Abstract
The application of animal manure and organic soil amendments as an alternative to expensive inorganic fertilizers is becoming more prevalent in the USA and worldwide. A field experiment was conducted on Bluegrass–Maury silty loam soil at the Kentucky State University Research Farm using the Kennebec variety of white potato (Solanum tuberosum) under Kentucky climatic conditions. The study involved 12 soil treatments in a randomized complete block design. The treatments included four types of animal manures (cow manure, chicken manure, vermicompost, and sewage sludge), biochar at three application rates (5%, 10%, and 20%), and native soil as control plots. Additionally, animal manures were supplemented with 10% biochar to assess the influence of combining biochar with animal manure on the accumulation of heavy metals in potato tubers. The study aimed to (1) determine the concentration of seven heavy metals (Cd, Cr, Ni, Pb, Mn, Zn, Cu) and two essential nutrients (K and Mg) in soils treated with biochar and animal manure, and (2) assess metal mobility from soil to potato tubers at harvest by determining the bioaccumulation factor (BAF). The results revealed that Cd, Pb, Ni, Cr, and Mn concentrations in potato tubers exceeded the FAO/WHO allowable limits. Whereas the BAF values varied among the soil treatments, with Cd, Cu, and Zn having high BAF values (>1), and Pb, Ni, Cr, and Mn having low BAF values (<1). This observation demonstrates that potato tubers can remediate Cd, Cu, and Zn when grown under the soil amended with biochar and animal manure.
1. Introduction
Potatoes (Solanum tuberosum) are a staple food crop globally, ranking fourth in consumption and contributing significantly to food security due to their adaptability, high yield, and potential for year-round cultivation [1]. In 2022, global potato production reached 375 million tons, with the USA contributing 17.8 million tons [2]. However, increasing soil contamination with heavy metals poses a threat to sustainable potato production and food safety.
Heavy metals have a profound influence on plant physiology and soil microbial activity. Essential micronutrients, such as Zn, Cu, Ni, Mn, K, and Mg, are required in trace amounts for plant growth and metabolic processes. Zn aids in photosynthesis and reproduction [3], Ni supports enzymatic activity and nitrogen metabolism [4,5], K mitigates stress [6], Mg contributes to chlorophyll production and metal stress tolerance [7], and Cu is absorbed in small amounts (5–20 µg g−1 dry matter) [8]. However, excessive accumulation can be toxic; for example, Zn levels above 300 µg g−1 in plants are harmful, and the tolerable intake limit for humans is 45 mg/day [9,10]. In contrast, non-essential metals like Pb and Cd have no biological function and persist in the environment. Pb interferes with nutrient uptake and is linked to severe health issues, including liver and brain damage [11,12,13]. At the same time, Cd is highly mobile in soil, harming plant growth and microbial communities [14,15,16,17,18].
Heavy metals, such as Cd, Cu, and Zn, can accumulate in agricultural soils through industrial discharge, irrigation with polluted water, and the excessive use of organic amendments, including animal manure [19,20]. While animal manure provides essential nutrients (N, P, K) and improves soil fertility [21,22,23], it often contains hazardous heavy metals, which may leach into groundwater or accumulate in crop tissues, including potato tubers [24,25,26]. The consumption of such contaminated crops poses severe risks to human health, as recognized by WHO and FAO guidelines [27,28]. Despite the agronomic benefits of animal manure, the unregulated use remains a global environmental concern due to its role in elevating toxic metal levels in soils. Therefore, safe and sustainable strategies are urgently needed to manage heavy metal accumulation in food crops, particularly in root vegetables such as potatoes, which are prone to absorbing metals from contaminated soil.
Among various remediation approaches [29,30], phytoremediation stands out as an environmentally friendly approach. The use of plants to extract or stabilize pollutants, combined with the application of biochar, has shown promise. Biochar, a carbon-rich product derived from pyrolysis, can immobilize heavy metals, enhance soil quality, and reduce metal uptake by plants [31,32,33]. However, the dual role of edible crops, such as potatoes, in phytoremediation and human consumption necessitates a careful evaluation of their safety and effectiveness.
While previous studies have explored the benefits of biochar and manure amendments individually, limited research has evaluated their combined effects on the accumulation of heavy metals in potatoes. Furthermore, the extent to which potatoes can safely function as both a food crop and a phytoremediator under these conditions remains underexplored. This study aims to fill this gap by assessing the accumulation of essential and non-essential metals in potato tubers grown in soils amended with animal manure and biochar. The objectives of this study were to (1) assess the impact of animal manure amended with biochar as an organic fertilizer on the accumulation of heavy metals in potato tubers; (2) investigate the potential of using three rates of biochar and biochar mixed with animal manure on the accumulation of essential and toxic metals in potato tubers at harvest, in relation to the FAO and WHO allowable limits; and (3) calculate the bioaccumulation factor (BAF) for the potential use of potatoes in phytoremediation technology.
2. Materials and Methods
2.1. Field Experimental Design
A field experiment consisting of 36 plots (three replicates of 12 treatments) was carried out during the summer of 2023 at the Harold and Benson Research and Demonstration Farm, Kentucky State University, in a randomized complete block design. Each plot measured 3.7 m in width and 22 m in length. The study included six soil management practices: cow manure obtained from Lowe’s (Frankfort, KY, USA); chicken manure sourced from Espoma Company (Millville, NJ, USA); vermicompost supplied by Wiggle Worm Soil Builder (UNCO Industries, INC, Union Grove, WI, USA); sewage sludge acquired from the Metropolitan Sewer District (Louisville, KY, USA); biochar applied at three rates (5%, 10%, and 20%) and acquired from Wakefield Agricultural Carbon, LLC (Valdosta, GA, USA); and a no-amendment control plots. Additionally, cow manure, vermicompost, chicken manure, and sewage sludge were each combined with 10% biochar, resulting in 12 treatments. Each treatment was replicated three times. The control treatment consisted of the native soil, classified as Bluegrass–Maury Silty Loam, with a composition of 56% silt, 38% clay, and 6% sand. The application rates for the soil amendments (excluding the unamended control) and their respective properties are detailed in Table 1A,B.

