Abstract
Surface-enhanced Raman spectroscopy (SERS) is a highly sensitive technique for detecting heavy metals through the plasmonic effect of metallic nanoparticles. In this study, TiO2 nanotubes were used as substrates due to their stability, large surface area, and ordered morphology. Silver nanostructures were electrodeposited to enhance the SERS response by generating hot spots. The influence of voltage and Ag concentration on electrodeposition was analyzed using methylene blue (1 × 10−5 M) as a probe molecule. Higher voltages and concentrations promoted dendritic growth, reaching Raman intensities above 70,000 a.u., optimizing sensitivity. All experiments were conducted in triplicate to ensure reproducibility.
1. Introduction
Currently, one of the most significant environmental and public health problems is the excessive presence of heavy metals in water bodies. These elements, although naturally occurring and with valuable industrial applications, such as lead in pipes or cadmium in alloys, can pose a significant risk to human health by accumulating in tissues and altering vital functions [1]. Elements such as lead, mercury, cadmium, and arsenic contribute to environmental pollution due to their high toxicity, persistence, and bioaccumulation capacity [2]. Several countries have reported the presence of heavy metals in foods of animal and plant origin, reflecting the magnitude of the problem. For example, contaminated fish have been detected in Mexico, Japan, and Chile; pork with lead in Australia; and salmon with cadmium in Norway [2].
Although the detection of heavy metals represents a critical environmental challenge, this study does not directly address their quantification. Instead, the study focuses on understanding how electrodeposition parameters affect the formation of Ag nanostructures on TiO2 nanotubes, aiming to optimize substrates that could later be applied in the SERS-based detection of such pollutants.
Given this situation, it is essential to have accurate analytical techniques that allow the presence of heavy metals in water sources near industrial areas to be quantified [3]. In this context, nanotechnology has become highly relevant because the most notable phenomena in matter occur at scales smaller than 100 nanometers. In this range, materials exhibit physical, chemical, and optical properties that are different from or improved upon those they exhibit in their macroscopic state. A historical example is the use of gold and silver nanoparticles in stained glass since the 10th century, where the color depended on the size of the particles [4]. This ability to manipulate matter at the nanometric level (1–100 nm) has made it possible to design materials with unique properties and advanced applications in fields such as catalysis, energy, and molecular detection [4].
This has led to the emergence of methods such as atomic absorption spectroscopy, as well as electrochemical and photometric techniques, which are widely used.
However, in recent years, surface-enhanced Raman spectroscopy (SERS) has gained interest due to its high sensitivity, selectivity, and ability to detect trace levels of analytes [3]. The performance of SERS substrates strongly depends on the material that supports the metallic nanostructures, as their morphology and chemical stability directly influence signal enhancement. In this context, semiconductor oxides such as titanium dioxide (TiO2) have attracted significant attention for their versatility and compatibility with metal deposition processes. TiO2 has established itself as one of the most studied semiconductors in nanoscience due to its chemical stability, amphoteric nature, and sensitivity to ultraviolet radiation. Its ability to crystallize in three phases—anatase, rutile, and brookite—gives it structural and functional versatility, with the anatase phase standing out for its high photocatalytic activity and large surface area [5]. These properties favor the adsorption and uniform distribution of metal ions on the surface, facilitating their subsequent reduction and nucleation. Consequently, TiO2 serves as an ideal substrate for the formation of metallic nanostructures, particularly silver, to enhance the SERS effect by generating hot spots that amplify the Raman signal.
However, the manufacturing of these substrates faces challenges associated with morphological control and the homogeneity of metal nanoparticles, factors strongly influenced by electrodeposition voltage and electrolyte concentration [6]. Therefore, this study focuses on evaluating the effect of voltage and Ag concentration on the formation of nanostructures on TiO2 nanotubes, with the aim of optimizing the synthesis conditions to obtain more sensitive and reproducible SERS substrates, with potential applicability in the detection of heavy metal indicators or other environmental contaminants.
