Abstract
The UV, UV/VUV photolysis and their combination with persulfate (S2O82−) were studied for the elimination of trimethoprim and 5-fluorouracil. Methods were examined in terms of transformation and mineralization rate and the matrix effect. The relative contribution of the direct UV photolysis and radical-based reactions (•OH, SO4•−) were also investigated. Without S2O82−, the efficiency of UV/VUV photolysis highly exceeds that of UV photolysis due to the •OH formation, while in the presence of S2O82−, the dominant reaction partner is SO4•−. However, SO4•−-based methods proved to be efficient for both transformation and mineralization; they are sensitive for the matrix components.
1. Introduction
The complete removal of pharmaceutical ingredients from water is one of the most challenging tasks in water purification today. The application of antibiotics remains the primary treatment for bacterial infections for humans and animals. However, the release of pharmaceuticals, especially antibiotics into the environment, has led to the emergence of antibiotic-resistant bacterial strains and created a global health emergency causing at least 700,000 deaths a year. In addition to antibiotics, we must also pay attention to removing other drugs used in large quantities that are difficult to biodegrade. The conventional wastewater treatment renders limited results in pharmaceuticals elimination; thus, additive water treatment processes are required to prevent them exceeding into the environment and decreasing the risk. Advanced oxidation processes (AOPs) are additional water treatment methods that use chemical or photochemical processes to decompose compounds that are not or are only slightly removed by biological water treatment. Most AOPs are based on the highly reactive hydroxyl radical (HO•) generation; however, during the last decade, the number of publications relating to the investigation of chlorine species or sulphate radical ion-based processes has been fluently increasing.
The low-pressure mercury vapor (LPM) lamp emitting at 254 nm is a widely used light source for water disinfection, but its effectiveness in eliminating organic pollutants and reducing toxicity is highly limited. The molar absorbance at 254 nm and the quantum yield of the transformation of the target compound determine the efficiency of UV photolysis. The LPM lamps, equipped with special high-purity quartz, emit at 254 and 185 nm and are suitable for high-purity water production due to the HO• via absorption 185 nm VUV light by water:
H2O + hν → H• + HO•. Φ(HO•)185nm = 0.33
The efficacy of UV photolysis can be enhanced by using various oxidizing agencies, such as ozone, hydrogen peroxide, or persulfate ion [1]. During persulfate-based processes, the reaction with sulfate radical ions (SO4•−) is the primary degradation pathway. Persulfate (S2O82−) is a strong oxidant but can be activated to generate highly reactive SO4•− characterized by a higher oxidative potential (E0 = 2.6 V) than that of S2O82− (E0 = 2.1 V). The 254 nm UV light-activated persulfate (S2O82−; ε254 nm = 20–22 M−1 cm−1 [2]) is a clean source for producing SO4•− with high quantum yield [2] via the homolytic bond cleavage [3]:
S2O82− + hν → 2 SO4•−. Φ(SO4•−)254nm = 1.4 ± 0.3
The SO4•− is a potent oxidizing agent, similar to the HO• (E0 = 2.8 V) [3]. The primary reaction mechanism of SO4•− initiated transformation of organic substances is the electron transfer opposite to the addition, which is characteristic of HO•-initiated transformations [4]. Based on the literature so far, the AOP methods based on SO4•− are adequate for the degradation and mineralization of organic micropollutants, similar to HO•-based methods [5,6], especially in the case of the aromatic molecules with an amino group [7]. Comparing the primary reactive radicals, HO• is less selective and more reactive towards saturated organic substances than SO4•−. The reaction rate constants of HO• with TRIM and 5-FU are 7.36 × 109 M−1 s−1 [8] and 1.52 × 109 M−1 s−1 [9], respectively, whereas the reaction rate constant with SO4•− is only available for TRIM, its value is 3.81 × 109 M−1 s−1 [10], just slightly lower than with HO•.
This research compares and studies the UV photolysis, UV/VUV photolysis-producing HO•, and their combinations with persulfate to eliminate and mineralize two commonly used pharmaceuticals, namely trimethoprim (TRIM), an antibiotic drug, and the 5-fluorouracil (5-FU), a chemotherapeutic agent. Both selected model components are present in detectable concentrations in urban and hospital wastewater [11,12].
2. Materials and Methods
2.1. Photochemical Experiments
Two low-pressure mercury vapor (LPMUV and LMPVUV) lamps were used as light sources. LPMUV emits 254 nm UV photons (GCL307T5L/CELL, produced by LightTech), while LPMVUV (GCL307T5VH/CELL produced by LightTech) emits both 254 nm UV and 185 nm VUV photons. Both lamps have the same electric (15 W) and geometric (227 mm arc length and 20.5 mm diameter) parameters. The envelope of LMPVUV lamp was made from synthetic quartz to transmit the VUV185nm photons. The UV photon flux was determined by ferrioxalate actinometry and found to be the same (3.70 × 10−6 molphoton s−1) for both LPM lamps. The flux of the 185 nm VUV photons was determined by methanol actinometry and found to be one magnitude lower; 3.23 × 10−7 molphoton s−1. The experiments were carried out in a 500 mL cylindrical glass reactor with a 45 mm inner diameter and 300 mm height. The thickness of the irradiated water layer was 13 mm, which is enough for the complete absorption of 185 nm photons [13]. The initial concentration of TRIM and 5-FU was 1.0 × 10−4 M in each case.
