef]
- Nielsen, S.; Sabioni, P.; Gowing, L.; Le Foll, B. Pharmacotherapies for cannabis use disorders: Clinical challenges and promising therapeutic agents. Handb. Exp. Pharmacol. 2020, 258, 355–372. [Google Scholar] [CrossRef]
- Vandrey, R.; Haney, M. Pharmacotherapy for cannabis dependence: How close are we? CNS Drugs 2009, 23, 543–553. [Google Scholar] [CrossRef]
- Pinapati, K.K.; Vidya, S.; Khan, M.F.; Mandal, D.; Banerjee, S. Gut bacteria, endocannabinoid system, and marijuana addiction: Novel therapeutic implications. Health Sci. Rev. 2024, 10, 100144. [Google Scholar] [CrossRef]
- Gindin, S.; Karia, E.; Petrushkevich, A.; Sadeghian, S. Facilitating the bloom of intestinal flora: Observing the influence of tetrahydrocannabinol (THC) on beneficial bacteria inhabiting the intestinal microbiota. Undergrad. Res. Nat. Clin. Sci. Technol. J. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, M.; Ou, Z.; Wu, D.; Deng, L.; Lu, Z.; Zhang, J.; Deng, G.; Chen, S.; et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe 2020, 61, 102138. [Google Scholar] [CrossRef]
- Cruz-Lebrón, A.; Johnson, R.; Mazahery, C.; Troyer, Z.; Joussef-Piña, S.; Quiñones-Mateu, M.E.; Strauch, C.M.; Hazen, S.L.; Levine, A.D. Chronic opioid use modulates human enteric microbiota and intestinal barrier integrity. Gut Microbes 2021, 13, 1946368. [Google Scholar] [CrossRef]
- Buckner, J.D. College cannabis use: The unique roles of social norms, motives, and expectancies. J. Stud. Alcohol Drugs 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Roditis, M.L.; Delucchi, K.; Chang, A.; Halpern-Felsher, B. Perceptions of social norms and exposure to pro-marijuana messages are associated with adolescent marijuana use. Prev. Med. 2016, 93, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Khademi, S.; Hallinan, C.M.; Conway, M.; Bonomo, Y. Using social media data to investigate public perceptions of cannabis as a medicine: Narrative review. J. Med. Internet Res. 2023, 25, e36667. [Google Scholar] [CrossRef]
- Spackman, E.; Haines-Saah, R.; Danthurebandara, V.M.; Dowsett, L.E.; Noseworthy, T.; Clement, F.M. Marijuana use and perceptions of risk and harm: A survey among Canadians in 2016. Healthc. Policy 2017, 13, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hurd, Y.L.; Manzoni, O.J.; Pletnikov, M.V.; Lee, F.S.; Bhattacharyya, S.; Melis, M. Cannabis and the developing brain: Insights into its long-lasting effects. J. Neurosci. 2019, 39, 8250–8258. [Google Scholar] [CrossRef]
- Henquet, C.; Di Forti, M.; Morrison, P.; Kuepper, R.; Murray, R.M. Gene-environment interplay between cannabis and psychosis. Schizophr. Bull. 2008, 34, 1111–1121. [Google Scholar] [CrossRef]
- Farrelly, K.N.; Wardell, J.D.; Marsden, E.; Scarfe, M.L.; Najdzionek, P.; Turna, J.; MacKillop, J. The impact of recreational cannabis legalization on cannabis use and associated outcomes: A systematic review. Subst. Abus. 2023, 17, 11782218231172054. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Yarnell, S.; Radhakrishnan, R.; Ball, S.A.; D’Souza, D.C. Marijuana legalization: Impact on physicians and public health. Annu. Rev. Med. 2016, 67, 453–466. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. A holistic review of fentanyl use and its impact on public health. Curr. Addict. Res. 2024, 8, 23–33. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Nix, C.A.; Hollier, J.; Sagrera, C.E.; Delacroix, B.M.; Abubakar, T.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Benzodiazepines: Uses, dangers, and clinical considerations. Neurol. Int. 2021, 13, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A. A current overview on adolescent alcohol misuse and its potential negative impacts. Alcohol. Drug Addict. Alkohol. I Narkom. 2025, 37, 205–231. [Google Scholar] [CrossRef]
- Dorotheo, E.U.; Arora, M.; Banerjee, A.; Bianco, E.; Cheah, N.P.; Dalmau, R.; Eissenberg, T.; Hasegawa, K.; Naidoo, P.; Nazir, N.T.; et al. Nicotine and cardiovascular health: When poison is addictive—A WHF policy brief. Glob. Heart 2024, 19, 14. [Google Scholar] [CrossRef]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of tobacco smoking on cardiovascular disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef]
