Initial Characterization of Low Molecular Weight Hydrocarbons in an Oil Sands Pit Lake
Abstract
1. Introduction
2. Materials and Methods
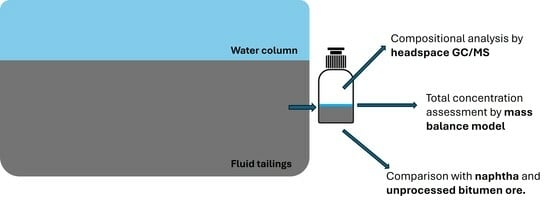
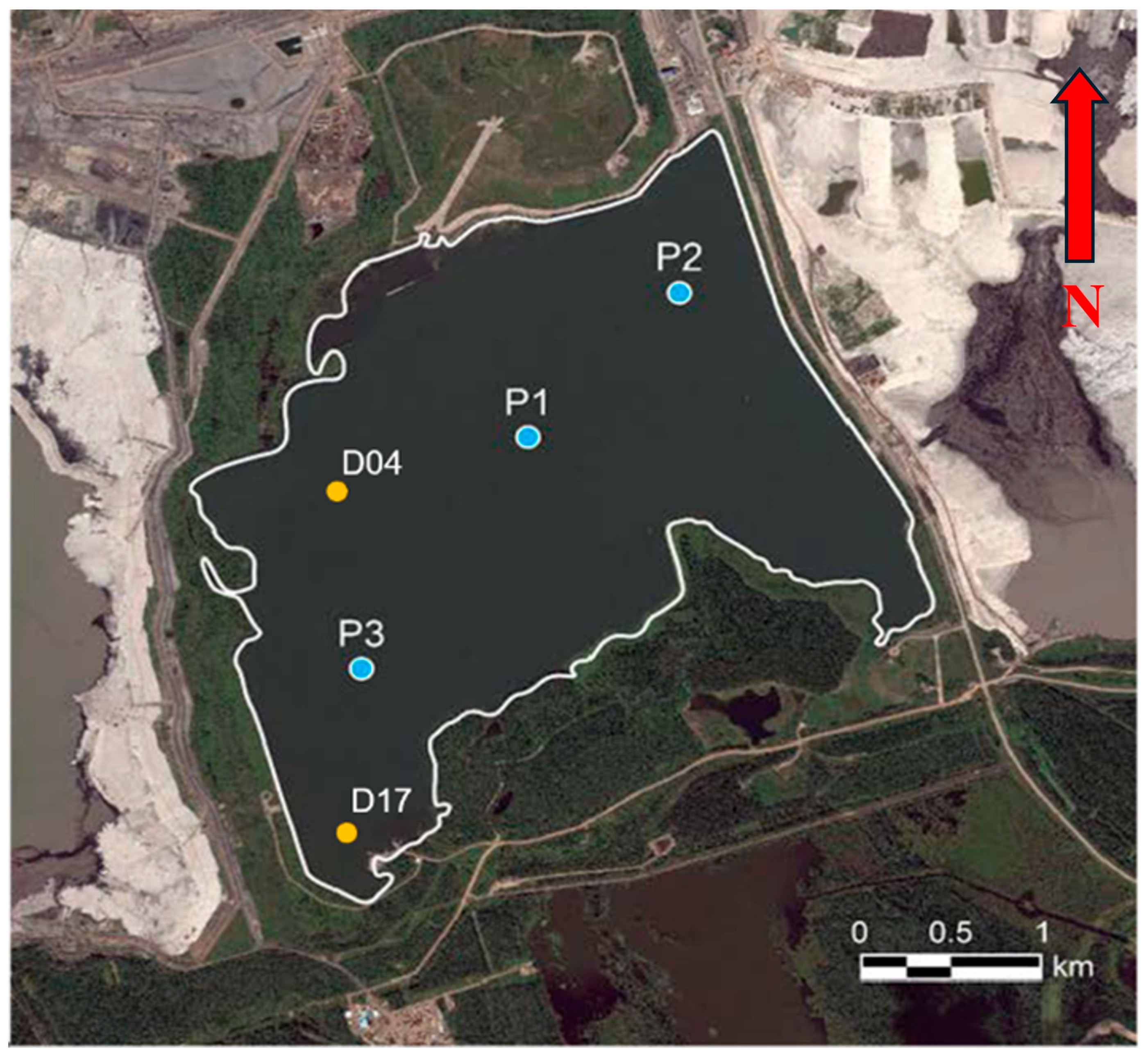
2.1. Sample Collection and Preparation
2.2. Chemical Reagents
2.3. Analytical Methodology
