Abstract
Diseases such are caries affect approximately 25% of the worldwide population. Such a state requires novel, antibacterial materials. This research aimed to synthesize and characterize the structures of two urethane-dimethacrylate monomers showing possible antibacterial activity for dental composite restorative materials (DCRMs). The monomers were based on isophorone diisocyanate (IPDI) and dicyclohexylmethane 4,4′-diisocyanate (CHMDI). The structures of the monomers and their key elements were confirmed with the application of spectroscopy methods. Nuclear Magnetic Resonance Spectroscopy (1H and 13C NMR) and Fourier Transform Infrared Spectroscopy (FTIR) were applied. The monomers were synthesized and their structures were confirmed with the abovementioned techniques.
1. Introduction
The urethane-dimethacrylate (UDMA) monomer, which is synthesized from trimethylhexamethylene diisocyanate and 2-hydroxyethyl methacrylate (HEMA), is commonly used in dental composite restorative materials (DCRMs) due to its excellent physicochemical and mechanical properties [1,2,3]. However, in recent years, there has been growing interest in DCRMs that possess antibacterial properties in response to the increasing demand for preventing secondary caries [4,5,6,7,8,9]. An effective strategy for achieving long-lasting antibacterial properties is covalent anchoring of a monomeric biocide within the polymeric matrix of a DCRM [10]. Dimethacrylate monomers with quaternary ammonium groups (QADMAs) were demonstrated to be especially effective [11,12,13,14,15]. Despite extensive research on QADMA-based DCRM matrices, the design and synthesis of novel monomers with tailored structures remain an important area of study.
Since 2019, our research group has been working on developing new urethane-dimethacrylates featuring two quaternary ammonium groups (QAUDMAs). These monomers were synthesized from 2-(methacryloyloxy)ethyl-2-alkylhydroxyethylmethylammonium bromide (QAHAMA-n, where n represents the carbon chain length of 8, 10, 12, 14, or 16) and aliphatic diisocyanates, including 2,4,4-trimethylhexamethylene diisocyanate (TMDI) [16,17] and 1,3-bis(1-isocyanato-1-methylethyl)benzene (TMXDI) [18]. We found that both the selection of the diisocyanate precursor and the length of the N-alkyl substituent significantly affected the final properties of the QAUDMAs and their copolymers with common dimethacrylates, specifically bisphenol A diglycidyl methacrylate (Bis-GMA) and triethyleneglycol dimethacrylate (TEGDMA).
In this study, we report the synthesis and structural characterization of new QAUDMA monomers that contain two quaternary ammonium groups. These monomers were obtained from 2-(methacryloyloxy)ethyl-2-dodecylhydroxyethylmethylammonium bromide (QAHAMA-12) and cycloaliphatic diisocyanates, including isophorone diisocyanate (IPDI) and dicyclohexylmethane 4,4′-diisocyanate (CHMDI).
2. Materials and Methods
2.1. Materials
Synthesis substrates, such as methyl methacrylate (MMA), N-methyldiethanolamine (MDEA), and dodecyl bromide (DDB), were purchased from Acros Organics (Geel, Belgium). Diisocyanates, isophorone diisocyanate (IPDI), and dicyclohexylmethane 4,4′-diisocyanate (CHMDI), as well as phenothiazine (PTZ), camphorquinone (CQ), N,N-dimethylaminoethyl methacrylate (DMAEMA), and tetramethylsilane (TMS), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dibutyltin dilaurate (DBTDL) was purchased from Fluka (Charlotte, NC, USA). Deuterated solvents, namely trichloromethane (CDCl3) and dichloromethane (CD2Cl2), were purchased from Deutero GMBH (Kastellaun, Germany). Potassium carbonate (K2CO3) and magnesium sulfate (MgSO4) were purchased from Chempur (Piekary Śląskie, Poland). Solvents such as toluene, trichloromethane (CHCl3), and dichloromethane (CH2Cl2) were purchased from Stan-lab (Lublin, Poland).
2.2. Synthesis of Monomers Possessing Quaternary Ammonium Groups
2.2.1. Synthesis of N,N-(2-hydroxyethyl)methylaminoethyl Methacrylate (HAMA)
Novel monomers possessing quaternary ammonium groups were obtained using a three-step process. The synthesis procedure has been described in the literature [16].