2.2. Materials and Methods
The concentration of model compounds was performed by Agilent 1100 HPLC system, equipped with a diode array detector (DAD). For the analysis of TRIM (≥99%, Sigma-Aldrich) and its degradation products, Gemini 3u C6-phenyl 110A column was used (thermostated at 40 °C). The eluent contains 10% methanol (HPLC grade, VWR) and 90% formate buffer (≥99%, Sigma-Aldrich); the flow rate was 0.4 mL min−1. In the case of 5-FU (≥99%, Sigma-Aldrich), the eluent consisted of 5.0 × 10−3 M sulfuric acid (HPLC grade, FLUKA), the flow rate was 0.8 mL min−1. The detection wavelength was 275 nm for TRIM and 210 nm for 5-FU.
Total organic carbon (TOC) measurements were performed using an Analytik Jena N/C 3100 analyzer. For the investigation of matrix effect, biologically treated wastewater was used with high ionic (c(HCO3−) = 526 mg L−1 and c(Cl−) = 120 mg L−1) and relatively low organic content (TOC = 5–6 mg L−1).
3. Results and Discussion
3.1. UV and UV/VUV Photolysis
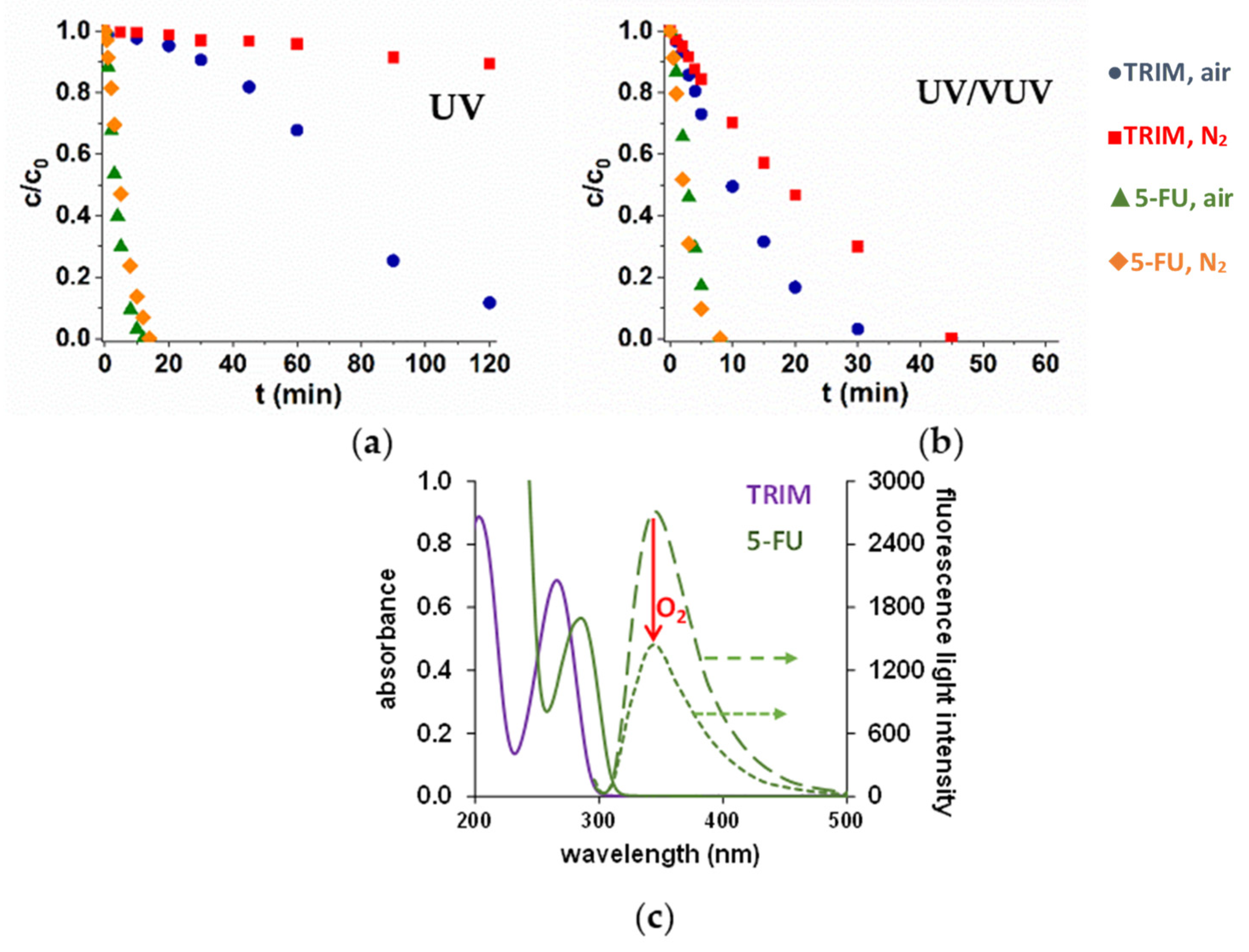
The efficiency of direct photolysis is determined by the molar absorbance of the target substance at 254 nm and the quantum efficiency of the transformation. However, the molar absorbances of the model compounds have a similar value (4045 M−1 cm−1 for TRIM and 5297 M−1 cm−1 for 5-FU), the transformation of 5-FU is significantly higher, with no more than 10 minutes required for the complete removal in both O2-free and O2-containing solutions. There is practically no transformation of TRIM in O2-free solution, and its transformation starts with a long induction period in the O2-containing one.
