Abstract
Accelerated urbanization and industrialization have significantly heightened water contamination risks, posing severe threats to public health and ecological balance. Polymeric membranes stand at the forefront of addressing this challenge, revolutionizing water and wastewater treatment. These membranes adeptly remove a broad spectrum of contaminants, including organic compounds and heavy metals, thereby playing a crucial role in mitigating environmental pollution. This research delves into the sophisticated mechanisms of polymeric membranes in filtering out pollutants, with a spotlight on the enhancements brought about by nanotechnology. This includes a detailed examination of their inherent antibacterial properties, showcasing their innovative design and potential for extensive application. The study further investigates advanced techniques like electrochemical processes and membrane distillation, particularly focusing on desalination. These methods are central to the advancement of water purification, emphasizing efficiency and environmental sustainability. However, challenges such as membrane fouling pose significant hurdles, necessitating ongoing research into surface modifications and antifouling strategies. This paper offers a comparative analysis of various membrane technologies, highlighting their manufacturing complexities and efficiency benchmarks. In summation, the paper underscores the importance of continuous innovation in membrane technology, aiming to develop sustainable and effective water treatment solutions. By bridging the gap between basic science and technological advancements, this review aims to guide practitioners and researchers towards a future where clean water is universally accessible, ensuring the preservation of our ecosystems.
1. Introduction
Water is vital for supporting life and ecosystems; however, rapid industrialization and urbanization have increased pollution risks. Branches of the economy, such as the textile and chemical industries, introduce pollution like dyes and heavy metals into city wastewater, resulting in pathogens and micro-pollution, which threaten health and ecosystems [1]. Conventional treatment processes cannot effectively remove newly identified contaminants and secondary products and are energy-intensive [2]. Polymeric membranes, made of polyethersulfone (PES), polyvinylidene fluoride (PVDF), and cellulose acetate (CA), in particular, provide an efficient approach owing to their mechanical robustness, chemical stability, and the possibility of being surface modified. Polymeric membranes are interesting alternatives to ceramic and metallic membranes for their low cost, simple fabrication procedure, and tunability for specific contaminant removals via surface functionalization and nanoparticle doping. PES, for example, with its good thermal stability and high mechanical strength, is appropriate for both ultrafiltration and nanofiltration, whereas PVDF displays outstanding chemical stability, resistance to fouling, etc. [3]. Nanotechnology has revised these membranes to counter challenges such as micropollutants and heavy metals, which further underscores their role as a key part of sophisticated water treatment technologies.
2. Mechanisms of Contaminant Removal
2.1. Basic Functioning and Types of Polymeric Membranes
Polymeric membranes act as selective barriers, separating contaminants based on size, charge, or solubility. They are composed of polymers that influence their durability, chemical resistance, and separation efficiency. Contaminant removal occurs through size exclusion, charge repulsion, or chemical interactions with the membrane surface [4]. The types of polymeric membranes are as follows:
- Ultrafiltration (UF): Polyethersulfone (PES) and cellulose acetate (CA) are the typical materials used for UF membranes. PES porous membranes have high thermal resistance and chemical strength to enable the removal of suspended solids, colloids, and pathogens. Cellulose acetate (CA) membranes made from renewable sources provide hydrophilic surfaces minimizing fouling propensity [3,5].
- Nanofiltration (NF): NF membranes are frequently made of polyvinylidene fluoride (PVDF) due to its excellent chemical resistance and fouling-resistant nature. Its compressed structure results in more effective contaminant rejection with a controlled permeability and is good for ion separation and partial desalination [6].
- Reverse Osmosis (RO): Polysulfone (PSU) and polyamide (PA) are typical materials for RO membranes. The mechanical strength and pressure durability of PSU and the selective layer of PA, with high salt and heavy metal rejection, are their critical attributes [7].
- Microfiltration (MF): Typically, PVDF, CA, etc., are used for MF membranes. These membranes have been favored due to their chemical and thermal resistances, whereas CA membranes are hydrophilic and their fouling by organics is less likely [8].
Of the membrane sources, RO offers the greatest contaminant rejection, yet at the cost of energy use and fouling potential. NF provides a compromise between rejection performance and permeability, but fouling is still an issue because of its compact structure. UF is better in terms of performance in the removal of big particles and under low-pressure operation; MF is more appropriate for pretreatment steps, especially in the wastewater treatment process. When deciding on the type of membrane, targeted contaminants, operation conditions, and desired treatment outcomes have to be taken into account, optimizing processes based on fouling potential, removal efficiency, and operational costs.