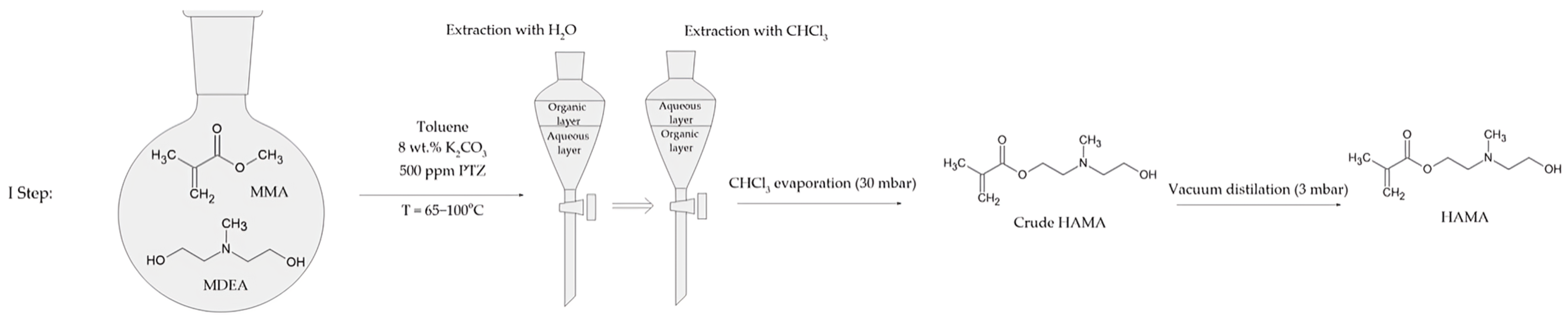
In the first step, MMA, MDEA, K2CO3, PTZ, and toluene were introduced to a 1000 mL round-bottom flask (Figure 1). The round-bottom flask was equipped with a standard distillation kit. The reaction was carried out for 2.5 h in the temperature range of 65–100 °C. The amounts of substrates and other substances used in the reaction are listed in Table 1.
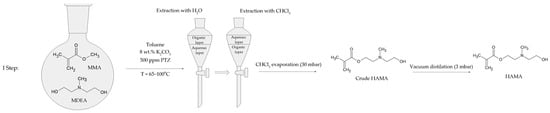
Figure 1.
Synthesis and preparation scheme of N,N-(2-hydroxyethyl)methylaminoethyl methacrylate.

Table 1.
The amounts of chemical substances used in the first step of synthesis.
After 2.5 h, the reaction mixture was cooled down and filtered. Then, the mixture was mixed with distilled water in a 2:1 ratio. Next, the aqueous phase was mixed with trichloromethane in a 3:1 ratio. The residual water was removed from the trichloromethane solution with MgSO4 overnight. The solvent was evaporated with a rotary evaporator under reduced pressure (30 mbar). The crude HAMA was purified by vacuum distillation (3 mbar), with fraction boiling at 110–130 °C. The yield of the reaction was 14%.
2.2.2. Synthesis of 2-(Methacryloyloxy)ethyl-2-dodecylhydroxyethylmethylammonium Bromide (QAHAMA-12)
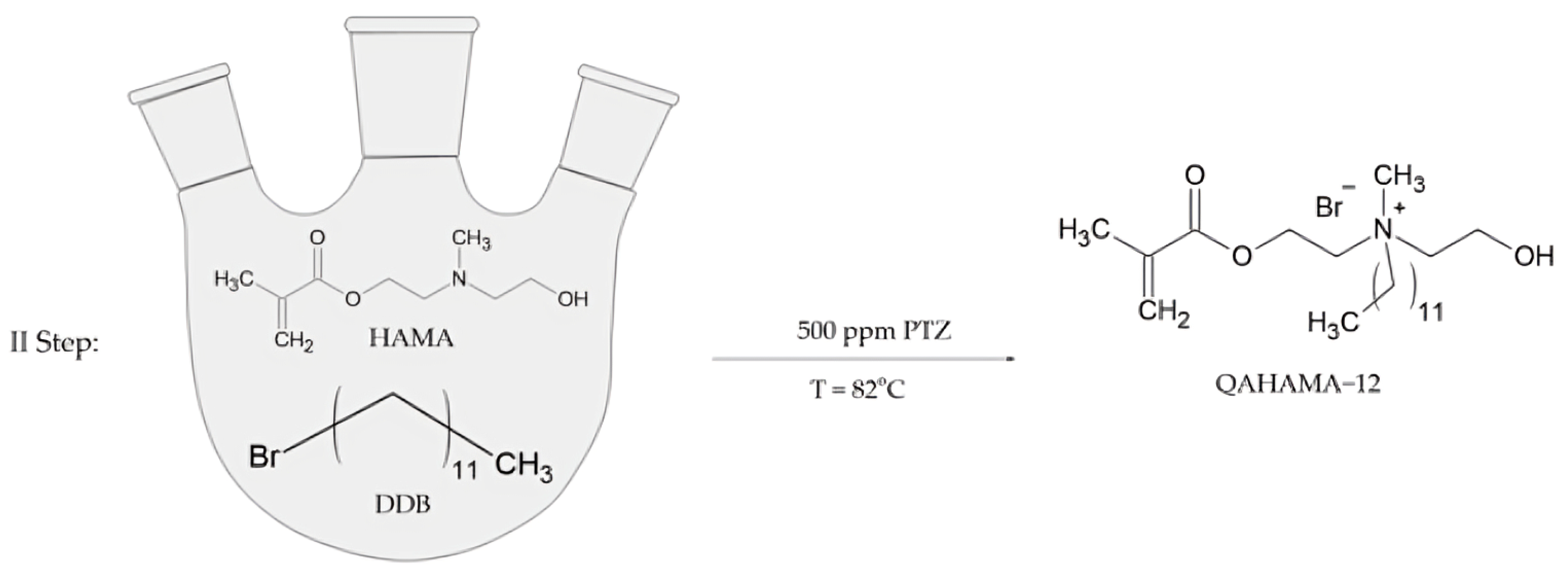
The second step of the synthesis was the N-alkylation of HAMA with alkyl bromide. HAMA, DDB, and PTZ were introduced to a 250 mL three-neck round-bottom flask (Figure 2). The exact amounts of chemical substances used in this step are presented in Table 2.