- Rehm, J. The risks associated with alcohol use and alcoholism. Alcohol Res. Health 2011, 34, 135–143. [Google Scholar]
- Varghese, J.; Dakhode, S. Effects of alcohol consumption on various systems of the human body: A systematic review. Cureus 2022, 14, e30057. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Pharmacogenomic and pharmacomicrobiomic aspects of drugs of abuse. Genes 2025, 16, 403. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. The bidirectional interplay between substances of abuse and gut microbiome homeostasis. Life 2025, 15, 834. [Google Scholar] [CrossRef] [PubMed]
- Romm, K.F.; Cohn, A.M.; Beebe, L.A.; Berg, C.J. Disparities in cannabis use outcomes, perceived risks and social norms across sexual orientation groups of US young adult women and men. Health Educ. Res. 2023, 38, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Dyar, C. A review of disparities in cannabis use and cannabis use disorder affecting sexual and gender minority populations and evidence for contributing factors. Curr. Addict. Rep. 2022, 9, 589–597. [Google Scholar] [CrossRef]
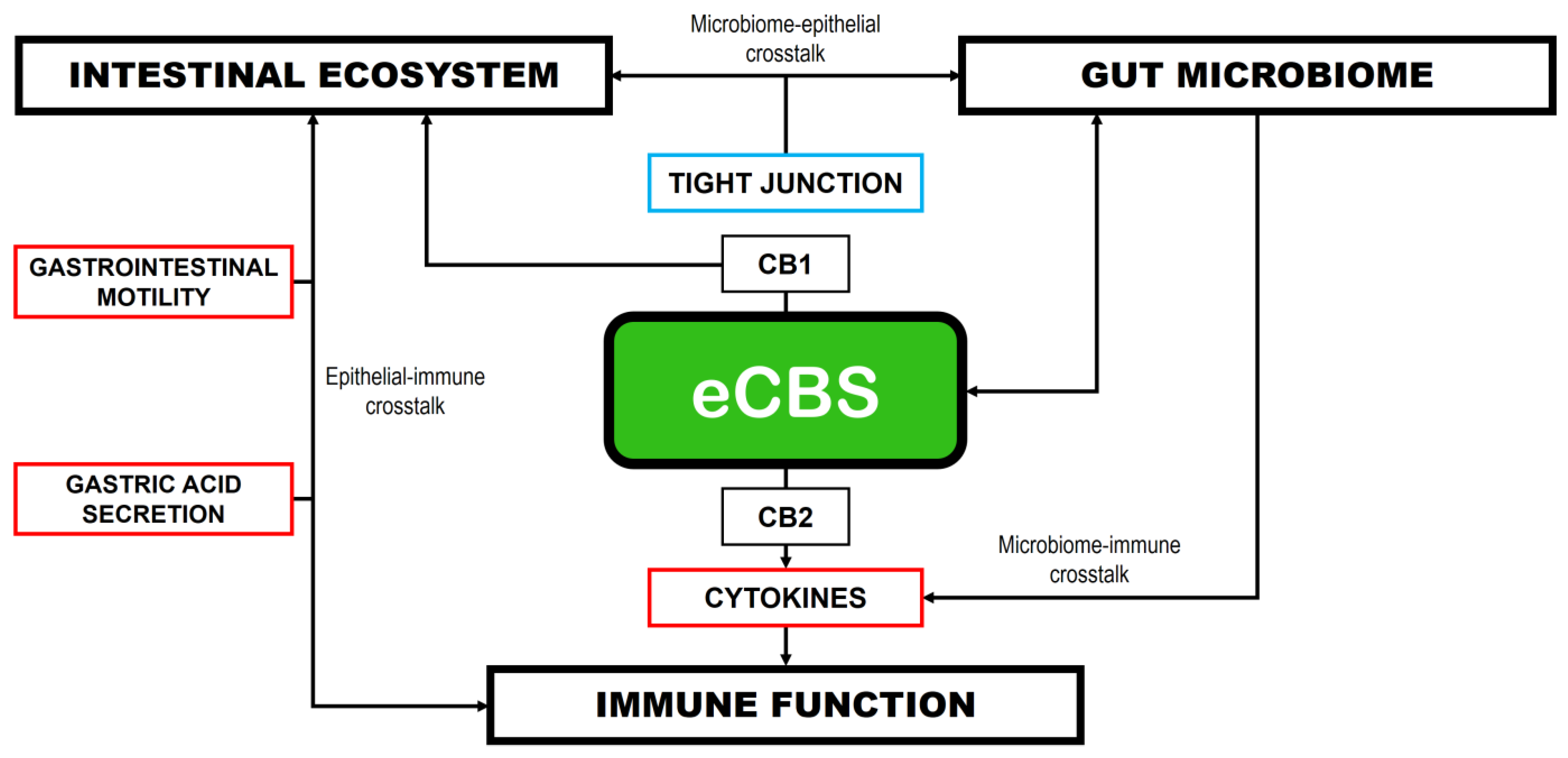
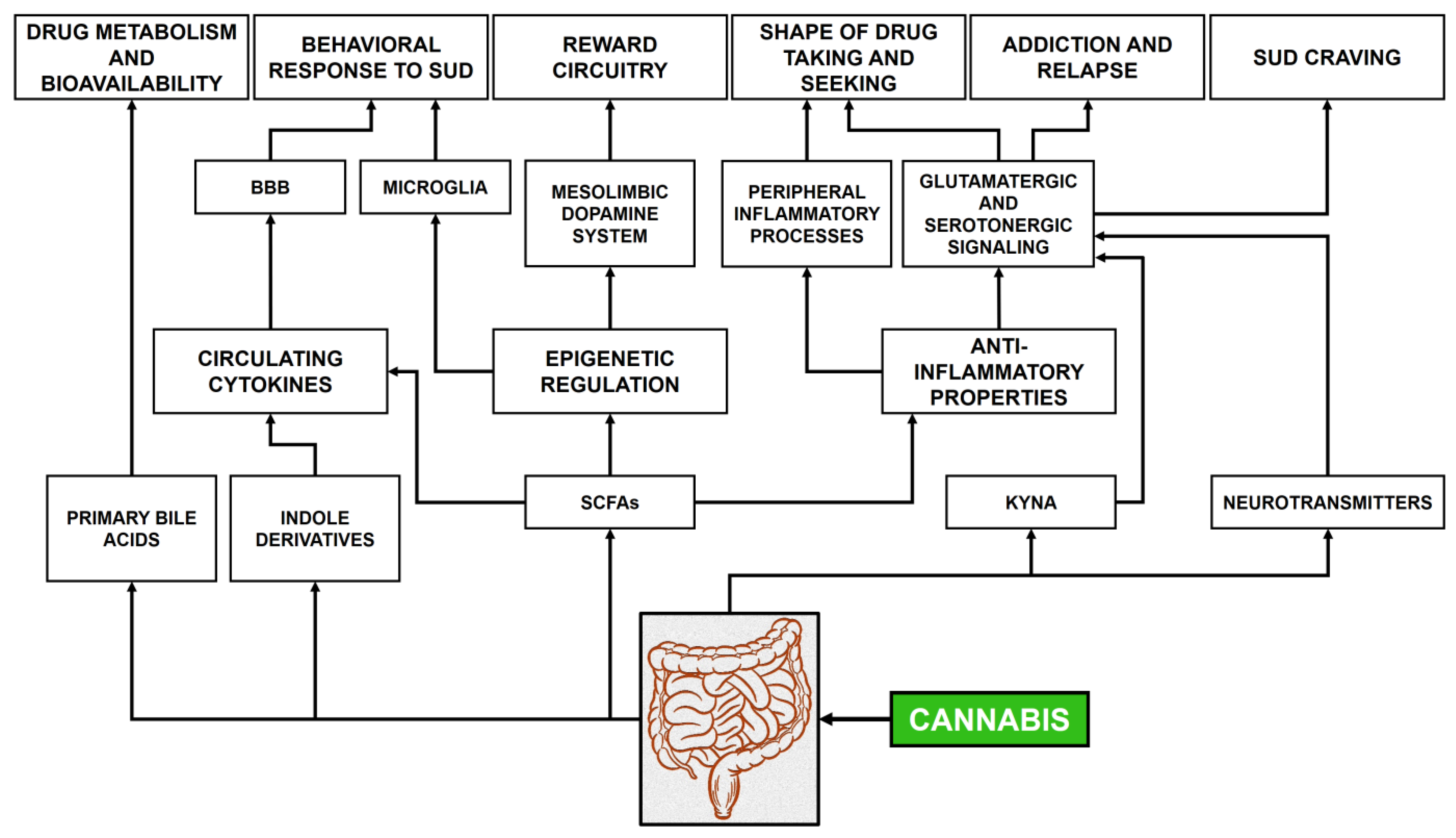
| Biological Species | Cannabinoids Produced | Bioactivities |
|---|---|---|
| Cannabis sativa | Tetrahydrocannabinols (THCs) Cannabidiols (CBDs) Cannabigerols (CBGs) Cannabichromenes (CBCs) Cannabicyclols (CBLs) Cannabielsoins (CBEs) Cannabinols (CBNs) Cannabinodiols (CBNDs) Cannabitriols (CBTs) Miscellaneous cannabinoids |
|
| Rhododendron adamsii R. anthopogonoides R. dauricum R. rubiginosum |
Chromane/chromene meroterpenoids (CBC, CBL) Grifolic acid (GFA) Daurichromenic acid (DCA) Confluentin (decarboxylated DCA) Rhododaurichromenic acids A-B Rubiginosins A-G Anthopogochromenes A-B Cannabigerorcynic acids Cannabiorcicyclolic acids |
|
|
Helichrysum umbraculigerum Glycyrrhiza foetida Amorpha fruticosa | Amorfrutins (bibenzyl cannabionoids) |
|
| Radula laxiramea R. marginata R. perrotteti | Perrottetinene (bibenzyl cannabinoid) |
|
|
Mycorrhizal fungi: Albatrellus dispansus Cylindrocarpon olidum |
GFA DCA Grifolin and neogrifolin Confluentin Cannabiorcichromenic acids |
|
| Reference/ Country | Sample Size and Age | Study Design | Summary of Findings |
|---|---|---|---|
| Albertella et al. [111]/Australia | N = 162 Age: 15–24 years | Longitudinal | Follow-up analyses showed that the early onset of cannabis use was associated with higher levels of introversive anhedonia in females only. |
| Bahorik et al. [112]/USA | N = 307 Age: >18 years | Longitudinal | Cannabis use led to poorer mental and physical health functioning. |
| Bechtold et al. [113]/USA | N = 506 Mean age: 13.9 years | Longitudinal | Four distinct subgroups of cannabis users were defined. However, these groups did not significantly differ in terms of their physical and mental health outcomes, including anxiety and suicide problems. |
| Chadi et al. [114]/USA | N = 26,821 Age: 12–18 years | Longitudinal | Cannabis use was associated with depression and suicidality. |
| Danielsson et al. [115]/Sweden | N = 8598 Age: 20–64 years | Longitudinal | No associations were found between cannabis use and the incidence of depression/anxiety or between depression/anxiety and later cannabis use onset. |
| Feingold et al. [116]/Israel, Canada, and Germany | N = 43,093 Age: >18 years | Longitudinal | The findings suggest that cannabis use and CUD are not associated with increased incidence of most anxiety disorders, and inversely, most anxiety disorders are not associated with increased incidence of cannabis use or CUD. |
| Floyd Campbell [117]/USA | N = 240 Age: 18–30 years | Cross-sectional | More than 20% of cannabis users experienced depression, which was higher in African American females compared to White females. |
| Han et al. [118]/USA | N = 281,650 Age: 18–34 years | Longitudinal | CUD was associated with a higher prevalence of past suicide ideation, plan, and attempt. The suicide plan among those with CUD and major depressive episode was higher for women than for men. |