2.2. Antimicrobial Properties of Nanoparticles in Membranes
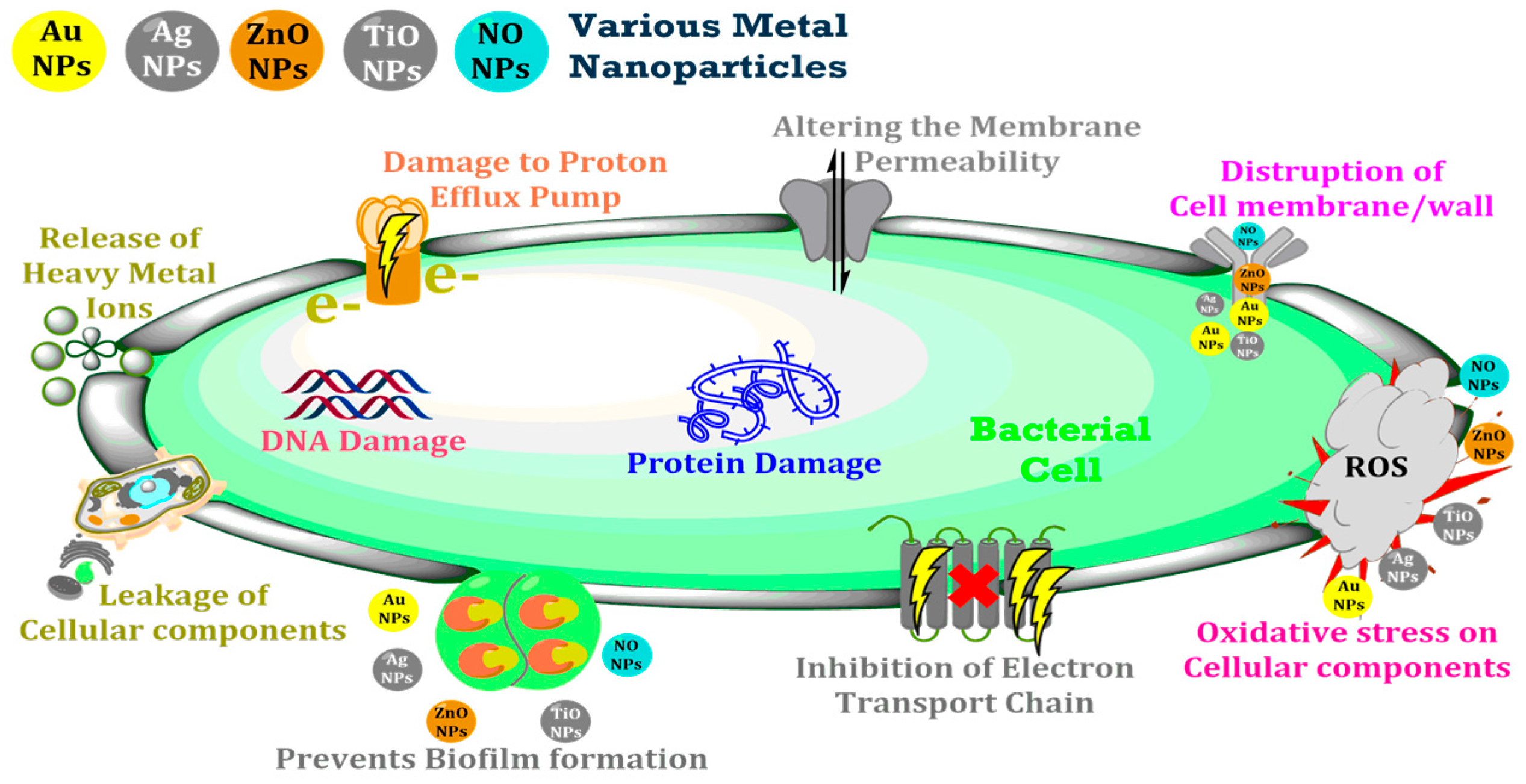
Nanoparticles (NPs) integrated into polymeric membranes enhance their antimicrobial properties, addressing biofouling issues. NPs combat microbes through mechanisms like reactive oxygen species (ROS) generation, metal ion release, and physical disruption of microbial cells [9]. For instance, silver and copper NPs release ions that inhibit microbial activity, while titanium dioxide generates ROS under ultraviolet (UV) light, disrupting microbial cells [10]. These mechanisms ensure prolonged membrane performance and effective contaminant removal. Figure 1 illustrates the antibacterial actions of nanoparticles, highlighting how NPs physically and chemically disrupt microbial cells to ensure effective antimicrobial activity.