Table 1.
(A) Application rate of soil amendments used for growing white potato at the Kentucky State University research farm. (B) Characteristics of the soil amendments and native soil (control) used in the experiment.
2.2. Cultivation Practices
Soil amendments were incorporated into the native soil at 5% N to minimize variations in potato tubers’ heavy metals content caused by differences in N composition among soil amendments (Table 1A,B). Each amendment was mixed into the top 15 cm of soil. White potato (Solanum tuberosum, var. Kennebec) was obtained from the Frankfort Service in Frankfort, KY, USA. The tubers were cut into appropriately sized pieces and planted in the prepared field at a depth of 4 inches, with a spacing of 30 cm between rows and plants. A drip irrigation system provided a consistent water supply and weed control measures were implemented as necessary. To prevent weed emergence, the pre-emergence herbicide trifluralin 4EC (Drexel Company, Memphis, TN, USA) was applied at a rate of 0.27 L hectare−1 [34].
2.3. Heavy Metals and Micronutrients Analyses
During the harvest, 10 tubers of similar size were randomly selected from each plot. A total of 30 tubers per treatment across three replicates were taken. The tubers were thoroughly washed with tap water, then deionized water, and then oven-dried at 65 °C for 48 h [35]. The dried samples were manually ground using a ceramic mortar and pestle to pass through a 1 mm non-metal sieve. It was followed by re-drying in an oven until a constant weight was achieved. For sample digestion, 1 g of each dried sample was mixed with 10 mL of concentrated nitric acid. The mixture was left to stand overnight and then heated for 4 h at 125 °C on a hot plate. The resulting mixture was diluted to 50 mL with double-distilled water and filtered using No. 1 filter paper. The concentrations of Cd, Pb, Cu, Cr, Ni, Zn, Mn, K, and Mg were measured using an inductively coupled plasma optical emission spectrometer (ICP-OES) (Model 720-ES, Perkin Elmer, Waltham, MA, USA) in standard mode, following USA EPA Method 6020a [36]. The detected metal levels in potato tubers were compared against the permissible limits established by the Codex Alimentarius Commission of the Joint FAO/WHO Food Standards [37] and the acceptable limits by the USA FDA [11].
2.4. Statistical Analysis
All data collected from this investigation were subjected to statistical analysis using SAS software (SAS Institute Inc., Cary, NC, USA, Version 9.1.3) [38]. A one-way analysis of variance (ANOVA) was performed to determine the effect of different soil amendment treatments on heavy metal accumulation in potato tubers. The assumptions of ANOVA (normality and homogeneity of variance) were checked using Shapiro–Wilk and Levene’s tests, respectively.
Where significant treatment effects (p ≤ 0.05) were detected, Duncan’s multiple range test (DMRT) was used for post-hoc mean separation to identify statistically distinct groups. The results are presented as mean ± standard error (SE) for each treatment. All figures displaying bioaccumulation factors and metal concentrations include error bars and significance groupings based on DMRT to facilitate visual interpretation.
3. Results and Discussions
Table 2 illustrates the elemental composition of soil amended with animal manure and a combination of manure and biochar used for white potato cultivation. The concentration of Pb was significantly higher (p ≤ 0.05) in soil treated with SS mixed with 10% Biochar (21.2 µg g−1) compared to soil amended with Vermi (17.5 µg g−1), while Pb levels varied among the other treatments. In contrast, Cd concentrations did not differ significantly across the treatments. However, Cd concentrations in potatoes cultivated with the soil amendments significantly surpassed the FDA/WHO permissible limit of 0.3 µg g−1 (Table 3).

Table 2.
Elemental composition (µg g−1 dry soil) of native soil and soil amended with animal manure and three biochar application rates used for growing white potato, Solanum tuberosum, var. Kennebec.
Ni levels were higher in soil amended with 10% Biochar than those treated with Cow mixed with 10% Biochar, Vermi combined with 10% Biochar, and 5% Biochar. Cr levels varied, with the highest concentration found in 10% Biochar combined with CM (16.4 µg g−1), which was significantly higher (p ≤ 0.05) than that in the non-amended vermicompost (13.08 µg g−1) (Table 2). Notably, the Cr levels in potato tubers surpassed the maximum allowable limit of 0.1 µg g−1 (Table 3). Mn concentration in the soil was found to be too high. The Mn concentration range was the greatest in 10% Biochar amended with SS (1459.4 µg g−1). There was no significant difference in Mn concentration among the treatments. Antonious et al. [39] observed that higher heavy metal concentrations in soil do not necessarily indicate their availability to plants.

Table 3.
The permissible limit of metals in potato, Solanum tuberosum tubers.
Table 3.
The permissible limit of metals in potato, Solanum tuberosum tubers.
| Heavy Metals | Permissible Limit in Potatoes, Mg kg−1 | Permissible Limit in Soil, Mg kg−1 |
|---|---|---|
| Cu | 10 [40] in plants | 100 |
| Zn | 0.6 [40] in plants | 300 |
| Ni | 0.1 [11] | 50 [41] |
| Cr | 0.1 [11] | 0.2 [42] |
| Mn | 2 [11] | 500 [43] |
| Cd | 0.3 [11] | 3 [42] |
| Pb | 1 [11] | 100 [42] |
| K | - | - |
| Mg | - | - |
Cu concentration was higher in soil amended with CM (11.41 µg g−1), which was significantly greater than 5% Biochar and Vermi. Zn concentration was higher in soil amended with CM (23.34 µg g−1). However, there was no significant difference in Zn concentration among the treatments. The K concentration was found to be high in the soil. K concentration was significantly greater (p < 0.05) in soil amended with 10% Biochar, 20% Biochar, CM, and Cow (317.3, 302.9, 324.8, and 307.1 µg g−1, respectively) than in 5% Biochar, 10% Biochar amended with Cow, and 10% Biochar amended with Vermi (205.1, 220.6, and 200.7 µg g−1, respectively). The study also found that Mg concentration was high in the soil. Mg concentration was significantly greater (p ≤ 0.05) in 20% Biochar (864.5 µg g−1) than in 5% Biochar, 10% Biochar amended with Cow, 10% biochar amended with CM, soil amended with 10% Biochar, Vermi and SS (725.4, 708.9, 732.5, 730.9, and 706.8 µg g−1, respectively).