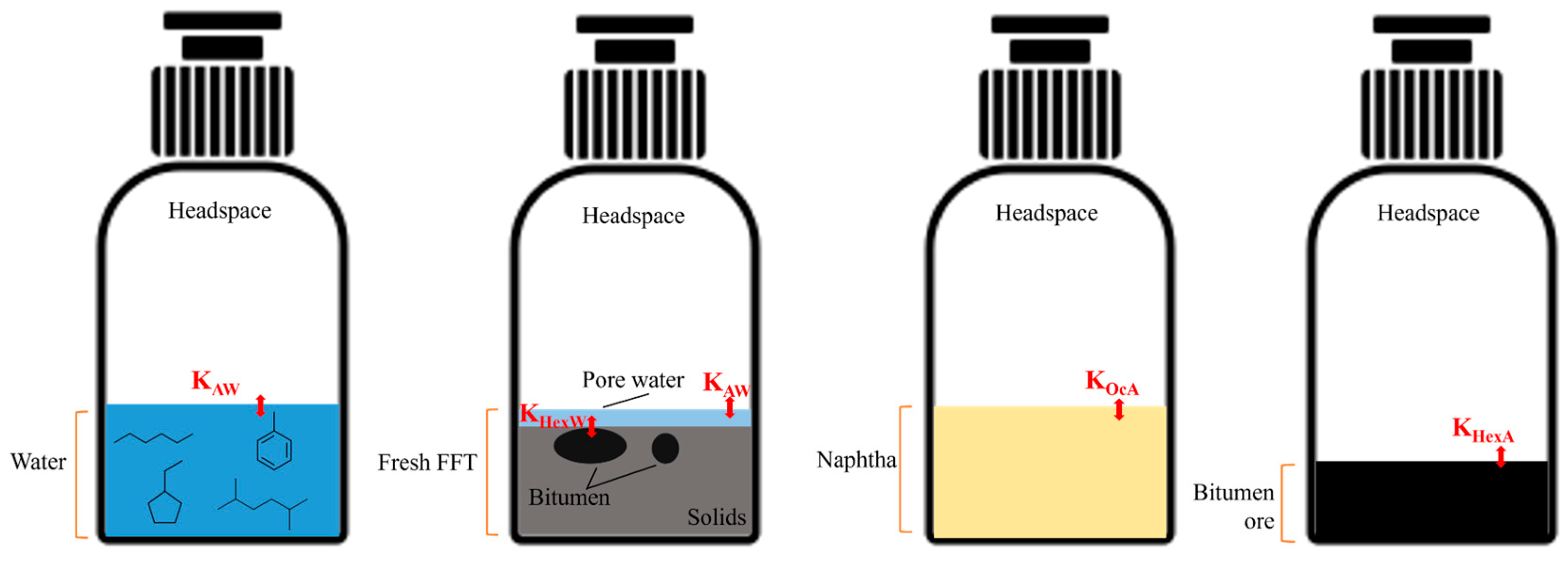
2.4. Equilibrium Mass Balance Modelling for Total LMWHCs Contents Estimation
2.5. Data Collection and Analysis
3. Results and Discussion
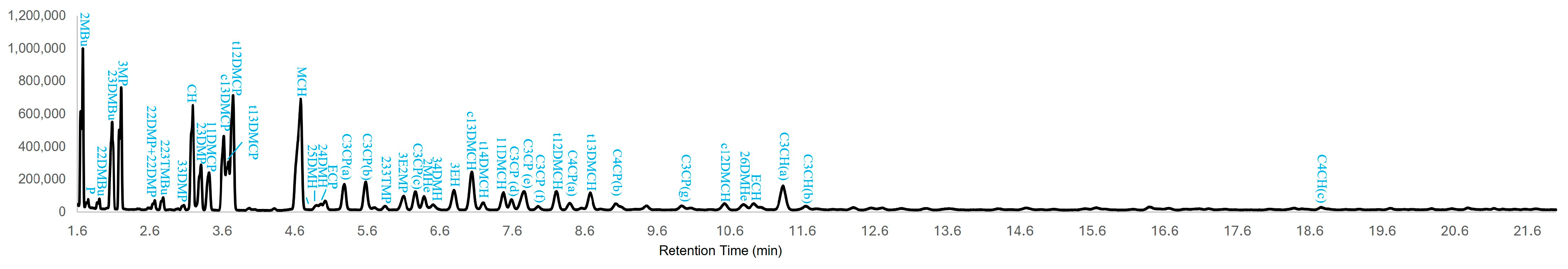
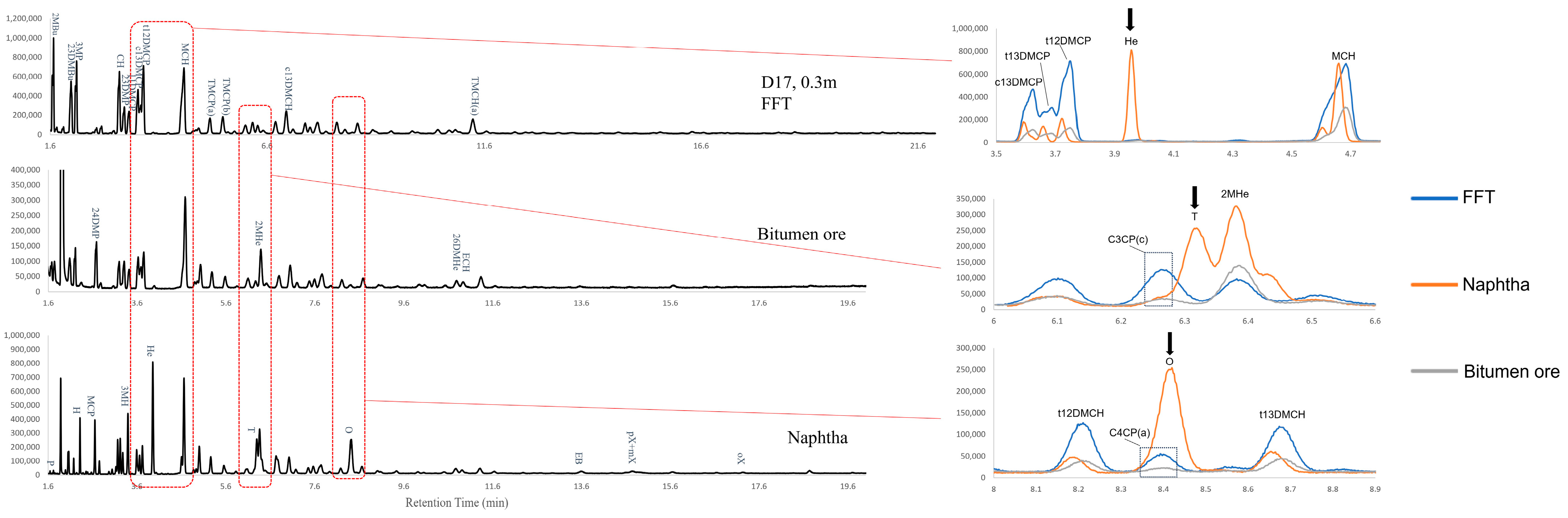