2. Materials and Methods
Materials and Process
For the synthesis of TiO2 nanotubes, these were obtained by anodization, using grade 2 (99% purity) titanium sheets with a thickness of 0.1 mm and dimensions of 2 × 1 cm. The active area exposed to the electrolyte during anodization and electrodeposition was 1 cm2, and only this region was used for Raman measurements. The samples were cleaned using an ultrasonic bath in acetone, distilled water, and ethanol (ACS grade, 99.5%, Meyer, México city, México) for 10 min each. Electrochemical anodization was then performed using a cell consisting of two electrodes, where the titanium sheet acted as the anode and the graphite rod as the cathode, with a distance of 2 cm between them. An electrolyte composed of ethylene glycol (98% Meyer, México city, México), water (3% by weight), and ammonium fluoride (2% by weight Meyer, México city, México) was used for a 100 mL solution. In addition, ethanol (ACS grade, 99.5%, Meyer, México city, México) and distilled water were used to rinse both the titanium sheets and the graphite rod. Experiments were conducted at temperatures ranging from 21.5 to 22.5 °C and relative humidity levels between 41% and 45%. Anodizing was carried out by applying a voltage of 25 V for 15 min. The samples obtained were subjected to heat treatment at 450 °C for 2 h to obtain the anatase phase.
Electrodeposition was carried out on TiO2 nanotubes with an electrolyte consisting of silver nitrate (AgNO3,99% purity, Fagalab, México city, México) and water, with concentrations of 1, 3, 6, and 12 mM. Voltages of 2 V and 5 V were applied for 30 s, using a DC power supply (Yisung, Shenzhen, China) and a cell with the TiO2 plate as the cathode and the graphite rod as the anode. The relative humidity (%RH) and temperature were monitored during each temperature ranging from 22.3 to 24.9 °C and relative humidity levels between 41.5% and 49.9%.
Morphological analysis was performed using a field emission scanning electron microscope (FE-SEM), model JSM-7600F (JEOL, Akishima, Tokyo, Japan). To verify the functionality of the substrates for SERS applications, a Raman microscope (QEPRO High-Performance Spectrometer, Ocean Insight, Dunedin, FL, USA) was used. A total of 20 μL of methylene blue with a concentration of 1 × 10−5 M was used with a 785 nm excitation laser, recording the characteristic vibrational modes of 454 cm−1, 504 cm−1, 1400 cm−1, and 1625 cm−1, and a total of 43 spectra were collected for analysis.
3. Results and Discussion
3.1. Morphological Characterization
According to morphological analysis using field emission electron microscopy, after electrochemical anodization and heat treatment at 450 °C, followed by silver electrodeposition at different concentrations, the surfaces obtained have a rough, nanostructured morphology characteristic of the formation of TiO2 nanotubes, as shown in Figure 1. This ordered tubular structure, observed homogeneously across the surface, coincides with what has been reported in the literature for nanotubes obtained by anodization in electrolytes containing fluoride ions, which facilitate localized dissolution of the oxide and subsequent self-organization of the nanotubes [7].
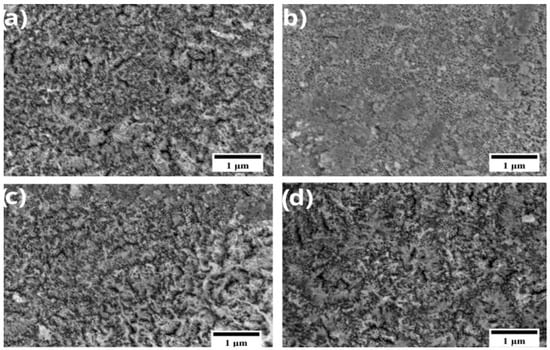
Figure 1.