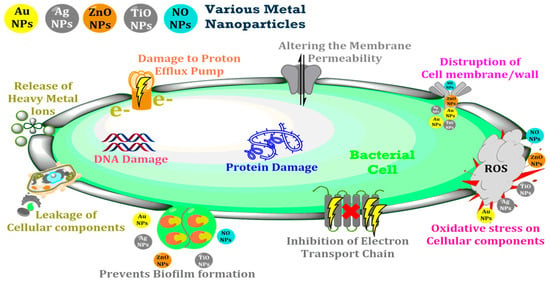
Figure 1.
Antibacterial mechanisms of nanoparticles integrated into membranes.
3. Advanced Techniques in Water Treatment
3.1. Electrochemical Processes
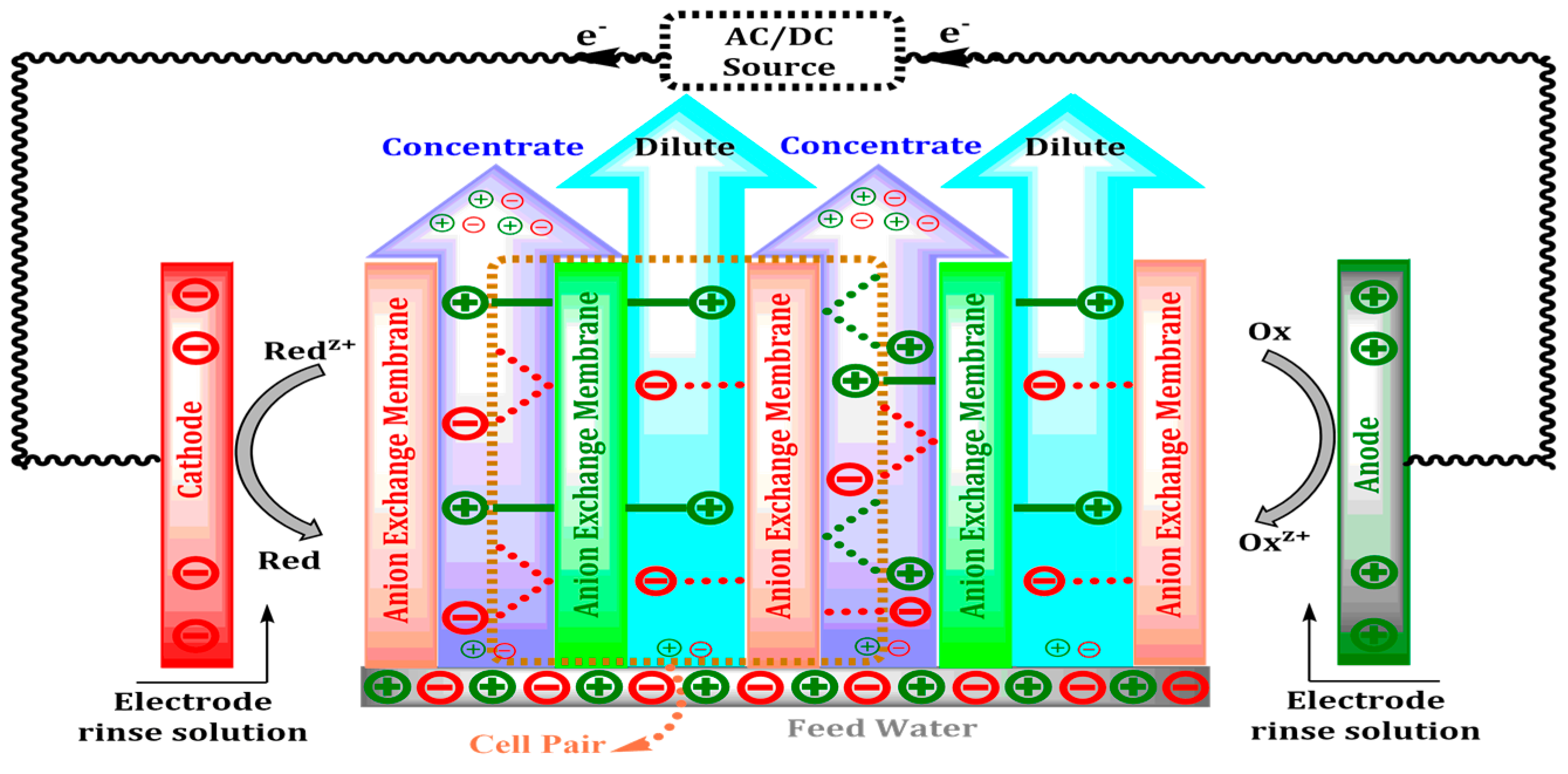
Electrodialysis (ED) uses ion selective membranes and a potential difference to extract ions such as Na+, Cl−, Ca2+, and SO42− from water and can remove up to 95% of Na+ and 88% of SO42− [11]. The ED stack, which is designed to contain the cation and anion exchange membranes alternately, can facilitate the movement of ions under an operating current density of 5–10 mA/cm2 and a voltage of 10–20 V, which will not only maximize separation but also reduce polarization [12]. Ion transport via exchange membranes, concentrate and dilute streams, and the oxidation–reduction area at the electrode chamber are shown in Figure 2.
