Abstract
The tasks and objectives of grassland management have changed significantly in recent decades. One of the key elements of adapting to climatic and economic challenges is the optimal use and future sustainability of grasslands. Ferenc Balázs’s plant stand assessment method is a fast, efficient and widely applicable method for evaluating the quantitative and qualitative characteristics of forage in grasslands, as well as the economic value of pastures. This study is based on a three-dimensional coenological survey which is low-cost, does not require technical infrastructure, and empirically considers the species’ preference by livestock. As a result of our extended criteria approach, we assigned modified forage value (k-value) categories to 2310 vascular plant species. Based on our investigations in the presented case study, the Balázs method was proven to be well suited for estimating the yield of grasslands and determining the relative forage value of grasslands with a high degree of confidence in practice. As this method is non-destructive and involves little trampling, it is particularly suitable for monitoring grassland habitats with a high density of protected plant and animal species.
1. Introduction
Maintaining grasslands is crucial for nature conservation, effective grassland management, and the provision of ecosystem services [1,2,3]. It is therefore essential to determine how many animals can be supported on our grasslands in the most sustainable way possible [4]. To this end, the biomass and yield of grasslands must be determined and estimated as accurately as possible.
The amount and quality of biomass produced and its species composition are closely related to grassland management practices too [5,6]. Numerous studies have found that different grazing intensities and their frequency can influence the quantity, composition and quality of herbaceous biomass available to livestock [7,8,9]. Additionally, the quality of forage is closely related to species composition [10,11]. Diverse grassland plant communities often provide better quality forage due to the diversity of nutrient profiles offered by different species [12]. Mowing influences the vegetative structure and growth cycle of plant species, thus also affecting the palatability and nutritional value of forage. Early mowing tends to reduce the flowering of some key forage species, affecting the availability of good-quality forage later in the growing season [13,14].
According to recent research, environmental stress factors, particularly drought and climate change, are garnering increasing attention due to their impact on biomass production. For example, it has been shown that an increase in the frequency and intensity of drought periods significantly reduces biomass production in Festuca arundinacea-dominated grasslands [15,16,17]. Studies have shown that different management practices can significantly influence biomass yield and nutritional value. For example, fertilisation of grasslands is associated with improved biomass production, which highlights the importance of nutrient management in improving feed quality [18,19].
In addition to the quantity of biomass, its quality is also essential. Therefore, successful management requires maximising biomass while harvesting at the optimum point of quality indicators and, among other things, applying rotational grazing strategies [20,21]. Research has also shown that the feed value of Festuca arundinacea varies depending on maturity and growth stage, particularly in terms of crude protein and digestibility. Early harvesting generally results in a higher protein content, while late harvesting can increase fibre content, which reduces digestibility [22,23]. The use of specific cultivation techniques to enhance digestibility and disease resistance further amplifies the potential of this grass species [24]. Laboratory testing of the feed value of grass involves various mechanical and chemical analyses. The most commonly used techniques include the determination of crude protein, fibre and mineral content. However, hyperspectral techniques are becoming more widespread, which can be used to identify different plant species and, with promising results, to determine specific feed quality indicators such as cellulose and lignin content [25]. These analyses provide important information on feed value and help to improve feed quality and optimise animal husbandry practices [25]. In addition, air pollutants, especially ozone, have been documented to adversely affect the quality of grass feed value, potentially reducing it [26].
Various methods are used to estimate the quantity and quality of pasture biomass. These methods for measuring and assessing the forage value of grasslands cover a broad spectrum, from laboratory analysis to remote sensing techniques, which fundamentally shape the future of forage production and evaluation. Several methods are available to assess biomass by mowing [27], but this is often not feasible in protected areas or when assessing protected species. In detail, for a given sampling area (typically 1 m2), it is necessary to obtain multiple replicate biomass samples to obtain a more accurate estimate of the grassland yield, which can be used to estimate it per hectare and even to collect information on the biomass quality of the area by sorting the cut biomass by grassland components. However, their drawbacks (different stubble heights, high manual labour and equipment requirements, small sample size, inaccurate sampling due to inflexing grasses, etc.) make their application difficult [28].
The practice of estimating the feeding value of grasslands based on the plant species that make up the grassland began in the mid-20th century and was primarily driven by economic interests. Ernst Ludwig Klapp [29] (Germany) and Ferenc Balázs [30] (Hungary) developed seemingly similar evaluation systems, but based on partially different principles [30]. Klapp et al. [29] published the relative forage value of about 350 grassland species. Species were ranked on a 10-point scale, with the most valuable species assigned a value of +8, sturgeon and other species not grazed by animals assigned a value of 0, and toxic species assigned a value of −1. The forage quality of the plant species was determined based on the following criteria:
- -
- Protein, fibre and mineral content, as determined by chemical analysis.
- -
- Tastiness and palatability to livestock.
- -
- Valuable plant parts (leaf, stem, flower, fruit).
- -
- Duration of the full value (as feed).
- -
- Usefulness and harvestability of the species.
- -
- Harmful and toxic properties.
- -
- Allowable proportion in the plant population (e.g., for toxic plants).
If worthless and toxic species are present in high proportions in the plant population, the overall value of the population will decrease accordingly. To quantify this, Klapp et al. [29] considered the following:
- Forage value of toxic plants up to 3% cover—1, between 3 and 10%—2, above 10% cover—3.
- The number of dicotyledonous species that contaminate hay is reduced by 1–2 values for cover greater than 10%.
- A separate assessment applies to grasses and weeds that are highly detrimental to feed value.
Some authors (like [29,31,32,33]) consider grasses more valuable forage than legumes (although their protein content is much lower) and apply only one negative category to all harmful species from the forage point of view. It is also unacceptable that the quality score for a species should decrease as its cover increases, because as the cover of the species increases, the species does not become more toxic or thorny. This distorts the estimated feed value of the species and thus also the value of the grassland. The main flaw of these methods is that they ignore the height difference between species. However, this has a significant impact on the quality of grassland forage. Thus, they can only perform the feed value estimation function with a strong bias, since they do not take into account the effect of the height difference of plant species on the relative mass of each species, from which we learn the contribution of each species to the feed value of grassland.
Similar errors have been found in, among others, the technique developed by Nitsche [34] and modified by Briemle [33,35] and Briemle and Ellenberg [36], using a 9-value scale (1–9), which has become known as “Futterwert (FW)”. During the development of the method of Briemle [31,32], in addition to 4 additional 9-valued scales (mowability, grazability, trampling tolerance and fallow deer), a functional trait was added, comprising sedges, other dicotyledonous species, herbaceous legumes, woody legumes, and woody shrubs. A habitat rating (extensive grassland, economic grassland, arable and garden, fallow, woodland edge species) was also made. This method was developed for 680 species. Although this method generally (and according to more recent scientific results) provides a good species relationship [37,38], ignoring the height of plant species may still strongly distort the results obtained.
Our study aims to review the process of forage value determination of species and to provide a dataset of 2310 vascular plant species in the Pannonian biogeographical region. Our approach joins the collection of a series of comparative European indicator values [39,40] with a section on grassland management.
2. Materials and Methods
2.1. Method for Estimating Production and Forage Value of Grasslands
During our revisional work, we went through Balázs’s [30] method step by step. Some parts were fully adopted, while others were supplemented and developed to obtain as accurate a picture as possible of the feed value of grasslands.
The first step is to employed the three-dimensional recording method developed by Balázs [30,41] to determine the amount of hay produced. This method is based on a coenological recording but the average height of the plant species is taken into consideration in addition to their cover. It is therefore a suitable method for estimating the relative yield of each species. The relative yield can be converted to an absolute value and dimension by multiplying by a mass coefficient. This method meets the needs of practical grassland farmers and grassland management specialists in determining the amount of green yield (aboveground biomass) or hay produced by the plant community. Using a classical 4 × 4 m quadrat method, we created coenological records to identify species and estimate their cover as accurately as possible, using DB values. These were obtained by dividing the quadrat area once or several times. 1 DB value as a unit is 1/32 of the quadrat area (3.125% cover). One record can have a maximum of 32 DB values, unless the association is multi-level, in each case each level must be recorded separately. The smallest DB value is 0.2, which represents 0.625% coverage.
The first step of the method is to calculate each plant species’ relative green mass.
t: relative green mass of the plant species, DB: cover of the species, m: average height of the plant species [cm].
t = DB × m
The m value is usually species-specific, but (because of the different growing conditions and weather conditions) it is always advisable to measure it in the field when recording. 1 ‘m’ corresponds to 0.01 m of plant height. Measuring the average height of the shoots (stem + leaf) is essential—protruding stems should be excluded.
Relative green mass of quadrats representing the grassland (T): sum of the relative green mass (t) of each plant species (T = ∑t).
The average height of the grassland (M) is obtained by dividing the sum of the relative green mass of the species in the grassland by the total cover expressed as ∑DB.
M: average height of the grassland [cm], T: relative green mass of the grassland, which is the sum of the species relative green mass meaning yield, ∑DB: total cover of the grassland [DB].
M = T/∑DB
In pastures, we have the opportunity to solely determine the cover values of each species as it is continuously grazed. In this case, we can only choose the average height of ungrazed plant individuals (e.g., outside the grazed paddock) as a control.
The B numbers are used to convert the relative yields into absolute quantities. B numbers express the real green mass of a t-value, and BM expresses the real green mass of a 0.01 m high section of 100% total cover grassland per hectare. Therefore, when using the B numbers, the DB values of the records must be converted to % cover (b).
Balázs (1951) [42] suggests the following values for Carpathian Basin pannonic grasslands:
Bt: for grasses: 0.125 t ha−1
for Medicago sativa: 0.147 t ha−1
BM: for grass: 0.400 t ha−1
for Medicago sativa: 0.470 t ha−1
There are two methods for calculating the yield.
- The average height of the grassland (M) is multiplied by BM and then multiplied by the actual cover [DB].
- The grassland’s relative green mass (T-value) is multiplied by [Bt].
The grassland yield cannot be fully utilised, so the stubble height (s) must be subtracted from the average height. Suppose the yield is to be converted into hay value. In that case, the resulting value must be divided by the drying factor (E), which typically ranges from 2.5 and 3.5, depending on the weather, plant species and state of development. For optimum utilisation time, this value is 2.5 for dry grassland, 3 for mesophilic grassland and alfalfa and 3.5 for red clover.
P = [(M − s) × BM × b] ×100−1 × E
P: amount of grass yield [kg × ha−1]
M: grassland height [cm]
s: stubble height [cm]
BM: weight of a 1 cm high grass section at 100% total cover; maturity: 0.4 [t × ha−1]
b: DB values in cover %
E: drying factor
The productivity of a grassland can only be accurately determined if the yields of all utilisation periods are added together. However, only the quantities calculated for the first growth are also suitable for comparing the yields of several grassland areas. The method used by Balázs (1960) [30] classifies pannonic grasslands into five classes of productivity:
- I.
- Class I: grassland producing > 6.0 t dry matter × ha−1
- II.
- Class II: grassland producing between 4.5 and 6.0 t dry matter × ha−1
- III.
- Class III: grassland producing between 3.0 and 4.5 t dry matter × ha−1
- IV.
- Class IV grassland producing between 1.5 and 3.0 t dry matter × ha−1
- V.
- Class V grassland producing between 0.0 and 1.5 t dry matter × ha−1
2.2. Forage Value of Species (k)
The forage value of grassland is determined by the proportion of grassland components (from the most valuable species of legumes and grasses to weed species that threaten animal health) present in it [10,43]. Therefore, each grassland species should be assessed separately for accurate evaluation, and grasslands should be managed as a mixture of these species. Classification should be carried out for both beneficial and harmful species. It is essential to highlight the latter species, as most methods [29,31,32] do not assign more than one quality category to them, despite the significant differences in the harmful effects of these species. Balázs [30,41,42] established the basis for a system to characterise the qualitative differences between different grass species and between different plant communities. The seasonal variations of these are also significant [44,45], and can be influenced by the nutrient supply of the plants [46] as well as by the palatability of the different plant groups [47,48,49,50], which can also be investigated using this method. The species quality value (k) is a relative score that indicates the relative role of plant species in forage production.
The forage value categories were developed based on the following criteria. The forage value of the best quality species is similar to that of the abstract feeds, and therefore, they were given a separate category. These species (+6 and +7) improve the forage value of the grassland. All species eaten by the livestock (regardless of taxonomic affiliation) and whose consumption does not have any detrimental consequences are classified on a scale of 1 to 5. Species that the animal does not eat or whose consumption may have harmful repercussions are considered dangerous. These species are given a negative sign and are scored on a scale of 1 to 3. Neutral species that cannot be classified in either group are assigned a value of 0.
Forage value (k-value) categories by Balázs:
Species of value +7: their presence improves the quality of grass fodder. They are high in protein, have high nutritional value, rich foliage, excellent digestibility (over 75% digestible organic matter in the last 10 days of May), slow senescence, rapid growth, are palatable to animals, very well adapted to different habitats, and are trampling-tolerant.
Species of value +6: Also species with excellent nutritional value (65–75% digestible organic matter in the last 10 days of May), slow to senesce, good yielding, but less adapted to the site (possibly shorter life).
Species of value +5: grassland plants that still provide excellent quality forage (but may contain more firming tissue), with a good leaf-stem ratio and 55–65% digestible organic matter in the last 10 days of May. They are not rough, contain little silica, have excellent palatability, are not flaky and do not contain unpleasant odours and flavours. They are suitable for rough grazing and mowing and can be used to make good-quality hay. They are good post-emergence species, but may have a shorter life span.
Species of value +4: also provides good-quality forage, but their leaf-to-stem ratio is worse than the previous group’s. They contain more solidifying tissue and yield less after grazing and mowing. Legume species which are of minor importance from a forage perspective are also included.
Species of value +3: They have a reduced forage value, but if used at the right time, they provide good-quality forage. They are usually slightly rough, flaky, or leathery, have a lot of firming tissue or are less palatable to animals. This category also includes other dicotyledonous species of the highest forage quality.
Species of value +2: At best forage straw quality, when mown young, they can be used as ballast fodder to “dilute” better-quality forage. The livestock usually grazes on them when young. Their nutritional value is relatively low. They have a relatively poor germination rate and tend to go stunted quickly. Most are rough, flaky, or hairy but lack strong, bulging stems. This group includes many dicotyledonous species that the livestock readily eat when young. In small quantities, they improve the palatability of the feed.
Species of value +1: They provide forage of litter quality at most. They are not grazed when young, senesce quickly and lose their foliage rapidly. They may contain silicic acid or other slightly harmful substances, but in small quantities, they do not cause any harm to the livestock. Their nutrient content is very low.
Species of value 0: These species are suitable for foraging at a particular stage of their development, but do not become particularly damaging later on. They are usually small species and are insignificant from a forage point of view.
Species of value −1: Unpleasant smelling, rough or hairy, stalky species, which multiply rapidly and take up a lot of space in front of valuable species. The livestock never eats them, but their possible consumption is not harmful. They are also only suitable for bedding out of necessity.
Species of value −2: These plants are already highly damaging in sward and forage. They usually also contain toxic substances that cause damage when added to fodder, or they are large, thorny plants that occupy a significant portion of the grassland.
Species of value −3: The most forage-poor plants in our grasslands. They are particularly dangerous in pastures because, unlike species favoured by animals, they can reproduce undisturbed without weed control mowing or due to the lack of grazing.
The following criteria were taken into account by Balázs [30] when determining the k-values of plant species:
- -
- Proportion of valuable plant parts (leaf, stem, flower, fruit).
- -
- The duration of wholesomeness (as feed).
- -
- Usefulness and harvestability of the species.
- -
- Pests and toxicity.
- -
- Permissible proportion in the plant population (e.g., for poisonous plants).
- -
- Grazability and regeneration time.
In addition to the above-mentioned features, during the refinement we also took into account the following characteristics, thereby extending the accuracy:
- -
- Protein, fibre, mineral content and protein/fibre ratio.
- -
- Digestibility of the main species and its variation in the first growth.
- -
- Palatability and preference by livestock.
The digestible organic matter (DOM) content of the samples taken from the trimmings of the plots was determined in vitro using rumen fluid digestion according to the method of Tilley and Terry [51].
As a result of our survey, we assigned modified k-values to 2310 species (Appendix A).
2.3. Relative Economic Value of Species (kt)
If the relative green yield (t) of a species is multiplied by the species-specific quality score, the relative economic value of the species in the grassland is obtained:
kt = k × t
The sign of the kt-value of a species can be + or −, depending on the forage value of the species. Species with a k-value of 0 will have a kt-value of 0. Subtracting the negative kt values from the positive kt values (∑kt+ −∑kt−) will give the difference in the relative kt value of the grassland (∑kt). If ∑kt− is greater than ∑kt+, then the grassland has no forage value. The grassland quality (K) is obtained from the value ∑kt by dividing by the average T-sum.