3.1. Tentative Identification of LMWHCs in Naphtha, Oil Sands Bitumen and BML Fluid Tailings
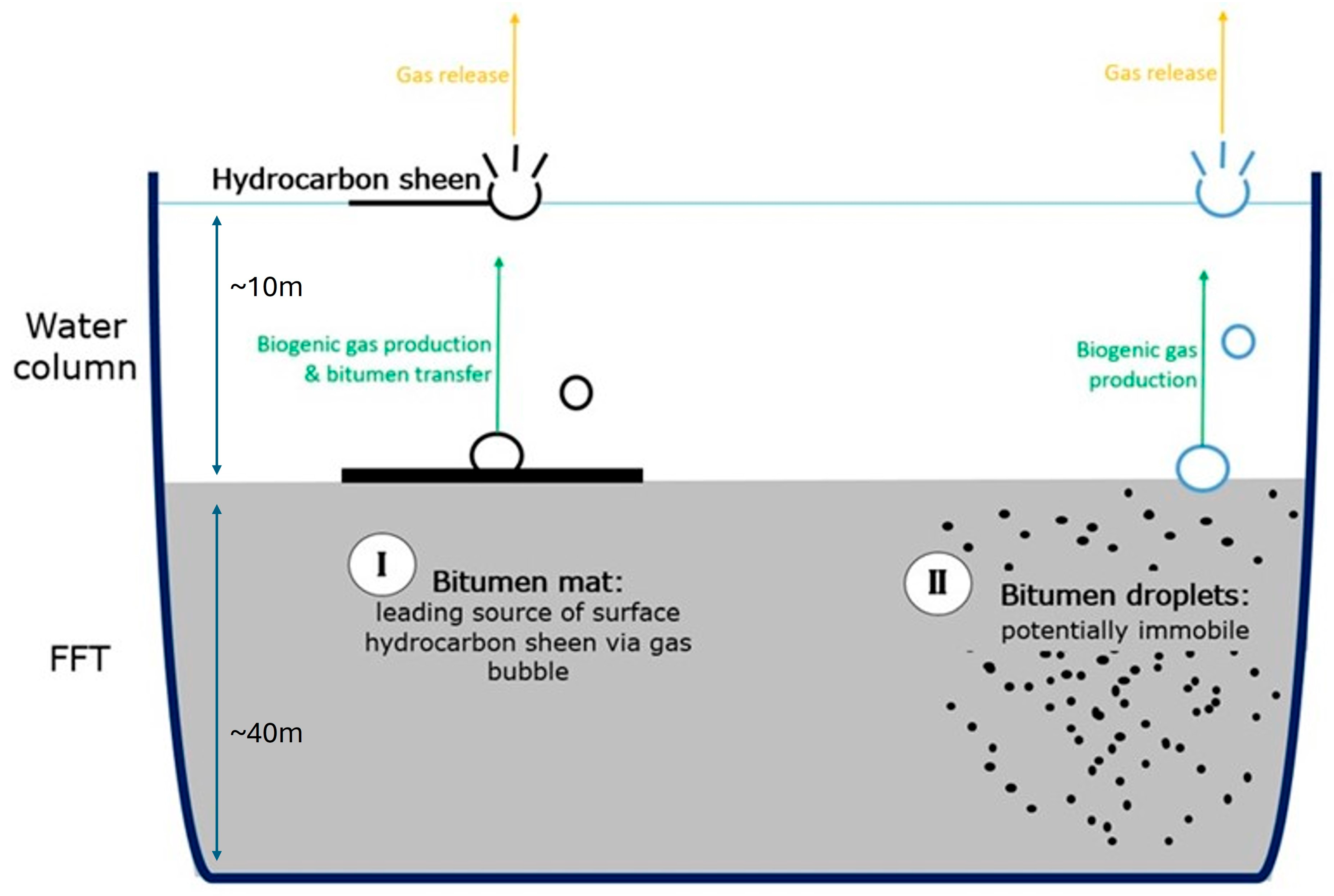
3.2. Assessment of LMWHCs Distributions Within BML Fluid Tailings
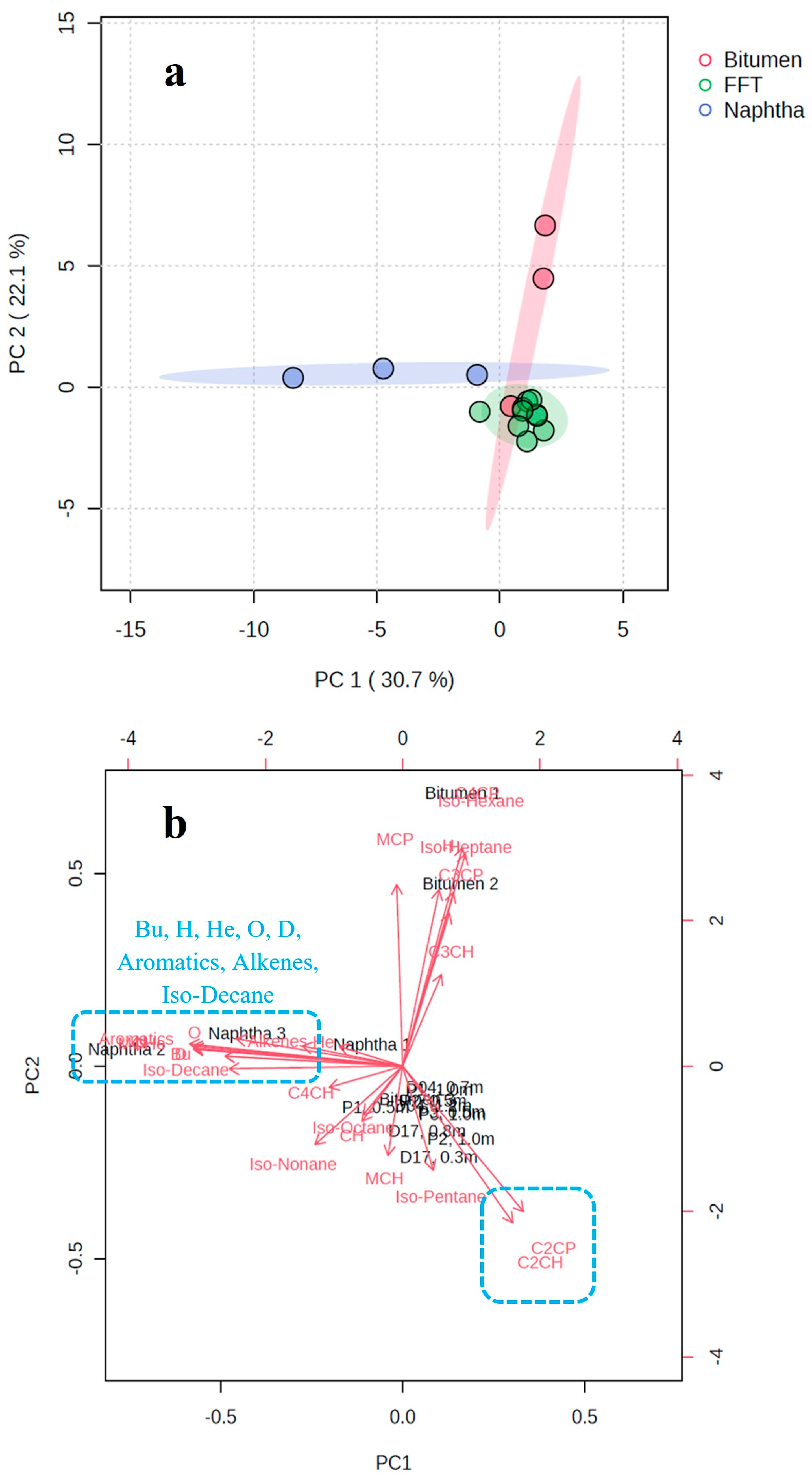
3.3. Exploring the Compositional Differences in LMWHCs Among Naphtha, Oil Sands Bitumen, and BML Fluid Tailings