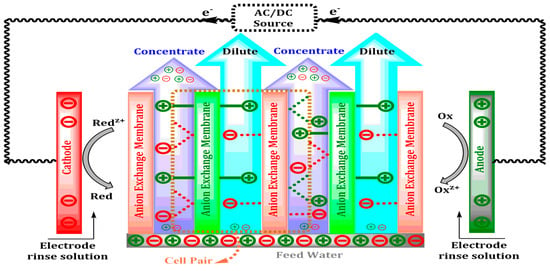
Figure 2.
Schematic representation of ion exchange, electrode reactions, and flow pathways in a typical electrodialysis (ED) stack.
3.2. Membrane Distillation Techniques for Desalination
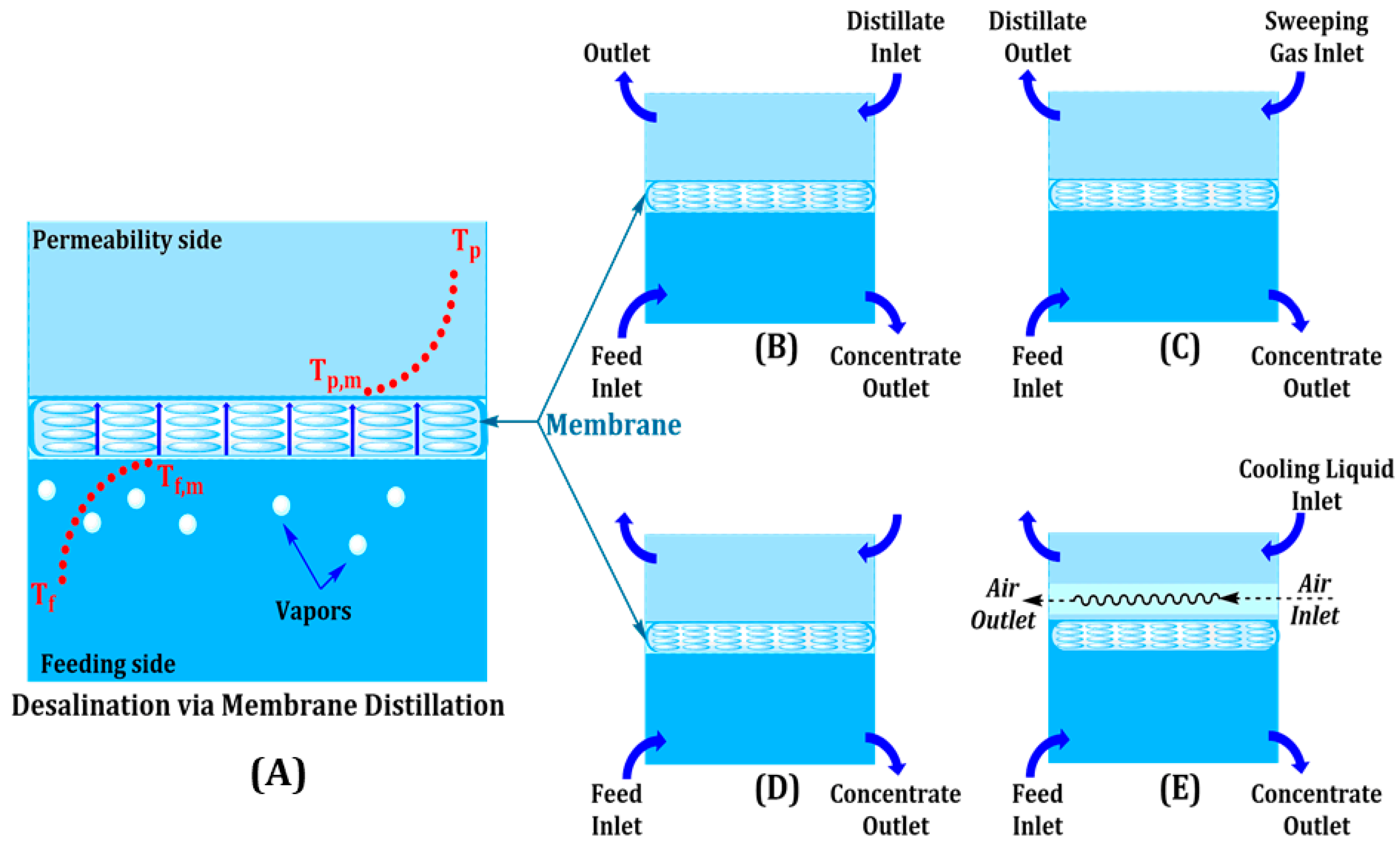
Membrane Distillation (MD) is one of the thermally driven separation processes where a hydrophobic membrane is used to selectively transport vapor while leaving non-volatile impurities behind. The vapor pressure gradient is sustained by operating with feed temperatures of between 60 and 80 °C, with membrane pressure below 100 mbar to allow water vapor permeation to take place. Direct Contact MD (DCMD), most commonly used in brine concentration processes, can recover 70–80% of water, whereas Air Gap MD (AGMD) indirectly enhances the thermal economy by decreasing heat loss across an air gap [13]. Vacuum MD (VMD) is conducted under reduced pressure to promote vapor flux, and Sweeping Gas MD (SGMD) uses a carrier gas to enhance the recovery of volatile organic compounds (VOCs) during industrial wastewater treatment [14]. The working modes and flow channels of the MD system are illustrated in Figure 3, which exhibits different channels for the feeding, permeability, and vapor.