K = (∑kt × T−1)
Semi-natural species rich grasslands always have a K-value below 5 because even if the dominant species has a K value of 5, the other, sub-ordinated species in the plant community will degrade the K value of the grassland. The quality of the green grass or hay produced can be determined regardless of quantity. Balázs [30] classifies grasslands into the following five classes according to their quality:
- I.
- Class: very good, high-quality grassland, K-value: >4.
- II.
- Class: good, quality grassland, K-value: 3–4.
- III.
- Class: medium, quality grassland, K-value: 2–3.
- IV.
- Class: poor, poor-quality grassland, K-value: 1–2.
- V.
- Class: bad, poor-quality grassland, K-value: 0–1.
The value of grass productivity (P)—based on the method of Balázs (1960)
The value of a grassland’s productivity is the grassland’s value-producing capacity divided by 100. This gives the point value of the economic value of the grassland.
P = kt ×100−1
Multiplying this by a given value in a given currency at current feed prices, the economic value produced by the grassland can be calculated.
2.4. Sample Area and Field Survey
In this study, we investigate various habitats where environmental factors differ significantly. One study site is a dry grassland where the thin-leaved Festuca pseudovina is the dominant species, and the other is a wet grassland where the broad-leaved Festuca arundinacea is the dominant species. Festuca pseudovina is a less-studied species, but Festuca arundinacea is a well-studied species due to their widespread use, which is attributed to their adaptability, drought tolerance, and high biomass production potential in temperate grassland ecosystems [52,53]. Festuca arundinacea-dominated grasslands make significant contributions to biodiversity and ecosystem stability. These grasslands are home to a diverse array of herbaceous species and contribute to soil organic carbon sequestration, thereby enhancing the overall ecosystem performance [15,16,17]. This species can improve forage quality in mixed pastures [54,55].
To test the method in our thesis, we selected two study sites (Figure 1):

Figure 1.
The sample areas: (a) Mende, wetland, with dominance of Festuca arundinacea, (b) Bösztör, dry grassland, with dominance of Festuca pseudovina.
- -
- A wetland dominated by Festuca arundinacea (Agrostio-Deschampsietum caesitosae Ujvárosi 1941) (Mende, Hungary).
- -
- A dry grassland dominated by Festuca pseudovina (Achilleo-Festucetum pseudovinae Soó (1933) 1947 corr. Borhidi 1996 (Bösztör, Hungary).
The 4 × 4 m sample quadrats were set up in a 7 × 7 Latin square layout. The quadrats were harvested by mowing the grass with separate mowing frequencies with a mower, leaving 0.04 m of stubble. Plots mowed 2 times a year were harvested on 30 June and 10 October; plots mowed 3 times a year were harvested on 18 May, 30 June and 10 October; and plots mowed 4 times a year were harvested on 18 May, 30 June, 5 August and 10 October. The test years were from 2016 to 2019.
2.5. Statistics
A non-parametric statistical method was used to analyse the cover value of species across different groups of complementary materials. According to the Shapiro–Wilk test, these variables were not normally distributed (p < 0.05). Therefore, the non-parametric Spearman test was used. All statistical analyses were performed using the XLSTAT statistical and data analysis software version 2024.4.1 [56].
3. Results
3.1. Case Study
The wet grassland dominated by Festuca arundinacea was investigated at the Mende sample site. The average cover was as follows: 55.5% grasses, 17.3% legumes, 18.7% forbs, 5.7% toxic species and 0.4% other species (Figure 2)
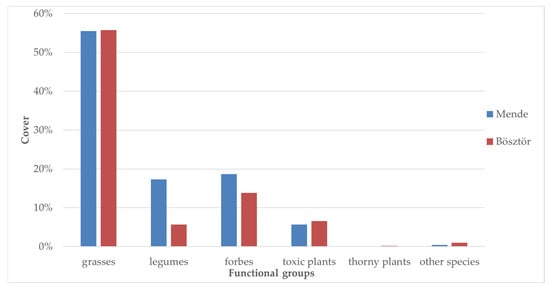
Figure 2.
Distribution of functional groups in the two sample areas.
Festuca pseudovina is the main constituent of the population in the other sample area in Bösztör. Grasses cover 55.7%, legumes cover 5.7%, forbs cover 13.8%, toxic species cover 6.6%, thorny species cover 0.2%, and other species cover 1.0%. Botriochloa ischaemum, a typical C4 grass species found in arid ecological sites, is often observed in drier years.
3.2. Result of the Yield Estimates
The Spearman correlation value of the green mass estimated by the Balázs method in the dry grassland at Bösztör was 0.44, indicating a moderately strong correlation with the values of the mown biomass samples. The wet meadow records at Mende had a Spearman correlation value of 0.96, indicating a robust correlation with the biomass sample data (Figure 3).
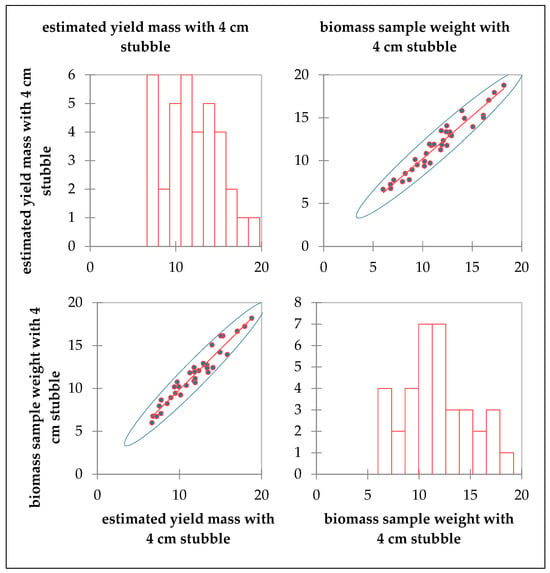
Figure 3.
Correlation of the weight estimation method of Balázs with the values of the mown biomass samples.
When examining the digestible organic matter yield, similar results were obtained, but the difference between treatments was even greater (Table 1). The productivity (P) in the table combines qualitative and quantitative indicators of the grassland yield in one number, allowing the quantitative and qualitative values of the yield of different grasslands to be standardised, and thus making it suitable for comparing grass or hay from different grasslands.

Table 1.
Procedure for calculating the digestible organic matter yield of grassland.
Table 1 shows the biomass estimation process. If the organic matter digestibility of the grassland is known, this method can also be used to calculate the yield of digestible organic matter per hectare of grassland. Our results showed that among the treatments tested, the 4 × mowed plots gave the highest production, while the 2 × mowed plots gave the lowest production.
3.3. Estimation of Forage Value of the Grassland
Table 2 shows the procedure for calculating the forage value of the grassland. The relative yield (t) obtained by multiplying the species cover (b) by its average height (m) is multiplied by the relative forage value (k) of the species. This is done for each species and the sum of the obtained values (Σkt) is divided by the sum of the relative yields (T) to obtain the average forage value (K) of the grassland, which in this case is 4.3, corresponding to a very good quality. Dividing Σkt (5752.7) by 100 gives the productivity of the grassland, which for the grassland under study was 57.5.

Table 2.
Forage value estimates.
4. Discussion
Determining the forage value of a grassland is a difficult task because, in addition to objective factors, it is also influenced by many subjective factors, such as habituation. Among the objective indicators, Balázs [30] considers the following to be the most important: the degree of development (age), nutritive value, protein content, starch value, fibre and silica content, digestibility, pungency, roughness, fluffiness, hardness, taste, odour, acidity, bitterness, toxicity, etc. The above characteristics indicate that the quality of grassland plants can significantly vary over a broad range. It is also clear that purely chemical analysis alone is insufficient to determine the value of forage, since, for example, species with negative morphological characteristics (e.g., stinginess) are not consumed by livestock even if they have excellent nutritional properties.
Our results demonstrate that the Balázs method is suitable with a high degree of confidence in practice. By changing the minimum DB value from 0.2 to 0.05 (0.156%). The process can be used to estimate the cover of rare, small species more accurately, which is particularly important in conservation and diversity studies. The method outlined above can also be used in conjunction with % cover estimation and altimetry. However, for those less experienced in cover estimation, DB value-based estimation is recommended for greater accuracy. During the course of our work, we have modified the Balázs k-values for several species based on recent results and have also significantly expanded the list of species with k-values based on this criterion to 2310 species (Appendix A) (Balázs reported 401 values).
The corrected Balázs three-dimensional recording method is well suited for estimating yield and grassland quality, as verified by direct yield estimation and the laboratory tests described above. The process requires virtually no equipment and is inexpensive to implement. The calculated productivity values represent the economic production of the studied grassland, providing a valuable tool for agricultural planning and management. Thanks to its quick and straightforward application, it can also be used for grassland mapping. It enables the monitoring of the temporal and spatial variation in stands as well as the effects of various treatments. In the future, the plan is to develop indicators that will allow for the expression of more accurate grasslands’ economic and forage value than hitherto. The method is also helpful for conservation purposes, as the three-dimensional coenological releves make it suitable for monitoring the management of protected areas [57]. The technique can measure the proportion of aboveground species production, which is an essential indicator of the carbon balance in grassland ecosystems, especially in lush grasslands [58,59,60]. It can therefore be used effectively in carbon balance studies without damaging the grasslands.
Natural grasslands, particularly in temperate and semi-arid regions, exhibit significant seasonality in biomass production, which can vary substantially due to climatic factors such as temperature and rainfall, as confirmed by our experiment.
The productivity of grassland is also closely related to the frequency of mowing. While increased mowing can increase direct biomass production by minimising interspecific competition, it can also reduce the overall long-term productivity of the grassland by depleting the necessary resources stored in the root system. In our study, 4 × mowing gave the highest productivity and the best forage quality. Regular mowing has been shown to minimise biomass accumulation due to the continuous removal of green tissue, which is essential for photosynthesis and energy storage [61]. Research also shows that optimal productivity can be achieved at specific mowing frequencies, such as every two years, which allows for the maintenance of adequate herbaceous cover while promoting biodiversity [62,63]. The correlation follows a bell curve where very low and very high mowing frequencies can reduce productivity, suggesting a “sweet spot” that balances the competitive dynamics between grassland species [64].
The frequency of mowing has a direct impact on the species richness and composition of grasslands. Studies show that higher mowing frequency is often associated with decreased species richness. In particular, grasslands subjected to intensive and frequent mowing may have reduced plant species abundance and diversity. This occurs because frequent mowing tends to favour disturbance-tolerant species over species less resistant to regular mowing, resulting in a homogenised plant community dominated by a few species [65,66]. For example, Socher et al. (2012) [67] found that more frequently mowed grasslands had lower species richness than those that were mowed less often, confirming a consistent trend observed in different studies [67]. Furthermore, Binder et al. 2018 [66] reported that while mowing intensity was positively correlated with species richness in controlled experiments, more frequent mowing tended to reduce overall diversity [66]. The interaction between mowing frequency and plant diversity is complex, and an intermediate approach, such as mowing every other year, has been proposed to maintain a richer plant community [62,68].
Natural arid grasslands, such as those found in semi-arid regions, typically show a high allocation of belowground biomass, and studies suggest that root mass accounts for 67% of total plant biomass in these ecosystems [69]. Belowground biomass is vital for drought resilience and resource acquisition, thereby increasing the overall resilience of the grassland [70]. For example, in alpine regions, aboveground biomass (AGB) averages around 68.8 g/m2, which varies with annual rainfall, suggesting that drier years result in decreased AGB due to limited water availability [71]. Furthermore, the dynamics of aboveground and belowground biomass production can be significantly influenced by climate, especially precipitation, which plays a crucial role in shaping productivity levels [71,72]. In a study of a northern dry grassland ecosystem, water availability was the primary limiting factor in dry years, reducing net primary productivity (NPP) under these conditions [73]. Dry grassland productivity is often resilient to short-term changes in precipitation, highlighting the importance of moisture patterns in determining biomass output [74,75].
Author Contributions
Conceptualisation, K.P., S.S. and Z.W.; methodology, K.P., Z.K. and S.S.; software, L.S. (László Sipos) and Z.W.; formal analysis, A.K., T.S.-S., I.T.-J. and E.S.-F.; investigation, K.P., E.S.-F., S.S. and I.T.-J.; writing—original draft preparation, K.P., M.B., E.S.-F., I.T.-J., D.B. and Z.K.; writing—review and editing, I.T.-J., K.P., L.S. (Leonárd Sári) and E.S.-F.; supervision, S.S. and K.P.; funding acquisition, K.P. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the strategic research fund of the University of Veterinary Medicine Budapest (Grant No. SRF-002), was supported by the Research Excellence Program of the Hungarian University of Agriculture and Life Sciences and OTKA K-147342.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due the data are part of an ongoing investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Forage values (k-values) of species.
Table A1.
Forage values (k-values) of species.