| Horwood et al. [119]/Australia | N = 6900 Age: 12–45 years | Longitudinal | The frequency of cannabis use was significantly associated with increasing depressive symptoms. |
| Kim et al. [120]/R. Korea | N = 234 Mean age: 41.8 years | Longitudinal | Cannabis use negatively affects the long-term clinical outcomes in patients with BD. |
| Leadbeater et al. [121]/USA | N = 36,309 Age: >18 years | Longitudinal | More frequent cannabis use was associated with increased psychotic symptoms, higher depression, and anxiety symptoms. Females exhibited a stronger association between CUD and mental health symptoms. |
| Levy & Weitzman [122]/USA | N = 527 Age: 14–18 years | Cross-sectional | Frequent cannabis use was linked to reports of symptoms of hallucinations, paranoia, or anxiety. |
| London-Nadeau et al. [123]/USA | N = 1538 Age: 13–17 years | Longitudinal | There is a bidirectional relationship between cannabis use and depression/anxiety symptoms in adolescents. Differences were found between heterosexual and LGBTQI participants; the latter showed a stronger association between cannabis use and depression. |
| Meier et al. [124]/USA | N = 506 Age: 15–26 years | Longitudinal | Increases in cumulative years of weekly cannabis use were linked to higher levels of depression symptoms and anxiety/depression problems. |
| Moitra et al. [125]/USA | N = 332 Age: 18–25 years | Longitudinal | A relationship between reductions in cannabis use and reductions in depressive symptoms was found. |
| Muñoz-Galán et al. [126]/Spain | N = 948 Age: 14–18 years | Longitudinal | From the 948 participants who commenced treatment for CUD, almost 20% developed a mental health disorder in the years following the onset of cannabis use. |
| Otten et al. [127]/The Netherlands | N = 1424 Age: 10–11 years | Longitudinal | Cannabis use is associated with an increase in symptoms of anxiety, but only in carriers of the short allele of the 5-HTTLPR genotype (a polymorphism in the promoter region of the serotonin transporter gene). |
| Patel et al. [128]/United Kingdom | N = 2026 Age: 16–25 years | Cross-sectional | Cannabis use reduces response to conventional antipsychotic treatment and increases compulsory hospital admissions. |
| Phillips et al. [129]/USA | N = 300 Age: 18–25 years | Cross-sectional | Among the three psychological factors tested (social anxiety, general anxiety, and depression), only depression was associated with cannabis use. |
| Rabin et al. [130]/Canada | N = 19 Age: 18–55 years | Cross-sectional | Verbal memory and learning improvements in schizophrenic subjects with cannabis abstinence for 28 days were found. |
| Rabin et al. [131]/Canada | N = 19 Age: 8–48 years | Cross-sectional | Short-term (28 days) cannabis abstinence is not associated with improvement in psychotic symptoms, but may be associated with improvement in depressive symptomatology in patients with schizophrenia. |
| Rasic et al. [132]/Canada | N = 976 Age: >16 years | Longitudinal | Illicit drug use, with and without cannabis use, among high school students increases the risk of depression, suicidal ideation, and suicidal attempts. Heavy cannabis use alone predicts depression but not suicidal ideation or attempts. |
| Richter et al. [133]/USA | N = 55,271 Age: 18–62 years | Cross-sectional | Anxiety was associated with a higher prevalence of CUD, while depression showed no significant association. |
| Sagar et al. [134]/USA | N = 12 Mean age: 28.6 years | Longitudinal | No evidence was found of an additive negative impact of BD and cannabis use on cognition. |
| Schoeler et al. [135]/United Kingdom | N = 285 Age: 18–55 years | Longitudinal | Frequent cannabis use during adolescence was a risk factor for later life depression. |
| Scholes-Balog et al. [136]/Australia | N = 927 Age: 15–19 years | Longitudinal | The rates of cannabis use increased with age, and were more common among males than females. Anxiety and depression are the most prevalent cannabis-related harms. |
| Tull et al. [137]/USA | N = 202 Age: 18–60 years | Cross-sectional | Findings suggest that patients with co-occurring PTSD and cannabis dependence may experience alterations in their emotional processing in response to a traumatic cue. |