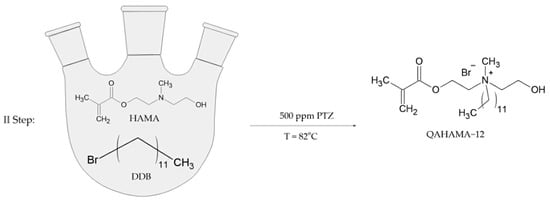
Figure 2.
Synthesis scheme of 2-(methacryloyloxy)ethyl-2-dodecylhydroxyethylmethylammonium bromide.

Table 2.
The amounts of chemical substances used in the reaction for obtaining QAHAMA-12.
The reaction was carried out for 5 days at 82 °C with the application of an oil bath and mechanical stirrer.
2.2.3. Synthesis of Quaternary Ammonium Urethane-Dimethacrylate Monomers (QA12 + IPDI and QA12 + CHMDI)
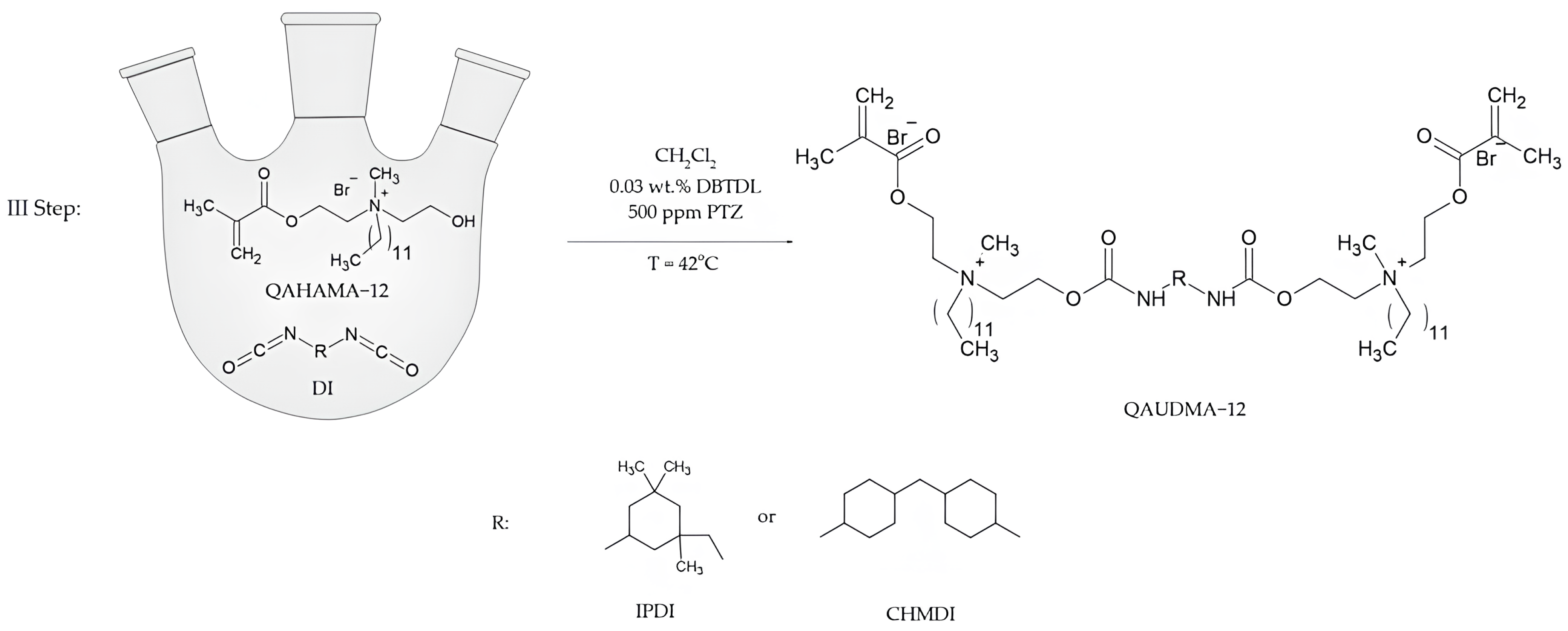
The final step of the synthesis was the addition of QAHAMA-12 to diisocyanate (Figure 3). A 50% solution of QAHAMA-12 and DBTDL in CH2Cl2 was introduced to a 250 mL three-neck round-bottom flask. The flask was equipped with a dropping funnel, reflux condenser, and thermometer. The mixture was brought to the boiling point of CH2Cl2 (approx. 42 °C), and then a 50% solution of diisocyanate was dropped into the boiling mixture for 1.5 h. The exact amounts of substrates and other chemical substances used in the final step are presented in Table 3.