| Species | k-Value | Species | k-Value |
|---|---|---|---|
| Abutilon theophrasti | −1 | Juncus tenageia | 0 |
| Acer campestre | −1 | Juncus tenuis | 0 |
| Acer negundo | −1 | Juniperus communis | −3 |
| Acer platanoides | −1 | Jurinea mollis | −1 |
| Acer pseudo-platanus | −1 | Jurinea glycacantha | −1 |
| Acer tataricum | −1 | Kickxia elatine | 0 |
| Achillea asplenifolia | 2 | Kickxia spuria | 0 |
| Achillea collina | 2 | Knautia arvensis | 1 |
| Achillea crithmifolia | 2 | Knautia dipsacifolia | 1 |
| Achillea distans | 2 | Knautia drymeia | 1 |
| Achillea horanszkyi | 2 | Knautia kitaibelii | 1 |
| Achillea millefolium | 2 | Kochia laniflora | −1 |
| Achillea nobilis | 2 | Kochia prostrata | −1 |
| Achillea ochroleuca | 2 | Kochia scoparia | −1 |
| Achillea pannonica | 2 | Koeleria cristata | 2 |
| Achillea ptarmica | 1 | Koeleria glauca | 2 |
| Achillea setacea | 2 | Koeleria grandis | 2 |
| Achillea tuzsonii | 2 | Koeleria javorkae | 2 |
| Acinos arvensis | 0 | Koeleria majoriflora | 2 |
| Aconitum anthora | −3 | Koeleria pyramidata | 2 |
| Aconitum moldavicum | −3 | Laburnum anagyroides | −3 |
| Aconitum variegatum | −3 | Lactuca perennis | −2 |
| Aconitum vulparia | −3 | Lactuca quercina | −2 |
| Acorus calamus | −1 | Lactuca saligna | −2 |
| Actaea spicata | −2 | Lactuca serriola | −2 |
| Adenophora liliifolia | 0 | Lactuca viminea | −2 |
| Adonis aestivalis | −2 | Lamium album | 0 |
| Adonis flammea | −2 | Lamium amplexicaule | 0 |
| Adonis vernalis | −2 | Lamium maculatum | 0 |
| Adonis x hybrida | −2 | Lamium orvala | 0 |
| Adoxa moschatellina | 0 | Lamium purpureum | 0 |
| Aegilops cylindrica | 0 | Lappula deflexa | −1 |
| Aegopodium podagraria | −1 | Lappula heteracantha | −1 |
| Aethionema saxatile | 0 | Lappula marginata | −1 |
| Aethusa cynapium | −3 | Lappula squarrosa | −1 |
| Agrimonia eupatoria | 2 | Lapsana communis | −1 |
| Agrimonia procera | 1 | Larix decidua | −1 |
| Agropyron cristatum | 5 | Laser trilobum | −2 |
| Agrostemma githago | −3 | Laserpitium latifolium | −2 |
| Agrostis canina | 3 | Laserpitium prutenicum | −2 |
| Agrostis capillaris | 2 | Lathraea squamaria | −1 |
| Agrostis stolonifera | 5 | Lathyrus aphaca | 2 |
| Agrostis vinealis | 3 | Lathyrus hirsutus | 2 |
| Ailanthus altissima | −3 | Lathyrus latifolius | 4 |
| Aira caryophyllea | 0 | Lathyrus linifolius | 3 |
| Aira elegantissima | 0 | Lathyrus niger | 3 |
| Ajuga chamaepitys | 0 | Lathyrus nissolia | 3 |
| Ajuga genevensis | 0 | Lathyrus pallescens | 4 |
| Ajuga laxmannii | 0 | Lathyrus palustris | 4 |
| Ajuga reptans | 0 | Lathyrus pannonicus | 2 |
| Alcea biennis | −2 | Lathyrus pisiformis | 4 |
| Alcea rosea | −2 | Lathyrus pratensis | 4 |
| Alchemilla acutiloba | 2 | Lathyrus sativus | 6 |
| Alchemilla crinita | 2 | Lathyrus sphaericus | 2 |
| Alchemilla glabra | 2 | Lathyrus sylvestris | 2 |
| Alchemilla glaucescens | 2 | Lathyrus tuberosus | 2 |
| Alchemilla hungarica | 2 | Lathyrus venetus | 2 |
| Alchemilla micans | 2 | Lathyrus vernus | 2 |
| Alchemilla monticola | 2 | Lavandula angustifolia | −1 |
| Alchemilla xanthochlora | 2 | Lavatera thuringiaca | −2 |
| Alisma gramineum | −2 | Lavatera trimestris | −2 |
| Alisma lanceolatum | −2 | Leersia oryzoides | 3 |
| Alisma plantago-aquatica | −2 | Legousia speculum-veneris | 1 |
| Alkanna tinctoria | −2 | Lembotropis nigricans | −2 |
| Alliaria petiolata | −2 | Lens culinaris | 3 |
| Allium angulosum | −2 | Leontodon autumnalis | 1 |
| Allium atropurpureum | −2 | Leontodon hispidus | 2 |
| Allium atroviolaceum | −2 | Leontodon incanus | 1 |
| Allium carinatum | −2 | Leontodon saxatilis | 1 |
| Allium flavum | −2 | Leonurus cardiaca | −1 |
| Allium lusitanicum | −2 | Leonurus marrubiastrum | −1 |
| Allium moschatum | −2 | Lepidium campestre | −2 |
| Allium oleraceum | −2 | Lepidium cartilagineum | −2 |
| Allium paniculatum | −2 | Lepidium densiflorum | −2 |
| Allium rotundum | −2 | Lepidium graminifolium | −2 |
| Allium scorodoprasum | −2 | Lepidium perfoliatum | −2 |
| Allium sphaerocephalon | −2 | Lepidium ruderale | −2 |
| Allium suaveolens | −2 | Lepidium virginicum | −2 |
| Allium ursinum | −2 | Leucanthemella serotinum | −2 |
| Allium vineale | −2 | Leucanthemum margaritae | −2 |
| Alnus glutinosa | −1 | Leucanthemum vulgare | 1 |
| Alnus incana | −1 | Leucojum aestivum | −3 |
| Alnus viridis | −1 | Leucojum vernum | −3 |
| Alopecurus aequalis | 3 | Libanotis pyrenaica | 1 |
| Alopecurus geniculatus | 3 | Ligularia sibirica | −2 |
| Alopecurus myosuroides | 3 | Ligustrum vulgare | −3 |
| Alopecurus pratensis | 4 | Lilium bulbiferum | −2 |
| Althaea cannabina | −1 | Lilium martagon | −2 |
| Althaea hirsuta | −1 | Limodorum abortivum | −1 |
| Althaea officinalis | −1 | Limonium gmelini | 1 |
| Alyssum alyssoides | 0 | Limosella aquatica | 0 |
| Alyssum desertorum | 0 | Linaria angustissima | −1 |
| Alyssum montanum | 0 | Linaria arvensis | −1 |
| Alyssum tortuosum | 0 | Linaria genistifolia | −1 |
| Amaranthus albus | 1 | Linaria vulgaris | −1 |
| Amaranthus blitoides | 1 | Linaria x kocianovichii | −1 |
| Amaranthus blitum | 1 | Lindernia procumbens | 0 |
| Amaranthus bouchonii | 1 | Linum austriacum | −1 |
| Amaranthus crispus | 1 | Linum catharticum | −1 |
| Amaranthus deflexus | 1 | Linum dolomiticum | −1 |
| Amaranthus graecizans | 1 | Linum flavum | −1 |
| Amaranthus patulus | 1 | Linum hirsutum | −1 |
| Amaranthus powellii | 1 | Linum perenne | −1 |
| Amaranthus retroflexus | 1 | Linum tenuifolium | −1 |
| Ambrosia artemisiifolia | −2 | Linum trigynum | −1 |
| Amelanchier ovalis | −1 | Linum usitatissimum | −1 |
| Ammannia verticillata | 1 | Liparis loeselii | −3 |
| Amorpha fruticosa | −3 | Listera ovata | −1 |
| Amygdalus communis | −1 | Lithospermum arvense | −1 |
| Amygdalus nana | −2 | Lithospermum officinale | −1 |
| Anacamptis pyramidalis | −1 | Lithospermum purpureo-coeruleum | −1 |
| Anagallis arvensis | 1 | Lolium multiflorum | 5 |
| Anagallis foemina | 1 | Lolium perenne | 6 |
| Anchusa azurea | −1 | Lolium remotum | −1 |
| Anchusa barrelieri | −1 | Lolium temulentum | −1 |
| Anchusa ochroleuca | −1 | Lonicera caprifolium | −2 |
| Anchusa officinalis | −1 | Lonicera nigra | −2 |
| Androsace elongata | 0 | Lonicera xylosteum | −2 |
| Androsace maxima | 0 | Loranthus europaeus | −3 |
| Anemone nemorosa | −2 | Lotus angustissimus | 6 |
| Anemone ranunculoides | −2 | Lotus borbasii | 7 |
| Anemone sylvestris | −2 | Lotus corniculatus | 7 |
| Anemone trifolia | −2 | Lotus tenuis | 6 |
| Anethum graveolens | 1 | Lotus uliginosus | 6 |
| Angelica archangelica | −2 | Ludwigia palustris | −2 |
| Angelica palustris | −2 | Lunaria annua | −1 |
| Angelica sylvestris | −2 | Lunaria rediviva | −1 |
| Antennaria dioica | 1 | Lupinus albus | −2 |
| Anthemis arvensis | −1 | Lupinus angustifolius | −2 |
| Anthemis austriaca | −1 | Lupinus luteus | −2 |
| Anthemis cotula | −1 | Lupinus polyphyllus | −2 |
| Anthemis ruthenica | −1 | Luzula campestris | −1 |
| Anthemis tinctoria | −1 | Luzula divulgata | −1 |
| Anthericum liliago | −2 | Luzula forsteri | −1 |
| Anthericum ramosum | −2 | Luzula luzuloides | −1 |
| Anthoxanthum odoratum | 2 | Luzula multiflora | −1 |
| Anthriscus caucalis | −2 | Luzula pallidula | −1 |
| Anthriscus cerefolium | −2 | Luzula pilosa | −1 |
| Anthriscus nitidus | −2 | Lychnis coronaria | −1 |
| Anthriscus sylvestris | −2 | Lychnis flos-cuculi | 1 |
| Anthyllis vulneraria | 4 | Lychnis viscaria | −1 |
| Apera interrupta | 1 | Lycium barbarum | −3 |
| Apera spica-venti | 1 | Lycium chinense | −3 |
| Aphanes arvensis | 0 | Lycopodium annotinum | −1 |
| Aphanes australis | 0 | Lycopodium clavatum | −1 |
| Apium graveolens | −2 | Lycopsis arvensis | −1 |
| Apium repens | −2 | Lycopus europaeus | −1 |
| Aquilegia vulgaris | −2 | Lycopus exaltatus | −1 |
| Arabidopsis thaliana | 0 | Lysimachia nummularia | 0 |
| Arabis alpina | 1 | Lysimachia punctata | −1 |
| Arabis auriculata | 1 | Lysimachia thyrsiflora | −1 |
| Arabis hirsuta | 1 | Lysimachia vulgaris | −1 |
| Arabis turrita | 1 | Lythrum hyssopifolia | −1 |
| Arctium lappa | −2 | Lythrum linifolium | −1 |
| Arctium minus | −2 | Lythrum salicaria | −2 |
| Arctium nemorosum | −2 | Lythrum thesioides | −1 |
| Arctium tomentosum | −2 | Lythrum tribracteatum | −1 |
| Aremonia agrimonoides | 1 | Lythrum virgatum | −1 |
| Arenaria leptoclados | 0 | Majanthemum bifolium | −1 |
| Arenaria procera | 0 | Malcolmia africana | 1 |
| Arenaria serpyllifolia | 0 | Malus domestica | −1 |
| Aristolochia clematitis | −2 | Malus sylvestris | −1 |
| Armeria elongata | 0 | Malva alcea | 1 |
| Armoracia lapathifolia | −2 | Malva moschata | 1 |
| Armoracia macrocarpa | −2 | Malva neglecta | 1 |
| Arnica montana | 1 | Malva pusilla | 1 |
| Arrhenatherum elatius | 4 | Malva sylvestris | 1 |
| Artemisia abrotanum | −1 | Malva verticillata | 1 |
| Artemisia absinthium | −2 | Marrubium peregrinum | −1 |
| Artemisia alba | −2 | Marrubium vulgare | −1 |
| Artemisia annua | −2 | Marrubium x paniculatum | −1 |
| Artemisia austriaca | −1 | Marsilea quadrifolia | −2 |
| Artemisia campestris | −1 | Matricaria chamomilla | −1 |
| Artemisia pontica | −1 | Matricaria discoidea | −1 |
| Artemisia santonicum | −1 | Matricaria maritima | −1 |
| Artemisia scoparia | −1 | Matricaria tenuifolia | −1 |
| Artemisia vulgaris | −2 | Matteuccia struthiopteris | −2 |
| Arum maculatum | −3 | Medicago arabica | 3 |
| Arum orientale | −3 | Medicago falcata | 6 |
| Aruncus sylvestris | −1 | Medicago lupulina | 5 |
| Asarum europaeum | −1 | Medicago minima | 4 |
| Asclepias syriaca | −3 | Medicago orbicularis | 3 |
| Asparagus officinalis | 1 | Medicago polymorpha | 4 |
| Asperugo procumbens | −1 | Medicago prostrata | 4 |
| Asperula arvensis | 1 | Medicago rigidula | 3 |
| Asperula cynanchica | 1 | Medicago sativa | 7 |
| Asperula orientalis | 1 | Medicago x varia | 7 |
| Asperula taurina | 1 | Melampyrum arvense | −2 |
| Asperula tinctoria | 1 | Melampyrum barbatum | −2 |
| Asphodelus albus | −1 | Melampyrum cristatum | −2 |
| Asplenium adiantum-nigrum | −2 | Melampyrum nemorosum | −2 |
| Asplenium fontanum | −2 | Melampyrum polyphyllus | −2 |
| Asplenium lepidum | −2 | Melampyrum pratense | −2 |
| Asplenium ruta-muraria | −2 | Melica altissima | 1 |
| Asplenium septentrionale | −2 | Melica ciliata | 1 |
| Asplenium trichomanes | −2 | Melica nutans | 2 |
| Asplenium viride | −2 | Melica picta | 1 |
| Aster amellus | 0 | Melica transsilvanica | 1 |
| Aster lanceolatus | −1 | Melica uniflora | 2 |
| Aster linosyris | 0 | Melilotus albus | 2 |
| Aster novae-angliae | −1 | Melilotus altissimus | 2 |
| Aster novi-belgii | −1 | Melilotus dentatus | 2 |
| Aster oleifolius | −1 | Melilotus officinalis | 2 |
| Aster sedifolius | −1 | Melissa officinalis | −1 |
| Aster tradescantii | −1 | Melittis carpatica | −1 |
| Aster tripolium | 0 | Melittis melissophyllum | −1 |
| Aster x salignus | −1 | Mentha aquatica | −1 |
| Aster x versicolor | −1 | Mentha arvensis | −1 |
| Astragalus asper | 2 | Mentha longifolia | −1 |
| Astragalus austriacus | 2 | Mentha pulegium | −1 |
| Astragalus cicer | 2 | Mentha x carinthiaca | −1 |
| Astragalus contortuplicatus | 2 | Mentha x dalmatica | −1 |
| Astragalus dasyanthus | 2 | Mentha x dumetorum | −1 |
| Astragalus exscapus | 3 | Mentha x gentilis | −1 |
| Astragalus glycyphyllos | 4 | Mentha x verticillata | −1 |
| Astragalus onobrychis | 2 | Menyanthes trifoliata | −3 |
| Astragalus sulcatus | 3 | Mercurialis annua | −2 |
| Astragalus varius | 2 | Mercurialis ovata | −2 |
| Astragalus vesicarius | 2 | Mercurialis perennis | −2 |
| Astrantia major | 1 | Mercurialis x paxii | −2 |
| Asyneuma canescens | 1 | Mespilus germanica | −2 |
| Athyrium filix-femina | −2 | Micropus erectus | 0 |
| Atriplex hortensis | −2 | Microrrhinum minus | 0 |
| Atriplex littoralis | −2 | Milium effusum | 3 |
| Atriplex oblongifolia | −2 | Minuartia fastigiata | 0 |
| Atriplex patula | −2 | Minuartia frutescens | 0 |
| Atriplex prostrata | −2 | Minuartia glomerata | 0 |
| Atriplex rosea | −2 | Minuartia setacea | 0 |
| Atriplex sagittata | −2 | Minuartia verna | 0 |
| Atriplex tatarica | −2 | Minuartia viscosa | 0 |
| Atropa bella-donna | −3 | Misopates orontium | 0 |
| Aurinia saxatilis | 1 | Moehringia muscosa | 0 |
| Avena barbata | 1 | Moehringia trinervia | 0 |
| Avena fatua | 1 | Moenchia mantica | 0 |
| Avena nuda | 1 | Molinia arundinacea | 1 |
| Avena sativa | 3 | Molinia coerulea | 1 |
| Avena sterilis | 1 | Morus alba | −1 |
| Avena strigosa | 1 | Morus nigra | −1 |
| Ballota nigra | −2 | Muscari botryoides | −2 |
| Barbarea stricta | 1 | Muscari comosum | −2 |
| Barbarea verna | 1 | Muscari racemosum | −2 |
| Barbarea vulgaris | 1 | Muscari tenuiflorum | −2 |
| Bassia sedoides | −1 | Myagrum perfoliatum | −1 |
| Beckmannia eruciformis | 4 | Mycelis muralis | −2 |
| Bellis perennis | 2 | Myosotis arvensis | 0 |
| Berberis vulgaris | −3 | Myosotis caespitosa | 0 |
| Berteroa incana | −1 | Myosotis discolor | 0 |
| Berula erecta | −2 | Myosotis nemorosa | 0 |
| Betonica officinalis | 1 | Myosotis palustris | 0 |
