1. Introduction
Although it is an irreplaceable and biogenic element, phosphorus can be viewed as a pollutant if it is present excessively in aquatic ecosystems. The presence of phosphates in surface water can cause a well-known phenomenon of eutrophication, i.e., the gradual “death” of the water body [
1,
2,
3]. There are different ways in which phosphates can reach surface water. The largest amount can occur as a result of leaching from agricultural areas, where the excess phosphate originates from added artificial fertilizers. In addition, large amounts of phosphates reach the water through the discharge of non-treated wastewater into recipients [
4]. According to the legal regulations of the Republic of Srpska, the maximum allowable concentration of phosphate (expressed as total phosphorus) ranges from 1 to 2 mg/L (depending on the wastewater treatment plant’s capacity), where the minimum reduction percentage should be 80% [
5]. Phosphate removal from wastewater is based on various physicochemical and biological processes. Enhanced Biological Phosphorus Removal (EBPR) is the most commonly used phosphorus removal process, in which microorganisms remove excess phosphate from wastewater [
6,
7]. Non-conventional procedures are often based on membrane processes and the use of certain sorbents [
8,
9,
10]. Sorption processes are gaining more importance, and numerous laboratory experiments are performed to prove the sorption properties of certain materials and to ensure the recovery of phosphorus at the same time, whether it is the sorption of phosphate from aqueous solutions [
11,
12,
13] or phosphate sorption from wastewater [
9,
14,
15,
16]. Those sorbents that are created as by-products of certain industrial processes or treatments are of particular importance. By using such materials for the sorption of specific pollutants from wastewater, their lifespan is extended, the use of commercial sorbents is reduced, the costs of wastewater treatment are lowered, and the pressure on the environment is minimized.
The focus of the paper is the use of the slag derived from the treatment of red mud (red mud slag—RMS) at high temperatures. Red mud is a highly alkaline byproduct that is produced in large quantities during the processing of bauxite ore using the Bayer process [
17]. The wider application of red mud has not yet gained traction, and red mud is mainly disposed of in open lakes and lagoons or stored in some other way [
18]. With certain treatments, red mud could be successfully used in construction, then as a source of useful elements, and in various applications related to environmental protection [
19]. Various studies have investigated the use of treated red mud, or products formed after certain treatments, for the sorption of phosphate from wastewater or aqueous solutions [
20,
21,
22,
23,
24].
Carbothermal reduction of red mud is a treatment process which is performed at a temperature of about 1600 °C. The goal of the treatment is to more efficiently extract certain useful elements from the slag, such as iron, sodium, titanium, etc. [
25,
26]. The treatment allows easier metal separation, with iron in the metallic form being separated using a relatively simple magnetic separation technology, and certain metals, such as titanium, being extracted by leaching from the resulting slag.
In this paper, a preliminary experiment was performed to determine whether RMS is suitable as a potential sorbent of phosphate from aqueous solutions (and later from wastewater), and these results are used only as a starting point for further research.
2. Materials and Methods
In this preliminary experiment, the red mud slag (RMS) is used as a potential sorbent for phosphate anions (orthophosphate—PO43−) from aqueous solutions. The RMS is obtained during the red mud treatment (carbothermal reduction) at 1600 °C, in a pilot-scale electric arc furnace (Institute of Process Metallurgy and Metal Recycling—IME, RWTH Aachen University, Aachen, Germany). Before the treatment, technical grade CaO (Otterbein, Großenlüder-Müs, Germany) is added as a fluxing agent. The primary product of the red mud treatment by carbothermal reduction consists of a metallic phase, which is separated magnetically, and the red mud slag.
A standard aqueous solution of phosphate was obtained by dissolving a certain amount of potassium dihydrogen phosphate (KH
2PO
4, Semikem LTD, Vogosca, Bosnia and Herzegovina) of analytical grade. Synthetic phosphate solution was made with an initial concentration of phosphate of 9.728 mg/L. The measurement of the phosphate concentration is conducted according to the standard Water quality—Determination of phosphorus—Ammonium molybdate spectrophotometric method (EN ISO 6878:2004) [
27].