| Weinberger et al. [138]/USA | N = 204,102 Age: 12–17 years | Cross-sectional | Youth with depression were more than twice as likely to report cannabis use compared to those without depression. |
| Welsh et al. [139]/USA | N = 483 Age: 12–24 years | Longitudinal | Cannabis use was associated with the development of externalizing behavior disorders and with ADHD. |
| Wilkinson et al. [140]/USA | N = 2276 Mean age: 51.7 years | Longitudinal | Initiation of cannabis use after treatment was associated with worse PTSD symptoms and more violent behavior. |
| Wilkinson et al. [141]/USA | N = 9816 Age: 18–32 years | Longitudinal | The frequency of cannabis use increases from adolescence to young adulthood, together with the rise in adolescent depressive symptoms. This association was found to be stronger in women. |
| Wong et al. [142]/USA | N = 73,183 Age: 12–15 years | Cross-sectional | Adolescents with a history of cannabis use showed an association with suicidal ideation or attempts in the previous year. |
| Zaman et al. [143]/USA | N = 483 Age: 12–18 years | Cross-sectional | Among adolescents with CUD, there was a high co-occurrence of alcohol and opioid abuse or dependence. These individuals also experienced significant psychiatric comorbidities. |
| Zorrilla et al. [144]/Spain | N = 1922 Age: 35.3–46.2 years | Longitudinal | BD patients who stopped using cannabis during manic/mixed episodes had similar clinical and functional outcomes to never users. |
| Neuropsychiatric Disorders | Treatment | Sample and Duration | Outcomes | Ref. |
|---|---|---|---|---|
| Epilepsy | Artisanal formulations of CBD. | N = 209. Age: >19 years. Treatment for 1.1–2.5 years. | With concomitant use of clobazam, 44% of patients had a 50% reduction in seizures upon addition of CBD compared with 33% in the population not treated pharmacologically. The most common reported side effect of CBD was sedation in less than 4% of patients, all of whom were also taking clobazam. | [166] |
| CBD-enriched cannabis oil: 1–10 mg/kg/d and 10–20 mg/kg/d. The selected formula contained CBD and THC at a ratio of 20:1 dissolved in olive oil. | N = 74. Age: 1–18 years. Treatment for 3–6 months. | CBD treatment yielded a significant positive effect on seizure load, with a significant reduction in seizure frequency. In addition, improvements in behavior and alertness, language, communication, motor skills, and sleep were found. AEs included somnolence, fatigue, gastrointestinal disturbances, and irritability. | [167] | |
| Dravet syndrome | Epidiolex® GWCARE1b: 20 mg/kg/d CBD. | N = 120. Age: 1–18 years. Treatment for 14 weeks. | The median reduction in convulsive seizures was 38.9%. AEs occurred often in the CBD group (93%) as well as in the placebo group (75%), most of the AEs were moderate or mild, including somnolence, diarrhea, decreased appetite, and fatigue. | [168] |
| Epidiolex® GWPCARE5 with a mean CBD dose of 21 mg/kg/d. | N = 264. Age: 2–55 years. Treatment for 274 days. | A sustained convulsive seizure reduction (between 37.5% and 44.3%) was achieved. AEs were common, including diarrhea, pyrexia, decreased appetite, and somnolence. | [169] | |
| Epidiolex® GWPCARE2: 10 mg/kg/d and 20 mg/kg/d CBD. | N = 198. Age: 2–18 years. Treatment for 14 weeks. | Median convulsive seizure reduction was 48.7% in the CBD 10 mg/kg/d group, 45.7% in the 20 mg/kg/d group, and 26.9% in the placebo group. The most common AEs were decreased appetite, diarrhea, somnolence, pyrexia, and fatigue. | [170] | |
| Lennox–Gastaut syndrome | Epidiolex® GWPCARE3: 10 mg/kg/d and 20 mg/kg/d CBD. | N = 225. Age: 2–55 years. Treatment for 14 weeks. | A significant reduction in drop seizures was found in the 10 mg/kg/d and 20 mg/kg/d groups. AEs frequently occurred in all groups, including somnolence, decreased appetite, and diarrhea. | [171] |
| Epidiolex® GWPCARE4: 20 mg/kg oral CBD daily. | N = 171. Age 2–55 years. Treatment for 14 weeks. | Significant reduction in drop seizures. AEs occurred often with diarrhea, somnolence, pyrexia, decreased appetite, and vomiting being the most common. | [172] | |