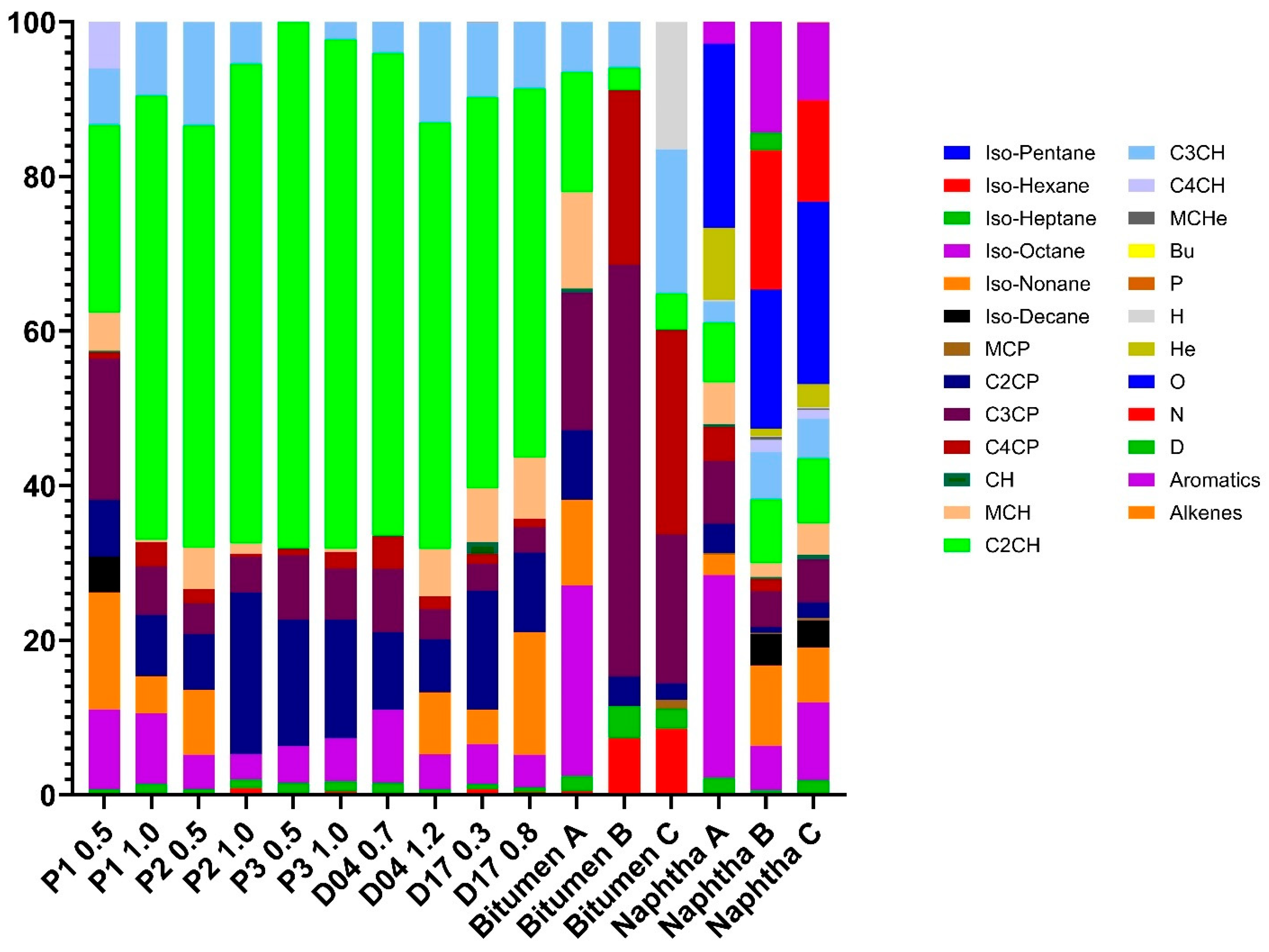
3.4. Relative Abundances Evaluation of LMWHCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Government of Alberta. Oil Sands Facts and Statistics. 2017. Available online: https://www.alberta.ca/oil-sands-facts-and-statistics (accessed on 23 January 2024).
- Speight, J.G. Oil Sand Production Processes; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Oil Sands Magazine. Naphtha Recovery Units. 2021. Available online: https://www.oilsandsmagazine.com/technical/mining/froth-treatment/naphthenic/nru-naphtha-recovery-unit (accessed on 23 January 2024).