Table 2 highlights variations in Mg, Cu, and K concentrations among soil treatments, while Zn levels remained statistically similar across all treatments.
The maximum allowable limits of Mg and K in potato tubers are not explicitly defined by the FAO or health organizations. The maximum concentration of copper in potato tubers can vary, but a study found that Cu concentrations in potato samples ranged from 0.505 to 2.729 mg/kg. This range indicates typical levels rather than a regulatory maximum.
3.1. BAF Values for Non-Essential Heavy Metals
Figure 1A revealed that the concentration of Cd in potato tubers of plants grown in Vermi and SS amended with 10% Biochar was greater compared to CW and Vermi amended with 10% Biochar. The bioaccumulation factor (BAF) of potato tubers, obtained by dividing the concentration of Cd in tubers by Cd concentration in soil, was found to exceed 1 (BAF > 1) in all the treatments tested reaching a maximum of 3.9 and 3.8 µg g−1 tissue, in Vermi and NA, respectively (Figure 1B). The BAF value in tubers of plants grown in Vermi was significantly higher compared to those grown in Vermi amended with 10% Biochar. In addition, all the treatments investigated successfully accumulated Cd in the tubers, with the most significant accumulation in plants grown in Vermi-amended soil. This observation proved that potato tubers can remediate soil contaminated with Cd. Accordingly, potatoes grown in such amendments are not edible and should be considered hazardous waste due to their higher concentrations of Cd. However, these findings have significant implications for the reclamation of soil contaminated with Cd.
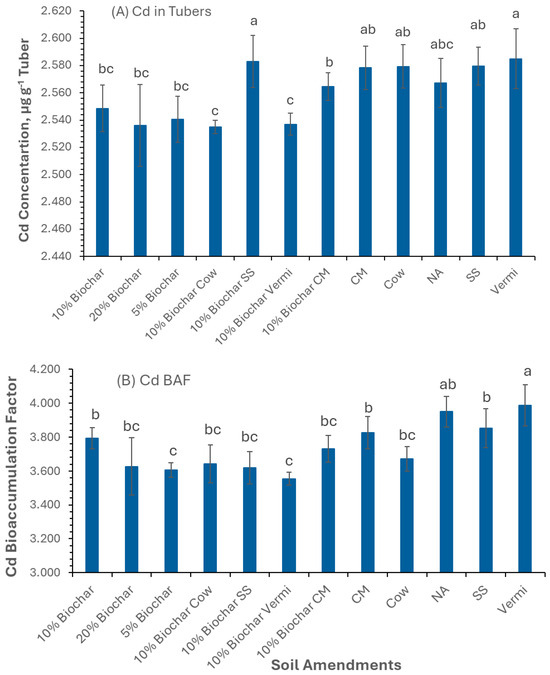
Figure 1.
Concentration of Cd in potato tubers grown under twelve soil management practices (A) and Cd bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
Figure 2A also revealed that the concentrations of Pb in potatoes grown under the studied soil amendments dramatically exceeded the FDA/WHO allowable limit of 1 µg g−1 for Pb (Table 3) in potato tubers at harvest. Significant concentrations of Pb were detected in the potato tubers grown in 10% Biochar, Cow, NA, and SS (5.7, 5.6, 5.5, and 5.6 µg g−1 tissue, respectively). On the contrary, mixtures of 20%, 5%, and 10% Biochar mixed with SS had the lowest concentrations of Pb. These results indicate that even NA is contaminated with Pb. Pb is the most common heavy metal and is resistant to corrosion. Food can be contaminated by naturally occurring Pb in the soil and by atmospheric fallout. However, the BAF of Pb in all tested tubers collected from the twelve soil treatments was below 1 (BAF < 1), reaching a maximum of 0.31 and 0.32 µg g−1 tissue in Vermi and SS, respectively (Figure 2B). The low BAF values of Pb in tubers grown in NA and soils amended with animal manure and Biochar treatments at three application rates indicate that Pb is not mobile from soil to potato tubers, a significant advantage for human safety.
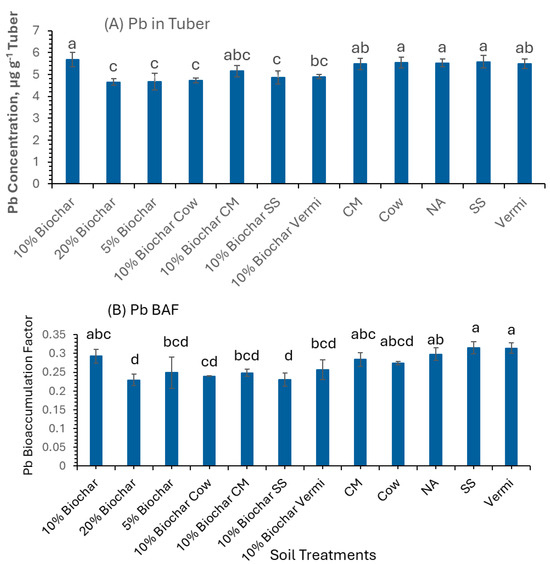
Figure 2.