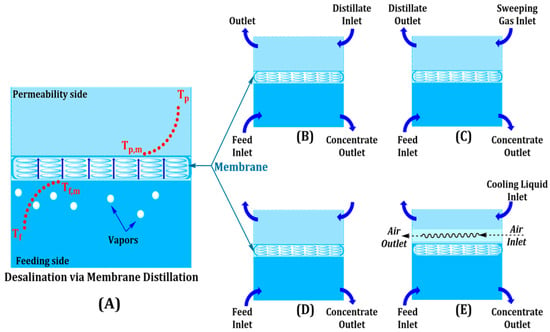
Figure 3.
Membrane distillation methods: (A) MD for desalination, (B) direct contact MD, (C) sweeping gas MD, (D) vacuum MD, and (E) air gap MD.
4. Challenges in Membrane Technologies
4.1. Membrane Fouling and Mitigation Strategies
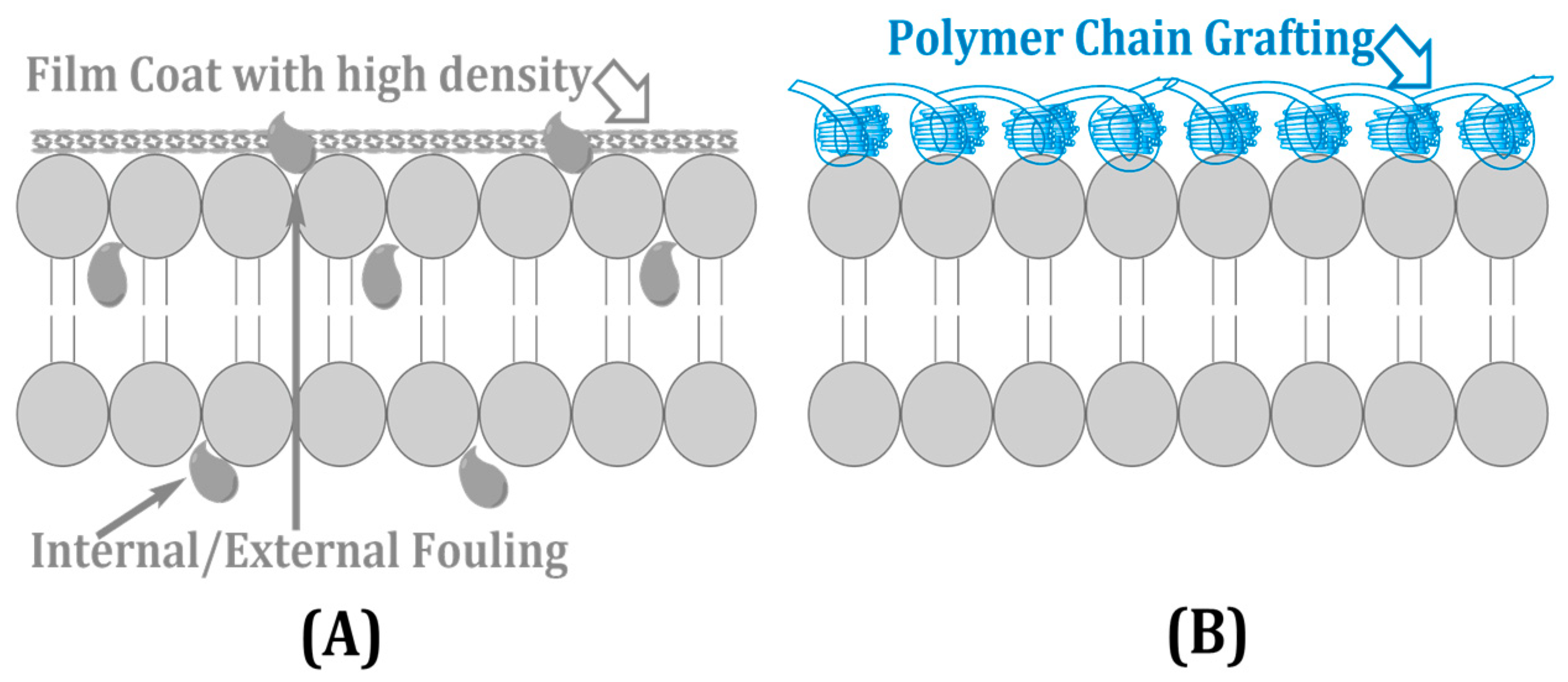
Membrane fouling through the deposition of natural organic material, inorganic particles, and microbiological slimes decreases water flux by as much as 40% and also increases operating expenditure [15]. Organic fouling arises due to protein and polysaccharide adhesion; whereas inorganic scaling deposits salts, such as CaCO3 and SO42−, biofouling results in microbial communities enhancing performance degradation. Mitigation solutions center around surface alteration and antifouling coatings/agents such as plasma-coated hydrophilics, zwitterionic polymers, and silver nanoparticles that decrease fouling incrustation between 20 and 30% [16]. Polymer grafting, as presented in Figure 4, creates dense coverage that prohibits foulant penetration (A), and with the polymer chains included (B), it can reduce the risk of microbial occurrence and particle attachment, prolonging membrane life [17].
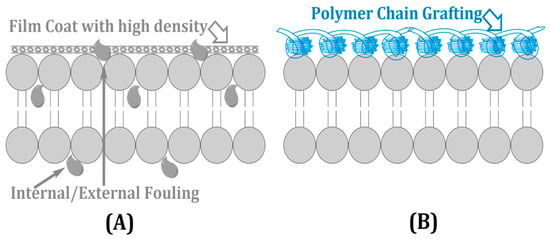
Figure 4.
Antifouling surface modification techniques: (A) dense nonporous coating and (B) polymer chain grafting for fouling resistance.
4.2. Limitations and Future Directions
Despite their advantages, polymeric membranes face challenges such as high fabrication costs, limited chemical stability under harsh conditions, and susceptibility to fouling [18]. Scaling up for industrial applications remains a significant barrier, particularly for advanced membrane technologies like membrane distillation. Future research should focus on developing low-cost, sustainable materials and improving membrane longevity. Innovations in nanotechnology, such as responsive membranes and bio-inspired designs, show promise for enhancing performance and adapting to varying water treatment needs. Additionally, integrating renewable energy sources with membrane systems could improve their economic and environmental feasibility [19].