- Du, J.; Cluett, W. Modelling of a naphtha recovery unit (NRU) with implications for process optimization. Processes 2018, 6, 74. [Google Scholar] [CrossRef]
- Syncrude Canada Ltd. 2022 Pit Lake Monitoring and Research Report (Base Mine Lake Demonstration Summary: 2012–2021); Syncrude Canada Ltd.: Calgary, AB, Canada, 2022. [Google Scholar]
- Dompierre, K.A.; Lindsay, M.B.J.; Cruz-Hernández, P.; Halferdahl, G.M. Initial geochemical characteristics of fluid fine tailings in an oil sands end pit lake. Sci. Total Environ. 2016, 556, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Tedford, E.; Halferdahl, G.; Pieters, R.; Lawrence, G.A. Temporal variations in turbidity in an oil sands pit lake. Environ. Fluid Mech. 2018, 19, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a unique Biological Group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef] [PubMed]
- Kavcar, P.C.; Wright, S.J. Effects of gas ebullition on cohesive sediment resuspension and cap stability. World Environ. Water Resour. Congr. 2009, 2009, 1–10. [Google Scholar] [CrossRef]
- Slater, G.F.; Goad, C.A.; Lindsay, M.B.; Mumford, K.G.; Colenbrander Nelson, T.E.; Brady, A.L.; Jessen, G.L.; Warren, L.A. Isotopic and chemical assessment of the dynamics of methane sources and microbial cycling during early development of an oil sands pit lake. Microorganisms 2021, 9, 2509. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.J.; Barbour, S.L.; Lindsay, M.B.J. Ebullition enhances chemical mass transport across the tailings-water interface of Oil Sands Pit Lakes. J. Contam. Hydrol. 2022, 245, 103938. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, D.; Nelson, T.C.; Risacher, F.F.; Morris, P.K.; Goad, C.; Slater, G.F.; Warren, L.A. The co-importance of physical mixing and biogeochemical consumption in controlling water cap oxygen levels in base mine lake. Appl. Geochem. 2019, 111, 104442. [Google Scholar] [CrossRef]
- Risacher, F.F.; Morris, P.K.; Arriaga, D.; Goad, C.; Nelson, T.C.; Slater, G.F.; Warren, L.A. The interplay of methane and ammonia as key oxygen consuming constituents in early stage development of base mine lake, the first demonstration oil sands pit lake. Appl. Geochem. 2018, 93, 49–59. [Google Scholar] [CrossRef]
- Clark, M.G.; Drewitt, G.B.; Carey, S.K. Energy and carbon fluxes from an oil sands pit lake. Sci. Total Environ. 2021, 752, 141966. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Fedorak, P.M.; Foght, J.M. Biodegradation of short-chain n-alkanes in oil sands tailings under methanogenic conditions. Environ. Sci. Technol. 2006, 40, 5459–5464. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Fedorak, P.M.; MacKinnon, M.D.; Foght, J.M. Metabolism of BTEX and naphtha compounds to methane in oil sands tailings. Environ. Sci. Technol. 2007, 41, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Duffin, L.; Wang, X.; Stoesz, J. Detection of volatile organic compounds in froth multiphase systems from oil sands operations using a headspace GC–MS method. Energy Fuels 2017, 31, 11925–11931. [Google Scholar] [CrossRef]
- Siddique, T.; Semple, K.; Li, C.; Foght, J.M. Methanogenic biodegradation of iso-alkanes and cycloalkanes during long-term incubation with oil sands tailings. Environ. Pollut. 2020, 258, 113768. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Boreham, C.J.; Minifie, S.A.; Teerman, S.C. The effect of minor to moderate biodegradation on C5 to C9 hydrocarbons in crude oils. Org. Geochem. 2002, 33, 1293–1317. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Yang, Z.; Hollebone, B.; Brown, C.E.; Landriault, M.; Fieldhouse, B. Chemical Fingerprints of Alberta oil sands and related petroleum products. Environ. Forensics 2011, 12, 173–188. [Google Scholar] [CrossRef]