| Epidiolex® GWPCARE5 with a mean CBD dose of 23 mg/kg/d. | N = 366. Age: 2–55 years. Treatment for 38 weeks. | Sustained reduction in seizures (between 47.7% and 57.4%) across all 12-week periods. AEs were common in all groups, including diarrhea, somnolence, and convulsions. | [173] | |
| Alzheimer’s disease | Nabilone: 1–2 mg/d. | N = 39. Age: >55 years. Treatment for 14 weeks. | Nabilone may be an effective treatment for agitation. However, sedation and cognition should be closely monitored. | [174] |
| Dementia | Oral THC: 1.5 mg twice daily. | N = 18. Mean age: 77 years. Treatment for 12 weeks. | Significant increases in dynamic balance, stride length, and gait velocity after THC administration compared to placebo. AEs included dizziness, somnolence, balance disorders, and falls. | [175] |
| Parkinson’s disease | CBD/THC treatment (dose not reported). | N = 15. Mean age: 67.5 years. Treatment for 3 months. | Patients who were already taking a CBD/THC product (N = 8) had lower global cognition scores, more non-motor symptoms, and improved pain levels and sleep, as well as reductions in anxiety. A few AEs, including sleepiness, concentration difficulties, and forgetfulness. | [176] |
| Multiple sclerosis | Oral cannabis extract (CE). 2-week dose titration phase from 5 mg to 25 mg of THC daily and a 10-week maintenance phase. | N = 144. Age: 18–64 years. Treatment for 12 weeks. | The rate of relief from muscle stiffness after 12 weeks was almost twice as high with CE as with placebo. | [177] |
| Autism spectrum disorder | Cannabis oil solution at a 20:1 ratio of CBD and THC. Sublingual administration 2 or 3 times/daily with CBD doses started at 1 mg/kg/d and titrated up to 10 mg/kg/d. | N = 60. Age: 5–17.5 years (mean age: 11.8 years). Treatment for 2–4 weeks. | Following the cannabis treatment, behavioral outbreaks were improved in 61% of patients. AEs included sleep disturbances (14%), irritability (9%), and loss of appetite (9%). | [178] |
| Recommended daily dose of CBD was 16 mg/kg (maximal daily dose 600 mg), and for THC daily dose of 0.8 mg/kg (maximal daily dose of 40 mg). | N = 53. Age: 4–22 years. Treatment for 66 days. | Self-injury and rage attacks, hyperactivity symptoms, sleep problems, and anxiety improved. AEs such as somnolence and a change in appetite were mild. | [179] | |
| Cannabis oil containing 45% olive oil, 30% CBD, and 1.5% THC. 1 sublingual oil drop 3 times per day (15 mg CBD and 0.75 mg THC). | N = 188. Mean age: 12.9 years. Treatment for 6 months. | After 6 months of treatment, 30.1% of the patients reported a significant improvement; 23 patients experienced at least one side effect. The most common was restlessness. | [180] | |
| Standardized CBD-enriched CE (with a CBD to THC ratio of 75/1). | N = 15. Age: 6–17 years (mean age: 10 years). Treatment for 5–24 months. | After 6–9 months of treatment, the strongest improvements were reported for seizures, attention deficit/hyperactivity symptoms, sleep disorders, and communication and social interaction deficits. The most frequently reported AEs were drowsiness, psychoactive symptoms, increased appetite, digestive disturbances, dry mouth, and lack of appetite. | [181] | |
| Rett syndrome | Epidyolex®: 100 mg/mL oral solution of CBD (38% patients were treated with CBD, and 50% in combination with clobazam). The median dose at their last follow-up was 15 mg/kg/d. | N = 26. Age: 7–32 years. Treatment for 13 months. | CBD reduced the incidence of seizures in 70%. A reduction in agitation or anxiety attacks, and an improvement in spasticity were reported. Only one patient experienced a transitory drooling and somnolence episode at the CBD initiation. Half of the patients showed a reduction in agitation and/or anxiety attacks, and an improvement in spasticity was reported in 40% of patients. | [182] |
| Tourette syndrome | A vaporized single 0.25 g dose of THC (10%), THC/CBD (9%/9%), CBD (13%), and placebo at 2-week intervals. | N = 12. Age: 22–54 years. Treatment for 6 weeks. | In terms of tics, there was no statistically significant difference for any of the cannabis products. The main AEs observed were sedation, psychomotor effects, dizziness, cough, burning throat, dry mouth, and feeling cold. | [183] |