The concentration of Pb in potato tubers grown under twelve soil management practices (A) and Pb bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
The Cr levels in potato tubers surpassed the maximum allowable limit of 0.1 µg g−1 (Table 3). As depicted in Figure 3A, potato tubers from plants cultivated in various treatments including 10% Biochar, a mix of 10% Biochar with Cow, CM, Cow alone, NA, SS, and Vermi showed significantly elevated (p ≤ 0.05) Cr concentrations (4.97, 4.8, 4.95, 4.96, 4.95, 4.96, and 4.95 µg g−1, respectively) compared to those grown with 5% Biochar (4.87 µg g−1) and 10% Biochar mixed with SS (4.90 µg g−1). Furthermore, the Cr bioaccumulation factor (BAF) in Vermi was significantly greater (p ≤ 0.05) than in the 10% Biochar combined with CM (Figure 3B). All BAF values for Cr were below 1, indicating that potato tubers do not accumulate chromium. Cr does not play a biological role in plant physiological processes [44], and elevated soil concentrations of Cr can hinder the uptake of essential nutrients like Fe, Mg, Ca, and P by forming insoluble complexes [45].
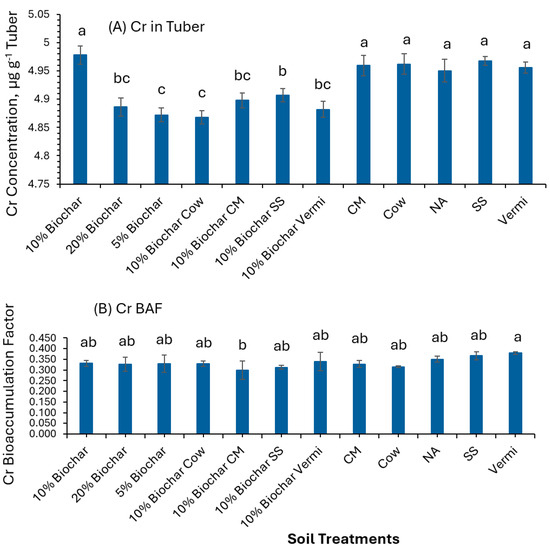
Figure 3.
Concentrations of Cr in potato tubers of plants grown under twelve soil management practices (A) and Cr bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
3.2. BAF Values for Essential Heavy Metals
Figure 4A shows that the Ni content in potato tubers surpassed the maximum permissible limit of 0.1 µg g−1. Tubers from plants grown with 10% biochar had a Ni concentration of 3.9 µg g−1, significantly higher (p ≤ 0.05) than those from plants grown with 20% Biochar, 5% Biochar, or 10% Biochar combined with Cow, CM, SS, or Vermi, which recorded concentrations of 3.42, 3.15, 3.07, 3.09, 3.38, and 3.11 µg g−1, respectively. No significant differences in Ni concentrations were noted among tubers from the other treatments (Figure 4A). Additionally, the Ni bioaccumulation factor (BAF) showed no significant variation across treatments (Figure 4B), with all values remaining below 1, suggesting that potato tubers do not act as accumulators of Ni.
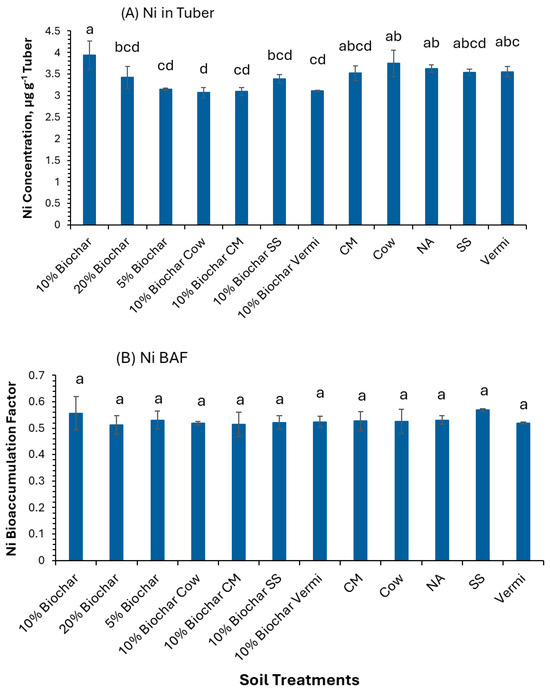
Figure 4.
Concentration of Ni in potato tubers grown under twelve soil management practices (A) and Ni bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
The results showed that Zn concentration in potato tubers in 10% Biochar was greater (22.8 µg g−1) compared to 20% Biochar, 5% Biochar, and 10% Biochar amended with SS (15.0, 14.0, and 13.9 µg g−1, respectively (Figure 5A). BAF values of Zn in tubers of plants grown in 10% Biochar, 10% Biochar mixed with Cow, NA, SS, and Vermi were above 1 (BAF > 1). These results indicate that potato tubers can be used to remediate soil contaminated with Zn. Whereas potato tubers grown in 20% Biochar and 10% Biochar mixed with SS had low BAF values (BAF < 1) (Figure 5B).
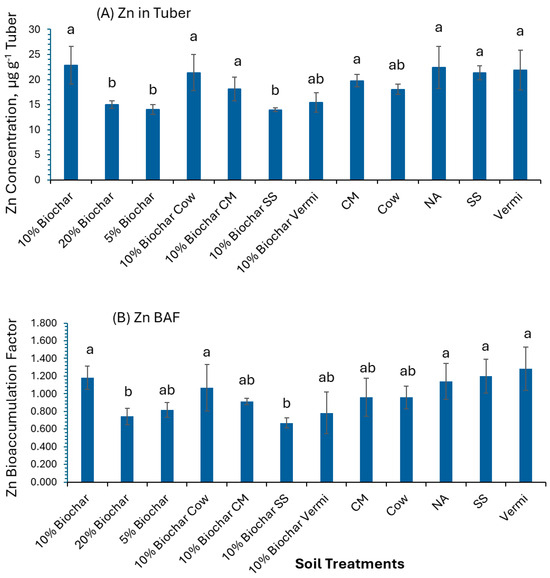
Figure 5.