The fluorescence behavior of the compounds was investigated using 254 nm as excitation wavelength. For 5-FU, no fluorescence was observed, while TRIM emitted an intensive fluorescent light with a maximum intensity at 340 nm. Thus, a significant part of the absorbed photons results in fluorescent light without chemical transformation. Consequently, the quantum yield related to the TRIM transformation is much lower (<0.002), than to the 5-FU transformation (0.08 and 0.061 in aerated and in O2-free solutions, respectively). The dissolved O2 decreased the intensity of fluorescent light, suggesting that O2 can quench the excited state of TRM. The formed singlet oxygen is probably responsible for the positive effect of the dissolved O2 on the transformation of TRIM in the case of UV photolysis.
In the case of UV/VUV photolysis, the low intensity 185 nm photons highly enhanced the transformation rate of TRIM both in aerated and O2-free solutions due to the formation of reactive H• and HO• (1) but only slightly affected the 5-FU transformation (Figure 1a,b). The manifestation of the degree of the positive effect of VUV photons depends on the relative contribution of direct UV photolysis and radical (mainly HO•)-based transformation. For 5-FU the contribution of direct UV photolysis remains significant even in the presence of VUV light-generated radicals, while for TRIM, the radical-based transformatin became the primary way.
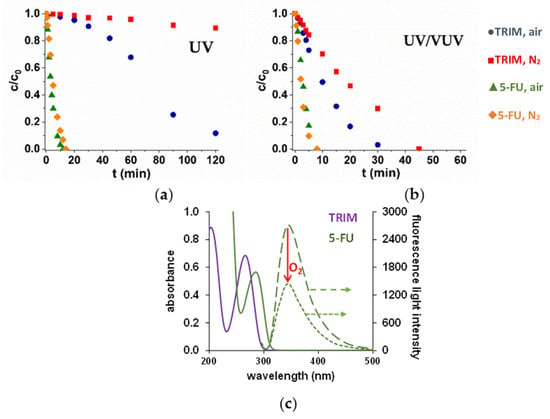
Figure 1.
Degradation of TRIM and 5-FU during UV (a) and UV/VUV (b) photolysis and their absorption and emission spectra in O2-free and O2-containing solutions (c).
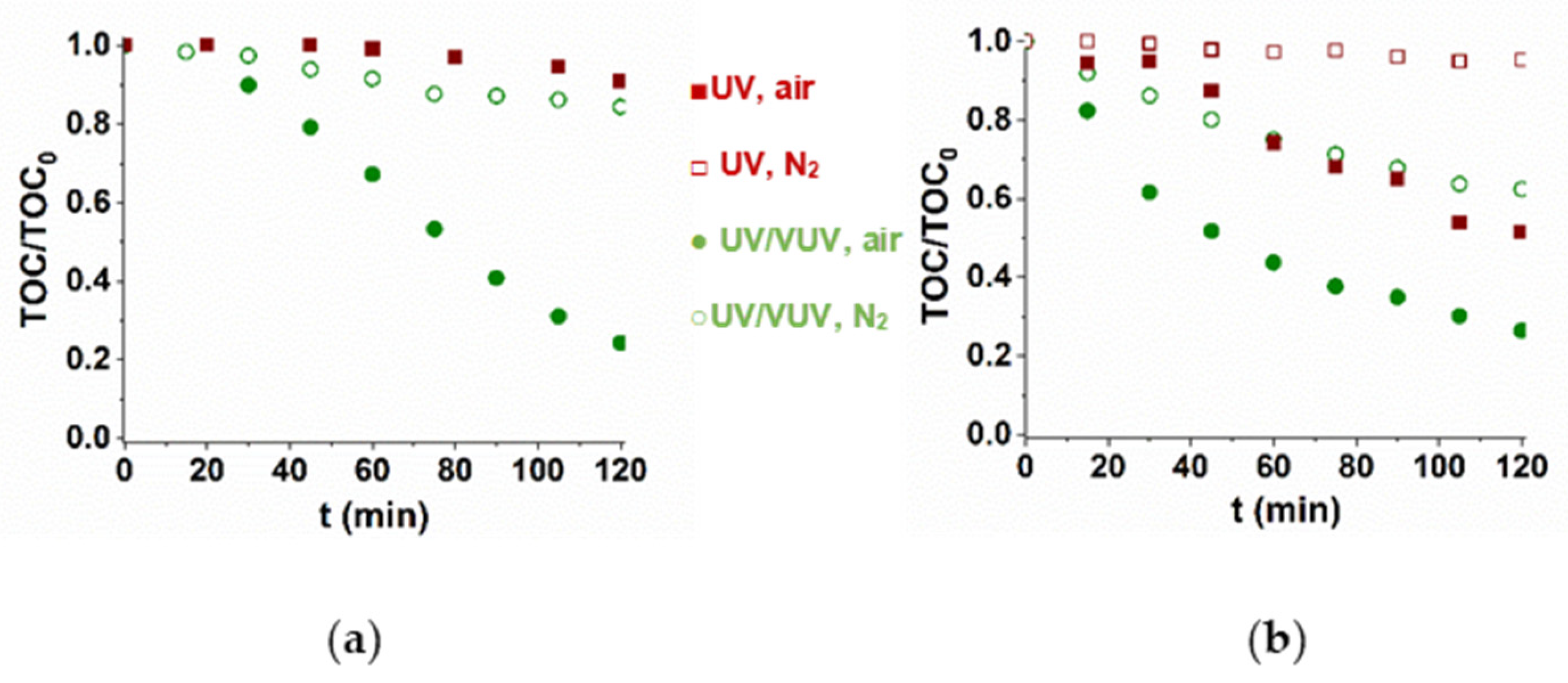
The presence of radicals produced by VUV photolysis of water also enhanced the mineralization rate. For TRIM, there is no observable mineralization in UV-radiated solution, while 40% of TOC can be removed for 5-FU (Figure 2). In UV/VUV light-irradiated O2-containing solutions about 80% of TOC was removed during 2 h. The results also showed that dissolved O2 has an essential role in mineralization most probably because of the formation organic peroxyl radicals (Figure 2).
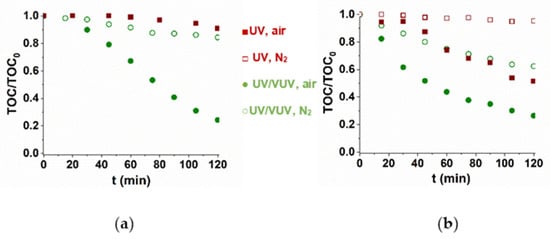
Figure 2.
Mineralization of TRIM (a) and 5-FU (b) during UV and UV/VUV photolysis.
3.2. Effect of S2O82−
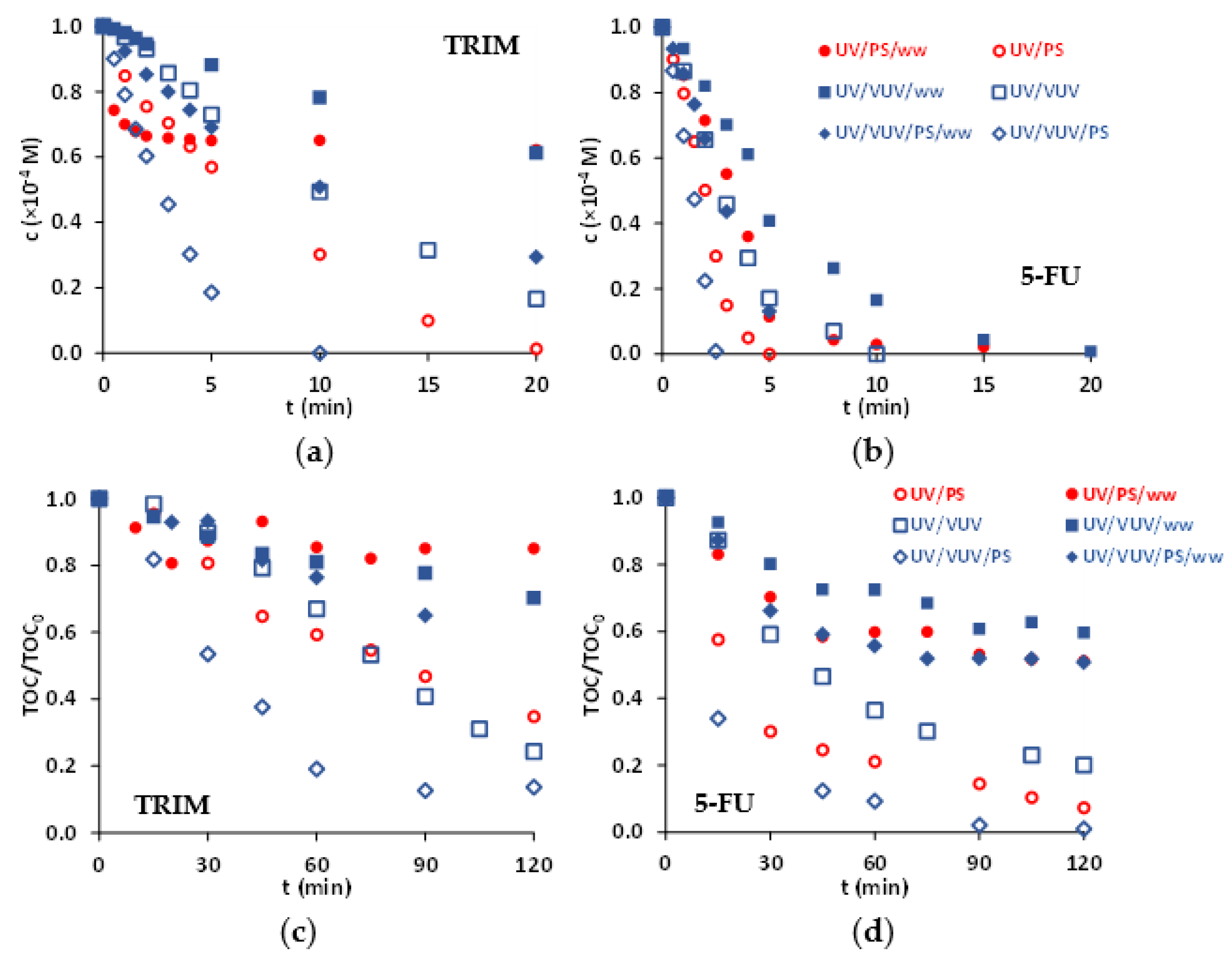
In the case of both pharmaceuticals and light sources the S2O82− addition enhanced the transformation rate independently on the presence of dissolved O2, due to the formation of reactive SO4•− via UV photolysis of S2O82−.