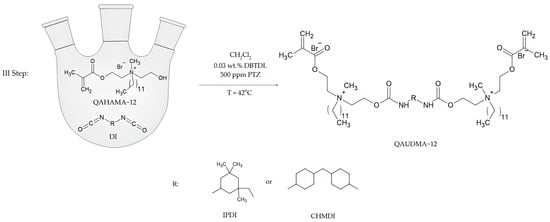
Figure 3.
Synthesis scheme of novel monomers based on IPDI and CHMDI.

Table 3.
The names of QAUDMA monomers and quantities of chemical substances used in the reaction of their synthesis.
After 1.5 h, the reaction was carried out for another 5 h, maintaining the boiling temperature of the solvent. Next, the mixture was cooled down and the solvent was evaporated with a rotary evaporator under reduced pressure (first 30 mbar, then 3 mbar). The obtained resins were placed in a laboratory dryer (SLW 53 STD, Wodzisław Śląski, Poland) at 42 °C for 24 h.
2.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
1H and 13C spectra were obtained with the application of a 300 MHz NMR spectrometer (UNITY/INOVA, Varian, Palo Alto, CA, USA). The 1H spectra were obtained with 512 scans and the 13C spectra with 40,000 scans. The deuterated solvents were CD2Cl2 and CDCl3, with TMS as the internal standard.