| Betula pendula | −1 | Myosotis ramosissima | 0 |
| Betula pubescens | −1 | Myosotis sparsiflora | 0 |
| Bidens cernua | −3 | Myosotis stenophylla | 0 |
| Bidens frondosa | −3 | Myosotis stricta | 0 |
| Bidens tripartita | −3 | Myosotis sylvatica | 0 |
| Bifora radians | −2 | Myosoton aquaticum | 1 |
| Biscutella laevigata | 1 | Myosurus minimus | 0 |
| Blackstonia acuminata | −2 | Narcissus angustifolius | −2 |
| Blechnum spicant | −2 | Narcissus poëticus | −2 |
| Blysmus compressus | 1 | Narcissus pseudonarcissus | −2 |
| Bolboschoenus maritimus | −1 | Nardus stricta | −1 |
| Borago officinalis | 1 | Nasturtium officinale | −2 |
| Bothriochloa ischaemum | 1 | Neottia nidus-avis | −1 |
| Botrychium lunaria | 0 | Nepeta cataria | −1 |
| Botrychium matricariifolium | 0 | Nepeta pannonica | −1 |
| Botrychium multifidum | 0 | Nepeta parviflora | −1 |
| Botrychium virginianum | −2 | Neslea paniculata | 1 |
| Brachypodium pinnatum | 2 | Nicandra physalodes | −3 |
| Brachypodium sylvaticum | 2 | Nicotiana rustica | −3 |
| Brassica elongata | 2 | Nicotiana tabacum | −3 |
| Brassica nigra | 1 | Nigella arvensis | −1 |
| Brassica oleracea | 3 | Nigella damascena | −1 |
| Brassica rapa | 3 | Nonea pulla | 1 |
| Brassica x juncea | 1 | Odontites lutea | −2 |
| Brassica x napus | 2 | Odontites vernus subsp. vernus | −2 |
| Briza media | 3 | Odontites vernus subsp. serotinus | −2 |
| Bromus arvensis | 1 | Oenanthe aquatica | −3 |
| Bromus benekenii | 1 | Oenanthe banatica | −3 |
| Bromus brachystachys | 1 | Oenanthe fistulosa | −3 |
| Bromus carinatus | 1 | Oenanthe silaifolia | −3 |
| Bromus catharticus | 1 | Oenothera biennis | −1 |
| Bromus commutatus | 1 | Oenothera glazioviana | −1 |
| Bromus erectus | 3 | Oenothera rubricaulis | −1 |
| Bromus hordaceus | 0 | Oenothera salicifolia | −1 |
| Bromus inermis | 5 | Oenothera suaveolens | −1 |
| Bromus japonicus | 0 | Oenothera x hoelscheri | −1 |
| Bromus lanceolatus | 1 | Omphalodes scorpioides | 1 |
| Bromus lepidus | 1 | Omphalodes verna | 1 |
| Bromus madritensis | 1 | Onobrychis arenaria | 4 |
| Bromus pannonicus | 3 | Onobrychis viciifolia | 4 |
| Bromus racemosus | 1 | Ononis arvensis | 3 |
| Bromus ramosus | 1 | Ononis pusilla | 1 |
| Bromus reptans | 3 | Ononis spinosa | −3 |
| Bromus rigidus | 1 | Ononis spinosiformis | 3 |
| Bromus secalinus | 0 | Onopordum acanthium | −3 |
| Bromus squarrosus | 0 | Onosma arenarium | −1 |
| Bromus sterilis | 1 | Onosma pseudarenarium | −1 |
| Bromus tectorum | 1 | Onosma tornense | −1 |
| Bryonia alba | −2 | Onosma visianii | −1 |
| Bryonia dioica | −2 | Ophioglossum vulgatum | 0 |
| Bulbocodium versicolor | −2 | Ophrys apifera | −1 |
| Bunias orientalis | −1 | Ophrys fuciflora | −1 |
| Buphthalmum salicifolium | −1 | Ophrys insectifera | −1 |
| Bupleurum affine | 1 | Ophrys scolopax | −1 |
| Bupleurum falcatum | 1 | Ophrys sphecodes | −1 |
| Bupleurum longifolium | 1 | Orchis coriophora | −1 |
| Bupleurum pachnospermum | 1 | Orchis laxiflora | −1 |
| Bupleurum praealtum | 1 | Orchis mascula subsp. signifera | −1 |
| Bupleurum rotundifolium | 1 | Orchis militaris | −1 |
| Bupleurum tenuissimum | 1 | Orchis morio | −1 |
| Butomus umbellatus | −1 | Orchis pallens | −1 |
| Calamagrostis arundinacea | 1 | Orchis purpurea | −1 |
| Calamagrostis canescens | 1 | Orchis simia | −1 |
| Calamagrostis epigeios | 1 | Orchis tridentata | −1 |
| Calamagrostis pseudophragmites | 1 | Orchis ustulata | −1 |
| Calamagrostis purpurea | 1 | Origanum vulgare | 1 |
| Calamagrostis stricta | 1 | Orlaya grandiflora | 1 |
| Calamagrostis varia | 1 | Ornithogalum boucheanum | −2 |
| Calamagrostis villosa | 1 | Ornithogalum comosum | −2 |
| Calamintha einseleana | 1 | Ornithogalum orthophyllum | −2 |
| Calamintha menthifolia | 1 | Ornithogalum pyramidale | −2 |
| Calamintha thymifolia | 1 | Ornithogalum refractum | −2 |
| Caldesia parnassifolia | −2 | Ornithogalum sphaerocarpum | −2 |
| Calepina irregularis | 1 | Ornithogalum umbellatum | −2 |
| Calla palustris | −2 | Ornithogalum x degenianum | −2 |
| Callitriche cophocarpa | 1 | Orobanche alba | −2 |
| Callitriche palustris | 0 | Orobanche alsatica | −2 |
| Calluna vulgaris | −2 | Orobanche arenaria | −2 |
| Caltha palustris | −2 | Orobanche caesia | −2 |
| Calystegia sepium | −2 | Orobanche caryophyllacea | −2 |
| Camelina alyssum | 1 | Orobanche cernua | −2 |
| Camelina microcarpa | 1 | Orobanche coerulescens | −2 |
| Camelina rumelica | 1 | Orobanche elatior | −2 |
| Camelina sativa | 1 | Orobanche flava | −2 |
| Campanula bononiensis | −1 | Orobanche gracilis | −2 |
| Campanula cervicaria | −1 | Orobanche hederae | −2 |
| Campanula glomerata | 1 | Orobanche loricata | −2 |
| Campanula latifolia | −1 | Orobanche lutea | −2 |
| Campanula macrostachya | −1 | Orobanche minor | −2 |
| Campanula moravica | 1 | Orobanche nana | −2 |
| Campanula patula | 1 | Orobanche picridis | −2 |
| Campanula persicifolia | 1 | Orobanche purpurea | −2 |
| Campanula rapunculoides | 1 | Orobanche ramosa | −2 |
| Campanula rapunculus | 1 | Orobanche reticulata | −2 |
| Campanula rotundifolia | 1 | Orobanche teucrii | −2 |
| Campanula sibirica | −1 | Osmunda regalis | −2 |
| Campanula trachelium | 1 | Ostrya carpinifolia | −1 |
| Campanula xylocarpa | 1 | Oxalis acetosella | −2 |
| Camphorosma annua | 1 | Oxalis corniculata | −2 |
| Cannabis sativa | −2 | Oxalis dillenii | −2 |
| Capsella bursa-pastoris | 1 | Oxalis fontana | −2 |
| Capsella rubella | 1 | Oxytropis pilosa | 3 |
| Cardamine amara | −1 | Padus avium | −1 |
| Cardamine bulbifera | −1 | Padus serotina | −1 |
| Cardamine enneaphyllos | −1 | Paeonia officinalis | −3 |
| Cardamine flexuosa | −1 | Panicum capillare | 1 |
| Cardamine glanduligera | −1 | Panicum miliaceum | 1 |
| Cardamine hirsuta | −1 | Panicum philadelphicum | 1 |
| Cardamine impatiens | −1 | Papaver argemone | −2 |
| Cardamine parviflora | −1 | Papaver dubium | −2 |
| Cardamine pratensis | −1 | Papaver hybridum | −2 |
| Cardamine trifolia | −1 | Papaver rhoeas | −2 |
| Cardamine waldsteinii | −1 | Papaver somniferum | −2 |
| Cardaminopsis arenosa | 1 | Parietaria officinalis | −1 |
| Cardaminopsis petraea | 1 | Paris quadrifolia | −3 |
| Cardaria draba | −1 | Parnassia palustris | −1 |
| Carduus acanthoides | −3 | Paronychia cephalotes | 0 |
| Carduus collinus | −3 | Parthenocissus inserta | −2 |
| Carduus crassifolius subsp. glaucus | −2 | Parthenocissus quinquefolia | −2 |
| Carduus crispus | −3 | Parthenocissus tricuspidata | −2 |
| Carduus hamulosus | −3 | Pastinaca sativa | −1 |
| Carduus nutans | −3 | Pedicularis palustris | −3 |
| Carex acuta | −1 | Peplis portula | 1 |
| Carex acutiformis | −1 | Persicaria amphibia | −2 |
| Carex alba | 1 | Persicaria bistorta | −2 |
| Carex appropinquata | −1 | Persicaria dubia | −2 |
| Carex bohemica | 1 | Persicaria hydropiper | −2 |
| Carex brevicollis | 0 | Persicaria lapathifolia | −2 |
| Carex brizoides | −1 | Persicaria maculosa | −2 |
| Carex buekii | −1 | Persicaria minor | −2 |
| Carex buxbaumii | −1 | Petasites albus | −2 |
| Carex caespitosa | 0 | Petasites hybridus | −2 |
| Carex canescens | 0 | Petrorhagia glumacea | 0 |
| Carex caryophyllea | 1 | Petrorhagia prolifera | 0 |
| Carex davalliana | 0 | Petrorhagia saxifraga | 0 |
| Carex diandra | −1 | Peucedanum alsaticum | −1 |
| Carex digitata | 1 | Peucedanum arenarium | −1 |
| Carex distans | 0 | Peucedanum carvifolia | −1 |
| Carex disticha | −1 | Peucedanum cervaria | −1 |
| Carex divisa | 1 | Peucedanum officinale | −1 |
| Carex divulsa | 0 | Peucedanum oreoselinum | −1 |
| Carex echinata | 1 | Peucedanum palustre | −1 |
| Carex elata | −1 | Peucedanum rochelianum | −1 |
| Carex elongata | −1 | Peucedanum verticillare | −1 |
| Carex ericetorum | 1 | Phacelia congesta | 2 |
| Carex flacca | 0 | Phacelia tanacetifolia | 2 |
| Carex flava | 0 | Phalaris arundinacea | 4 |
| Carex fritschii | 0 | Phalaris canariensis | 3 |
| Carex halleriana | 1 | Phaseolus vulgaris | 4 |
| Carex hartmannii | 0 | Phleum bertolonii | 5 |
| Carex hirta | 0 | Phleum paniculatum | 3 |
| Carex hordeistichos | 1 | Phleum phleoides | 3 |
| Carex hostiana | 1 | Phleum pratense | 5 |
| Carex humilis | 1 | Phlomis tuberosa | −1 |
| Carex lasiocarpa | −1 | Pholiurus pannonicus | 1 |
| Carex lepidocarpa | 0 | Phragmites australis | −1 |
| Carex limosa | 1 | Phyllitis scolopendrium | −2 |
| Carex liparicarpos | 1 | Physalis alkekengi | −2 |
| Carex melanostachya | −1 | Physocaulis nodosus | −2 |
| Carex michelii | 1 | Physospermum cornubiense | −2 |
| Carex montana | 1 | Phyteuma orbiculare | 1 |
| Carex nigra | 1 | Phyteuma spicatum | 1 |
| Carex otrubae | −1 | Phytolacca americana | −3 |
| Carex ovalis | 1 | Picea abies | −1 |
| Carex pairaei | 1 | Picris hieracioides | −1 |
| Carex pallescens | 0 | Pimpinella major | 2 |
| Carex panicea | 1 | Pimpinella saxifraga | 2 |
| Carex paniculata | −1 | Pinguicula alpina | −1 |
| Carex pendula | −1 | Pinguicula vulgaris | −1 |
| Carex pilosa | 0 | Pinus nigra | −1 |
| Carex pilulifera | 1 | Pinus sylvestris | −1 |
| Carex praecox | 1 | Piptatherum miliaceum | 1 |
| Carex pseudocyperus | −1 | Piptatherum virescens | 1 |
| Carex remota | 0 | Pisum elatius | 4 |
| Carex repens | −1 | Pisum sativum | 4 |
| Carex riparia | −1 | Plantago altissima | 2 |
| Carex rostrata | −1 | Plantago indica | 1 |
| Carex secalina | 1 | Plantago argentea | 2 |
| Carex spicata | 0 | Plantago lanceolata | 3 |
| Carex stenophylla | 1 | Plantago major | 2 |
| Carex strigosa | 0 | Plantago maritima | 2 |
| Carex supina | 1 | Plantago maxima | 2 |
| Carex sylvatica | 0 | Plantago media | 2 |
| Carex tomentosa | 1 | Plantago schwarzenbergiana | 2 |
| Carex umbrosa | 0 | Plantago stepposa | 2 |
| Carex vesicaria | −1 | Plantago tenuiflora | 2 |
| Carex viridula | 1 | Platanthera bifolia | −1 |
| Carex vulpina | 0 | Platanthera chlorantha | −1 |
| Carlina acaulis | −3 | Pleurospermum austriacum | −1 |
| Carlina vulgaris | −3 | Poa angustifolia | 5 |
| Carpesium abrotanoides | −1 | Poa annua | 2 |
| Carpesium cernuum | −1 | Poa badensis | 2 |
| Carpinus betulus | −1 | Poa bulbosa | 2 |
| Carpinus orientalis | −1 | Poa compressa | 1 |
| Carthamus lanatus | −3 | Poa humilis | 6 |
| Carum carvi | 2 | Poa nemoralis | 2 |
| Castanea sativa | −1 | Poa palustris | 5 |
| Catabrosa aquatica | 3 | Poa scabra | 2 |
| Caucalis latifolia | −1 | Poa pratensis | 6 |
| Caucalis platycarpos | −1 | Poa remota | 3 |
| Celtis australis | −1 | Poa stiriaca | 3 |
| Celtis occidentalis | −1 | Poa supina | 2 |
| Cenchrus incertus | −3 | Poa trivialis | 5 |
| Centaurea arenaria | 1 | Podospermum canum | 2 |
| Centaurea calcitrapa | −1 | Podospermum laciniatum | 2 |
| Centaurea cyanus | 1 | Polycnemum arvense | −1 |
| Centaurea diffusa | 1 | Polycnemum heuffelii | −1 |
| Centaurea indurata | 1 | Polycnemum majus | −1 |
| Centaurea jacea subsp. angustifolia | 2 | Polycnemum verrucosum | −1 |
| Centaurea jacea subsp. banatica | 2 | Polygala amara | 0 |
| Centaurea jacea subsp. jacea | 2 | Polygala amarella | 0 |
| Centaurea jacea subsp. macroptilon | 1 | Polygala comosa | 0 |
| Centaurea mollis | 2 | Polygala major | 0 |
| Centaurea nigrescens | 2 | Polygala nicaeensis subsp. carniolica | 0 |
| Centaurea pseudophrygia | 1 | Polygala vulgaris | 0 |
| Centaurea salonitana | 1 | Polygonatum latifolium | −2 |
| Centaurea scabiosa subsp. fritschii | 1 | Polygonatum multiflorum | −2 |
| Centaurea scabiosa subsp. scabiosa | 1 | Polygonatum odoratum | −2 |
| Centaurea scabiosa subsp. spinulosa | 1 | Polygonatum verticillatum | −2 |
| Centaurea scabiosa subsp.sadleriana | 1 | Polygonum arenarium | −2 |
| Centaurea solstitialis | −1 | Polygonum arenastrum | −2 |
| Centaurea stenolepis | 2 | Polygonum aviculare | −2 |
| Centaurea stoebe subsp. micranthos | 1 | Polygonum bellardii | −2 |
| Centaurea stoebe subsp. stoebe | 1 | Polygonum graminifolium | −2 |
| Centaurea triumfetti | 2 | Polygonum rurivagum | −2 |
| Centaurium erythraea | 1 | Polypodium interjectum | −2 |
| Centaurium littorale | 1 | Polypodium vulgare | −2 |
| Centaurium pulchellum | 0 | Polystichum aculeatum | −2 |
| Centunculus minimus | 0 | Polystichum braunii | −2 |
| Cephalanthera damasonium | −1 | Polystichum lonchitis | −2 |
| Cephalanthera longifolia | −1 | Polystichum setiferum | −2 |
| Cephalanthera rubra | −1 | Populus alba | −1 |
| Cephalaria pilosa | −2 | Populus deltoides | −1 |
| Cephalaria transsylvanica | −2 | Populus nigra | −1 |
| Cerastium arvense | 1 | Populus simonii | −1 |
| Cerastium brachypetalum | 1 | Populus tremula | −1 |
| Cerastium dubium | 1 | Populus x canadensis | −1 |
| Cerastium fontanum | 1 | Populus x canescens | −1 |
| Cerastium glomeratum | 1 | Portulaca oleracea | 0 |
| Cerastium pumilum | 0 | Potentilla alba | 2 |
| Cerastium semidecandrum | 1 | Potentilla anserina | −1 |
| Cerastium subtetrandrum | 1 | Potentilla arenaria | 1 |
| Cerastium sylvaticum | 1 | Potentilla argentea | 1 |
| Cerasus avium | −1 | Potentilla collina | 2 |
| Cerasus fruticosa | −1 | Potentilla erecta | −1 |
| Cerasus mahaleb | −1 | Potentilla heptaphylla | 1 |