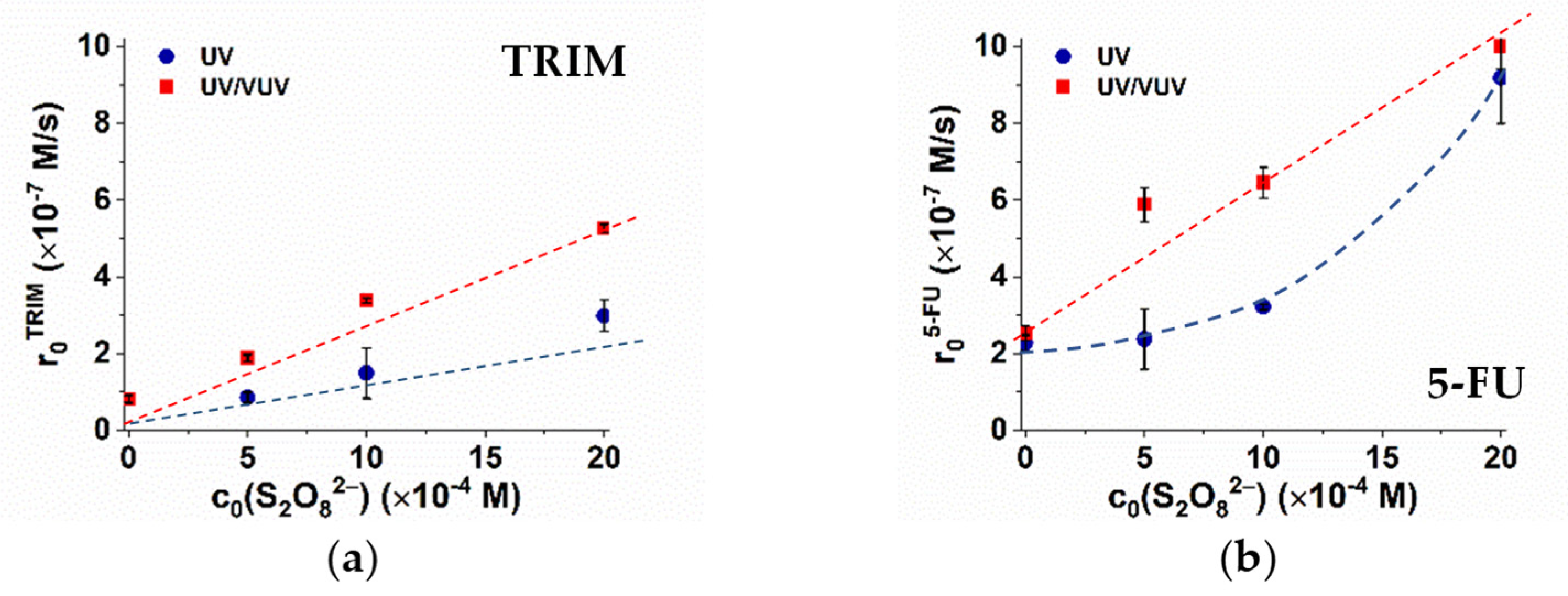
In the case of UV/S2O82− process, the transformation of organic substances can take place via direct UV photolysis or due to the reaction with SO4•−. For UV/VUV/S2O82−, additionally, the reactions with HO• must be taken into consideration. Comparing the molar absorbance of TRIM (4045 M−1 cm−1), 5-FU (5297 M−1 cm−1), and S2O82− (30 M−1 cm−1), the contribution of direct photolysis is likely to remain significant for 5-FU and negligible for TRIM transformation besides SO4•−- and/or HO•-initiated reactions at the given concentrations. Except in the case of UV/S2O82− process for 5-FU elimination, the transformation rate increased linearly with the initial concentration of added S2O82−. In the case of the UV/S2O82− process for the 5-FU elimination, the transformation rate changes exponentially (Figure 3).

Figure 3.
Effect of S2O82− dosage on the degradation of TRIM (a) and 5-FU (b) in the case of UV and UV/VUV photolysis.