5. Applications and Comparative Analysis
5.1. Effectiveness in Heavy Metal and Dye Removal
Polymeric membranes demonstrate remarkable efficiency in removing heavy metals and dyes from contaminated water sources. Their selective separation capabilities target pollutants such as arsenic, lead, cadmium, and synthetic dyes, which pose severe health and environmental risks [20]. Advanced modifications, such as the incorporation of nanomaterials like graphene oxide (GO) and titanium dioxide (TiO2), further enhance removal efficiency by improving adsorption and selectivity [21]. Table 1 demonstrates the effectiveness of polymeric membranes in dye removal. Ultrafiltration (UF) membranes using CA/PSU achieved 82% removal for methylene blue, improving to 94% with the addition of nano zero-valent iron (nZVI). Nanofiltration (NF) membranes, such as PES with GO-TiO2, achieved high removal rates, including 99.4% for reactive green 19 and 95.4% for direct yellow 12. These results highlight the significant role of nanomaterials in enhancing removal efficiencies for industrial dyes.

Table 1.
Efficiency of polymeric membranes in removing dyes from water.
5.2. Advantages and Drawbacks of Wastewater Treatment Methods
Various wastewater treatment methods offer distinct advantages and limitations. Polymeric membranes, while highly efficient, face challenges such as fouling and operational costs. In contrast, conventional methods like coagulation are cost-effective but generate significant sludge, while advanced oxidation processes effectively degrade pollutants but are energy-intensive [27,28,29]. An overview of wastewater treatment methods is shown in Table 2, including their merits and drawbacks, especially with respect to BOD (Biochemical Oxygen Demand) and COD (Chemical Oxygen Demand) as indicators for the assessment of treatment efficiency. BOD determines how much oxygen microorganisms require to break down organic matter, and COD quantifies oxygen needed for the chemical oxidation of pollutants to represent the extent of contamination.

Table 2.
Key advantages and disadvantages of wastewater treatment processes.