The process of phosphate sorption from aqueous solutions using the red mud slag was carried out under batch laboratory conditions, at the Faculty of Technology Zvornik. The experiment was carried out in triplicate. A volume of 20 mL of solution with known phosphate concentrations was pipetted into 50 mL falcon plastic tubes. Then, previously measured masses of three categories of the RMS in the amount of 5 g/L (0.1 g/20 mL) were added to the falcon tubes. The prepared tubes were placed on a horizontal laboratory shaker, where the shaking speed was set to 120 rpm for 24 h. To separate the sorbent from the sample solution, a laboratory centrifuge (Centric 322A, Tehtnica, Železniki, Slovenia) was used for 15 min at 6000 rpm. To separate the red mud slag from the sample solution more efficiently, after centrifugation, the decantate was filtered using quantitative filter paper (blue ribbon).
A Shimadzu UV/VIS spectrophotometer (Kyoto, Japan) was used to determine the phosphate concentration in the sample solution, according to the standard method BAS ISO 6878:2006 [
27]. All experiments were performed at room temperature, with three different pH values. The first one was set as acidic (pH ≈ 3), the second one as alkaline (pH ≈ 11), and the third group of the samples had a pH around neutral. This step aims to determine which conditions regarding the pH will be most favourable for the phosphorus sorption onto red mud slag.
3. Results and Discussion
Table 1 shows the chemical composition of the red mud and the RMS (after magnetic separation).
Based on the chemical composition, it is possible to notice a significant increase in calcium content, due to the addition of CaO during the treatment of the red mud.
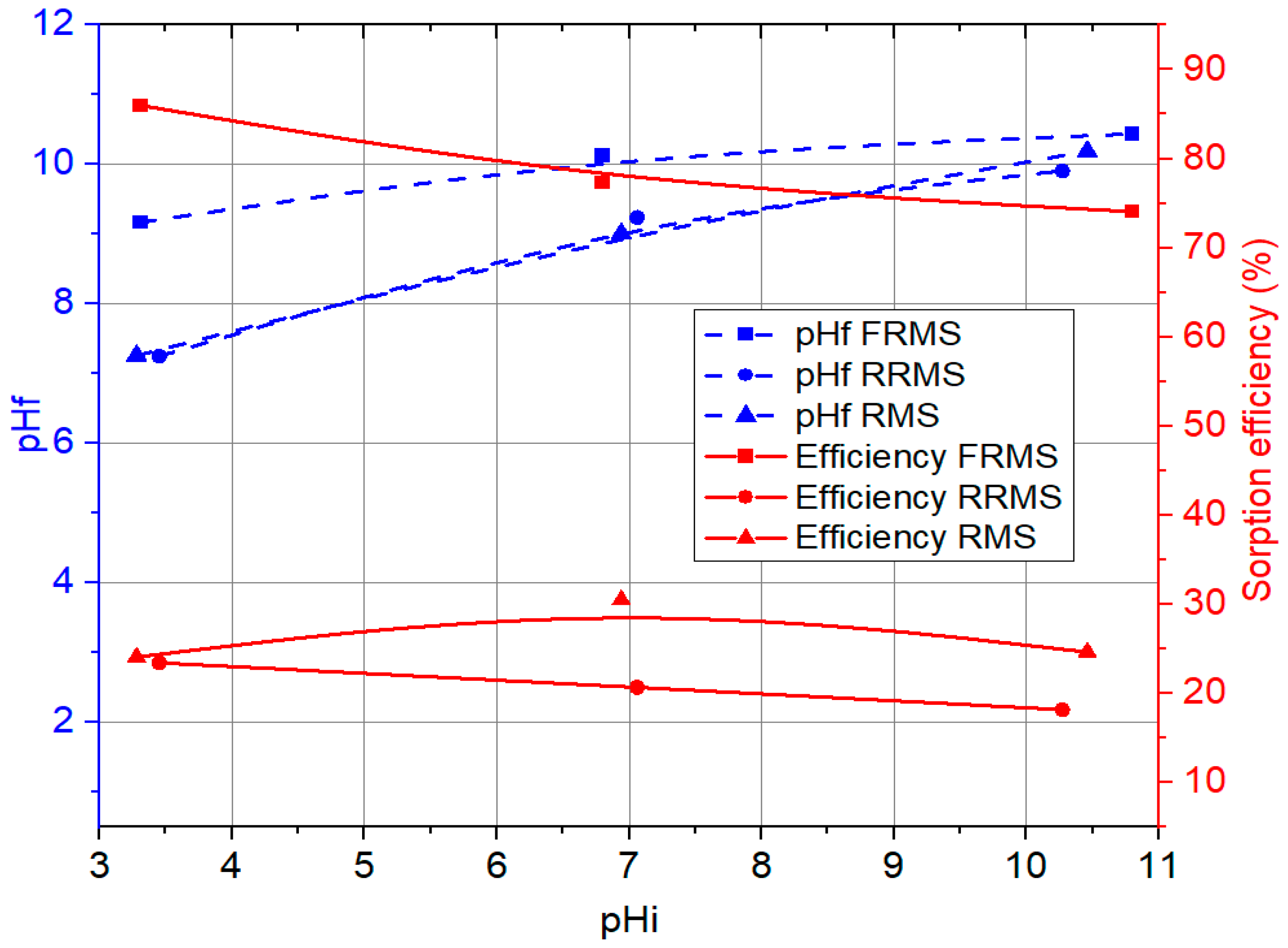
Figure 1 shows the comparative results of the change in the final pH value and the phosphate sorption efficiency depending on the initial pH value and the type of added sorbent. The results are shown for three sorbents: finely ground red mud slag (FRMS), i.e., the fine fraction obtained by sieving the slag through a 150 μm sieve; slag remaining on the sieve after sieving (RRMS); and unsieved raw red mud slag (RMS).