For UV/VUV/S2O82−, the transformation rates are higher (approximately doubled) than for the UV/S2O82− process, which suggest that the additive effect due to the VUV light most likely remains important even in the presence of SO4•− (Table 1). The primary reaction mechanism of SO4•−-initiated transformation of organic substances (S) is the electron transfer, which results in SO4−. In VUV irradiated solutions, water is the main absorbent because of its high concentration (55 M). However, absorbance of SO4− is not negligible (ε185nm = 160 M−1 cm−1) and gives a possibility for the VUV photolysis of SO4−, regenerating the SO4•− [14], with a relatively high quantum yield.
SO4•− + S → SO4− + S•−
SO42− + hν185nm → SO4•− + eaq− Φ(SO4•−)185nm = 0.64

Table 1.
The initial transformation rate of TRIM and 5-FU using various processes (c(S2O82−) = 1.0 × 10−3 M).
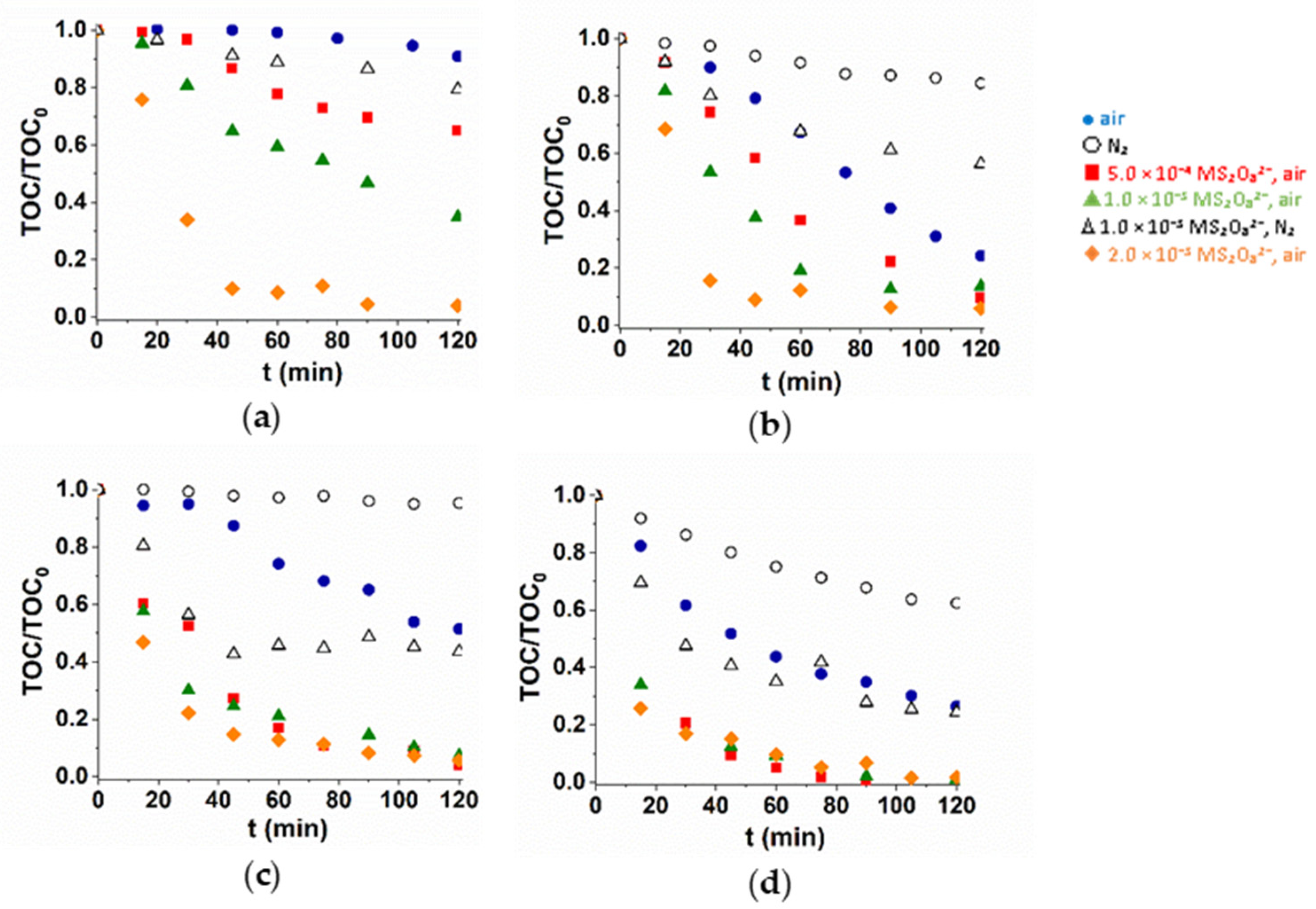
In many cases, the intermediates formed in the transformation of pharmaceuticals still have biological activity, so it is particularly important to study not only the conversion of the parent compound but also its mineralization. The mineralization was investigated with three different S2O82− concentrations (Figure 4). Both dissolved O2 and S2O82− enhanced the mineralization rate. The additive positive effect of VUV light is well manifested not only for transformation but also for mineralization efficiency. Using UV radiation, the mineralization rate increased with S2O82− concentration, but in UV/VUV irradiated solutions it was practically independent on that. At 1.0 × 10−3 M S2O82− concentrations, using UV radiation, the mineralization efficiency is close to that measured in UV/VUV photolysis.