| Cerasus vulgaris | −1 | Potentilla impolita | 2 |
| Ceratophyllum demersum | −2 | Potentilla inclinata | 2 |
| Ceratophyllum submersum | −2 | Potentilla leucopolitana | 2 |
| Cerinthe minor | −1 | Potentilla micrantha | 1 |
| Ceterach javorkaeanum | 0 | Potentilla neumanniana | 1 |
| Ceterach officinarum | 0 | Potentilla palustris | 1 |
| Chaerophyllum aromaticum | −1 | Potentilla patula | 1 |
| Chaerophyllum aureum | −1 | Potentilla pedata | 2 |
| Chaerophyllum bulbosum | −1 | Potentilla pusilla | 1 |
| Chaerophyllum hirsutum | −1 | Potentilla recta | 1 |
| Chaerophyllum temulum | −2 | Potentilla reptans | −1 |
| Chamaecytisus albus | −2 | Potentilla rupestris | 2 |
| Chamaecytisus austriacus | −2 | Potentilla supina | 2 |
| Chamaecytisus ciliatus | −2 | Potentilla thyrsiflora | 2 |
| Chamaecytisus heuffelii | −2 | Potentilla wiemanniana | 2 |
| Chamaecytisus ratisbonensis | −2 | Prenanthes purpurea | 1 |
| Chamaecytisus rochelii | −2 | Primula auricula | −1 |
| Chamaecytisus supinus | −2 | Primula elatior | −1 |
| Chamaecytisus triflorus | −2 | Primula farinosa | −1 |
| Chamaecytisus virescens | −2 | Primula veris | −1 |
| Chelidonium majus | −2 | Primula vulgaris | −1 |
| Chenopodium album subsp. album | −2 | Prunella grandiflora | 1 |
| Chenopodium album subsp. borbasii | −2 | Prunella laciniata | 1 |
| Chenopodium album subsp. pedunculare | −2 | Prunella vulgaris | 1 |
| Chenopodium ambrosioides | −3 | Prunus cerasifera | −1 |
| Chenopodium aristatum | −1 | Prunus domestica | −1 |
| Chenopodium bonus-henricus | −1 | Prunus spinosa | −3 |
| Chenopodium botrys | −1 | Pseudolysimachion incanum | 2 |
| Chenopodium chenipodioides | −1 | Pseudolysimachion longifolium | 2 |
| Chenopodium ficifolium | −1 | Pseudolysimachion orchideum | 2 |
| Chenopodium foliosum | −1 | Pseudolysimachion spicatum | 2 |
| Chenopodium giganteum | −2 | Pseudolysimachion spurium | 2 |
| Chenopodium glaucum | −1 | Pteridium aquilinum | −3 |
| Chenopodium hybridum | −3 | Puccinellia distans | 4 |
| Chenopodium murale | −1 | Puccinellia limosa | 3 |
| Chenopodium opulifolium | −1 | Puccinellia peisonis | 4 |
| Chenopodium polyspermum | −1 | Pulicaria dysenterica | −2 |
| Chenopodium pumilio | −1 | Pulicaria vulgaris | −2 |
| Chenopodium rubrum | −1 | Pulmonaria angustifolia | −1 |
| Chenopodium scheraderianum | −1 | Pulmonaria mollis | −1 |
| Chenopodium strictum subsp. striatiforme | −1 | Pulmonaria obscura | −1 |
| Chenopodium strictum subsp. strictum | −1 | Pulmonaria officinalis | −1 |
| Chenopodium suecicum | −1 | Pulsatilla grandis | −2 |
| Chenopodium urbicum | −2 | Pulsatilla patens | −2 |
| Chenopodium vulvaria | −1 | Pulsatilla pratensis | −2 |
| Chondrilla juncea | −1 | Pyrus communis | −1 |
| Chorispora tenella | 1 | Pyrus magyarica | −1 |
| Chrysopogon gryllus | −1 | Pyrus nivalis | −1 |
| Chrysosplenium alternifolium | −1 | Pyrus pyraster | −1 |
| Cichorium intybus | 3 | Pyrus x austriaca | −1 |
| Cicuta virosa | −3 | Quercus cerris | −1 |
| Circaea alpina | −2 | Quercus dalechampii | −1 |
| Circaea lutetiana | −2 | Quercus frainetto | −1 |
| Circaea x intermedia | −2 | Quercus petraea | −1 |
| Cirsium arvense | −3 | Quercus polycarpa | −1 |
| Cirsium boujartii | −3 | Quercus pubescens | −1 |
| Cirsium brachycephalum | −3 | Quercus robur | −1 |
| Cirsium canum | −3 | Quercus rubra | −1 |
| Cirsium eriophorum | −3 | Quercus virgiliana | −1 |
| Cirsium erisithales | −3 | Radiola linoides | 0 |
| Cirsium furiens | −3 | Ranunculus acris | −2 |
| Cirsium oleraceum | −3 | Ranunculus aquatilis | −3 |
| Cirsium palustre | −3 | Ranunculus arvensis | −2 |
| Cirsium pannonicum | −2 | Ranunculus auricomus | −2 |
| Cirsium rivulare | −3 | Ranunculus baudotii | −3 |
| Cirsium vulgare | −3 | Ranunculus bulbosus | −2 |
| Cladium mariscus | −2 | Ranunculus cassubicus | −2 |
| Cleistogenes serotina | 1 | Ranunculus circinatus | −3 |
| Clematis alpina | −3 | Ranunculus cymbalaria | −2 |
| Clematis integrifolia | −3 | Ranunculus fallax | −2 |
| Clematis recta | −3 | Ranunculus flammula | −3 |
| Clematis vitalba | −3 | Ranunculus fluitans | −3 |
| Clematis viticella | −3 | Ranunculus illyricus | −2 |
| Clinopodium vulgare | 1 | Ranunculus lanuginosus | −2 |
| Cnidium dubium | 2 | Ranunculus lateriflorus | −2 |
| Coeloglossum viride | 0 | Ranunculus lingua | −3 |
| Colchicum arenarium | −3 | Ranunculus parviflorus | −2 |
| Colchicum autumnale | −3 | Ranunculus pedatus | −2 |
| Colchicum hungaricum | −3 | Ranunculus peltatus | −3 |
| Colutea arborescens | −1 | Ranunculus polyanthemos | −2 |
| Commelina communis | 1 | Ranunculus polyphyllus | −2 |
| Conium maculatum | −3 | Ranunculus psilostachys | −2 |
| Conringia austriaca | 1 | Ranunculus repens | −3 |
| Conringia orientalis | 1 | Ranunculus rhipiphyllus | −3 |
| Consolida orientalis | −2 | Ranunculus rionii | −3 |
| Consolida regalis | −2 | Ranunculus sardous | −3 |
| Convallaria majalis | −3 | Ranunculus sceleratus | −3 |
| Convolvulus arvensis | −1 | Ranunculus strigulosus | −2 |
| Convolvulus cantabrica | −1 | Ranunculus trichophyllus | −3 |
| Conyza canadensis | −3 | Raphanus raphanistrum | −2 |
| Corallorhiza trifida | −1 | Raphanus sativus | −2 |
| Coriandrum sativum | −1 | Rapistrum perenne | −2 |
| Corispermum canescens | −1 | Reseda inodora | −1 |
| Corispermum nitidum | −1 | Reseda lutea | −1 |
| Cornus mas | −1 | Reseda luteola | −1 |
| Cornus sanguinea | −1 | Reseda phyteuma | −1 |
| Coronilla coronata | 2 | Rhamnus catharticus | −2 |
| Coronilla vaginalis | 3 | Rhamnus saxatilis | −2 |
| Coronopus didymus | 1 | Rhinanthus alectorolophus | −2 |
| Coronopus squamatus | 1 | Rhinanthus borbasii | −2 |
| Corothamnus procumbens | −1 | Rhinanthus minor | −2 |
| Corydalis cava | −2 | Rhinanthus rumelicus | −2 |
| Corydalis intermedia | −2 | Rhinanthus serotinus | −2 |
| Corydalis pumila | −2 | Rhinanthus wagneri | −2 |
| Corydalis solida | −2 | Ribes alpinum | −1 |
| Corylus avellana | −1 | Ribes aureum | −1 |
| Corylus colurna | −1 | Ribes nigrum | −1 |
| Corynephorus canescens | 2 | Ribes petraeum | −1 |
| Cotinus coggygria | −3 | Ribes rubrum | −1 |
| Cotoneaster integerrimus | −3 | Ribes uva-crispa | −1 |
| Cotoneaster matrensis | −3 | Ricinus communis | −3 |
| Cotoneaster niger | −3 | Robinia pseudo-acacia | −3 |
| Cotoneaster tomentosus | −3 | Rorippa amphibia | −2 |
| Crambe tataria | −1 | Rorippa austriaca | −2 |
| Crataegus calycina | −2 | Rorippa palustris | −2 |
| Crataegus laevigata | −3 | Rorippa sylvestris | −2 |
| Crataegus monogyna | −3 | Rorippa x anceps | −2 |
| Crataegus nigra | −1 | Rorippa x armoracioides | −2 |
| Crepis biennis | −2 | Rorippa x astylis | −2 |
| Crepis capillaris | −1 | Rorippa x hungarica | −2 |
| Crepis nicaeënsis | −1 | Rosa agrestis | −3 |
| Crepis paludosa | −1 | Rosa arvensis | −3 |
| Crepis pannonica | −2 | Rosa caesia | −3 |
| Crepis praemorsa | −1 | Rosa canina | −3 |
| Crepis pulchra | −2 | Rosa corymbifera | −3 |
| Crepis rhoeadifolia | −1 | Rosa dumalis | −3 |
| Crepis setosa | −1 | Rosa elliptica | −3 |
| Crepis taraxicifolia | −1 | Rosa rugosa | −3 |
| Crepis tectorum | −1 | Rosa gallica | −3 |
| Crocus albiflorus | −2 | Rosa gizellae | −3 |
| Crocus heuffelianus | −2 | Rosa glauca | −3 |
| Crocus reticulatus | −2 | Rosa hungarica | −3 |
| Crocus sativus | −2 | Rosa inodora | −3 |
| Crocus tommasinianus | −2 | Rosa kmetiana | −3 |
| Cruciata glabra | 1 | Rosa jundzillii | −3 |
| Cruciata laevipes | 1 | Rosa majalis | −3 |
| Cruciata pedemontana | 1 | Rosa micrantha | −3 |
| Crupina vulgaris | 1 | Rosa tomentella | −3 |
| Crypsis aculeata | 0 | Rosa pendulina | −3 |
| Cucubalus baccifer | 2 | Rosa polyacantha | −3 |
| Cuscuta approximata | −2 | Rosa rubiginosa | −3 |
| Cuscuta australis | −2 | Rosa villosa | −3 |
| Cuscuta campestris | −2 | Rosa scabriuscula | −3 |
| Cuscuta epilinum | −2 | Rosa sherardii | −3 |
| Cuscuta epithymum subsp. epithymum | −2 | Rosa spinosissima | −3 |
| Cuscuta epithymum subsp. kotschi | −2 | Rosa subcanina | −3 |
| Cuscuta europaea | −2 | Rosa subcollina | −3 |
| Cuscuta lupuliformis | −2 | Rosa szaboi | −3 |
| Cyclamen purpurascens | −2 | Rosa tomentosa | −3 |
| Cydonia oblonga | −1 | Rosa zagrebiensis | −3 |
| Cymbalaria muralis | 0 | Rosa zalana | −3 |
| Cynodon dactylon | 2 | Rubus caesius | −3 |
| Cynoglossum hungaricum | −2 | Rubus fruticosus agg. | −3 |
| Cynoglossum officinale | −2 | Rubus idaeus | −3 |
| Cynosurus cristatus | 4 | Rubus saxatilis | −3 |
| Cynosurus echinatus | 2 | Rumex acetosa | −1 |
| Cyperus difformis | 1 | Rumex acetosella | −1 |
| Cyperus flavescens | −1 | Rumex aquaticus | −2 |
| Cyperus fuscus | 1 | Rumex confertus | −1 |
| Cyperus glaber | −1 | Rumex conglomeratus | −1 |
| Cyperus glomeratus | −1 | Rumex crispus | −2 |
| Cyperus longus | −1 | Rumex dentatus | −1 |
| Cyperus pannonicus | 0 | Rumex hydrolapathum | −2 |
| Cypripedium calceolus | −2 | Rumex kerneri | −2 |
| Cystopteris fragilis | −2 | Rumex maritimus | −1 |
| Dactylis glomerata | 5 | Rumex obtusifolius | −2 |
| Dactylis polygama | 3 | Rumex palustris | −1 |
| Dactylorhiza fuchsii | −1 | Rumex patientia | −2 |
| Dactylorhiza incarnata | −1 | Rumex pseudonatronatus | −2 |
| Dactylorhiza maculata | −1 | Rumex pulcher | −1 |
| Dactylorhiza majalis | −1 | Rumex sanguineus | −1 |
| Dactylorhiza sambucina | −1 | Rumex stenophyllus | −1 |
| Danthonia alpina | 2 | Rumex thyrsiflorus | −2 |
| Danthonia decumbens | 1 | Ruscus aculeatus | −3 |
| Daphne cneorum | −3 | Ruscus hypoglossum | 1 |
| Daphne laureola | −3 | Sagina apetala subsp. apetala | 0 |
| Daphne mezereum | −3 | Sagina apetala subsp. erecta | 0 |
| Datura stramonium | −3 | Sagina nodosa | 0 |
| Daucus carota | 1 | Sagina procumbens | 0 |
| Deschampsia cespitosa | 1 | Sagina sabuletorum | 0 |
| Deschampsia flexuosa | 1 | Sagina saginoides | 0 |
| Descurainia sophia | −1 | Sagina subulata | 0 |
| Dianthus arenarius | 2 | Sagittaria sagittifolia | −3 |
| Dianthus armeria | 2 | Salicornia prostrata | 1 |
| Dianthus barbatus | 2 | Salix alba | −2 |
| Dianthus carthusianorum | 2 | Salix aurita | −2 |
| Dianthus collinus | 2 | Salix caprea | −2 |
| Dianthus deltoides | 2 | Salix cinerea | −2 |
| Dianthus diutinus | 2 | Salix elaeagnos | −2 |
| Dianthus giganteiformis | 2 | Salix fragilis | −2 |
| Dianthus plumarius subsp praecox | 2 | Salix myrsinifolia | −2 |
| Dianthus plumarius subsp. lumnitzeri | 2 | Salix pentandra | −2 |
| Dianthus plumarius subsp. regis-stephani | 2 | Salix purpurea | −2 |
| Dianthus pontederae | 2 | Salix rosmarinifolia | −2 |
| Dianthus serotinus | 2 | Salix triandra | −2 |
| Dianthus superbus | 2 | Salix viminalis | −2 |
| Dichostylis micheliana | 1 | Salix x multinervis | −2 |
| Dictamnus albus | −2 | Salsola kali | −1 |
| Digitalis ferruginea | −3 | Salsola soda | −1 |
| Digitalis grandiflora | −3 | Salvia aethiopis | 1 |
| Digitalis lanata | −3 | Salvia austriaca | 1 |
| Digitalis purpurea | −3 | Salvia glutinosa | 1 |
| Digitaria ciliaris | 1 | Salvia nemorosa | 2 |
| Digitaria ischaemum | 0 | Salvia nutans | 1 |
| Digitaria sanguinalis | 1 | Salvia officinalis | 1 |
| Diphasium complanatum | −2 | Salvia pratensis | 2 |
| Diphasium issleri | −2 | Salvia sclarea | 1 |
| Diphasium tristachyum | −2 | Salvia verbenaca | 2 |
| Diplotaxis erucoides | 1 | Salvia verticillata | 2 |
| Diplotaxis muralis | 1 | Sambucus ebulus | −3 |
| Diplotaxis tenuifolia | 1 | Sambucus nigra | −3 |
| Dipsacus laciniatus | −3 | Sambucus racemosa | −3 |
| Dipsacus sylvestris | −3 | Samolus valerandi | 1 |
| Doronicum austriacum | 1 | Sanguisorba minor | 2 |
| Doronicum hungaricum | 1 | Sanguisorba officinalis | 2 |
| Doronicum orientale | 1 | Sanicula europaea | 2 |
| Dorycnium germanicum | 0 | Saponaria officinalis | −1 |
| Dorycnium herbaceum | 0 | Sarothamnus scoparius | −3 |
| Draba lasiocarpa | 0 | Satureja hortensis | 1 |
| Draba muralis | 1 | Saxifraga adscendens | 0 |
| Draba nemorosa | 1 | Saxifraga bulbifera | 0 |
| Dracocephalum austriacum | −2 | Saxifraga granulata | 0 |
| Dracocephalum moldavica | −2 | Saxifraga paniculata | 0 |
| Dracocephalum ruyschiana | −2 | Saxifraga tridactylites | 0 |
| Drosera anglica | −2 | Scabiosa canescens | 2 |
| Drosera rotundifolia | −2 | Scabiosa columbaria | 2 |
| Dryopteris carthusiana | −2 | Scabiosa ochroleuca | 2 |
| Dryopteris cristata | −2 | Scabiosa triandra | 2 |
| Dryopteris dilatata | −2 | Scandix pecten-veneris | 1 |
| Dryopteris expansa | −2 | Schoenoplectus lacustris | −2 |
| Dryopteris filix-mas | −2 | Schoenoplectus litoralis | −2 |
| Dryopteris pseudo-mas | −2 | Schoenoplectus mucronatus | −1 |
| Ecballium elaterium | −3 | Schoenoplectus setaceus | −1 |
| Echinochloa crus-galli | 1 | Schoenoplectus supinus | −1 |
| Echinochloa eruciformis | 1 | Schoenoplectus tabernaemontani | −2 |
| Echinochloa occidentalis | 1 | Schoenoplectus triqueter | −2 |
| Echinochloa oryzoides | 1 | Schoenus ferrugineus | −1 |
| Echinochloa phyllopogon | 1 | Schoenus nigricans | −1 |
| Echinocystis lobata | −3 | Scilla autumnalis | 0 |
| Echinops ruthenicus | −3 | Scilla drunensis | 0 |
| Echinops sphaerocephalus | −3 | Scilla kladnii | 0 |