SEM micrograph of samples anodized at 25 V for 15 min and electrodeposited with AgNO3 concentrations of (a) 1 mM, (b) 3 mM, (c) 6 mM, and (d) 12 mM, magnified 20,000×.
In Figure 1a, corresponding to a concentration of 1 mM, a low density of nanoparticles can be seen, suggesting limited deposition due to the lower availability of silver ions in the solution. On the other hand, in Figure 1b, at a concentration of 3 mM, an increase in the number of particles is observed, indicating a more active nucleation process. In Figure 1c, at 6 mM, the Ag nanoparticles exhibit a uniform and homogeneous distribution across the TiO2 nanotube surface, suggesting that this concentration favors more controlled and efficient nucleation. Finally, at 12 mM, a clear increase in agglomeration and particle clustering is observed. This behavior is consistent with ion oversaturation in the electrolyte, which promotes the uncontrolled growth and coalescence of silver deposits rather than the formation of well-defined nanostructures. Taken together, these results indicate that increasing the concentration of AgNO3 promotes greater coverage of the nanoparticles on the TiO2 nanotubes, improving the coating, although with some agglomeration at high concentrations.
A quantitative analysis of Ag nanostructures was, at 1 mM AgNO3, the nanoparticles exhibited an average diameter of 40.73 ± 10.91 nm. For 3 mM, the diameter increased to 82.67 ± 19.44 nm, while at 6 mM a value of 61.33 ± 16.89 nm was obtained. Finally, at 12 mM the nanoparticles presented an average diameter of 62.53 ± 11.37 nm. These quantitative measurements confirm that Ag concentration influences nanoparticle growth, with a marked increase between 1 and 3 mM and a tendency toward saturation at higher concentrations.
In one of the samples electrodeposited with a concentration of 6 mM AgNO3 at a voltage of 2 V for 30 s, dendrites can be seen (Figure 2), suggesting irregular metal growth, possibly caused by a non-homogeneous distribution of current on the electrode surface [8]. According to the model proposed by Wranglen, this type of morphology appears when the local current density exceeds a critical threshold, which can be attributed to irregularities on the electrode surface or to ion transport limited by diffusion [8].
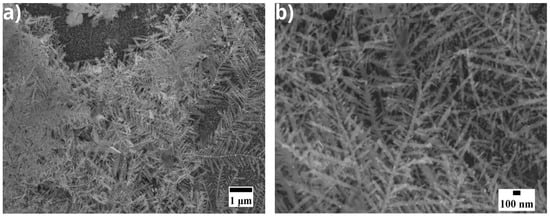
Figure 2.
SEM micrographs at (a) ×10,000 and (b) ×30,000, electroplated at 2 V for 30 s with 6 mM AgNO3.
3.2. Surface-Enhanced Raman Spectroscopy (SERS)
According to Figure 3, the characteristic vibrational modes of the methylene blue molecule can be observed in the bands at 454 cm−1, 504 cm−1, 1400 cm−1, and 1625 cm−1, corresponding to the molecular bonds C N-C, C-NC, C-H, and C-C, respectively [9]. Likewise, the spectra of the samples treated at a voltage of 2 V and 5 V, respectively, are shown. The use of TiO2 nanotubes is essential because their high surface area, ordered morphology, and semiconducting nature facilitate uniform Ag nucleation and create well-distributed hot spots. In contrast, flat Ti surfaces provide significantly fewer anchoring sites for metal growth, leading to lower SERS activity. Therefore, the presence of TiO2 nanotubes directly enhances the density and localization of plasmonic regions compared to Ag deposition on flat Ti. Concentrations of 1 mM (Figure 3a) and 3 mM AgNO3 (Figure 3b) recorded intensities of 15,366 a.u. and 31,948 a.u., respectively, while substrates deposited at 2 V reached only 2204 a.u. and 2367 a.u. Similarly, samples prepared with concentrations of 6 mM (Figure 4a) and 12 mM (Figure 4b) showed intensities of 2542 a.u. and 5546 a.u. at 2 