Although traditional technologies such as coagulation and activated sludge are cost-effective on a large scale, membrane technologies offer significantly better contaminant removal and less sludge production, especially for micro-pollutants and heavy metals. But their elevated fouling potential and cost to operate also requires optimization strategies to achieve a compromise between efficiency and sustainability.
6. Conclusions
Polymeric membranes have proven to be transformative in addressing the challenges of water contamination. Their ability to selectively remove a wide range of pollutants, from organic compounds to heavy metals, has established them as a cornerstone of modern water treatment technologies. Enhanced by nanotechnology, these membranes offer superior contaminant removal, antimicrobial properties, and operational flexibility, making them indispensable in applications like desalination, industrial wastewater treatment, and dye removal. Their innovative designs and adaptability ensure they remain at the forefront of sustainable water purification efforts. To further advance the role of polymeric membranes, innovation is critical. Future developments should focus on creating cost-effective and durable membrane materials capable of withstanding harsh operating conditions. Addressing challenges such as fouling through advanced surface modifications and integrating renewable energy sources into membrane-based systems will enhance their environmental and economic sustainability. Additionally, the development of responsive and bio-inspired membranes can optimize performance in varying conditions, ensuring efficient and adaptable solutions. By prioritizing these advancements, polymeric membranes can play a pivotal role in achieving universal access to clean and safe water.
Author Contributions
Conceptualization, B.K.S. and B.P.; literature review and data analysis, T.P. and S.V.; software and visualization, S.S.; writing—original draft preparation, B.K.S.; writing—review and editing, B.P. and T.P.; supervision, B.K.S.; project administration, S.S.; resources and funding acquisition, Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smith, J.L.; Kumar, P.; Lee, A. Application of polymeric membranes in the purification of water contaminated with dyes. J. Adv. Membr. Sci. 2022, 12, 45–60. [Google Scholar]
- Shukla, B.K.; Teeli, M.A.; Shukla, S.K.; Chandra, R.; Bharti, N.; Singh, U. A Comprehensive Overview of Vital Water Quality Parameters. Lect. Notes Civ. Eng. 2024, 439, 1–20. [Google Scholar]
- Kim, J.Y.; Lee, K.H.; Lee, J.W.; Khan, I.A.; Kim, J.O. Structural and performance variation of PES/PVDF membranes after exposure to the pretreated feed water of CECs. Chemosphere 2023, 335, 139096. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.B.; Mehta, S.; Wang, Y. Polymeric membranes: A review on their chemical structure and influence on filtration performance. J. Membr. Stud. 2022, 45, 101–115. [Google Scholar]
- Gayatri, R.; Yuliwati, E.; Jaafar, J.; Fizal, A.N.S.; Hossain, M.S.; Zulkifli, M.; Yahaya, A.N.A.; Taweepreda, W. Polymer-based nanocomposite membranes for industrial wastewater treatment: A review. J. Environ. Chem. Eng. 2024, 12, 113276. [Google Scholar] [CrossRef]
- Austria, H.F.M.; Sardome, R.P.; Setiawan, O.; Huang, T.H.; Lei, W.C.; Tian, X.Y.; Hu, C.C.; Lee, K.R.; Lai, J.Y.; Tayo, L.L.; et al. Efficient membrane fouling mitigation in self-cleaning piezoelectric PVDF-graphene loose nanofiltration membranes for sustainable textile wastewater treatment. Sep. Purif. Technol. 2024, 346, 127317. [Google Scholar] [CrossRef]
- Castro, K.; Abejón, R. Removal of Heavy Metals from Wastewaters and Other Aqueous Streams by Pressure-Driven Membrane Technologies: An Outlook on Reverse Osmosis, Nanofiltration, Ultrafiltration and Microfiltration Potential from a Bibliometric Analysis. Membranes 2024, 14, 180. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Nkambule, T.I.; Mamba, B.B.; Hai, F.I. Strategies for mitigating challenges associated with trace organic compound removal by high-retention membrane bioreactors (HR-MBRs). npj Clean Water 2024, 7, 18. [Google Scholar] [CrossRef]
- Chauhan, M.; Shekhar, S.; Yadav, B.; Garg, V.; Dutt, R.; Sonali; Singh, R.P. Nanoparticles: A promising tool to promote reactive oxygen species in cancer therapy. Curr. Protein Pept. Sci. 2021, 22, 827–830. [Google Scholar] [CrossRef]