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra were obtained using the Spectrum Two spectrometer (Perkin-Elmer, Waltham, MA, USA) with 128 scans at a resolution of 1 cm−1.
3. Results
3.1. 1H NMR
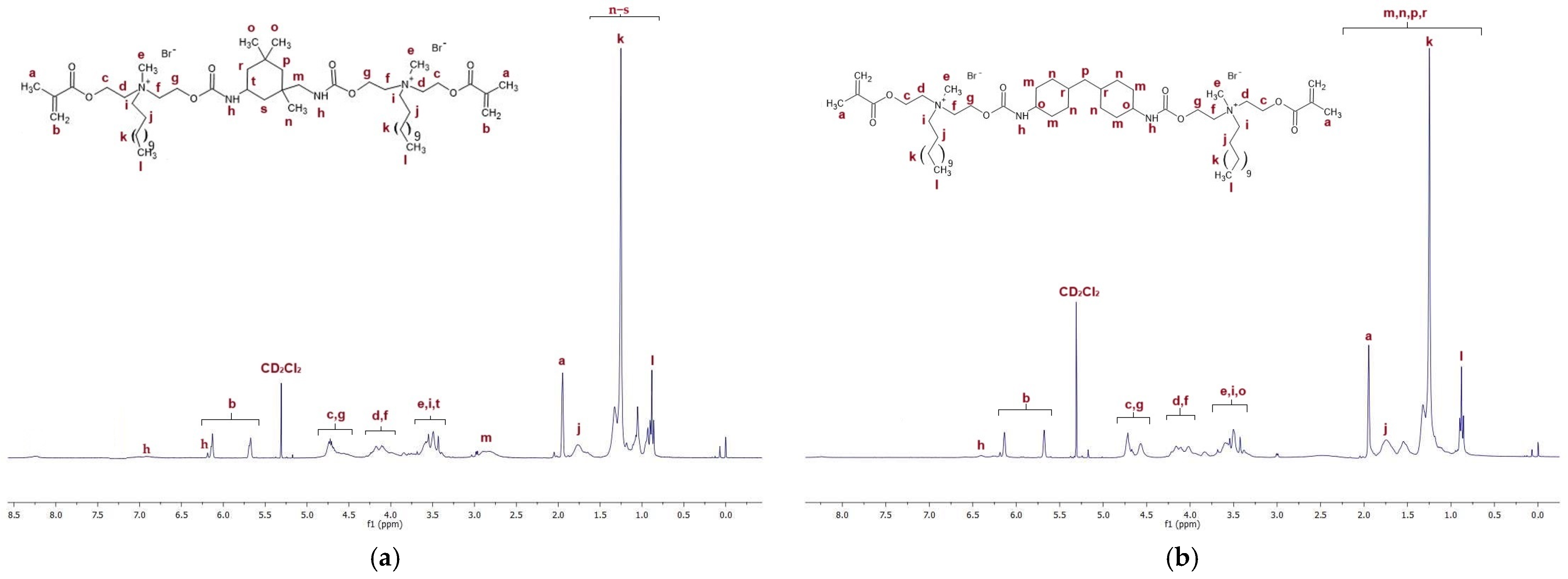
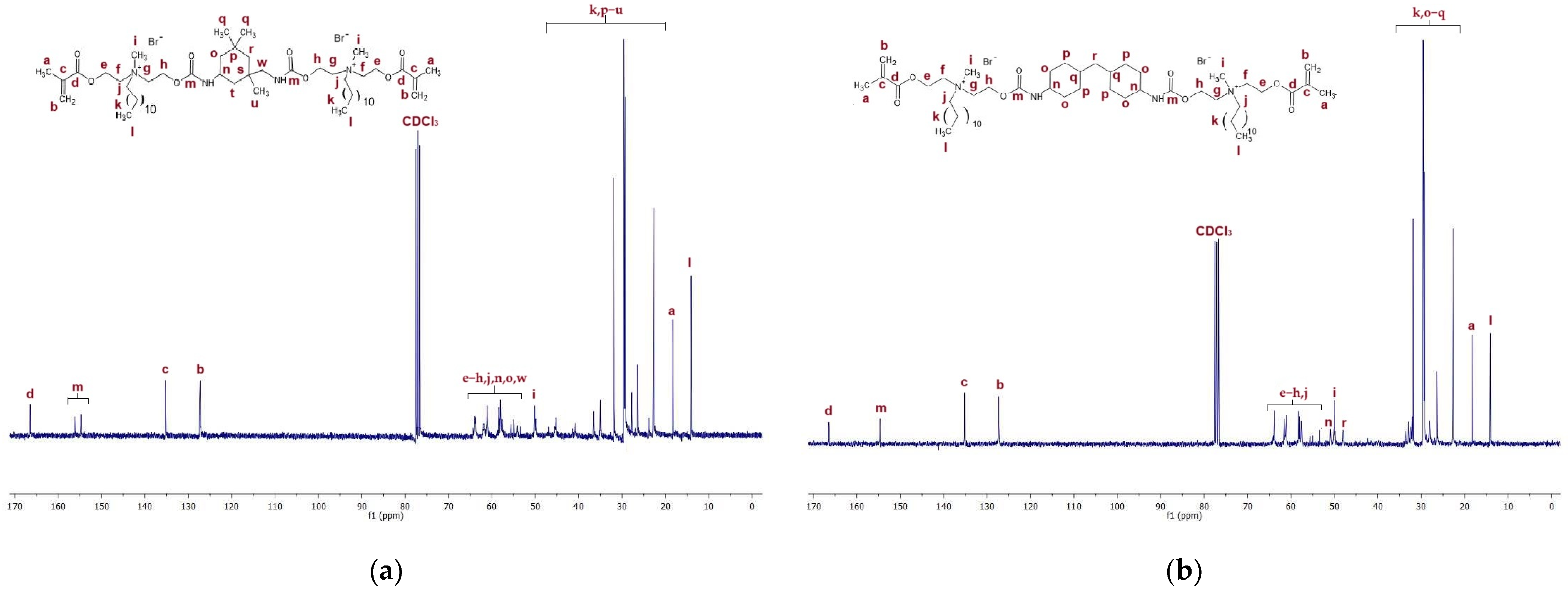
Figure 4a presents the 1H NMR spectra of QA12 + IPDI. Table S1 in the Supplementary Materials lists all peaks visible in the NMR spectra for QA12 + IPDI. Figure 4b shows the 1H NMR spectra of QA12 + CHMDI; all peaks are also presented in Table S2 (in the Supplementary Materials).
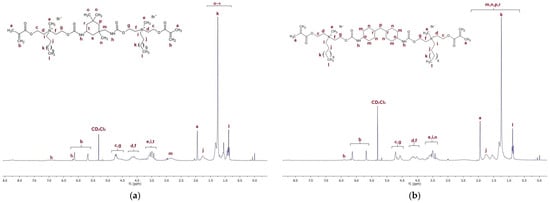
Figure 4.
1H NMR spectra of (a) QA12 + IPDI and (b) QA12 + CHMDI.
3.2. 13C NMR
Figure 5a,b present the 13C NMR spectra of QA12 + IPDI and QA12 + CHMDI. Tables S3 and S4 in the Supplementary Materials list all peaks visible in the 13C NMR spectra, respectively, for QA12 + IPDI and QA12 + CHMDI.