- Foght, J.M.; Gieg, L.M.; Siddique, T. The microbiology of oil sands tailings: Past, present, future. FEMS Microbiol. Ecol. 2017, 93, fix034. [Google Scholar] [CrossRef]
- Speight, J.G. The Chemistry and Technology of Petroleum, 4th ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Strausz, O.P.; Morales-Izquierdo, A.; Kazmi, N.; Montgomery, D.S.; Payzant, J.D.; Safarik, I.; Murgich, J. Chemical Composition of athabasca bitumen: The saturate fraction. Energy Fuels 2010, 24, 5053–5072. [Google Scholar] [CrossRef]
- El-Waraky, M. Assessment of the Sources and Biodegradation Potential of Hydrocarbons and Naphthenic Acids in an Oil Sand End Pit Lake. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2021. [Google Scholar]
- Strausz, O.; Lown, E.M. The Chemistry of Alberta Oil Sands, Bitumens and Heavy Oils; Alberta Energy Research Institute: Calgary, AB, Canada, 2003. [Google Scholar]
- Siddique, T.; Mohamad Shahimin, M.F.; Zamir, S.; Semple, K.; Li, C.; Foght, J.M. Long-term incubation reveals methanogenic biodegradation of C5 and C6 iso-alkanes in oil sands tailings. Environ. Sci. Technol. 2015, 49, 14732–14739. [Google Scholar] [CrossRef] [PubMed]
| Abbreviation | GC Retention Time (Minutes) | Compound Name | Occurrence | ||
|---|---|---|---|---|---|
| Fluid Tailings | Bitumen Ore | Naphtha | |||
| Bu | 1.241 * | Butane | O | ||
| 2MBu⋆ | 1.676 | 2-Methylbutane | O | O | O |
| P⋆ | 1.751 | Pentane | O | ||
| 22DMBu | 1.906 | 2,2-Dimethylbutane | O | O | O |
| CPTe | 1.991 | Cyclopentene | O | ||
| 23DMBu | 2.049 | 2,3-Dimethylbutane | O | O | O |
| 2MP | 2.064 | 2-Methylpentane | O | ||
| 3MP | 2.209 | 3-Methylpentane | O | O | O |
| 1HXe | 2.243 | 1-Hexene | O | ||
| H⋆ | 2.35 | Hexane | O | O | |
| 3HXe | 2.384 | 3-Hexene | O | ||
| 3MCPTe | 2.454 | 3-Methylcyclopentene | O | ||
| 4MCPTe | 2.488 | 4-Methylcyclopentene | O | ||
| 22DMP | 2.604 | 2,2-Dimethylpentane | O | O | O |
| 24DMP | 2.67 | 2,4-Dimethylpentane | O | O | |
| MCP⋆ | 2.678 | Methylcyclopentane | O | O | |
| 223TMBu | 2.784 | 2,2,3-Trimethylbutane | O | O | O |
| 1MCPTe | 2.968 | 1-Methylcyclopentene | O | ||
| 33DMP | 3.071 | 3,3-Dimethylpentane | O | O | O |
| B | 3.196 | Benzene | O | ||
| CH | Cyclohexane | O | O | O | |
| 2MH | 3.223 | 2-Methylhexane | O | ||
| 23DMP | 3.31 | 2,3-Dimethylpentane | O | O | O |
| 11DMCP | 3.42 | 1,1-Dimethylcyclopentane | O | O | |
| 3MH | 3-Methylhexane | O | |||
| c13DMCP | 3.624 | cis-1,3-Dimethylcyclopentane | O | O | O |
| 3EP | 3.655 | 3-Ethylpentane | O | O | |
| t13DMCP | 3.685 | trans-1,3-Dimethylcyclopentane | O | O | O |
| t12DMCP | 3.751 | trans-1,2-Dimethylcyclopentane | O | O | O |
| He | 3.957 | Heptane | O | ||
| c12DMCP | 4.64 | cis-1,2-Dimethylcyclopentane | O | O | O |
| MCH⋆ | 4.687 | Methylcyclohexane | O | O | O |
| 25DMH⋆ | 4.901 | 2,5-Dimethylhexane | O | O | O |
| 24DMH | 4.96 | 2,4-Dimethylhexane | O | O | O |
| ECP⋆ | 5.023 | Ethylcyclopentane | O | O | O |
| C3CP (a) | 5.284 | C3-cyclopentane (a) | O | O | O |
| C3CP (b) | 5.583 | C3-Cyclopentane (b) | O | O | O |
| 233TMP | 5.853 | 2,3,3-Trimethylpentane | O | O | O |
| 3E2MP | 6.092 | 3-Ethyl-2-methylpentane | O | O | O |
| 23DMH | 4.131 * | 2,3-Dimethylhexane | O | ||
| C3CP (c) | 6.27 | C3-Cyclopentane (c) | O | O | |
| T⋆ | 6.299 | Toluene | O | ||
| 2MHe | 6.385 | 2-Methylheptane | O | O | O |
| 34DMH | 6.514 | 3,4-Dimethylhexane | O | O | |
| 4MHe | 4.335 * | 4-Methylheptane | O | ||
| 3MHe | 6.734 | 3-Methylheptane | O | ||
| 3EH | 6.795 | 3-Ethylhexane | O | O | |
| C3CP (d) | C3-Cyclopentane (d) | O | O | ||
| c13DMCH | 7.046 | cis-1,3-Dimethylcyclohexane | O | O | O |
| t14DMCH | 7.199 | trans-1,4-Dimethylcyclohexane | O | O | O |
| 11DMCH | 7.48 | 1,1-Dimethylcyclohexane | O | O | O |
| C3CP (e) | 7.596 | C3-Cyclopentane (e) | O | O | O |
| C3CP (f) | 7.767 | C3-Cyclopentane (f) | O | O | O |