| Medical cannabis treatment, including THC and CBD. | N = 18. Age: 20–50 years. Treatment for 12 weeks. | After 12 weeks of treatment, a significant average reduction of YGTSS and of PUTS was observed. Common side effects were dry mouth, fatigue, and dizziness. Three patients suffered from psychiatric side effects, including worsening of obsessive-compulsive disorder, panic attacks, and anxiety. Six patients reported cognitive side effects regarding time perception, visuospatial disorientation, confusion, slow processing speed, and attention. | [184] | |
| Attention deficit/Hyperactivity disorder | Sativex Oromucosal Spray: THC (2.7 mg) and CBD (2.5 mg). | N = 30. Age: 18–55 years. Treatment for 6 weeks. | The treated group showed an improvement in Qb Test scores that approached significance. Nominally significant improvements in attention deficit/hyperactivity symptoms were also found for the treated group compared to the placebo. Concerns about cognitive impairment were alleviated by lower THC formulas. | [185] |
| Task-based fMRI data from young adults with and without cannabis use. Go/NoGo behavioral and fMRI data were evaluated for main and interaction effects of disorder diagnosis and cannabis use. | N = 73. Age: 21–27 years (mean age: 24.6 years). Treatment for 1 year. | Patients made significantly more commission errors on NoGo trials than controls, and also had less frontoparietal and frontostriatal activity, independent of cannabis use. No main effects of cannabis use on response inhibition or functional brain activation were observed. An interaction of disorder diagnosis and cannabis use was found in the right hippocampus and cerebellar vermis, with increased recruitment of these regions in cannabis-using controls during correct response inhibition. | [186] | |
| Bipolar disorder | Both patients received a placebo for the initial 5 days and CBD from the 6th to the 30th day. The initial oral dose of 600 mg reaches 1200 mg/d. From the 6th to the 20th day, the first patient received adjunctive olanzapine (oral dose of 10–15 mg). On day 31, CBD treatment was discontinued and replaced by a placebo for 5 days. | N = 2. Age: 35 and 36 years. Treatment for 40 days. | One patient showed improvements in YMRS and BPRS scores while on CBD plus olanzapine, but no additional improvement during CBD monotherapy. The second patient had no symptom improvement with any dose of CBD. CBD appears not to be effective in attenuating mania. No side effects reported. | [187] |
| Social anxiety disorder | CBD (600 mg). | N = 24. Mean age: 24.6 years. Treatment for 1.5 hours before a simulated public speaking test. | Pre-test CBD administration in patients versus placebo resulted in significantly reduced anxiety, cognitive impairment, and discomfort in speech performance, and significantly decreased hyper-alertness in anticipatory speech. CBD and control groups, however, did not differ, reflecting similar response profiles during the public speaking test. | [188] |
| CBD (400 mg). | N = 10. Age: 20–33 years (mean age: 24.2 years). Treatment for 140 min. | CBD compared to placebo resulted in significantly lower subjective anxiety, and modulated blood flow in the left parahippocampal gyrus, hippocampus, and inferior temporal gyrus, and right posterior cingulate gyrus. | [189] | |
| Anxiety | Oral CBD (600 mg). | N = 32. Mean age: 26 years. Treatment for 130 min prior to entering virtual reality. | Immersion in the virtual reality session elicited anxiety as indexed by the BAI, as well as increased cortisol concentration, heart rate, and systolic blood pressure. However, CBD had no impact upon any of these effects, except for a strong trend to increase anxiety. CBD had no effect on persecutory ideation as assayed by the CAPE questionnaire or the SSPS. | [190] |
| CBD 25 mg/d in capsules. | N = 72. Age: 18–70 years (mean age: 34 years). Treatment for 1 month. | Anxiety scores decreased within the first month of treatment in 57 patients (79.2%) and remained decreased during the study duration. | [191] | |