Concentration of Zn in potato tubers grown under twelve soil management practices (A) and Zn bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different(p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
The permissible limit of Zn in edible plants is 0.6 mg g−1 as recommended by WHO [40]. Zn reduces heavy metal toxicity by restricting its availability as it plays a significant role in heavy metal homeostasis [46].
Figure 6A shows that potato plants grown in 10% Biochar (22.5 µg g−1) and NA (21.91 µg g−1) had significantly greater (p< 0.05) concentrations of Cu in their tubers compared to tubers of plants grown in 20% Biochar, 5% Biochar, 10% Biochar mixed with Cow, 10% Biochar mixed with SS, 10% Biochar mixed CM and 10% biochar mixed with Vermi (7.97, 7.75, 8.1, 7.5, 8.35, and 7.6 µg g−1 tissue, respectively). The BAF values of Cu in tubers grown in the NM control and 10% Biochar treatments show a BAF > 1 that is significantly greater than (p < 0.05) compared to tubers of plants grown in 20% Biochar, 5% Biochar, 10% Biochar mixed with Cow, 10% Biochar mixed with SS, 10% Biochar mixed CM, 10% Biochar mixed with Vermi and CM (Figure 6B). These high (>1) BAF values indicate that potato tubers of plants grown in the twelve treatments are Cu accumulators.
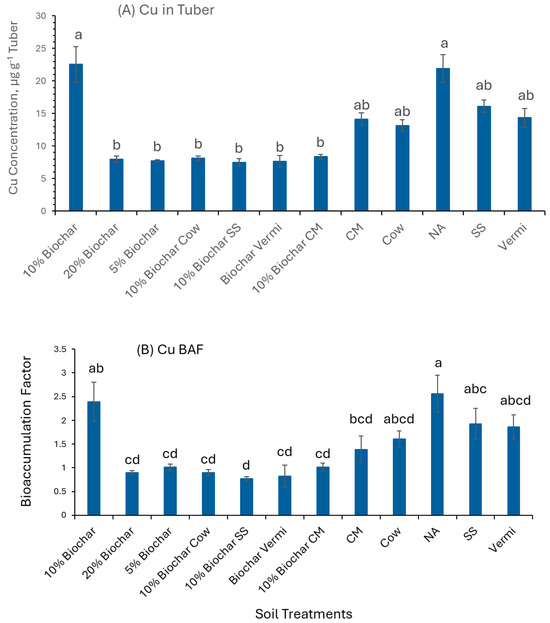
Figure 6.
Concentrations of Cu in potato tubers of plants grown under twelve soil management practices (A) and Cu bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple test for mean comparisons.
Figure 7A also shows that soil amended with SS (13.8 µg g−1) had a significantly higher (p ≤ 0.05) Mn concentration in potato tubers than 20% Biochar, 5% Biochar, 10% Biochar mixed with Cow, 10% Biochar mixed with Vermi, CM, Cow, and NA (11.62, 11.07, 9.95, 11.09, 10.96, 10.68, and 11.03 µg g−1 tissue, respectively). In addition, the BAF value of Mn in all the soil treatments investigated was below 1 (BAF < 1) (Figure 7B). Soil amended with SS had significantly greater (p ≤ 0.05) BAF than the other treatments. These Mn BAF values indicate that potato tubers are not Mn accumulators. Mn is both a heavy metal and an essential micronutrient. It is a part of photosynthetic enzymes and proteins in plants [47].
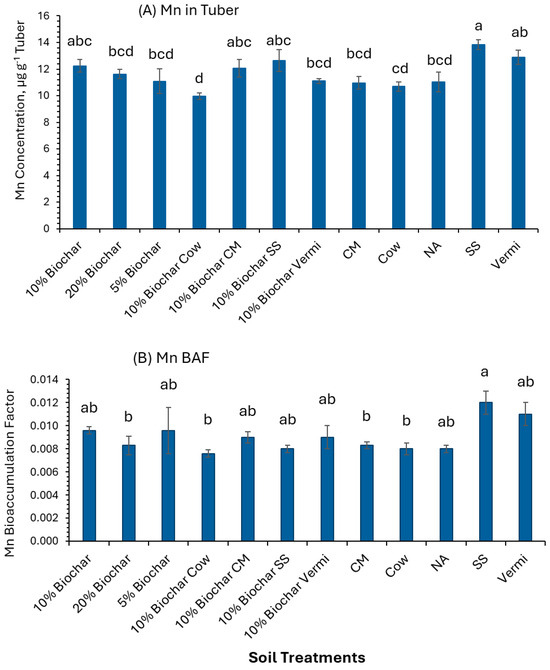
Figure 7.
Concentrations of Mn in potato tubers grown under twelve soil management practices (A) and Mn bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
3.3. BAF Values for Essential Nutrients
Figure 8A shows that K concentration in potato tubers was significantly greater (p< 0.05) in soil amended with CM (18,738 µg g−1) than 20% Biochar, 5% Biochar, 10% Biochar mixed with Cow, and 10% Biochar mixed with Vermi (14,517, 12,512, 15,989, and 12,840 µg g−1, respectively). Whereas, the BAF values of K were exceedingly higher than 1, there was no significant difference in BAF values among the treatments (Figure 8B). This suggests that plants absorb and accumulate K in higher concentrations, which may be due to its importance as a macronutrient.