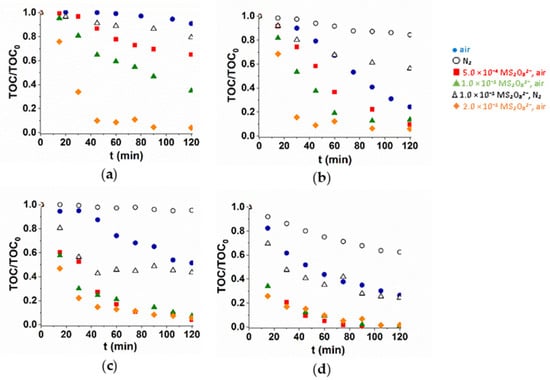
Figure 4.
Mineralization of TRIM (a,b) and 5-FU (c,d) during UV (a,c) and UV/VUV photolysis (b,d).
3.3. Radical Scavenger
The role of HO• radical was investigated with a commonly used HO• scavenger, tert-butanol (t-BuOH). The t-BuOH reacts three orders of magnitude faster with HO• (kHO• = 6.0 × 108 M−1 s−1) [15] than with SO4•− (kSO4–• = 4.0 × 105 M−1 s−1) [16]. Applying 3.0 × 10−2 M t-BuOH ensured that 95% of HO• and no more than 3% of SO4•− reacts with t-BuOH. At first, the effect of radical scavenger was investigated in the case of UV/VUV photolysis and found to be significant for TRIM, while for 5-FU the effect was moderated because of the relatively high contribution of the direct photolysis to the transformation (Table 2). During S2O82−-based processes, the t-BuOH just slightly inhibited the transformation, which means that the SO4•− is the dominant reaction partner. It also confirmed that, in the presence S2O82−, the positive effect of VUV is most probably attributable to the regeneration of SO4•− (4) and not to the HO• formation, opposite to the simple UV/VUV photolysis.

Table 2.
Effect of t-BuOH (3.0 × 10−2 M) on the degradation of TRIM and 5-FU.
3.4. Matrix Effect
In the development of additive water treatment processes, it is important to examine the effect of matrices and energy consumption because of several practical reasons. In this study, a biologically treated domestic wastewater was used, having low organic content (5–6 mg L−1) and relatively high Cl− (3.4 × 10−3 M) and HCO3− (8.6 × 10−3 M) concentration.
The biologically treated domestic wastewater has no significant absorbance at 254 nm, thus, it has no effect on the UV photolysis (Figure 5). For UV/VUV photolysis, the organic content and HCO3− behaves as an HO• scavenger (k = 8.5 × 106 M−1 s−1, [16]) and inhibits the transformation and mineralization. The effect of Cl− is not completely clarified. It can react with HO• (k = 4.3 × 109 M−1 s−1, [17]), but several studies described that no significant radical scavenging effect of Cl− because of the fast backward reaction.
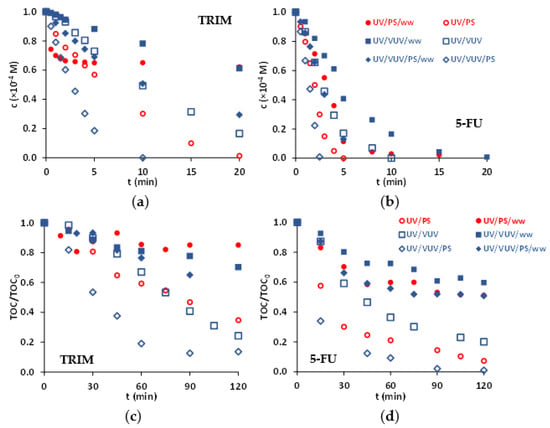
Figure 5.
Effect of biologically treated domestic wastewater (ww) as matrix of the transformation (a,b) and mineralization (c,d) of TRIM (a,c) and 5-FU (b,d) (c(S2O82−) = 1.0 × 10−3 M).
The negative effect of matrix manifested well for UV/S2O82− process (Figure 5). For TRIM, the transformation and mineralization were completely inhibited after a short period, while in MilliQ water, both were extremely fast. For 5-FU in terms of transformation, the effect is moderated, however, mineralization is inhibited similarly to TRIM. For UV/VUV/S2O82− similar trends were observed, but the inhibitory effect of the matrix is less strong than for UV/S2O82−.
For S2O82−-based processes, the main reaction partner is SO4•−. The SO4•− reacts moderately quickly with both Cl− (k = 3.6 × 108 M−1 s−1) and HCO3− (k = 1.6 × 106 M−1 s−1) via electron transfer reaction:
SO4•− + Cl− → SO4− + Cl•
Cl• + Cl− → Cl2•−
SO4•− + HCO3− → SO42− + CO3•− + H+
The further transformation of Cl2•− can finally result in HO• at a neutral pH from the Cl•/Cl2•− reaction with water. The formed CO3•− is a quite selective reactive species, its reaction rate constant with TRIM is 1.3 × 107 M−1 s−1 [18]. By increasing the concentration of persulfate, good efficiency can be obtained even in such a complex matrix, as the biologically treated wastewater. The required PDS concentration is determined by the analytical parameters of the matrix, especially the TOC content and the inorganic ion concentration.