| Post-traumatic stress disorder | Three active concentrations of smoked cannabis (i.e., high THC, 12% THC and <0.05% CBD; high CBD, 11% CBD and 0.50% THC; and THC + CBD, 7.9% THC and 8.1% CBD). | N = 80 (stage 1) and N = 74 (stage 2). Mean age: 44.9 years. Treatment for 3 weeks in stage 1, and after a 2-week washout period, treatment for 3 weeks in stage 2. | The study did not find a significant difference in symptom severity changes between the active cannabis concentrations and placebo by the end of stage 1. However, THC + CBD and high THC led to decreased depression and social anxiety in stage 2. | [192] |
| Oral CBD (22–28 mg/capsule). | N = 11. Mean age: 39.9 years. Treatment for 8 weeks. | Ten patients experienced a decrease in symptom severity, as evidenced by a lower PCL-5 score at 8 weeks than at initial baseline. | [193] | |
| Medical cannabis. | N = 80. Age: >18 years. Analyzed retrospectively, CAPS data were collected. | Patients reported a >75% decrease in CAPS scores when they were using cannabis compared to periods when they were not. In addition, the treatment reduced anxiety and improved sleep disturbances. | [194] | |
| Nabilone tablets treatment (0.5 mg). | N = 10. Mean age: 43.6 years. Treatment for 7 weeks. | Reduction in nightmares as measured by the CAPS scores in treated patients. | [195] | |
| Sleep disturbances | CBD (50 mg/d). | N = 387. Age: 25–54 years. Treatment for 3–6 months. | CBD use improves general health and well-being, stress, post-workout sore muscles, anxiety, skin conditions, and sleep problems. | [196] |
| CBD capsules (25 mg) + liquid (12–24 mg). | N = 1. Age: 10 years. Treatment for 5 months with CBD capsules + 1 month with CBD liquid. | SDSC scores decreased over the 5-month period, indicating an increase in sleep quality and quantity. | [197] | |
| Nabilone (0.5–1.0 mg) compared to amitriptyline (10–20 mg). | N = 31. Age: >18 years. Treatment for 2 weeks. | Although sleep was improved by both nabilone and amitriptyline, nabilone was superior to amitriptyline. The most common AEs for nabilone were dizziness, nausea, and dry mouth. | [198] | |
| Pain | Nabiximols: THC (2.7 mg) and CBD (2.5 mg) in sprays. Three doses: low (1–4 sprays), medium (6–10 sprays), or high (11–16 sprays). | N = 263. Mean age: 58 years. Treatment for 5 weeks. | Reports of pain relief were significantly greater for nabiximols than placebo overall, especially in the low- and medium-dose groups. There were no other significant group differences. AEs were dose-related, with only the high-dose group reporting a decrease in mood. | [199] |
| Dronabinol: THC. | N = 240. Age: 18–70 years. Treatment for 16 weeks. | The primary endpoint was the change in pain intensity on the 11-point NRS over a 16-week treatment period. Pain intensity during 16-week dronabinol and placebo treatment was reduced by 1.92 and 1.81 points, without a significant difference. AEs: Restlessness, irritability, sleep interference, decreased appetite, and excessive sweating. | [200] | |
| Inhaling medium dose (3.53%) and low dose (1.29%) of THC. | N = 39. Mean age: 50 years. Treatment for 2 h. | Cannabis has analgesic efficacy, with the low dose being as effective as the medium dose for pain relief. Pain relief appears to be maximal after the second dosing at 180 min post-baseline, but the peak effect drops off 1 to 2 h later. | [201] | |
| Fibromyalgia | THC-rich cannabis oil (24.44 mg/mL of THC and 0.51 mg/mL of CBD). | N = 17. Mean age: 51.9 years. Treatment for 8 weeks. | After the intervention, the cannabis group presented a significant decrease in FIQ score in comparison with the placebo group. The cannabis group presented significant improvement on the “feel good”, “pain”, “do work”, and “fatigue” scores. | [202] |
| Single vapor inhalation of Bedrocan (22.4-mg THC, <1 mg CBD); Bediol (13.4-mg THC, 17.8 mg CBD); Bedrolite (18.4 mg CBD, <1 mg THC). | N = 20. Mean age: 39 years. Treatment: mean of 22 inhalations. | None of the treatments had an effect greater than placebo on spontaneous or electrical pain responses, although more subjects receiving Bediol displayed a 30% decrease in pain scores compared to placebo. AEs: deterioration in mood and alertness, sore throat and sour taste, coughed during inhalation, nausea without vomiting. | [203] | |