Regarding the pH change, a more significant change in the final pH value was recorded for samples in an acidic environment. The finely ground sample (FRMS) showed the most important change in the final pH value (pHf ≈ 9), while the RRMS and RMS fractions were comparable (pHf ≈ 7). In the case of the experiment at neutral initial conditions, the final pH increased to approximately 9 for RRMS and RMS sorbents, and in the case of FRMS, the final pH value was approximately 10. In alkaline conditions, pH change was not significant, and all tested sorbents showed approximately the same final pH value (pHf ≈ 0 ± 0.40). Since the red mud is a highly alkaline material, it could be assumed that slag itself also has alkaline properties. Sorption experiments showed that the sample of finely ground slag showed significantly better efficiency in removing phosphate from the solution than the other two tested samples. For the sorption reaction, acidic conditions are more favourable.
In addition to the change in the final pH value, changes in the sorption efficiency are also evident. The residual phosphate concentration in the solution depended more on the type of added sorbent and its characteristics than on the pH value of the solution. The lowest phosphate sorption efficiency was obtained in the case of applying the RRMS. Given that the RMS was generated by red mud treatment at high temperatures, a certain metallic phase was obtained in the slag. That metallic phase was primarily separated; however, a percentage of it remained in the slag, and after sieving, it remained in the RRMS. For the RRMS, a low sorption efficiency was expected. Sorption processes are generally more effective with smaller particle sizes, which have a higher surface area for interaction. The maximum sorption efficiency for the RRMS samples was about 20%. The RMS also did not give much better sorption efficiency. The maximum sorption efficiency in this case was about 30%. However, the fine fraction (FRMS) proved to be an effective phosphate sorbent in this screening experiment. Given that the fine fraction consists of particles that are smaller than 150 μm, it is expected that their adsorption capacity is higher, due to the development of the specific surface and the availability of a greater number of sorption sites. The maximum sorption efficiency for the FRMS ranged from 74% to 86%, which was reached in an acidic environment. In such conditions, the best removal of phosphate from the aqueous solution was achieved, where from the initial concentration of 9.728 mg/L, the phosphate concentration was lowered to 1.364 mg/L.