| Echium italicum | −2 | Scilla spetana | 0 |
| Echium maculatum | −2 | Scilla vindobonensis | 0 |
| Echium vulgare | −2 | Scirpoides holoschoenus | −1 |
| Elaeagnus angustifolia | −1 | Scirpus pungens | −1 |
| Elatine alsinastrum | 1 | Scirpus radicans | −1 |
| Elatine hexandra | 1 | Scirpus sylvaticus | −1 |
| Elatine hungarica | 1 | Scleranthus annuus | 0 |
| Elatine hydropiper | 1 | Scleranthus dichotomus | 0 |
| Elatine triandra | 1 | Scleranthus perennis | 0 |
| Eleocharis acicularis | 1 | Scleranthus polycarpos | 0 |
| Eleocharis austriaca | 1 | Scleranthus verticillatus | 0 |
| Eleocharis carniolica | 1 | Sclerochloa dura | 0 |
| Eleocharis mamillata | 1 | Scopolia carniolica | −3 |
| Eleocharis ovata | 1 | Scorzonera austriaca | 1 |
| Eleocharis palustris | 1 | Scorzonera hispanica | 1 |
| Eleocharis quinqueflora | 1 | Scorzonera humilis | 1 |
| Eleocharis uniglumis | 1 | Scorzonera parviflora | 1 |
| Eleusine indica | 1 | Scorzonera purpurea | 1 |
| Elymus caninus | 3 | Scrophularia nodosa | −2 |
| Elymus hispidus | 3 | Scrophularia scopolii | −2 |
| Elymus repens | 3 | Scrophularia umbrosa | −2 |
| Ephedra distachya | −2 | Scrophularia vernalis | −2 |
| Epilobium ciliatum | −1 | Scutellaria altissima | −2 |
| Chamaenerion angustifolium | −2 | Scutellaria columnae | −2 |
| Epilobium collinum | −1 | Scutellaria galericulata | −2 |
| Chamaenerion dodonaei | −1 | Scutellaria hastifolia | −2 |
| Epilobium hirsutum | −2 | Secale sylvestre | 1 |
| Epilobium lanceolatum | −1 | Securigera elegans | 5 |
| Epilobium montanum | −1 | Securigera varia | 5 |
| Epilobium obscurum | −1 | Sedum acre | −2 |
| Epilobium palustre | −1 | Sedum album | −2 |
| Epilobium parviflorum | −2 | Sedum caespitosum | −2 |
| Epilobium roseum | −1 | Sedum hispanicum | −2 |
| Epilobium tetragonum | −1 | Hylotelephium telephium subsp. maxium | −2 |
| Epipactis atrorubens | −2 | Sedum neglectum | −2 |
| Epipactis helleborine | −2 | Sedum reflexum | −2 |
| Epipactis leptochila | −2 | Sedum sartorianum | −2 |
| Epipactis microphylla | −2 | Sedum sexangulare | −2 |
| Epipactis muelleri | −2 | Sedum spurium | −2 |
| Epipactis palustris | −2 | Selaginella helvetica | 0 |
| Epipactis pontica | −2 | Selinum carvifolia | 1 |
| Epipactis purpurata | −2 | Sempervivum marmoreum | 0 |
| Epipactis voethii | −2 | Sempervivum tectorum | 0 |
| Epipactis exilis | −2 | Senecio aquaticus | −2 |
| Epipactis mecsekensis | −2 | Tephroseris aurantiaca | −2 |
| Epipactis albensis | −2 | Senecio doria | −2 |
| Epipactis placentina | −2 | Senecio erraticus | −2 |
| Epipactis muelleri | −2 | Senecio erucifolius | −2 |
| Epipactis nordeniorum | −2 | Senecio sarracenicus | −2 |
| Epipactis tallosii | −2 | Senecio inaeequidens | −2 |
| Epipactis bugacensis | −2 | Tephroseris integrifolia | −2 |
| Epipactis greuterii | −2 | Senecio jacobaea | −2 |
| Epipogium aphyllum | −2 | Senecio germanicus | −2 |
| Equisetum arvense | −2 | Senecio ovatus | −2 |
| Equisetum fluviatile | −2 | Tephroseris longifolia | −2 |
| Equisetum hyemale | −2 | Senecio paludosus | −2 |
| Equisetum palustre | −2 | Senecio rupestris | −2 |
| Equisetum ramosissimum | −2 | Senecio sylvaticus | −2 |
| Equisetum sylvaticum | −2 | Senecio umbrosus | −2 |
| Equisetum telmateia | −2 | Senecio vernalis | −2 |
| Equisetum variegatum | −2 | Senecio viscosus | −2 |
| Equisetum x moorei | −2 | Senecio vulgaris | −2 |
| Eragrostis cilianensis | 1 | Serratula lycopifolia | 2 |
| Eragrostis minor | 1 | Serratula radiata | 2 |
| Eragrostis parviflora | 1 | Serratula tinctoria | 2 |
| Eragrostis pilosa | 2 | Seseli annuum | 2 |
| Eranthis hyemalis | 0 | Seseli hippomarathrum | 2 |
| Erechtites hieraciifolia | −2 | Seseli leucospermum | 2 |
| Erigeron acris | −2 | Seseli osseum | 2 |
| Erigeron annus | −2 | Seseli varium | 2 |
| Eriophorum angustifolium | −1 | Sesleria albicans | 1 |
| Eriophorum gracile | −1 | Sesleria heuflerana | 2 |
| Eriophorum latifolium | −1 | Sesleria hungarica | 1 |
| Eriophorum vaginatum | −1 | Sesleria sadlerana | 1 |
| Erodium ciconium | −1 | Sesleria uliginosa | 1 |
| Erodium cicutarium | −1 | Setaria italica | 1 |
| Erodium neilreichii | −1 | Setaria pumila | 1 |
| Erophila praecox | 0 | Setaria verticillata | 1 |
| Erophila spathulata | 0 | Setaria verticilliformis | 1 |
| Erophila verna | 0 | Setaria viridis | 1 |
| Eruca sativa | −2 | Sherardia arvensis | 1 |
| Erucastrum gallicum | 1 | Sicyos angulatus | −2 |
| Erucastrum nasturtiifolium | 1 | Sideritis montana | 0 |
| Eryngium campestre | −3 | Silaum peucedanoides | −1 |
| Eryngium planum | −3 | Silaum silaus | −1 |
| Erysimum cheiranthoides | −3 | Silene alba | 1 |
| Erysimum crepidifolium | −3 | Silene armeria | 1 |
| Erysimum diffusum | −3 | Silene borysthenica | 1 |
| Erysimum hieracifolium | −3 | Silene conica | 0 |
| Erysimum odoratum | −3 | Silene dichotoma | 1 |
| Erysimum pallidiflorum | −3 | Silene dioica | 1 |
| Erysimum repandum | −3 | Silene gallica | 0 |
| Erythronium dens-canis | −3 | Silene longiflora | 1 |
| Euclidium syriacum | 0 | Silene multiflora | 1 |
| Euonymus europaea | −2 | Silene nemoralis | 1 |
| Euonymus verrucosa | −2 | Silene noctiflora | 1 |
| Eupatorium cannabinum | −2 | Silene nutans | 1 |
| Euphorbia amygdaloides | −2 | Silene otites | 1 |
| Euphorbia angulata | −2 | Silene viridiflora | 1 |
| Euphorbia carpatica | −2 | Silene viscosa | 1 |
| Euphorbia cyparissias | −2 | Silene vulgaris | 1 |
| Euphorbia dulcis | −2 | Sinapis alba | −2 |
| Euphorbia epithymoides | −2 | Sinapis arvensis | −2 |
| Euphorbia esula | −2 | Sisymbrium altissimum | −2 |
| Euphorbia exigua | −2 | Sisymbrium loeselii | −2 |
| Euphorbia falcata | −2 | Sisymbrium officinale | −2 |
| Euphorbia helioscopia | −2 | Sisymbrium orientale | −2 |
| Euphorbia humifusa | −2 | Sisymbrium polymorphum | −2 |
| Euphorbia lucida | −2 | Sisymbrium strictissimum | −2 |
| Euphorbia maculata | −2 | Sium latifolium | −2 |
| Euphorbia nutans | −2 | Sium sisarum | −2 |
| Euphorbia palustris | −2 | Smyrnium perfoliatum | −1 |
| Euphorbia glareosa | −2 | Solanum alatum | −3 |
| Euphorbia peplus | −2 | Solanum dulcamara | −3 |
| Euphorbia platyphyllos | −2 | Solanum villosum | −3 |
| Euphorbia salicifolia | −2 | Solanum nigrum | −3 |
| Euphorbia segetalis | −2 | Solanum rostratum | −3 |
| Euphorbia seguierana | −2 | Solidago canadensis | −2 |
| Euphorbia stricta | −2 | Solidago gigantea | −2 |
| Euphorbia taurinensis | −2 | Solidago virgaurea | −1 |
| Euphorbia verrucosa | −2 | Sonchus arvensis | −1 |
| Euphorbia villosa | −2 | Sonchus asper | −1 |
| Euphorbia virgata | −2 | Sonchus oleraceus | −1 |
| Euphrasia kerneri | −1 | Sonchus palustris | −2 |
| Euphrasia rostkoviana | −1 | Sorbus aria | −1 |
| Euphrasia stricta | −1 | Sorbus aucuparia | −1 |
| Euphrasia tatarica | −1 | Sorbus austriaca | −1 |
| Fagopyrum esculentum | −1 | Sorbus domestica | −1 |
| Fagus sylvatica | −2 | Sorbus graeca | −1 |
| Falcaria vulgaris | −1 | Sorbus torminalis | −1 |
| Fallopia convolvulus | −1 | Sorbus x danubialis | −1 |
| Fallopia dumetorum | −1 | Sorghum bicolor | 3 |
| Fallopia japonica | −3 | Sorghum halepense | 3 |
| Fallopia sachalinensis | −3 | Sorghum sudanense | 3 |
| Fallopia x bohemica | −3 | Sparganium emersum | −2 |
| Ferula sadlerana | 1 | Sparganium erectum | −2 |
| Festuca altissima | 3 | Sparganium minimum | −2 |
| Festuca amethystina | 2 | Spergula arvensis | 0 |
| Festuca arundinacea | 4 | Spergula pentandra | 0 |
| Festuca dalmatica | 3 | Spergularia marina | 0 |
| Festuca drymeia | 2 | Spergularia maritima | 0 |
| Festuca filiformis | 2 | Spergularia rubra | 0 |
| Festuca gigantea | 3 | Spiraea crenata | −1 |
| Festuca heterophylla | 2 | Spiraea media | −1 |
| Festuca nigrescens | 3 | Spiraea salicifolia | −1 |
| Festuca ovina | 2 | Spiranthes aestivalis | −1 |
| Festuca pallens | 2 | Spiranthes spiralis | −1 |
| Festuca pannonica | 2 | Stachys alpina | −1 |
| Festuca pratensis | 6 | Stachys annua | 1 |
| Festuca pseudodalmatica | 3 | Stachys byzantina | −2 |
| Festuca pseudovaginata | 3 | Stachys germanica | −1 |
| Festuca pseudovina | 3 | Stachys palustris | 1 |
| Festuca rubra | 4 | Stachys recta | 1 |
| Festuca rupicola | 3 | Stachys sylvatica | −1 |
| Festuca tenuifolia | 3 | Staphylea pinnata | −1 |
| Festuca vaginata | 2 | Stellaria graminea | 1 |
| Festuca valesiaca | 3 | Stellaria holostea | 1 |
| Festuca vojtkoi | 2 | Stellaria media | 1 |
| Festuca x stricta | 2 | Stellaria nemorum | 1 |
| Festuca x wagneri | 3 | Stellaria palustris | 1 |
| Ficaria verna | 1 | Stellaria uliginosa | 1 |
| Filago arvensis | 0 | Sternbergia colchiciflora | 0 |
| Filago minima | 0 | Stipa borysthenica | −2 |
| Filago vulgaris | 0 | Stipa bromoides | −2 |
| Filipendula ulmaria | 1 | Stipa capillata | −2 |
| Filipendula vulgaris | 1 | Stipa crassiculmis | −2 |
| Foeniculum vulgare | −1 | Stipa dasyphylla | −2 |
| Fragaria moschata | 2 | Stipa eriocaulis | −1 |
| Fragaria vesca | 2 | Stipa pennata | −2 |
| Fragaria viridis | 2 | Stipa pulcherrima | −2 |
| Frangula alnus | −1 | Stipa tirsa | −1 |
| Fraxinus angustifolia | −1 | Stratiotes aloides | −3 |
| Fraxinus excelsior | −1 | Suaeda maritima | 1 |
| Fraxinus ornus | −1 | Suaeda pannonica | 1 |
| Fraxinus pennsylvanica | −1 | Succisa pratensis | 2 |
| Fritillaria meleagris | −2 | Succisella inflexa | 2 |
| Fumana ericoides | 0 | Symphytum officinale | −1 |
| Fumana procumbens | 0 | Symphytum tuberosum | −1 |
| Fumaria officinalis | −1 | Syrenia cana | −3 |
| Fumaria parviflora | −1 | Syringa vulgaris | −3 |
| Fumaria rostellata | −1 | Taeniatherum asperum | 1 |
| Fumaria schleicheri | −1 | Tamarix gallica | −2 |
| Fumaria vaillantii | −1 | Tamarix ramosissima | −2 |
| Gagea bohemica | 0 | Tamarix tetrandra | −2 |
| Gagea lutea | 0 | Tamus communis | −1 |
| Gagea minima | 0 | Tanacetum corymbosum | −2 |
| Gagea pratensis | 0 | Tanacetum parthenium | −2 |
| Gagea pusilla | 0 | Tanacetum vulgare | −2 |
| Gagea spathacea | 0 | Taraxacum bessarabicum | 1 |
| Gagea szovitsii | 0 | Taraxacum laevigatum | 1 |
| Gagea villosa | 0 | Taraxacum officinale | 3 |
| Galanthus nivalis | −3 | Taraxacum palustre | 2 |
| Galega officinalis | 2 | Taraxacum serotinum | 2 |
| Galeobdolon luteum | −2 | Taxus baccata | −3 |
| Galeopsis bifida | −2 | Teesdalia nudicaulis | 1 |
| Galeopsis ladanum | −2 | Telekia speciosa | −2 |
| Galeopsis pubescens | −2 | Tetragonolobus maritimus | 4 |
| Galeopsis segetum | −2 | Teucrium botrys | 1 |
| Galeopsis speciosa | −2 | Teucrium chamaedrys | 1 |
| Galeopsis tetrahit | −2 | Teucrium montanum | 1 |
| Galinsoga ciliata | −1 | Teucrium scordium | 1 |
| Galinsoga parviflora | −1 | Teucrium scorodonia | 1 |
| Galium abaujense | −1 | Thalictrum aquilegiifolium | −2 |
| Galium album | −1 | Thalictrum flavum | −2 |
| Galium aparine | −1 | Thalictrum foetidum | −2 |
| Galium austriacum | −1 | Thalictrum lucidum | −2 |
| Galium boreale | −1 | Thalictrum minus | −2 |
| Galium divaricatum | 1 | Thalictrum simplex | −2 |
| Galium elongatum | −1 | Thelypteris palustris | −2 |
| Galium glaucum | −1 | Thesium arvense | 0 |
| Galium humifusum | −1 | Thesium bavarum | 0 |
| Galium lucidum | −1 | Thesium dollineri | 0 |
| Galium mollugo | −1 | Thesium linophyllon | 0 |
| Galium odoratum | −1 | Thladiantha dubia | −2 |
| Galium palustre | −1 | Thlaspi alliaceum | 1 |
| Galium parisiense | 1 | Thlaspi arvense | 1 |
| Galium pumilum | 1 | Thlaspi coerulescens | 1 |
| Galium rivale | −1 | Thlaspi goesingense | 1 |
| Galium rotundifolium | 1 | Thlaspi jankae | 1 |
| Galium rubioides | −1 | Thlaspi kovatsii | 1 |
| Galium schultesii | −1 | Thlaspi montanum | 1 |
| Galium spurium | 1 | Thlaspi perfoliatum | 1 |
| Galium sylvaticum | −1 | Thymelaea passerina | 0 |
| Galium tenuissimum | 1 | Thymus caespitosus | −1 |
| Galium tricornutum | 1 | Thymus glabrescens | −1 |
| Galium uliginosum | −1 | Thymus pannonicus | −1 |
| Galium verum | −1 | Thymus praecox | −1 |
| Gaudinia fragilis | 2 | Thymus pulegioides | −1 |
| Genista germanica | −2 | Thymus serpyllum | −1 |
| Genista ovata | −2 | Thymus vulgaris | −1 |
| Genista pilosa | −2 | Tilia cordata | −1 |
| Genista pilosa | −2 | Tilia platyphyllos | −1 |
| Genista tinctoria | −2 | Tilia tomentosa | −1 |
| Genistella sagittalis | −1 | Tofieldia calyculata | 1 |
| Gentiana asclepiadea | 1 | Tordylium maximum | 1 |
| Gentiana cruciata | 1 | Torilis arvensis | −1 |
| Gentiana pneumonanthe | 1 | Torilis japonica | −1 |
| Gentianella austriaca | 0 | Torilis ucranica | −1 |
| Gentianella amarella subsp. livonica | 0 | Tragopogon dubius | 2 |
| Gentianopsis ciliata | 0 | Tragopogon floccosus | 2 |
| Geranium bohemicum | 1 | Tragopogon orientalis | 2 |
| Geranium columbinum | 0 | Tragus racemosus | −1 |
| Geranium dissectum | 0 | Traunsteinera globosa | −1 |
| Geranium divaricatum | 1 | Tribulus terrestris | −3 |
| Geranium lucidum | 0 | Trifolium alpestre | 4 |
| Geranium molle | 0 | Trifolium angulatum | 4 |
| Geranium palustre | 1 | Trifolium arvense | 1 |
| Geranium phaeum | 1 | Trifolium aureum | 5 |
| Geranium pratense | 1 | Trifolium campestre | 5 |