| Migraine | Inhaled medical cannabis. | N = 653. Age: 18–74 years. Treatment: 7441 sessions. | There were significant reductions in headache and migraine ratings after cannabis use (56%). Men reported larger reductions in headache than women, and the use of concentrates was associated with larger reductions in headache than flowers. | [204] |
| Dried Cannabis flower. | N = 582. Treatment: the average user entered 21 sessions (median = 5 sessions) over 125 days (median = 65 days). | Dried Cannabis flower may be an effective medication for the treatment of migraine- and headache-related pain, but the effectiveness differs according to characteristics of the Cannabis plant, the combustion methods, and the age and gender of the patient. | [205] | |
| Schizophrenia | CBD (600–800 mg). | N = 42. Treatment over 4 weeks. | Both treatments were effective in reducing PANSS and BPRS scores at each time point. CBD was tolerated better, with fewer side effects reported. Anandamide levels were higher in the CBD group post-treatment. | [206] |
| CBD (600 mg). | N = 39. Age: 18–50 years. Treatment for 6 weeks. | Both groups showed improvement on PANSS scores, and only the placebo group improved on the MCCB. Similar AEs were noted between the groups, with more sedation evident in the CBD group. | [207] | |
| CBD (1000 mg). | N = 88. Age: 18–65 years. Treatment for 6 weeks. | The CBD group reported lower positive symptom scores (PANSS, CGI-S) and was more likely to be rated as improved and less severely ill than the placebo group. The CBD group also showed improvements in the cognitive domain of motor speed compared to placebo (BACS and GAF scores). Similar AEs were reported between groups. | [208] |
| Bacterial Phyla, Families, and Genera | Increased | Decreased | Reference |
|---|---|---|---|
| Clinical studies | |||
| ACTINOMYCETOTA | |||
| Bifidobacterium | X | Vigay et al. [260] | |
| Collinsella | X | Vigay et al. [260] | |
| BACILLOTA | |||
| Peptostreptococcaceae | X | Castonguay-Paradis et al. [257] | |
| Acidaminococcus | X | Fulcher et al. [258] | |
| Anaerostipes | X | Fulcher et al. [258] | |
| Clostridum | X | Fulcher et al. [258] | |
| Coprococcus | X | Vigay et al. [260] | |
| Dialister | X | Fulcher et al. [258] | |
| Dorea | X | Fulcher et al. [258] | |
| Faecalibacterium | X | Vigay et al. [260] | |
| Ruminococcus | X | Fulcher et al. [258] | |
| Solobacterium | X | Fulcher et al. [258] | |
| Veillonella | X | Castonguay-Paradis et al. [257] | |
| BACTEROIDOTA | |||
| Bacteroides | X X | Panee et al. [259] Zhuang et al. [261] | |
| Prevotella | X X | Fulcher et al. [258] Panee et al. [259] | |
| FUSOBACTERIODOTA | |||
| Fusobacterium | X | Fulcher et al. [258] | |
| PSEUDOMONADOTA | |||
| Escherichia/Shigella | X | Vigay et al. [260] | |
| VERRUCOMICROBIOTA | |||
| Akkermansiaceae | X | Castonguay-Paradis et al. [257] | |
| Preclinical studies | |||
| BACILLOTA | |||
| Erysipelotrichaceae | X | Mehrpouya-Bahrami et al. [263] | |
| Lachnospiraceae | X | Mehrpouya-Bahrami et al. [263] | |
| Ruminococcus | X | Mohammed et al. [264] | |
| VERRUCOMICROBIOTA | |||
| Akkermansia | X X | Al-Ghezi et al. [262] Mehrpouya-Bahrami et al. [263] | |
| X | Mohammed et al. [264] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego-Ruiz, A.; Borrego, J.J. A Holistic Review of Cannabis and Its Potential Risks and Benefits in Mental Health. Psychiatry Int. 2025, 6, 92. https://doi.org/10.3390/psychiatryint6030092
Borrego-Ruiz A, Borrego JJ. A Holistic Review of Cannabis and Its Potential Risks and Benefits in Mental Health. Psychiatry International. 2025; 6(3):92. https://doi.org/10.3390/psychiatryint6030092
Chicago/Turabian StyleBorrego-Ruiz, Alejandro, and Juan J. Borrego. 2025. "A Holistic Review of Cannabis and Its Potential Risks and Benefits in Mental Health" Psychiatry International 6, no. 3: 92. https://doi.org/10.3390/psychiatryint6030092
APA StyleBorrego-Ruiz, A., & Borrego, J. J. (2025). A Holistic Review of Cannabis and Its Potential Risks and Benefits in Mental Health. Psychiatry International, 6(3), 92. https://doi.org/10.3390/psychiatryint6030092