4. Conclusions
This paper examines the potential for the red mud slag usage as a phosphate sorbent in aqueous solutions. The red mud slag is obtained by carbothermal reduction of red mud at high temperatures. Primary slag treatment involves the magnetic separation of the metallic phase obtained by red mud reduction. By the subsequent sieving of the RMS, the resulting fractions are finely ground slag (FRMS) and residue after sieving (RRMS). These two fractions and the raw slag sample (RMS) were subjected to a screening experiment to remove phosphate from an aqueous solution with an initial concentration of 9.728 mg/L PO43−. After 24 h of contact between the sorbent and the solution in three different pH ranges (acidic, neutral, and alkaline), the obtained results indicated the best option regarding the type of sorbent and pH conditions. It was visible that the concentration of residual phosphate in the solution depended more on the type of added sorbent than on the pH value of the solution. The best results in terms of efficiency were achieved for the FRMS sorbent, i.e., the finely ground fraction of the slag, for the acidic initial conditions (pH ≈ 3). In this case, a maximum sorption efficiency of 86% was achieved. These results indicate that RMS can be used as a potential sorbent for phosphate from water with certain treatments (sieving), because the fine fraction (RRMS), due to its larger specific surface area and smaller particle size, has a better ability to be a sorbent for phosphate ions compared to unsieved slag. Further investigation should be performed to determine the sorption properties, kinetics, and chemistry of the phosphate sorption using the red mud slag.
Author Contributions
Conceptualization, J.V. and S.N.S.; methodology, J.V. and S.N.S.; software, D.K.; validation, M.P., S.S. and N.V.; formal analysis, N.V.; investigation, J.V.; resources, D.K. and S.S.; data curation, S.N.S.; writing—original draft preparation, J.V., S.N.S. and D.K.; writing—review and editing, M.P. and J.V.; visualization, D.K.; supervision, S.N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Commission, grant number 101135077 (EURO-TITAN).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mekonnen, M.M.; Hoekstra, A.Y. Global anthropogenic phosphorus loads to fresh water and associated grey water footprints and water pollution levels: A high-resolution global study. Wat. Resour. Res. 2018, 54, 345–358. [Google Scholar] [CrossRef]
- Schaum, C. Phosphorus: Polluter and Resource of the Future: Motivations, Technologies and Assessment of the Elimination and Recovery of Phosphorus from Wastewater; IWA Publishing: London, UK, 2018. [Google Scholar]
- Drizo, A. Phosphorus Pollution Control: Policies and Strategies; John Wiley & Sons: Edinburgh, UK, 2019. [Google Scholar]
- Sathiyamurthi, S.; Nalini, S.; Sivasakthi, M. Phosphate: Sources, Method of Analysis and Treatment. In Handbook of Water Pollution; Wiley: Hoboken, NJ, USA, 2024; pp. 235–279. [Google Scholar] [CrossRef]
- Pravilnik o uslovima ispuštanja otpadnih voda u površinske vode (Sl. glasnik RS broj 44/01). Available online: www.voders.org/images/PDF/pravilnici/Pravilnik_ispustanje-povrs-vode_44_01.pdf (accessed on 6 June 2025).