| C3CP (g) | 7.962 | C3-Cyclopentane (g) | O | O | |
| t12DMCH | 8.21 | trans-1,2-Dimethylcyclohexane | O | O | O |
| C4CP (a) | 8.392 | C4-Cyclopentane (a) | O | O | |
| O⋆ | 8.42 | Octane | O | ||
| t13DMCH | 8.679 | trans-1,3-Dimethylcyclohexane | O | O | O |
| C4CP (b) | 9.034 | C4-Cyclopentane (b) | O | O | O |
| C3CP (h) | 9.937 | C3-Cyclopentane (h) | O | O | |
| 24DMHe | 10.064 | 2,4-Dimethylheptane | O | O | |
| c12DMCH | 10.535 | cis-1,2-Dimethylcyclohexane | O | O | |
| 26DMHe | 10.779 | 2,6-Dimethylheptane | O | O | |
| C4CP (c) | 10.782 | C4-Cyclopentane (c) | O | ||
| C3CP (i) | 7.117 * | C3-Cyclopentane (i) | O | ||
| ECH | 10.932 | Ethylcyclohexane | O | O | O |
| C3CH (a) | 11.335 | C3-Cyclohexane (a) | O | O | O |
| C3CH (b) | 11.653 | C3-Cyclohexane (b) | O | O | O |
| C4CP (d) | 7.738 * | C4-Cyclopentane (d) | O | ||
| C4CP (e) | 7.882 * | C4-Cyclopentane (e) | O | ||
| C4CP (f) | 8.219 * | C4-Cyclopentane (f) | O | ||
| C3CH (c) | 12.97 | C3-Cyclohexane (c) | O | O | |
| C3CH (d) | 13.321 | C3-Cyclohexane (d) | O | O | |
| 23DMHe | 13.548 | 2,3-Dimethylheptane | O | ||
| MCHe | 8.697 * | Methylcycloheptane | O | ||
| EB | 13.593 | Ethylbenzene | O | ||
| pX | 14.753 | p-Xylene | O | ||
| mX | m-Xylene | O | |||
| 3MO | 15.656 | 3-Methyloctane | O | O | |
| C3CH (e) | 16.385 | C3-Cyclohexane (e) | O | ||
| C3CH (f) | 10.773 * | C3-Cyclohexane (f) | O | ||
| C4CH (a) | 16.89 | C4-Cyclohexane (a) | O | ||
| C4CP (g) | 11.211 * | C4-Cyclopentane (g) | O | ||
| C4CP (h) | 11.383 * | C4-Cyclopentane (h) | O | ||
| oX | 17.229 | o-Xylene | O | ||
| C4CH (b) | 17.069 | C4-Cyclohexane (b) | O | ||
| N | 18.733 | Nonane | O | ||
| C3B (a) | 15.345 * | C3-Benzene (a) | O | ||
| C3CH (g) | 16.228 * | C3-Cyclohexane (g) | O | ||
| 3MN | 17.285 * | 3-Methylnonane | O | ||
| C3B (b) | 19.139 * | C3-Benzene (b) | O | ||
| C3B (c) | 19.804 * | C3-Benzene (c) | O | ||
| C4CH (c) | 18.756 | C4-Cyclohexane (c) | O | O | O |
| C3B (d) | 20.37 * | C3-Benzene (d) | O | ||
| C3B (e) | 21.552 * | C3-Benzene (e) | O | ||
| 26DMO | 21.391 | 2,6-Dimethyloctane | O | ||
| D | 22.556 * | Decane | O | ||
| Compounds | Percent Concentration (by Volume) in Fluid Tailings | Percent Concentration (by Volume) in Pore Water |
|---|---|---|
| 2-Methylbutane | 99.8 | 0.2 |
| Pentane | 99.8 | 0.2 |
| Benzene | 95.1 | 4.9 |
| Methylcyclohexane | >99.9 | <0.1 |
| 3-Methylhexane | >99.9 | <0.1 |
| cis-1,3-Dimethylcyclopentane | >99.9 | <0.1 |
| Heptane | >99.9 | <0.1 |
| Toluene | 98.8 | 1.2 |
| cis-1,2-Dimethylcyclohexane | >99.9 | <0.1 |
| Compound Name | Henry’s Law Constant (Pa·m3/mol) (at 25 °C) | Hexadecane-Water Partitioning Coefficient (log KHexW) (at 25 °C) |
|---|---|---|
| Heptane | 2.1 × 105 | 4.94 |
| 3,3-Dimethylpentane | 1.9 × 105 | 4.94 |
| Octane | 3.2 × 105 | 5.57 |
| 2,5-Dimethylhexane | 3.4 × 105 | 5.57 |
| Toluene | 6.7 × 102 | 2.77 |
| 2-Methylheptane | 3.7 × 105 | 5.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, H.; Wang, C.; Steven, B.S.H.; Slater, G.F. Initial Characterization of Low Molecular Weight Hydrocarbons in an Oil Sands Pit Lake. Earth 2025, 6, 44. https://doi.org/10.3390/earth6020044
Bao H, Wang C, Steven BSH, Slater GF. Initial Characterization of Low Molecular Weight Hydrocarbons in an Oil Sands Pit Lake. Earth. 2025; 6(2):44. https://doi.org/10.3390/earth6020044
Chicago/Turabian StyleBao, Han, Chenlu Wang, Bridget S. H. Steven, and Greg F. Slater. 2025. "Initial Characterization of Low Molecular Weight Hydrocarbons in an Oil Sands Pit Lake" Earth 6, no. 2: 44. https://doi.org/10.3390/earth6020044
APA StyleBao, H., Wang, C., Steven, B. S. H., & Slater, G. F. (2025). Initial Characterization of Low Molecular Weight Hydrocarbons in an Oil Sands Pit Lake. Earth, 6(2), 44. https://doi.org/10.3390/earth6020044