| Geranium pusillum | 0 | Trifolium diffusum | 4 |
| Geranium pyrenaicum | 1 | Trifolium dubium | 5 |
| Geranium robertianum | 1 | Trifolium fragiferum | 6 |
| Geranium rotundifolium | 0 | Trifolium hybridum | 7 |
| Geranium sanguineum | 1 | Trifolium incarnatum | 4 |
| Geranium sibiricum | 1 | Trifolium medium | 4 |
| Geranium sylvaticum | 1 | Trifolium micranthum | 5 |
| Geum aleppicum | 1 | Trifolium montanum | 4 |
| Geum urbanum | 1 | Trifolium ochroleucon | 4 |
| Gladiolus byzantinus | −2 | Trifolium ornithopodioides | 3 |
| Gladiolus imbricatus | −2 | Trifolium pallidum | 4 |
| Gladiolus palustris | −2 | Trifolium pannonicum | 5 |
| Glaucium corniculatum | −2 | Trifolium patens | 5 |
| Glaucium flavum | −2 | Trifolium pratense | 7 |
| Glaux maritima | 0 | Trifolium repens | 7 |
| Glechoma hederacea | −2 | Trifolium resupinatum | 5 |
| Glechoma hirsuta | −2 | Trifolium retusum | 4 |
| Gleditsia triacanthos | −3 | Trifolium rubens | 5 |
| Globularia cordifolia | 1 | Trifolium striatum | 5 |
| Globularia punctata | 1 | Trifolium strictum | 5 |
| Glyceria declinata | −1 | Trifolium subterraneum | 2 |
| Glyceria fluitans | −1 | Trifolium vesiculosum | 4 |
| Glyceria maxima | −1 | Triglochin maritimum | −1 |
| Glyceria nemoralis | −1 | Triglochin palustre | −1 |
| Glyceria notata | −1 | Trigonella caerulea | 3 |
| Glycyrrhiza echinata | 2 | Trigonella foenum-graecum | 3 |
| Glycyrrhiza glabra | 2 | Trigonella gladiata | 3 |
| Gnaphalium luteo-album | 0 | Trigonella monspeliaca | 3 |
| Gnaphalium sylvaticum | 0 | Trigonella procumbens | 3 |
| Gnaphalium uliginosum | 0 | Trinia glauca | −1 |
| Goodyera repens | 0 | Trinia ramosissima | −1 |
| Gratiola officinalis | −2 | Trisetum flavescens | 4 |
| Gymnadenia conopsea | −1 | Trollius europaeus | −2 |
| Gymnadenia odoratissima | −1 | Tulipa sylvestris | −2 |
| Gymnocarpium dryopteris | −2 | Turritis glabra | 1 |
| Gymnocarpium robertianum | −2 | Tussilago farfara | −2 |
| Gypsophila fastigiata | 1 | Typha angustifolia | −1 |
| Gypsophila muralis | 1 | Typha latifolia | −1 |
| Gypsophila paniculata | 1 | Typha laxmannii | −1 |
| Haynaldia villosa | 2 | Typha minima | −1 |
| Hedera helix | −3 | Typha shuttleworthii | −1 |
| Heleochloa alopecuroides | 1 | Ulmus glabra | −1 |
| Heleochloa schoenoides | 0 | Ulmus laevis | −1 |
| Helianthemum canum | 0 | Ulmus minor | −1 |
| Helianthemum nummularium | 0 | Ulmus procera | −1 |
| Helianthemum ovatum | 0 | Urtica dioica | 1 |
| Helianthus annuus | −2 | Urtica kioviensis | 1 |
| Helianthus decapetalus | −2 | Urtica pilulifera | 1 |
| Helianthus rigidus | −2 | Urtica urens | 1 |
| Helianthus tuberosus | −2 | Vaccaria hispanica | 2 |
| Helichrysum arenarium | −1 | Vaccinium myrtillus | −1 |
| Helictotrichon adsurgens | 2 | Vaccinium oxycoccos | −1 |
| Helictotrichon compressum | 2 | Vaccinium vitis-idaea | −1 |
| Helictotrichon pratense | 2 | Valeriana dioica | −1 |
| Helictotrichon pubescens | 1 | Valeriana excelsa | −1 |
| Heliotropium europaeum | −2 | Valeriana officinalis | −1 |
| Heliotropium supinum | −2 | Valeriana stolonifera | −1 |
| Helleborus dumetorum | −3 | Valeriana tripteris | −1 |
| Helleborus odorus | −3 | Valerianella carinata | 1 |
| Helleborus purpurascens | −3 | Valerianella coronata | 1 |
| Helleborus viridis | −3 | Valerianella dentata | 1 |
| Helminthia echioides | −1 | Valerianella locusta | 1 |
| Hemerocallis fulva | −3 | Valerianella pumila | 1 |
| Hemerocallis lilio-asphodelus | −3 | Valerianella rimosa | 1 |
| Hepatica nobilis | −1 | Ventenata dubia | 1 |
| Heracleum mantegazzianum | −3 | Veratrum album | −3 |
| Heracleum sphondylium | −3 | Veratrum nigrum | −3 |
| Herniaria glabra | 0 | Verbascum austriacum | −1 |
| Herniaria hirsuta | 0 | Verbascum blattaria | −2 |
| Herniaria incana | 0 | Verbascum densiflorum | −2 |
| Hesperis matronalis | −1 | Verbascum lychnitis | −1 |
| Hesperis sylvestris | −1 | Verbascum nigrum | −1 |
| Hesperis tristis | 1 | Verbascum phlomoides | −2 |
| Hibiscus trionum | 1 | Verbascum phoeniceum | 0 |
| Hieracium pilosella | 1 | Verbascum pulverulentum | −1 |
| Hieracium aurantiacum | 1 | Verbascum speciosum | −2 |
| Hieracium bauhinii | 1 | Verbascum thapsus | −2 |
| Hieracium bifidum | 1 | Verbena officinalis | 1 |
| Hieracium bupleuroides | 1 | Verbena supina | 1 |
| Hieracium caespitosum | 1 | Veronica acinifolia | 0 |
| Hieracium cymosum | 1 | Veronica agrestis | 0 |
| Hieracium echioides | 1 | Veronica anagallis-aquatica | 2 |
| Hieracium lachenalii | 1 | Veronica anagalloides | 1 |
| Hieracium lactucella | 1 | Veronica arvensis | 0 |
| Hieracium laevigatum | 1 | Veronica austriaca | 1 |
| Hieracium macranthum | 1 | Veronica beccabunga | 1 |
| Hieracium murorum | 1 | Veronica catenata | 1 |
| Hieracium piloselloides | 1 | Veronica chamaedrys | 1 |
| Hieracium racemosum | 1 | Veronica dillenii | 0 |
| Hieracium sabaudum | 1 | Veronica hederifolia | 0 |
| Hieracium schmidtii | 1 | Veronica montana | 1 |
| Hieracium staticifolium | 1 | Veronica officinalis | 1 |
| Hieracium umbellatum | 1 | Veronica opaca | 0 |
| Hierochloë australis | 1 | Veronica peregrina | 0 |
| Hierochloë repens | 1 | Veronica persica | 0 |
| Himantoglossum adriaticum | −1 | Veronica polita | 0 |
| Himantoglossum caprinum | −1 | Veronica praecox | 0 |
| Hippocrepis comosa | 2 | Veronica prostrata | 0 |
| Hippocrepis emerus | 2 | Veronica scardica | 1 |
| Hippophaë rhamnoides | −3 | Veronica scutellata | 1 |
| Holcus lanatus | 2 | Veronica serpyllifolia | 1 |
| Holcus mollis | 1 | Veronica teucrium | 1 |
| Holosteum umbellatum | 1 | Veronica triphyllos | 0 |
| Hordelymus europaeus | 1 | Veronica verna | 0 |
| Hordeum hystrix | 1 | Viburnum lantana | −2 |
| Hordeum marinum | 1 | Viburnum opulus | −2 |
| Hordeum murinum | 1 | Vicia angustifolia | 3 |
| Hornungia petraea | 0 | Vicia articulata | 3 |
| Hottonia palustris | −2 | Vicia biennis | 4 |
| Humulus lupulus | −1 | Vicia cassubica | 4 |
| Humulus scandens | −1 | Vicia cracca | 3 |
| Huperzia selago | 0 | Vicia dumetorum | 4 |
| Hydrocharis morsus-ranae | −2 | Vicia ervilia | 3 |
| Hydrocotyle vulgaris | −2 | Vicia faba | 3 |
| Hyoscyamus niger | −3 | Vicia grandiflora | 3 |
| Hypericum barbatum | 1 | Vicia hirsuta | 3 |
| Hypericum elegans | 1 | Vicia lathyroides | 3 |
| Hypericum hirsutum | 1 | Vicia lutea | 3 |
| Hypericum humifusum | 1 | Vicia narbonensis | 3 |
| Hypericum maculatum | 1 | Vicia oroboides | 4 |
| Hypericum montanum | 1 | Vicia pannonica | 3 |
| Hypericum perforatum | 1 | Vicia peregrina | 3 |
| Hypericum tetrapterum | 1 | Vicia pisiformis | 4 |
| Hypochoeris maculata | 1 | Vicia sativa | 4 |
| Hypochoeris radicata | 1 | Vicia sepium | 4 |
| Hyssopus officinalis | 1 | Vicia sparsiflora | 4 |
| Impatiens balfouri | −2 | Vicia sylvatica | 4 |
| Impatiens glandulifera | −2 | Vicia tenuifolia | 3 |
| Impatiens noli-tangere | −2 | Vicia tenuissima | 3 |
| Impatiens parviflora | −2 | Vicia tetrasperma | 3 |
| Inula britannica | 1 | Vicia villosa | 4 |
| Inula conyza | 2 | Vinca herbacea | −2 |
| Inula ensifolia | 2 | Vinca major | −2 |
| Inula germanica | −1 | Vinca minor | −2 |
| Inula helenium | 2 | Vincetoxicum hirundinaria | −3 |
| Inula hirta | −1 | Vincetoxicum pannonicum | −3 |
| Inula oculus-christi | −1 | Viola alba | 1 |
| Inula salicina | 2 | Viola ambigua | 1 |
| Inula spiraeifolia | 2 | Viola arvensis | 0 |
| Ipomoea purpurea | −1 | Viola biflora | 1 |
| Iris aphylla | −2 | Viola canina | 1 |
| Iris arenaria | −2 | Viola collina | 1 |
| Iris germanica | −2 | Viola cyanea | 1 |
| Iris graminea | −2 | Viola elatior | 1 |
| Iris pseudacorus | −3 | Viola hirta | 1 |
| Iris pumila | −2 | Viola kitaibeliana | 0 |
| Iris sibirica | −2 | Viola mirabilis | 1 |
| Iris spuria | −2 | Viola montana | 1 |
| Iris variegata | −2 | Viola odorata | 1 |
| Isatis tinctoria | −1 | Viola palustris | 1 |
| Isopyrum thalictroides | −2 | Viola pumila | 1 |
| Jasione montana | 1 | Viola riviniana | 1 |
| Jovibarba hirta | 0 | Viola rupestris | 0 |
| Jovibarba sobolifera | 0 | Viola stagnina | 1 |
| Juglans nigra | −1 | Viola suavis | 1 |
| Juglans regia | −1 | Viola sylvestris | 1 |
| Juncus alpinus | 0 | Viola tricolor | 1 |
| Juncus articulatus | 0 | Viscum album | −2 |
| Juncus atratus | −1 | Vitis rupestris | −1 |
| Juncus bufonius | 0 | Vitis sylvestris | −1 |
| Juncus bulbosus | 0 | Vitis vinifera | −1 |
| Juncus capitatus | 0 | Vitis vulpina | −1 |
| Juncus compressus | 0 | Vulpia bromoides | 1 |
| Juncus conglomeratus | −1 | Vulpia myuros | 1 |
| Juncus effusus | −1 | Waldsteinia geoides | 1 |
| Juncus gerardii | −1 | Xanthium italicum | −3 |
| Juncus inflexus | −1 | Xanthium spinosum | −3 |
| Juncus maritimus | −2 | Xanthium strumarium | −3 |
| Juncus sphaerocarpus | 0 | Xeranthemum annuum | −1 |
| Juncus subnodulosus | −1 | Xeranthemum cylindraceum | −1 |
References
- Huber, R.; Le’Clec’h, S.; Buchmann, N.; Finger, R. Economic Value of Three Grassland Ecosystem Services When Managed at the Regional and Farm Scale. Sci. Rep. 2022, 12, 4194. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland Ecosystem Services: A Systematic Review of Research Advances and Future Directions. Landsc. Ecol. 2020, 35, 793–814. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More Important for Ecosystem Services than You Might Think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Boval, M.; Dixon, R.M. The Importance of Grasslands for Animal Production and Other Functions: A Review on Management and Methodological Progress in the Tropics. Animal 2012, 6, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Moog, D.; Poschlod, P.; Kahmen, S.; Schreiber, K.-F. Comparison of Species Composition between Different Grassland Management Treatments after 25 Years. Appl. Veg. Sci. 2002, 5, 99–106. [Google Scholar] [CrossRef]
- Hoekstra, N.J.; De Long, J.R.; Jansma, A.P.; Iepema, G.; Manhoudt, A.; van Eekeren, N. Differences in Grassland Sward Biodiversity and Management Regime Lead to Mixed Effects on Ecosystem Services. Eur. J. Agron. 2023, 149, 126886. [Google Scholar] [CrossRef]
- Frank, D.A.; Wallen, R.L.; White, P.J. Ungulate Control of Grassland Production: Grazing Intensity and Ungulate Species Composition in Yellowstone Park. Ecosphere 2016, 7, e01603. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Cheng, G.; Wu, J.; Hong, J.; Niu, S. Effects of Grazing Regimes on Plant Traits and Soil Nutrients in an Alpine Steppe, Northern Tibetan Plateau. PLoS ONE 2014, 9, e108821. [Google Scholar] [CrossRef]
- Alves, F.G.S.; Gomes, E.C.; Faria, A.F.G.; Pompeu, R.C.F.F.; Coutinho, D.N.; Cândido, M.J.D. The Effects of Grazing Frequency and Intensity on Herbage and Structural Characteristics of Irrigated ‘Tifton 85’ Bermudagrass Grazed by Sheep. N. Z. J. Agric. Res. 2025, 68, 2131–2142. [Google Scholar] [CrossRef]
- French, K.E. Species Composition Determines Forage Quality and Medicinal Value of High Diversity Grasslands in Lowland England. Agric. Ecosyst. Environ. 2017, 241, 193–204. [Google Scholar] [CrossRef]
- Komac, B.; Domènech, M.; Fanlo, R. Effects of Grazing on Plant Species Diversity and Pasture Quality in Subalpine Grasslands in the Eastern Pyrenees (Andorra): Implications for Conservation. J. Nat. Conserv. 2014, 22, 247–255. [Google Scholar] [CrossRef]
- Blaix, C.; Chabrerie, O.; Alard, D.; Catterou, M.; Diquelou, S.; Dutoit, T.; Lacoux, J.; Loucougaray, G.; Michelot-Antalik, A.; Pacé, M.; et al. Forage Nutritive Value Shows Synergies with Plant Diversity in a Wide Range of Semi-Natural Grassland Habitats. Agric. Ecosyst. Environ. 2023, 347, 108369. [Google Scholar] [CrossRef]
- Humbert, J.-Y.; Pellet, J.; Buri, P.; Arlettaz, R. Does Delaying the First Mowing Date Benefit Biodiversity in Meadowland? Environ. Evid. 2012, 1, 9. [Google Scholar] [CrossRef]
- Stolz, C.; Deppe, U.; Kämmer, G.; Kuhwald, M.; Nass, D.; Sönnichsen, L. The Development of Nutrient Contents on a New Conservation Area in the Far North of Germany Concerning Different Types of Use. A Proposal for a Sustainable Development in Nature Conservation Practice. Pol. J. Soil Sci. 2018, 51, 133. [Google Scholar] [CrossRef]
- Rayburn, E.B.; Blaser, R.E.; Fontenot, J.P. In Vivo Quality of Tall Fescue as Influenced by Season, Legumes, Age, and Canopy Strata. Agron. J. 1980, 72, 872–876. [Google Scholar] [CrossRef]
- Nosalewicz, A.; Siecińska, J.; Kondracka, K.; Nosalewicz, M. The Functioning of Festuca Arundinacea and Lolium Perenne under Drought Is Improved to a Different Extend by the Previous Exposure to Water Deficit. Environ. Exp. Bot. 2018, 156, 271–278. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Darkalt, A. Changes in the Physiological and Morphometric Characteristics and Biomass Distribution of Forage Grasses Growing under Conditions of Drought and Silicon Application. Plants 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, T.; De Boever, J.L.; Haesaert, G.; Cougnon, M.; Debevere, S.; Vandaele, L.; Goossens, K. Management Strategies to Improve the Nitrogen Use Efficiency in Grasslands and the Protein Quality in Grass Silages: Lessons from a Practical Study on Dairy Farms in Flanders. Grassl. Res. 2024, 3, 18–32. [Google Scholar] [CrossRef]