4. Conclusions
In this work, UV (254 nm) and UV/VUV (254 and 185 nm) photolysis was combined with persulfate (S2O82−) addition. Commonly used drugs, trimethoprim (TRIM) and 5-fluorouracil (5-FU), were used as target substances. Opposite to the similar molar absorbances, UV photolysis was effective only for 5-FU transformation, while the low intensity VUV photons enhanced the transformation and mineralization rates of both components. The addition of S2O82− highly enhanced the transformation and mineralization efficiencies and linearly with the S2O82− concentration. The effect of t-BuOH as a radical scavenger proved that the main reaction partner the SO4•−, even in the case of the UV/VUV/S2O82− process. In biologically treated domestic wastewater as a matrix, the efficacy of UV/S2O82− and UV/VUV/S2O82− processes are significantly reduced, partly due to the organic matter and mainly due to the reaction of SO4•− with inorganic ions.
Author Contributions
L.F.: experimental work, writing—original draft preparation; A.S. and A.C.: experimental work; T.A.: conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Tünde Alapi is thankful for the support of the János Bolyai Research Scholarship (Hungarian Academy of Sciences) and for the New National Excellence Program of the Ministry for Innovation and Technology (ÚNKP-21-5-SZTE-594). Luca Farkas is thankful for the financial support of the Mecenatúra Sponsorship (New National Excellence Program of the Ministry for Innovation and Technology, MEC_R 141283) and for the National Talent Programme (NTP-NFTÖ-21-B-0064). This work was sponsored by the National Research, Development and Innovation Office-NKFI Fund OTKA, project number FK132742.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used is included in the article.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hsieh, S.; Lai, W.W.; Lin, A.Y. Kinetics and mechanism of 4-methylbenzylidene camphor degradation by UV-activated persulfate oxidation. Environ. Sci. Pollut. Res. 2021, 28, 18021–18034. [Google Scholar] [CrossRef] [PubMed]
- Mark, G.; Schuchmann, M.N.; Schuchmann, H.P.; von Sonntag, C. The photolysis of potassium peroxodisulphate in aqueous solution in the presence of tert-butanol: A simple actinometer for 254 nm radiation. J. Photochem. Photobiol. A 1990, 55, 157–168. [Google Scholar] [CrossRef]
- Karpel Vel Leitner, N. Sulfate radical ion—Based AOPs. In Advanced Oxidation Processes for Water Treatment; Stefan, M.I., Ed.; IWA Publishing: London, UK, 2017; pp. 429–460. [Google Scholar]
- Criquet, J.; Leitner, N.K.V. Degradation of acetic acid with sulfate radical generated by persulfate ion photolysis. Chemosphere 2009, 77, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, G.; Zoh, K. Benzophenone-3 degradation via UV/H2O2 and UV/persulfate reactions, Benzophenone-3 degradation via UV/H2O2 and UV/persulfate reaction. J. Hazard. Mater. 2021, 403, 123591. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Lu, S.; Wang, Z.; Wang, Y.; Zhang, G.; Guo, X.; Guo, W.; Zhang, T.; Xi, B. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Wojnárovits, L.; Tóth, T.; Takács, E. Critical evaluation of rate coefficients for hydroxyl radical reactions with antibiotics: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 575–613. [Google Scholar] [CrossRef]
- Ganzenko, O.; Oturan, N.; Sirés, I.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Fast and complete removal of the 5-fluorouracil drug from water by electro-Fenton oxidation. Environ. Chem. Lett. 2018, 16, 281–286. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yao, H.Y.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef] [PubMed]
- Mahdi-Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Mahnik, S.N.; Lenz, K.; Weissenbacher, N.; Mader, R.M.; Fuerhacker, M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 2007, 66, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharabli, S.; Engeßer, P.; Gera, D.; Klein, S.; Oppenländer, T. Engineering of a highly efficient Xe2*-excilamp (xenon excimer lamp, λmax = 172 nm, η = 40%) and qualitative comparison to a low-pressure mercury lamp (LP-Hg, λ=185/254 nm) for water purification. Chemosphere 2016, 144, 811–815. [Google Scholar] [CrossRef]
- Han, M.; Mohseni, M. Influence of sulfate and the interactions of major organic and inorganic solutes on the formation of nitrite during VUV photolysis of nitrate-rich water. J. Environ. Chem. Eng. 2021, 9, 105756. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1247. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J. Chem. Soc. Faraday Trans. 1 1973, 69, 1597–1607. [Google Scholar] [CrossRef]
- Wols, B.A.; Harmsen, D.J.H.; Beerendonk, E.F.; Hofman-Caris, C.H.M. Predicting pharmaceutical degradation by UV (LP)/H2O2 processes: A kinetic model. Chem. Eng. J. 2014, 255, 334–343. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).