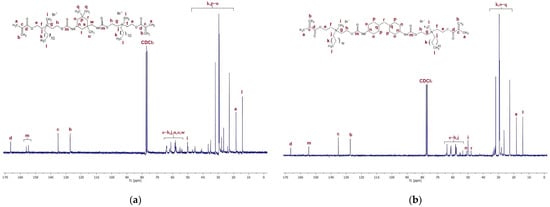
Figure 5.
13C NMR spectra of (a) QA12 + IPDI and (b) QA12 + CHMDI.
3.3. FTIR Spectra
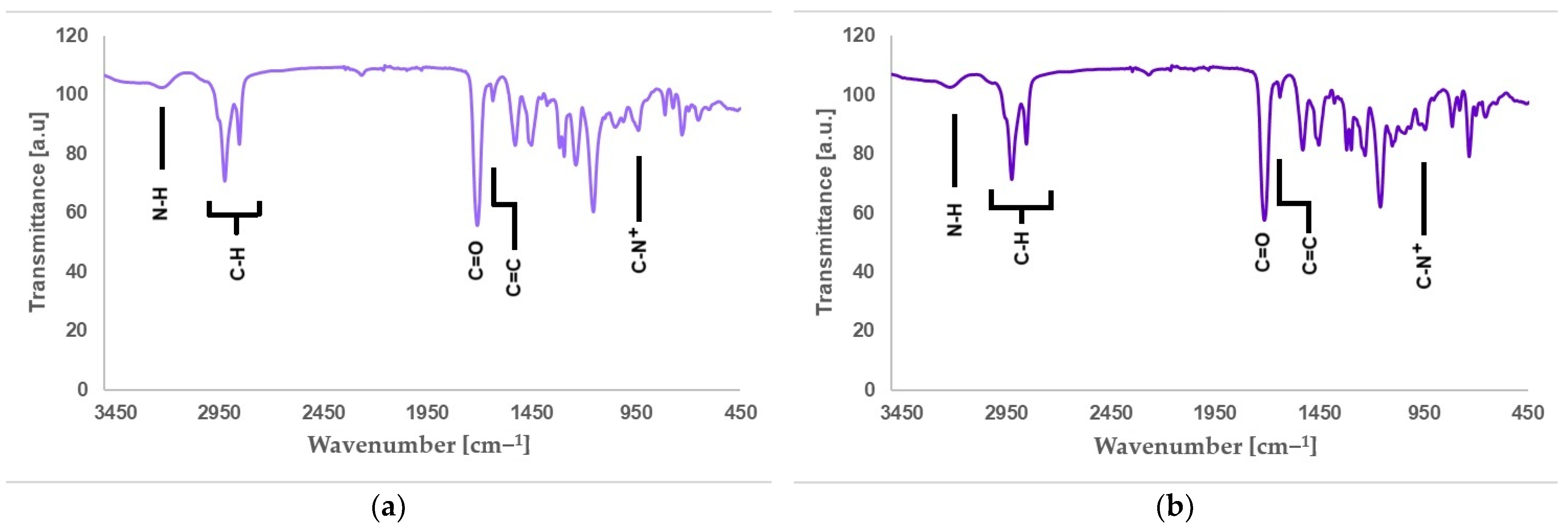
Figure 6a shows the FTIR spectra of QA12 + IPDI, and Figure 6b presents the FTIR spectra of QA12 + CHMDI. Table S5 (in the Supplementary Materials) lists all peaks visible in the FTIR spectra of QA12 + IPDI, and Table S6 (in the Supplementary Materials) collects all peaks of QA12 = CHMDI present in the FTIR spectra.
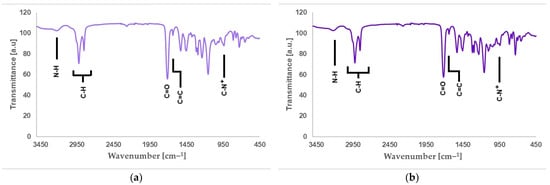
Figure 6.
FTIR spectra of (a) QA12 + IPDI and (b) QA12 + CHMDI.
4. Discussion
Two novel monomers were synthesized. The procedure known from the literature was adapted. This procedure was developed to obtain monomers based on 1,6-diisocyanato-2,2,4-trimethylhexane (TMDI) possessing various lengths of the N-alkyl substituent [16]. In this research, instead of TMDI, isophorone diisocyanate (IPDI) and dicyclohexylmethane 4,4′-diisocyanate (CHMDI) were used, as well as brododecane instead of alkyl bromides of various lengths. The synthesis procedure required modification. The time of the addition reaction of QAHAMA-12 to diisocyante was extended from 3 h to 5 h (Figure 3). A new step was also added to the procedure, namely thermal treatment for 24 h at 40 °C, due to the presence of an isocyanate group peak in FTIR after the evaporation of dichloromethane. The result was QA12 + IPDI and QA12 + CHMDI (Figure 7)—two yellowish, viscous liquids.