V, compared to 37,776 a.u. and 77,890 a.u. obtained at 5 V (Figure 4). These differences confirm that an increase in voltage favors the formation of more efficient nanostructures for SERS amplification, where it was observed that for both voltages, the increase in AgNO3 concentration led to a notable increase in the intensity of the Raman peaks. In particular, the 12 mM sample showed almost twice the intensity of the 6 mM sample, suggesting that a greater availability of Ag+ ions in the solution promotes the formation of denser metal deposits with better coverage on the substrate [10]. The results showed that the condition of 5 V and 12 mM AgNO3 produced the most intense and defined Raman signals compared to those obtained at 2 V, where the intensities were significantly lower. This difference highlights the influence of electrodeposition conditions, particularly the applied voltage. Likewise, a positive correlation was observed between the concentration of AgNO3 and the SERS intensity, indicating that a higher concentration of silver nitrate favors the formation of more efficient structures for Raman amplification, provided that the applied voltage remains within an optimal range. This can be explained by the fact that increasing the voltage from 2 V to 5 V produces a more intense electric field that favors the migration and reduction of Ag+ ions, promoting faster and more uniform nucleation on the substrate surface [11,12]. In contrast, at low voltages, the electric field is insufficient to effectively drive metal deposition, limiting the growth of nanostructures and, therefore, the efficiency of SERS amplification [11,12]. It is important to note that no vibrational modes associated with carbon contamination, nitrates, nitrites, or environmental impurities were detected in the Raman spectra. Only the characteristic peaks of methylene blue were present, and no additional bands attributable to graphite residues or AgNO3 remnants were observed.
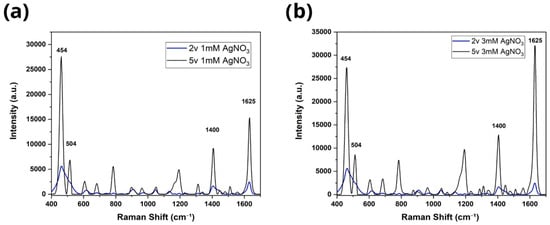
Figure 3.
Evaluation of the Raman spectra of 2 V and 5 V electrodeposited TiO2 at concentrations of (a) 1 mM and (b) 3 mM with the blue methylene molecule at 1 × 10−5.
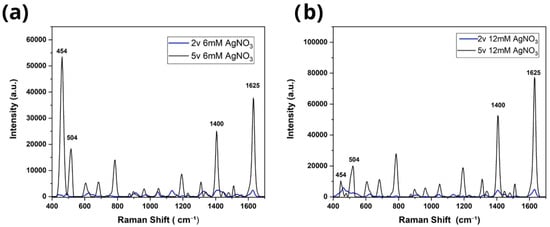
Figure 4.
Evaluation of the Raman spectra of TiO2 electrodeposited at 2 V and 5 V in concentrations of (a) 6 mM and (b) 12 mM with the blue methylene molecule at 1 × 10−5.
4. Conclusions
According to the results obtained, it was possible to obtain SERS substrates using electrochemical anodization and electrodeposition techniques. The TiO2 nanotubes presented an orderly and reproducible morphology, attributed to the action of fluoride ions in the electrolyte, while heat treatment favored the crystallization of the anatase phase, providing a suitable surface for metal deposition. SEM analysis revealed the formation of silver nanoparticles and dendritic structures on TiO2 nanotubes, whose distribution and size depended directly on the applied voltage and concentration.
The response in the SERS showed that both the increase in AgNO3 concentration and voltage favored the intensity of the Raman signal, reaching values above 70,000 a.u. for samples treated at 5 V and 12 mM. The results showed that concentration significantly influences the Raman response. As the AgNO3 concentration increased, an increase in signal intensity was also observed, regardless of the applied voltage. However, in substrates treated at 2 V, the maximum intensities did not exceed 6000 a.u., while at 5 V, values above 70,000 a.u. were reached, demonstrating the decisive role of voltage in the performance of the SERS substrate.