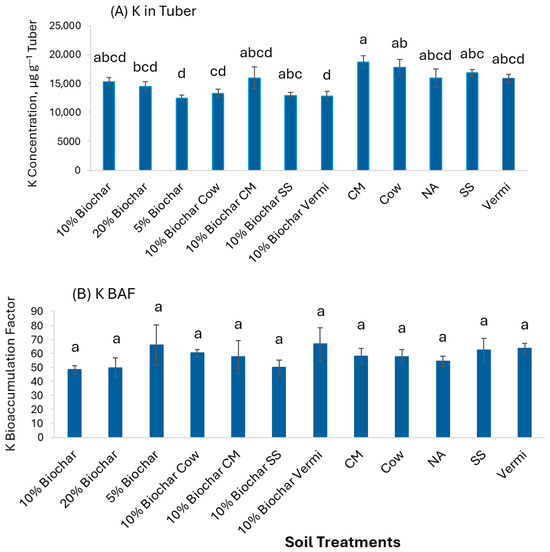
Figure 8.
Concentrations of K in potato tubers grown under twelve soil management practices (A) and K bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
Figure 9A shows that Mg concentration in potato tubers was significantly greater (p< 0.05) in CM and Cow (1020.21 and 999.18 µg g−1) than in the other treatments. Meanwhile, 10% Biochar mixed with SS and 5% Biochar had the lowest Mg content in potato tubers compared to all other treatments. BAF values of Mg were significantly greater (p ≤ 0.05) in 10% Biochar mixed with CM, SS, and CM (BAF > 1) compared to soil amended with 10% Biochar, 20% Biochar, 5% Biochar, 10% Biochar mixed with SS and NA (Figure 9B). This indicates that potato tubers may accumulate Mg at different levels depending on the type of soil amendment.
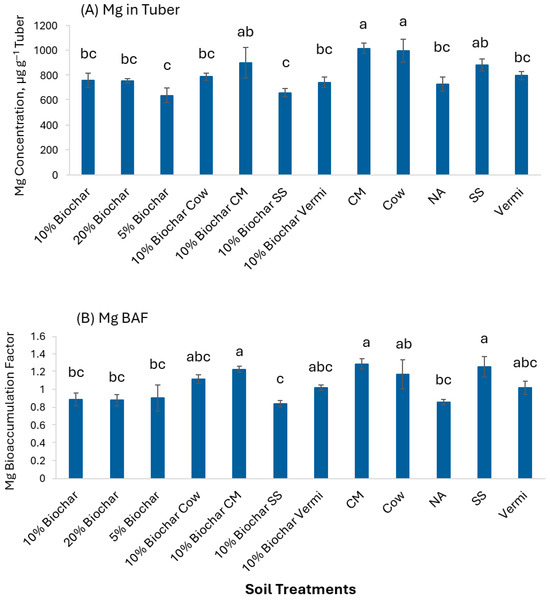
Figure 9.
Concentration of Mg in potato tubers grown under twelve soil management practices (A) and Mg bioaccumulation factors (B). Statistical analysis was carried out among the soil treatments using analysis of variance (ANOVA). Values accompanied by a different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple tests for mean comparisons.
Heavy metals BAF values below 1 imply that the potato tubers do not accumulate heavy metals at a higher concentration than in the soil, and vice versa for BAF values greater than 1 [48]. Our study found that BAF values for Cd, Cu, and Zn were greater than 1, indicating that potato tubers are good accumulators of these heavy metals. Conversely, BAF values for Pb, Ni, Cr, and Mn were less than 1. However, if edible crops are used for phytoremediation, one should check the permissible limit of specific heavy metals before consumption. If the accumulated concentration of heavy metals in the potato tuber is below the allowable limit, then the tubers can be deemed edible. In this study, Ni, Cr, Mn, Cd, and Pb concentrations were above the permissible limit. This shows that the potato tubers used in this research cannot be used for consumption purposes but can be used for phytoremediation.
There has been little research on the bioaccumulation of heavy metals using potato tubers grown under animal manure. Orellana-Mendoza et al. [49] conducted research on bioaccumulation of heavy metals by potato tubers using CM as a soil amendment and found that CM increases soil concentrations of Cu and Zn above the permissible limit and leads to higher accumulation of Zn, Cu, and Pb in potato tubers compared to other fertilizers. They further found BAF less than 1 for Cd, Cu, Pb, Cr, and Zn in potato tubers and deemed them safe for human consumption.
Heavy metal uptake by crops depends on how the roots of crops interact with different heavy metals in the soil [50]. The concentration of heavy metals is primarily shaped by root activities within the rhizosphere. These activities can significantly influence the solubility and availability of nutrients and heavy metals through root exudates and their interactions in the soil. Various processes, including adsorption, desorption, precipitation, dissolution, oxidation, and reduction, regulate the bioavailability and toxicity of metal ions in the soil solution. Root exudates can modify the leachability of elements [50], affecting their mobilization and immobilization. Furthermore, the solubility and bioaccumulation of heavy metals in soil are significantly influenced by the levels of organic matter present in the animal manures [41,51,52]. In our study, the interaction between potato tubers and the specific heavy metals and the variation in the organic matter present in the animal manures and biochar might have made differences in the accumulated heavy metal concentration in the potato tubers.
Again, monitoring heavy metal uptake in crops grown in recycled waste is essential for identifying appropriate animal manures for organic fertilizers and understanding how metals are absorbed and moved within plants. Animal manures that increase metal accumulation in edible plants should be avoided to reduce the risk of human exposure to heavy metals through the food chain. Higher BAF values are expected for essential plant nutrients such as Mg, K, Cu, and Zn. The ways in which different plant species and animal manures influence bioaccumulation can vary significantly. There is a clear relationship between the types of crops and the soil amendments employed in metal remediation. Additionally, careful consideration should be given to the final disposal of plants and other residues after achieving phytoremediation activities.