Figure 7.
The novel QAUDMA monomers in Petri dishes: (a) QA12 + IPDI; (b) QA12 + CHMDI.
The spectroscopic analysis of the novel monomer structures, with the application of NMR and IR techniques, confirmed the structures of QA12 + IPDI and QA12 + CHMDI. Key elements of these monomer structures are methacrylate groups, N-alkyl substituents, quaternary ammonium groups, urethane bonds, and a diisocyanate core. In the 1H NMR spectra, the signals of the methacrylate groups are visible at 1.94 ppm (-CH3) as well as 5.67 ppm and 6.12 ppm (=CH2) for both monomers (Figure 4a; Figure 4b; Table S1; Table S2). In the 13C NMR spectra, the signals of the methacrylate groups are visible at 19 ppm (-CH3) and 127 ppm and 128 ppm (=CH2) for QA12 + IPDI and QA12 + CHMDI, respectively; 135 ppm and 136 ppm (=CH2-C<) for QA12 + IPDI and QA12 + CHMDI, respectively; and 166 ppm (-COO-) for both monomers (Figure 5a; Figure 5b; Table S3; Table S4). Signals coming from the N-alkyl substituents visible in the 1H NMR spectra are at 3.30–3.70 ppm (N+-CH2-CH2-(CH2)9-CH3) for QA12 + IPDI and QA12 + CHMDI, 1.55–1.71 ppm (N+-CH2-CH2-(CH2)9-CH3) for both monomers, 1.25–1.32 ppm (N+-CH2-CH2-(CH2)9-CH3) for both monomers, and 0.86 ppm and 0.85 ppm (N+-CH2-CH2-(CH2)9-CH3) for QA12 + IPDI and QA12 + CHMDI, respectively (Figure 4a; Figure 4b; Table S1; Table S2). In the case of the 13C NMR spectra, the signals of the N-alkyl substituents are visible at 58–66 ppm for QA12 + IPDI and 55–66 ppm for QA12 + CHMDI (-N+-CH2-(CH2)10-CH3), 23–46 ppm and 23–35 ppm (-N+-CH2-(CH2)10-CH3) for QA12 + IPDI and QA12 + CHMDI, respectively, and 14 ppm (-N+-CH2-(CH2)10-CH3) for both monomers (Figure 5a; Figure 5b; Table S3; Table S4). The hydrogen of the urethane bond is visible at 6.20 ppm and 6.93 ppm for QA12 + IPDI and at 6.27 ppm and 6.44 ppm for QA12 + CHMDI (Figure 4a; Figure 4b; Table S1; Table S2); in the 13C NMR spectra, the carbon of the urethane bond is present at 155 ppm for both monomers (Figure 5a; Figure 5b; Table S3; Table S4). The IPDI core signals in the 1H NMR spectra are visible at 0.86–1.90 ppm (CH3-, -CH2-), 2.89 ppm (-CH2-NH-), and 3.30–3.70 ppm (>CH-NH-) (Figure 4a; Table S1), and in the 13C NMR spectra, the signals are visible at 23–46 ppm (CH3-, -CH2-, >C<) and 58–66 ppm (-CH2-, >CH-) (Figure 5a; Table S3). In the 1H NMR spectra, the signals of the CHMDI core are visible at 0.85–1.94 ppm (-CH2-; >CH-) and 3.30–3.70 ppm (>CH-) (Figure 4b; Table S2); in the 13C NMR spectra, the signals are present at 23–55 ppm (-CH2-, >CH-), 49 ppm (-CH2-), and 51 ppm (>CH-) (Figure 5b; Table S4). In the FTIR spectra, the 1urethane bond is visible at 3215 cm−1, as well as at 1534 cm−1 (QA12 + IPDI) and 1536 cm−1 (QA12 + CHMDI), while quaternary ammonium groups are present at 941 cm−1 for both monomers (Figure 6a; Figure 6b; Table S5; Table S6).
5. Conclusions
In the scope of this research, two novel monomers based on isophorone diisocyanate (IPDI) and dicyclohexylmethane 4,4′-diisocyanate (CHMDI) were synthesized. The monomers possessed key elements in their structures: two methacrylate groups, two N-alkyl substituents possessing 12 carbon atoms, two quaternary ammonium groups, two urethane bonds, and a core based on the abovementioned diisocyanates. The structures of these monomers were confirmed with Proton and Carbon Nuclear Magnetic Resonance Spectroscopy (1H and 13C NMR) and Fourier Transform Infrared Spectroscopy (FTIR). All of the key elements of the structures were present in the obtained spectra.