- Bryan, W.B.; Mata, D.J.; Gekara, O.J. Manure and Management Affect Grassland Production and Soil Quality in Organic Lamb Production. Agrosyst. Geosci. Environ. 2019, 2, 190058. [Google Scholar] [CrossRef]
- Chang, S.; Xie, K.; Du, W.; Jia, Q.; Yan, T.; Yang, H.; Hou, F. Effects of Mowing Times on Nutrient Composition and In Vitro Digestibility of Forage in Three Sown Pastures of China Loess Plateau. Animals 2022, 12, 2807. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Q.; Cheng, L.; Zhong, R. Effects of Mowing Regimes on Forage Yield and Crude Protein of Leymus Chinensis (Trin.) Tzvel in Songnen Grassland. Grassl. Sci. 2021, 67, 275–284. [Google Scholar] [CrossRef]
- Andueza, D.; Cruz, P.; Farruggia, A.; Baumont, R.; Picard, F.; Michalet-Doreau, B. Nutritive Value of Two Meadows and Relationships with Some Vegetation Traits. Grass Forage Sci. 2010, 65, 325–334. [Google Scholar] [CrossRef]
- Andueza, D.; Rodrigues, A.M.; Picard, F.; Rossignol, N.; Baumont, R.; Cecato, U.; Farruggia, A. Relationships between Botanical Composition, Yield and Forage Quality of Permanent Grasslands over the First Growth Cycle. Grass Forage Sci. 2016, 71, 366–378. [Google Scholar] [CrossRef]
- Chen, L.; Auh, C.-K.; Dowling, P.; Bell, J.; Chen, F.; Hopkins, A.; Dixon, R.A.; Wang, Z.-Y. Improved Forage Digestibility of Tall Fescue (Festuca arundinacea) by Transgenic down-Regulation of Cinnamyl Alcohol Dehydrogenase. Plant Biotechnol. J. 2003, 1, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.D.; Rayburn, E.B.; Griggs, T.C. Grassland Ecology and Ecosystem Management for Sustainable Livestock Performance. Agronomy 2023, 13, 1380. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil Carbon Sequestration Accelerated by Restoration of Grassland Biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef]
- Sala, O.E.; Austin, A.T. Methods of Estimating Aboveground Net Primary Productivity. In Methods in Ecosystem Science; Sala, O.E., Jackson, R.B., Mooney, H.A., Howarth, R.W., Eds.; Springer: New York, NY, USA, 2000; pp. 31–43. ISBN 978-1-4612-1224-9. [Google Scholar]
- Nagy, G.; Pető, K. A Lábonálló Gyepek Termésének Mérése. [Measuring the Yield of Standing Grasslands]. Állattanyésztés é S Takarmányozás 2001, 50, 139–154. [Google Scholar]
- Klapp, E.; Boecker, P.; König, F.; Stählin, A. Wertzahlen der Grünlandpflanzen. [Feed and ecological values of grassland species]. Das. Grünland 1953, 2, 38–40. [Google Scholar]
- Balázs, F. A gyepek botanikai és gazdasági értékelése. [Botanical and economic evaluation of grasslands]. A Keszthelyi Mezőgazdasági Akadémia Kiadványai 1960, 8, 3–23. [Google Scholar]
- Briemle, G.; Nietsche, S.; Nitsche, L. Grünlandpflanzen Und Ihre Nutzungswertzahlen. [Grassland Species and Their Utility Values]. Jahrb. Naturschutz Hess. 2003, 8, 81–96. [Google Scholar]
- Briemle, G.; Nitsche, S.; Nitsche, L. Nutzungswertzahlen Für Gefäßpflanzen. [The Utility Values of Vascular Plants]. Schriftenreihe Veg. 2002, 38, 203–225. [Google Scholar]
- Briemle, G. Möglichkeiten Und Grenzen Fer Anwendbarkeit von Wertzahlen Im Grünland. Das Wirtschaftseigene Futter 43 1997, 141–164. [Google Scholar]
- Nitsche, L. Vegetations-Bestanderfassungen Nach Dem Hessischen Biotoppflegesystem Für Magerrasen. [Vegetation Surveys According to the Hessian Biotope Management System for Nutrient-Poor Grasslands]. Naturschutz Und Landschaftsplanung 1993, 25, 17–23. [Google Scholar]
- Briemle, G. Zur Anwendbarkeit Ökologischer Wertzahlen Im Grünland. [On the Applicability of Ecological Values in Grassland]. Angew. Bot. 1997, 71, 219–228. [Google Scholar]
- Briemle, G.; Ellenberg, H. Zur Mahdverträglichkeit von Grünlandpflanzen. Möglichkeiten Der Praktischen Anwendung von Ziegerwerten. [On the Mowing Compatibility of Grassland Plants. Practical Applications of Forage and Ecological Values]. Nat. Landschaft 1994, 69, 139–147. [Google Scholar]
- Čarni, A.; Ćuk, M.; Krstonošić, D.; Škvorc, Ž. Study of Forage Quality of Grasslands on the Southern Margin of the Pannonian Basin. Agronomy 2021, 11, 2132. [Google Scholar] [CrossRef]
- Cebrián-Piqueras, M.A.; Trinogga, J.; Trenkamp, A.; Minden, V.; Maier, M.; Mantilla-Contreras, J. Digging into the Roots: Understanding Direct and Indirect Drivers of Ecosystem Service Trade-Offs in Coastal Grasslands via Plant Functional Traits. Environ. Monit. Assess. 2021, 193, 271. [Google Scholar] [CrossRef]
- Tichý, L.; Axmanová, I.; Dengler, J.; Guarino, R.; Jansen, F.; Midolo, G.; Nobis, M.P.; Van Meerbeek, K.; Aćić, S.; Attorre, F.; et al. Ellenberg-Type Indicator Values for European Vascular Plant Species. J. Veg. Sci. 2023, 34, e13168. [Google Scholar] [CrossRef]
- Midolo, G.; Herben, T.; Axmanová, I.; Marcenò, C.; Pätsch, R.; Bruelheide, H.; Karger, D.N.; Aćić, S.; Bergamini, A.; Bergmeier, E.; et al. Disturbance Indicator Values for European Plants. Glob. Ecol. Biogeogr. 2023, 32, 24–34. [Google Scholar] [CrossRef]
- Balázs, F. A gyepek termésbecslése növényszociológia alapján. [Estimating grass yields based on plant sociology]. Agrártudomány 1949, 1, 26–35. [Google Scholar]
- Balázs, F. Mérések a B Szám Megállapítására. [Measurements to Determine the B Number]. Mosonmagyaróvári Mezőgazdasági Kísérleti Intézet Évkönyve 1951, 1, 29–38. [Google Scholar]
- Bettin, K.; Komainda, M.; Tonn, B.; Isselstein, J. Relationship between Concentrate Feeding Strategy and Grassland Phytodiversity on Dairy Farms. Agric. Ecosyst. Environ. 2023, 344, 108293. [Google Scholar] [CrossRef]
- Gilhaus, K.; Hölzel, N. Seasonal Variations of Fodder Quality and Availability as Constraints for Stocking Rates in Year-Round Grazing Schemes. Agric. Ecosyst. Environ. 2016, 234, 5–15. [Google Scholar] [CrossRef]
- Sölter, U.; Hopkins, A.; Sitzia, M.; Goby, J.P.; Greef, J.M. Seasonal Changes in Herbage Mass and Nutritive Value of a Range of Grazed Legume Swards under Mediterranean and Cool Temperate Conditions. Grass Forage Sci. 2007, 62, 372–388. [Google Scholar] [CrossRef]
- King, C.; McEniry, J.; Richardson, M.; O’Kiely, P. Yield and Chemical Composition of Five Common Grassland Species in Response to Nitrogen Fertiliser Application and Phenological Growth Stage. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2012, 62, 644–658. [Google Scholar] [CrossRef]
- Chirilă, S.D.; Răileanu, S.; David, L.O.; Covaliov, S.; Doroftei, M. An Analysis of Plant Palatability on Pastures of the Delta: Case Study, Danube Delta Area, Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13568. [Google Scholar] [CrossRef]
- Nakano, T.; Bat-Oyun, T.; Shinoda, M. Responses of Palatable Plants to Climate and Grazing in Semi-Arid Grasslands of Mongolia. Glob. Ecol. Conserv. 2020, 24, e01231. [Google Scholar] [CrossRef]
- Scharenberg, A.; Arrigo, Y.; Gutzwiller, A.; Soliva, C.R.; Wyss, U.; Kreuzer, M.; Dohme, F. Palatability in Sheep and in Vitro Nutritional Value of Dried and Ensiled Sainfoin (Onobrychis viciifolia) Birdsfoot Trefoil (Lotus corniculatus), and Chicory (Cichorium intybus). Arch. Anim. Nutr. 2007, 61, 481–496. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Wyse, D.L.; Ehlke, N.J. Palatability and Nutritive Value of Native Legumes. Nativ. Plants J. 2009, 10, 224–231. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A Two-Stage Technique for the in Vitro Digestion of Forage Crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Liu, D.; Semenchuk, P.; Essl, F.; Lenzner, B.; Moser, D.; Blackburn, T.M.; Cassey, P.; Biancolini, D.; Capinha, C.; Dawson, W.; et al. The Impact of Land Use on Non-Native Species Incidence and Number in Local Assemblages Worldwide. Nat. Commun. 2023, 14, 2090. [Google Scholar] [CrossRef] [PubMed]
- Anacker, B.L.; Seastedt, T.R.; Halward, T.M.; Lezberg, A.L. Soil Carbon and Plant Richness Relationships Differ among Grassland Types, Disturbance History and Plant Functional Groups. Oecologia 2021, 196, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Tatarko, A.R.; Knops, J.M.H. Nitrogen Addition and Ecosystem Functioning: Both Species Abundances and Traits Alter Community Structure and Function. Ecosphere 2018, 9, e02087. [Google Scholar] [CrossRef]
- Hayes, F.; Mills, G.; Jones, L.; Abbott, J.; Ashmore, M.; Barnes, J.; Neil Cape, J.; Coyle, M.; Peacock, S.; Rintoul, N.; et al. Consistent Ozone-Induced Decreases in Pasture Forage Quality across Several Grassland Types and Consequences for UK Lamb Production. Sci. Total Environ. 2016, 543, 336–346. [Google Scholar] [CrossRef]
- Lumivero XLSTAT Statistical and Data Analysis Solution 2024. Available online: https://www.xlstat.com/download (accessed on 20 October 2024).
- Pereña-Ortiz, J.F.; Salvo-Tierra, Á.E.; Sánchez-Mata, D. Application of Phytosociological Information in the Evaluation of the Management of Protected Areas. Plants 2023, 12, 406. [Google Scholar] [CrossRef]
- Flombaum, P.; Sala, O.E. A Non-Destructive and Rapid Method to Estimate Biomass and Aboveground Net Primary Production in Arid Environments. J. Arid. Environ. 2007, 69, 352–358. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Jiang, M.; Tong, S.; Lu, X. Spatiotemporal Change of Aboveground Biomass and Its Response to Climate Change in Marshes of the Tibetan Plateau. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102385. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Zhang, J.; Li, Z.; Wang, L.; Wang, Z.; Wu, Y.; Wang, Y.; Liang, C. Effect of Grazing Types on Community-Weighted Mean Functional Traits and Ecosystem Functions on Inner Mongolian Steppe, China. Sustainability 2020, 12, 7169. [Google Scholar] [CrossRef]
- Serba, D.D.; Hejl, R.W.; Burayu, W.; Umeda, K.; Bushman, B.S.; Williams, C.F. Pertinent Water-Saving Management Strategies for Sustainable Turfgrass in the Desert U.S. Southwest. Sustainability 2022, 14, 12722. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, J.; Gu, R.; Liang, Y.; Yan, Y.; Gao, Q.; Yang, J. Influence of Different Mowing Systems on Community Characteristics and the Compensatory Growth of Important Species of the Stipa Grandis Steppe in Inner Mongolia. Sustainability 2016, 8, 1121. [Google Scholar] [CrossRef]
- Koyama, A.; Kubo, D.; Yoshihara, Y.; Jamsran, U.; Okuro, T. Response of Degraded Vegetation to Introduction of Prescribed Burning or Mowing Management in a Mongolian Steppe. Grassl. Sci. 2016, 62, 37–44. [Google Scholar] [CrossRef]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. Mowing: A Meta-Analysis of Biodiversity Benefits for Grassland Management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Winkler, J.; Malovcová, M.; Adamcová, D.; Ogrodnik, P.; Pasternak, G.; Zumr, D.; Kosmala, M.; Koda, E.; Vaverková, M.D. Significance of Urban Vegetation on Lawns Regarding the Risk of Fire. Sustainability 2021, 13, 11027. [Google Scholar] [CrossRef]
- Binder, S.; Isbell, F.; Polasky, S.; Catford, J.A.; Tilman, D. Grassland Biodiversity Can Pay. Proc. Natl. Acad. Sci. USA 2018, 115, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Klaus, V.H.; Hölzel, N.; Fischer, M. Direct and Productivity-Mediated Indirect Effects of Fertilization, Mowing and Grazing on Grassland Species Richness. J. Ecol. 2012, 100, 1391–1399. [Google Scholar] [CrossRef]
- Uchida, K.; Ushimaru, A. Biodiversity Declines Due to Abandonment and Intensification of Agricultural Lands: Patterns and Mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Ran, L.; Wang, X.; Li, S.; Zhou, Y.; Xu, Y.-J.; Chan, C.N.; Fang, N.; Xin, Z.; Shen, H. Integrating Aquatic and Terrestrial Carbon Fluxes to Assess the Net Landscape Carbon Balance of a Highly Erodible Semiarid Catchment. J. Geophys. Res. Biogeosci. 2022, 127, e2021JG006765. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Sun, J.; Qiu, J.; Pei, W.; Zhang, K.; Xu, X.; Liu, D. Dynamic of Grassland Degradation and Its Driving Forces from Climate Variation and Human Activities in Central Asia. Agronomy 2023, 13, 2763. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Xu, J.; Ji, Y.; Du, X.; Gao, J. Precipitation Dominates the Allocation Strategy of Above- and Belowground Biomass in Plants on Macro Scales. Plants 2023, 12, 2843. [Google Scholar] [CrossRef]
- Li, X.; Zuo, X.; Qiao, J.; Hu, Y.; Wang, S.; Yue, P.; Cheng, H.; Song, Z.; Chen, M.; Hautier, Y. Context-Dependent Impact of Changes in Precipitation on the Stability of Grassland Biomass. Funct. Ecol. 2024, 38, 1185–1198. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Ma, W.; Wang, W. Relationship between Variability in Aboveground Net Primary Production and Precipitation in Global Grasslands. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Yan, H.; Liang, C.; Li, Z.; Liu, Z.; Miao, B.; He, C.; Sheng, L. Impact of Precipitation Patterns on Biomass and Species Richness of Annuals in a Dry Steppe. PLoS ONE 2015, 10, e0125300. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, H.; Duan, L. Resistance of Grassland Productivity to Drought and Heatwave over a Temperate Semi-Arid Climate Zone. Sci. Total Environ. 2024, 951, 175495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).