4. Conclusions
This study provides critical insights into the phytoremediation potential of potato tubers for Cd, Cu, and Zn in soils mixed with animal manures and biochar. High BAF values (BAF > 1) for Cd, Cu, and Zn across treatments confirm the capacity of potato tubers to uptake and accumulate these metals from the contaminated soils. However, accumulating Cd and Pb beyond the permissible limits in all treatments makes the tubers unfit for human consumption. This reinforces the need to distinguish between crops used for food and crops employed for phytoremediation.
Further, the low mobility (BAF < 1) of Pb, Ni, Cr, and Mn in most treatments suggests a limited risk of transfer of these metals to potato tubers. This highlights biochar-manure combinations as promising tools for reducing metal translocation in the food chain. These findings underscore the dual role of certain animal manures and amendments in both enhancing soil fertility and remediating metal uptake. However, there is a pressing need to identify and standardize the optimal manure-biochar mixing formulations that maximize the phytoremediation efficiency while minimizing food safety risks. Further studies should focus on the identification of such formulations. More research should also explore the molecular and physiological mechanisms driving uptake and transport in potatoes, especially regarding the application of animal manure. Studies should also refine using non-food crops or inedible crop varieties for phytoremediation to avoid safety conflicts.
Author Contributions
Writing, original ideas, field design, methodology, review, and editing: G.F.A.; field, laboratory work, and data collection report: A.N. and B.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was funded by the United States Department of Agriculture, National Institute of Food and Agriculture (USDA/NIFA), under a grant to Kentucky State University, Agreement #KYX-10-23-80P, Accession 7005611.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author at george.antonious@kysu.edu.
Acknowledgments
We appreciate E. Turley and the farm crew at Kentucky State University Research Farm for their support during the fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeliha, L.; Aksoy, A.; Akgul, G. Accumulation and effects of heavy metals on potatoes in Nevsehir, Turkey. Fresenius Environ. Bull. 2017, 26, 7083–7090. [Google Scholar]
- FAOSTAT Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023.
- Samman, S. Trace elements. In Essentials of Human Nutrition, 2nd ed.; Mann, I., Truswell, S., Eds.; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Shahzad, B.; Tanveera, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Nickel, S. Nickel whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium, and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Farid, M.; Farooq, M.A.; Fatima, A.; Abubakar, M.; Ali, S.; Raza, N.; Alhaithloul, H.A.; Soliman, M.H. Copper-Induced Responses in Different Plant Species. In Approaches to the Remediation of Inorganic Pollutants; Hasanuzzaman, M., Ed.; Springer: Singapore, 2021; pp. 259–280. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in Soils, Water and Food Crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex General Standard for Contaminants and Toxins in Food and Feed (CXS 193-1995); Adopted 1995; Revised 1997, 2006, 2008, 2009; Amended 2009, 2010. Available online: https://www.fao.org/fileadmin/user_upload/agns/pdf/CXS_193e.pdf (accessed on 21 March 2025).
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Effect of Lead on Oxidative Status, Antioxidative Response and Metal Accumulation in Coronopus didymus. Plant Physiol. Biochem. 2016, 105, 290–296. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Lead, Identification of Dangerous Levels of Lead, Final Rule. Fed. Regist. 2001, 66, 1206–1240. [Google Scholar]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Yang, Y.; Hassan, M.F.; Ali, W.; Zou, H.; Liu, Z.; Ma, Y. Effects of cadmium pollution on human health: A narrative review. Atmosphere 2025, 16, 225. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. Adv. Agron. 2013, 120, 275–379. [Google Scholar] [CrossRef]
- Karaca, A.; Cetin, S.C.; Turgay, Q.C.; Kizilkaya, R. Effects of heavy metals on soil enzyme activities. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Chen, L.; Guo, H.; Luo, S.; Xiao, X.; Xi, Q.; Wei, W.; He, Y. Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Bioresour. Technol. 2010, 101, 8599–8605. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Pal, A.; Chen, C.W.; Pandey, A.; Dong, C.D. Advances on Tailored Biochar for Bioremediation of Antibiotics, Pesticides, and Polycyclic Aromatic Hydrocarbon Pollutants from Aqueous and Solid Phases. Sci. Total Environ. 2022, 838, 153054. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Muhammad, Z. An overview of the common bacterial diseases of potato in Pakistan, associated crop losses and control stratagems. J. Plant Pathol. 2020, 102, 3–10. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef]
- Antonious, G.F.; Dawood, M.H.; Turley, E.T.; Paxton, R.B. Biochar and animal manures increased the yield of three varieties of turnips. Int. J. Appl. Agric. Sci. 2022, 8, 50–56. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Gyawali, R.B.; Freeman, A.C. Influence of biochar and animal manures application on ammonia and nitrate concentrations in the root and shoot of three varieties of turnips. Agriculture 2023, 13, 137. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Bhattacharyya, P.; Chakraborty, A. Effects of metal-contaminated organic wastes on microbial biomass and activities: A review. In Heavy Metal Contamination of Soil; Ahmed, I., Hayat, S., Pichtel, J., Eds.; Science Publishers, Inc.: Plymouth, UK, 2005; pp. 195–204. [Google Scholar]