Future research should focus on the characterization of novel monomers and copolymerization with dental monomers used in DCRM matrices. Further research should determine the physicochemical and mechanical properties, as well as the antibacterial activity, of the copolymers.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/engproc2025087020/s1: Table S1: 1H NMR signals of QA12 + IPDI monomer; Table S2: 1H NMR signals of QA12 + CHMDI monomer; Table S3: 13C NMR signals of QA12 + IPDI monomer; Table S4: 13C NMR signals of QA12 + CHMDI monomer; Table S5: The interpretation of FTIR spectra of QA12 + IPDI monomer; Table S6: The interpretation of FTIR spectra of QA12 + CHMDI monomer.
Author Contributions
Conceptualization, I.B.-R. and P.D.; methodology, I.B.-R. and P.D.; formal analysis, P.D.; investigation, P.D. and P.K.; resources, I.B.-R., P.D. and P.K.; writing—original draft preparation, I.B.-R., P.D. and P.K.; writing—review and editing, I.B.-R. and P.D.; visualization, P.D.; supervision, I.B.-R.; project administration, I.B.-R.; funding acquisition, P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Polish Budget Funds for Scientific Research in 2024 as core funding for research and development activities at the Silesian University of Technology—funding for young scientists, grant number: 04/040/BKM24/0286.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
References
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef]
- Gajewski, V.E.; Pfeifer, C.S.; Fróes-Salgado, N.R.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Badr, S.; Abdulrahman, A.; Abdullah, A.; Essam, A.; Mohammad, Y.; Shahzeb, H. Effect of various antibacterial materials in dental composites: A systematic review. Ann. Dent. Spec. 2021, 9, 39–44. [Google Scholar]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M.; Montiel-Company, J.M. Antibacterial Properties of Nanoparticles in Dental Restorative Materials. A Systematic Review and Meta-Analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef]
- Chan, D.; Hu, W.; Chung, K.; Larsen, R.; Jensen, S.; Cao, D.; Gaviria, L.; Ong, J.; Whang, K.; Eiampongpaiboon, T. Reactions: Antibacterial and bioactive dental restorative materials: Do they really work? Am. J. Dent. 2018, 15, 32B–36B. [Google Scholar]
- Chen, L.; Suh, B.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar] [PubMed]
- Farrugia, C.; Camilleri, J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review. Dent. Mater. 2015, 31, e89–e99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Hu, Y.; Huang, F.; Xiao, Y. Quaternary ammonium compounds in dental restorative materials. Dent. Mater. J. 2018, 37, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Pringle, N.; Dube, A.; Adam, R.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Chen, J.H.; Ma, S.; Izutani, N. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn. Dent. Sci. Rev. 2012, 48, 115–125. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, Y.H.; Xing, X.D.; Li, F.; Ma, S.; Qi, L.L.; Chen, J.H. Antibacterial Activity and Cytotoxicity of Two Novel Cross-Linking Antibacterial Monomers on Oral Pathogens. Arch. Oral Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, F.; Sadeghi, B.; Najafi, F.; Mosslemin, M.H.; Niakan, M. Synthesis and Characterization of Novel Polymerizable Bis-Quaternary Ammonium Dimethacrylate Monomers with Antibacterial Activity as an Efficient Adhesive System for Dental Restoration. Polym. Bull. 2019, 76, 1295–1315. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Q.; Liu, F.; He, J.; Lin, Z. Synthesis of Novel Antibacterial Monomers (UDMQA) and Their Potential Application in Dental Resin. J. Appl. Polym. Sci. 2013, 129, 3373–3381. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and Characterization of Photo-Curable Bis-Quaternary Ammonium Dimethacrylate with Antimicrobial Activity for Dental Restoration Materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Chrószcz, M.W.; Barszczewska-Rybarek, I.M. Synthesis and Characterization of Novel Quaternary Ammonium Urethane-Dimethacrylate Monomers—A Pilot Study. Int. J. Mol. Sci. 2021, 22, 8842. [Google Scholar] [CrossRef] [PubMed]
- Chrószcz-Porębska, M.; Kazek-Kęsik, A.; Chladek, G.; Barszczewska-Rybarek, I. Novel mechanically strong and antibacterial dimethacrylate copolymers based on quaternary ammonium urethane-dimethacrylate analogues. Dent. Mater. 2023, 39, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Drejka, P.; Chrószcz-Porębska, M.; Kazek-Kęsik, A.; Chladek, G.; Barszczewska-Rybarek, I. Chemical Modification of Dental Dimethacrylate Copolymer with Tetramethylxylylene Diisocyanate-Based Quaternary Ammonium Urethane-Dimethacrylates—Physicochemical, Mechanical, and Antibacterial Properties. Materials 2024, 17, 298. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).