- Kamo, A.; Ozcan, A.; Sonmezoglu, O.A.; Sonmezoglu, S. Understanding antibacterial disinfection mechanisms of oxide-based photocatalytic materials. Nanocomposite Nanohybrid Mater. Process. Appl. 2023, 17, 195. [Google Scholar]
- Honarparvar, S.; Zhang, X.; Chen, T.; Alborzi, A.; Afroz, K.; Reible, D. Frontiers of membrane desalination processes for brackish water treatment: A review. Membranes 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Oddonetto, T.L.; Deemer, E.; Lugo, A.; Cappelle, M.; Xu, P.; Santiago, I.; Walker, W.S. Assessment of salt-free electrodialysis metathesis: A novel process for brine management in brackish water desalination using monovalent selective ion exchange membranes. Desalination 2024, 592, 118160. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H. Integration of proton exchange membrane fuel cell with air gap membrane distillation for sustainable electricity and freshwater cogeneration: Performance, influential mechanism, multi-objective optimization and future perspective. Renew. Sustain. Energy Rev. 2024, 199, 114523. [Google Scholar] [CrossRef]
- Saffarimiandoab, F.; Gul, B.Y.; Tasdemir, R.S.; Ilter, S.E.; Unal, S.; Tunaboylu, B.; Menceloglu, Y.Z.; Koyuncu, I. A review on membrane fouling: Membrane modification. Desalination Water Treat. 2021, 216, 47–70. [Google Scholar] [CrossRef]
- Horseman, T.; Yin, Y.; Christie, K.S.; Wang, Z.; Tong, T.; Lin, S. Wetting, scaling, and fouling in membrane distillation: State-of-the-art insights on fundamental mechanisms and mitigation strategies. ACS EST Eng. 2020, 1, 117–140. [Google Scholar] [CrossRef]
- Shukla, B.K.; Sharma, P.K.; Yadav, H.; Singh, S.; Tyagi, K.; Yadav, Y.; Rajpoot, N.K.; Rawat, S.; Verma, S. Advanced membrane technologies for water treatment: Utilization of nanomaterials and nanoparticles in membranes fabrication. J. Nanoparticle Res. 2024, 26, 222. [Google Scholar] [CrossRef]
- Subasinghe, P.; Said, K.A.M.; Rahman, M.R.; Kuok, K.K. Polyvinylidene fluoride gravity-driven membranes modification and membrane fouling: A review. Groundw. Sustain. Dev. 2024, 26, 101215. [Google Scholar] [CrossRef]
- Seah, M.Q.; Chua, S.F.; Ang, W.L.; Lau, W.J.; Mansourizadeh, A.; Thamaraiselvan, C. Advancements in Polymeric Membranes for Challenging Water Filtration Environments: A Comprehensive Review. J. Environ. Chem. Eng. 2024, 12, 112628. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Jahan, I.; Foyez, T.; Imran, A.B. Bio-inspired nanomaterials for micro/nanodevices: A new era in biomedical applications. Micromachines 2023, 14, 1786. [Google Scholar] [CrossRef]
- Jadoun, S.; Fuentes, J.P.; Urbano, B.F.; Yáñez, J. A review on adsorption of heavy metals from wastewater using conducting polymer-based materials. J. Environ. Chem. Eng. 2023, 11, 109226. [Google Scholar] [CrossRef]
- Mohamat, R.; Bakar, S.A.; Mohamed, A.; Muqoyyanah, M.; Othman, M.H.D.; Mamat, M.H.; Malek, M.F.; Ahmad, M.K.; Yulkifli, Y.; Ramakrishna, S. Incorporation of graphene oxide/titanium dioxide with different polymer materials and its effects on methylene blue dye rejection and antifouling ability. Environ. Sci. Pollut. Res. 2023, 30, 72446–72462. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Jackcina Stobel Christy, E.; Ida Celine Mary, G.; Jayaraj, K.; Pius, A. Cellulose acetate based biopolymeric mixed matrix membranes with various nanoparticles for environmental remediation-A comparative study. J. Environ. Chem. Eng. 2019, 7, 103278. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Ngang, H.; Ooi, B.; Ahmad, A.; Lai, S. Preparation of PVDF–TiO2 mixed-matrix membrane and its evaluation on dye adsorption and UV-cleaning properties. Chem. Eng. J. 2012, 197, 359–367. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N, Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Gong, W.; Bai, L.; Liang, H. Membrane-based technologies for removing emerging contaminants in urban water systems: Limitations, successes, and future improvements. Desalination 2024, 590, 117974. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, A.K.; Bhatnagar, A. Treatment of pharmaceutical wastewater using photocatalytic reactor and hybrid system integrated with biofilm based process: Mechanistic insights and degradation pathways. J. Environ. Chem. Eng. 2023, 11, 109141. [Google Scholar] [CrossRef]
- Robertson, M.; Jameson, L. The Future of Membrane Technologies in Water Treatment. J. Adv. Filtr. Syst. 2019, 14, 400–412. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).