- Qi, J.; Yang, H.; Wang, X.; Zhu, H.; Wang, Z.; Zhao, C.; Li, B.; Liu, Z. State-of-the-art on animal manure pollution control and resource utilization. J. Environ. Chem. Eng. 2023, 11, 110462. [Google Scholar] [CrossRef]
- Khan, A.; Malik, S.; Ali, N.; Bilal, M.; El-Shazly, M.; Iqbal, H.M. Biopolymer-based sorbents for emerging pollutants. In Sorbent Materials for Controlling Environmental Pollution; Elsevier: Amsterdam, The Netherlands, 2021; pp. 463–491. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing systems. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Zeng, L.; Zhongren, N.; Chuanyang, Z. Potato absorption and phytoavailability of Cd, Ni, Cu, Zn, and Pb in sierozem soils amended with municipal sludge compost. J. Arid. Land. 2018, 10, 638–652. [Google Scholar] [CrossRef]
- Yin, J.; Hong, Z.S.; Gao, Y.F. Yielding characteristics of natural soft Lianyungang clay. J. Southeast Univ. Nat. Sci. Ed. 2009, 39, 1059–1064. [Google Scholar]
- Antonious, G.F. Distribution of seven heavy metals among hot pepper plant parts. J. Environ. Sci. Health Part B 2016, 51, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, R.; Pant, D.; Malviya, P. Engineered algal biochar for contaminant remediation and electrochemical applications. Sci. Total Environ. 2021, 774, 145676. [Google Scholar] [CrossRef]
- Brown, R.C. The role of pyrolysis and gasification in a carbon-negative economy. Processes 2021, 9, 882. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Low, C.Y. Use of biochar as a carbon-sequestering additive in cement mortar. Cem. Concr. Compos. 2018, 87, 110–129. [Google Scholar] [CrossRef]
- Shin, J.; Jang, E.; Park, S.; Ravindran, B.; Chang, S.W. Agro-environmental impacts, carbon sequestration, and profit analysis of blended biochar pellet application in the paddy soil-water system. J. Environ. Manag. 2019, 244, 92–98. [Google Scholar] [CrossRef]
- University of Kentucky Cooperative Extension Service. Vegetable Production Guide for Commercial Growers; University of Kentucky College of Agriculture, Food and Environment: Lexington, KY, USA; Available online: https://publications.ca.uky.edu/files/ID36.pdf (accessed on 10 January 2025).
- Antonious, G.F.; Snyder, J.C. Accumulation of heavy metals in plants and potential phytoremediation of lead by potato, Solanum tuberosum L. J. Environ. Sci. Health A 2007, 42, 811–816. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Method 6020a: Inductively Coupled Plasma-Mass Spectrometry; USEPA: Washington, DC, USA, 1998.
- Codex Alimentarius Commission, Joint FAO/WHO. Food Standards; Codex Alimentarius Commission: The Hague, The Netherlands, 2006; Available online: https://www.fao.org/4/a1100e/a1100e00.pdf (accessed on 15 January 2025).
- SAS Institute Inc. SAS/STAT Guide; Version 9.1.3; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Antonious, G.F.; Silitonga, M.R.; Tsegaye, T.; Unrine, J.M.; Coolong, T.; Snyder, J.C. Elevated concentrations of trace-elements in soil do not necessarily reflect the metals available plants. J. Environ. Sci. Health Part B 2013, 48, 219–225. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Permissible Limits for Heavy Metals in Soil and Plants; WHO: Geneva, Switzerland, 1996; Available online: https://scirp.org/reference/referencespapers?referenceid=2696523 (accessed on 9 June 2025).
- Nepal, A.; Antonious, G.F.; Bebe, F.N.; Webster, T.C.; Gyawali, B.R.; Neupane, B. Heavy Metal Accumulation in Three Varieties of Mustard Grown under Five Soil Management Practices. Environments 2024, 11, 77. [Google Scholar] [CrossRef]
- Ediene, V.F.; Umoetok, S.B.A. Concentration of heavy metals in soils at the municipal dumpsite in Calabar metropolis. Asian J. Environ. Ecol. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Anjali, R.; Kumar, K. Manganese: Affecting our Environment (Water, Soil and Vegetables). Int. J. Innov. Res. Sci. Technol. 2018, 4, 1–7. [Google Scholar]
- Appenroth, K.J. Definition of “Heavy Metals” and Their Role in Biological Systems. In Soil Heavy Metals; Soil Biology, Vol. 19; Sherameti, I., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 9–14. [Google Scholar] [CrossRef]
- Reale, L.; Ferranti, F.; Mantilacci, S.; Corboli, M.; Aversa, S.; Landucci, F.; Baldisserotto, C.; Ferroni, L.; Pancaldi, S.; Venanzoni, R. Cyto-histological and morpho-physiological responses of common duckweed (Lemna minor L.) to chromium. Chemosphere 2016, 145, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Osu, C.I.; Onyema, M.O. Vanadium inhibition capacity on nutrients and heavy metal uptake by Cucumis sativus. J. Am. Sci. 2016, 12, 1–7. [Google Scholar]
- White, P.J.; Greenwood, D.J. Properties and management of cationic elements for crop growth. Soil Cond. Plant Growth 2013, 12, 160–194. [Google Scholar]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical speciation, plant uptake, and toxicity of heavy metals in agricultural soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef]
- Orellana-Mendoza, E.; Camel, V.; Yallico, L.; Quispe-Coquil, V.; Cosme, R. Effect of Fertilization on the Accumulation and Health Risk for Heavy Metals in Native Andean Potatoes in the Highlands of Perú. Toxicol. Rep. 2024, 12, 594–606. [Google Scholar] [CrossRef]
- Wenzel, W.W. Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 2009, 321, 385–408. [Google Scholar] [CrossRef]
- Riaz, U.; Aslam, A.; uz Zaman, Q.; Javeid, S.; Gul, R.; Iqbal, S.; Javid, S.; Murtaza, G.; Jamil, M. Cadmium contamination, bioavailability, uptake mechanism and remediation strategies in soil-plant-environment system: A critical review. Curr. Anal. Chem. 2021, 17, 49–60. [Google Scholar] [CrossRef]
- Nepal, A.; Antonious, G.F.; Gyawali, B.R.; Webster, T.C.; Bebe, F. Assessing the Bioaccumulation of Heavy Metals in Cabbage Grown under Five Soil Amendments. Pollutants 2024, 4, 58–71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).