Author Contributions
Conceptualization, T.O.; methodology, M.T.; formal analysis, L.Z., J.H.; investigation, G.M.; resources, L.Z.; data curation, O.V., L.G.; writing—original draft preparation, G.M.; writing—review and editing, A.M., L.P.; supervision, L.P.; project administration, O.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that all data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors thank the Facultad de Ciencias Químicas, Universidad Veracruzana and the Center for Research in Micro and Nanotechnology (MICRONA) for providing facilities and equipment that supported this research.
Conflicts of Interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
References
- Contaminación por Metales Pesados. SciELO Bolivia- Scientific Electronic Library Online. Available online: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1817-74332009000100013 (accessed on 13 July 2025).
- Londoño Franco, L.F.; Londoño Muñoz, P.T.; Muñoz Garcia, F.G. Los riesgos de los metales pesados en la salud humana y animal. Biotecnol. En El Sect. Agropecu. Y Agroindustrial 2016, 14, 145–153. [Google Scholar] [CrossRef]
- Pabón Guerrero, S.E.; Benítez Benítez, R.; Sarria Villa, R.A.; Gallo Corredor, J.A. Contaminación del agua por metales pesados, métodos de análisis y tecnologías de remoción. Una revisión. Entre Cienc. E Ing. 2020, 14, 9–18. [Google Scholar] [CrossRef]
- Mendoza Uribe, G.; Rodríguez López, J.L. La nanociencia y la nanotecnología: Una revolución en curso. Perfiles Latinoam. 2007, 14, 161–186. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. (Eds.) Titanium and Titanium Alloys; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, L.; Xie, K.; Lai, Y.; Liu, B.; Ren, B.; Lin, C. SERS study of Ag nanoparticles electrodeposited on patterned TiO2 nanotube films. J. Raman Spectrosc. 2010, 42, 986–991. [Google Scholar] [CrossRef]
- Feng, Y.; Rijnaarts, H.H.M.; Yntema, D.; Gong, Z.; Dionysiou, D.D.; Cao, Z.; Miao, S.; Chen, Y.; Ye, Y.; Wang, Y. Applications of anodized TiO2 nanotube arrays on the removal of aqueous contaminants of emerging concern: A review. Water Res. 2020, 186, 116327. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.K.; Niu, Y.; Hussain, T.; Tabassum, H.; Tang, W.; Xu, M.; Ahuja, R. How to avoid dendrite formation in metal batteries: Innovative strategies for dendrite suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Anastasopoulos, J.A.; Soto Beobide, A.; Manikas, A.C.; Voyiatzis, G.A. Quantitative surface-enhanced resonance Raman scattering analysis of methylene blue using silver colloid. J. Raman Spectrosc. 2017, 48, 1762–1770. [Google Scholar] [CrossRef]
- Sulka, G.D. Introduction to anodization of metals. In Nanostructured Anodic Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–34. [Google Scholar] [CrossRef]
- Kaniyankandy, S.; Nuwad, J.; Thinaharan, C.; Dey, G.K.; Pillai, C.G.S. Electrodeposition of silver nanodendrites. Nanotechnology 2007, 18, 125610. [Google Scholar] [CrossRef]
- Maytorena-Sánchez, A.; Hernández-Torres, J.; Zamora-Peredo, L.; López-Huerta, F.; Orozco-Cruz, R.; García-González, L. Impacto del Voltaje y Temperatura en Recubrimientos de TiO2 Obtenidos Mediante Anodización Electroquímica Utilizando HCl Como Electrolito. RIIIT 2022. Available online: https://riiit.com.mx/apps/site/idem.php?module=Catalog&action=ViewItem&id=6671&item_id=85456 (accessed on 1 March 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).