- Izadi, P.; Izadi, P.; Eldyasti, A. Design, operation and technology configurations for enhanced biological phosphorus removal (EBPR) process: A review. Rev. Environ. Sci. Biotechnol. 2020, 19, 561–593. [Google Scholar] [CrossRef]
- Zhang, C.; Guisasola, A.; Baeza, J.A. A review on the integration of mainstream P-recovery strategies with enhanced biological phosphorus removal. Water Res. 2022, 212, 118102. [Google Scholar] [CrossRef] [PubMed]
- Zahed, M.A.; Salehi, S.; Tabari, Y.; Farraji, H.; Ataei-Kachooei, S.; Zinatizadeh, A.A.; Mahjouri, M. Phosphorus removal and recovery: State of the science and challenges. ESPR 2022, 29, 58561–58589. [Google Scholar] [CrossRef]
- Usman, M.O.; Aturagaba, G.; Ntale, M.; Nyakairu, G.W. A review of adsorption techniques for removal of phosphates from wastewater. Water Sci. Technol. 2022, 86, 3113–3132. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Wang, Z.; Lv, M.; Tang, A.; Yu, Y.; Li, A. Insight into the synthesis and adsorption mechanism of adsorbents for efficient phosphate removal: Exploration from synthesis to modification. Chem. Eng. J. 2022, 442, 136147. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Kowalkowska, A.; Filipkowska, U.; Struk-Sokołowska, J.; Bolozan, L.; Gache, L.; Ilie, M. Recovery of phosphorus as soluble phosphates from aqueous solutions using chitosan hydrogel sorbents. Sci. Rep. 2021, 11, 16766. [Google Scholar] [CrossRef]
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A comprehensive review on nitrate and phosphate removal and recovery from aqueous solutions by adsorption. AQUA—Water Infrastruct. Ecosyst. Soc. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Zhang, Z.; Liu, Q.; Jiang, K.; Tian, J.; Ni, F. Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar. J. Environ. Chem. Eng. 2021, 9, 105079. [Google Scholar] [CrossRef]
- Zhang, K.; Van Dyk, L.; He, D.; Deng, J.; Liu, S.; Zhao, H. Synthesis of zeolite from fly ash and its adsorption of phosphorus in wastewater. Green Process. Synth. 2021, 10, 349–360. [Google Scholar] [CrossRef]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A comprehensive review on technological advances of adsorption for removing nitrate and phosphate from waste water. J. Water Process Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Jucherski, A.; Walczowski, A.; Bugajski, P.; Jóźwiakowski, K.; Rodziewicz, J.; Janczukowicz, W.; Mielcarek, A. Long-term operating conditions for different sorption materials to capture phosphate from domestic wastewater. Sustain. Mater. Technol. 2022, 31, e00385. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Rai, S.; Bahadure, S.; Chaddha, M.J.; Agnihotri, A. Disposal Practices and Utilization of Red Mud (Bauxite Residue): A Review in Indian Context and Abroad. J. Sustain. Metall. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Vuković, J.; Perušić, M.; Stopić, S.; Kostić, D.; Smiljanić, S.; Filipović, R.; Damjanović, V. A review of the red mud utilization possibilities. OUAC 2024, 35, 165–173. [Google Scholar] [CrossRef]
- Liu, C.J.; Li, Y.Z.; Luan, Z.K.; Chen, Z.Y.; Zhang, Z.G.; Jia, Z.P. Adsorption removal of phosphate from aqueous solution by active red mud. J. Environ. Sci. 2007, 19, 1166–1170. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, Q.; Li, Q.; Xu, X.; Yang, Z.; Wang, X.; Yu, H. Characterization of red mud granular adsorbent (RMGA) and its performance on phosphate removal from aqueous solution. Chem. Eng. J. 2012, 193, 161–168. [Google Scholar] [CrossRef]
- Lin, J.Y.; Kim, M.; Li, D.; Kim, H.; Huang, C. The removal of phosphate by thermally treated red mud from water: The effect of surface chemistry on phosphate immobilization. Chemosphere 2020, 247, 125867. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef]
- Tao, L.; Huang, H.; Chen, X.; Chen, J.; Evrendilek, F.; Liu, J. Valorizing calcium-loaded red mud composites for phosphorus removal and recovery. Process Saf. Environ. Prot. 2025, 194, 542–554. [Google Scholar] [CrossRef]
- Feng, H.; She, X.F.; You, X.M.; Zhang, G.Q.; Wang, J.S.; Xue, Q.G. Carbothermal reduction of red mud for iron extraction and sodium removal. HTMP 2022, 41, 161–171. [Google Scholar] [CrossRef]
- Stopic, S.; Kostić, D.; Schneider, R.; Sievers, M.; Wegmann, F.; Emil Kaya, E.; Friedrich, B. Recovery of Titanium from Red Mud Using Carbothermic Reduction and High Pressure Leaching of the Slag in an Autoclave. Minerals 2024, 14, 1151. [Google Scholar] [CrossRef]
- EN ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrophotometric Method. International Standard Organization: Geneva, Switzerland, 2004.
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).