Abstract
The purpose of the present investigation was to formulate a mouth-dispersing film of a Clopidogrel Hydrogen Sulphate dosage form for a rapid onset of action, which is very easy for administration, without the issue of swallowing and using water. The Mouth Dissolving Film of Clopidogrel Hydrogen Sulphate was prepared through the Solvent Casting Method and its in vitro characterization was evaluated. The folding endurance is 173.6 ± 0.22, and the drug content is 96.33 ± 1.15. The drug release rate of the optimized formula A4 is 96.00% in 5 min, and it was concluded according to this result that the film prepared using an HPMC E5 polymer forms superior film that offers rapid drug release.
1. Introduction
Because of all of its benefits, Mouth Dissolving Film is a popular drug administration technique. When MDF comes into contact with saliva, it dissolves instantly and does not require any water. As a result, the formulation is enhanced for usage in patients of all ages and patient compliance is increased [1]. MDFs are preparations that come in the form of strips and include active chemicals that have been dissolved or dispersed in materials that create films [2]. It enables fast drug solubility, absorption, and instant bioavailability due to the high blood flow and permeability of buccal mucosa 4–1000 times greater than skin [3]. In conditions of various peripheral vascular disease, coronary artery disease, and cerebrovascular disease, clopidogrel is an oral, thienopyridine-class anti-platelet medication used to block blood clots and reduce the risk of myocardial infarction and stroke. For the drug clopidogrel, the preparation of a Mouth Dissolving Film was chosen since it has a low bioavailability. By creating the film, we may boost the drug’s bioavailability by avoiding first-pass metabolism, which also accelerates the drug’s onset of action.
2. Materials
Zentiva Pharmaceuticals, Ankleshwar, provided Clopidogrel Hydrogen Sulphate as a gift sample. Polyvinylpyrrolidone (PVP) and Hydroxy Propyl Methyl Cellulose (HPMC) E5 of an analytical grade were used. All of the testing was conducted using distilled water.
3. Method
3.1. Method of Preparation of Mouth Dissolving Film
The Solvent Casting Method is used for preparation of MDF of Clopidogrel Hydrogen Sulphate.
3.2. Steps for Preparation of MDF of Clopidogrel Hydrogen Sulphate [4]
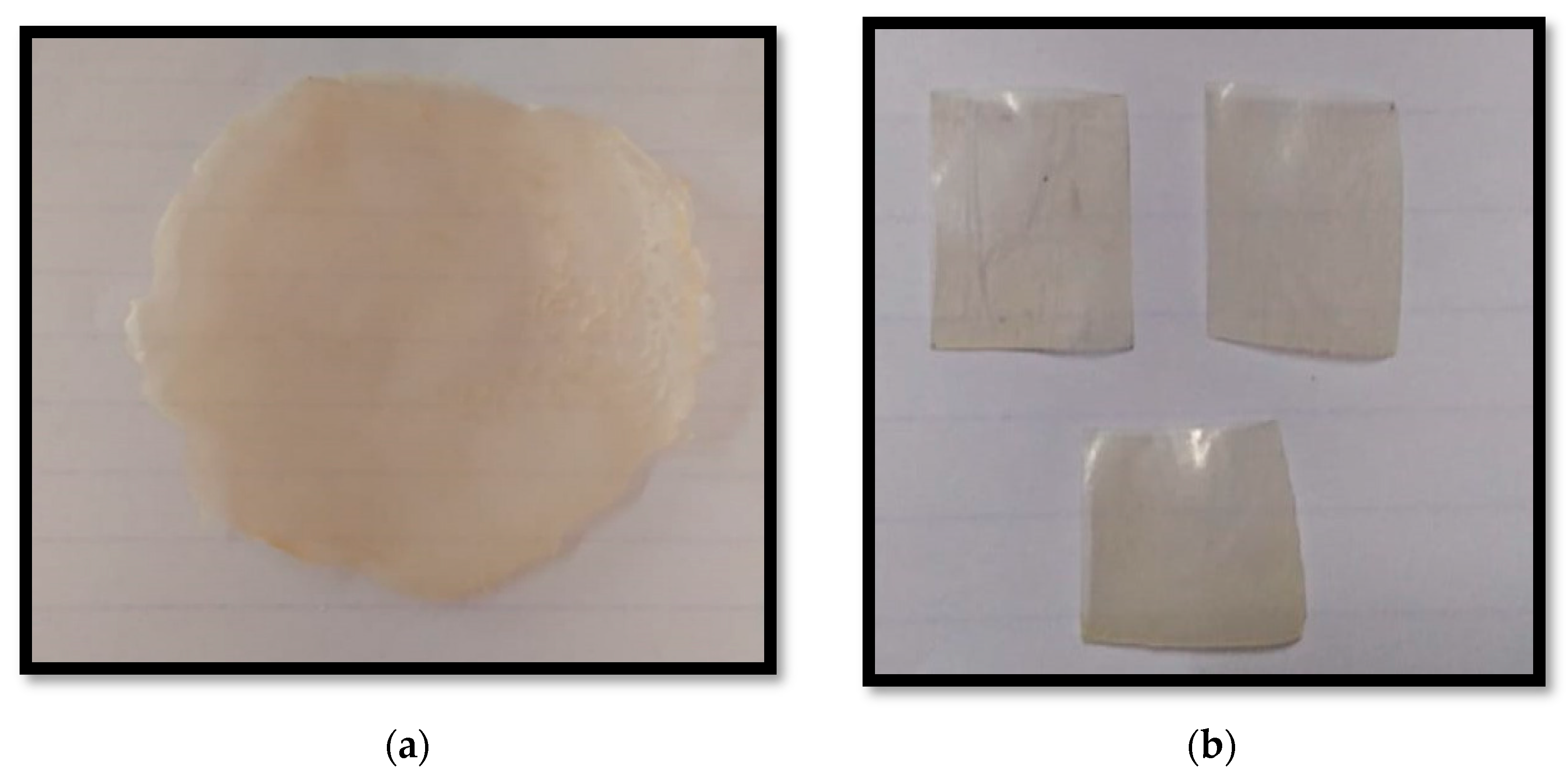
Films were prepared using the Solvent Casting Method in accordance with the formula (A4) shown in the Table 1. A sufficient amount of HPMC E5 was precisely weighed, then dissolved in water by continually stirring and sonicated for 15 min to produce a transparent solution. The required quantity of a plasticizer was added to the above solution. Clopidogrel Hydrogen Sulphate was dissolved in Methanol according to a requirement by continually stirring and also sonicated for 15 min to produce a transparent result. The remaining quantity of water was mixed with the other excipients, and the mixture was also sonicated for 15 min. The three solutions were combined and stirred continuously for five minutes. For 24 h, the solution was set down to allow trapped air bubbles to get out. The solution was then poured onto a glass Petri dish and allowed to dry for 24 h in a 50 °C oven. The films were carefully removed from the Petri plate after drying, cut into the required shape (2 cm2), and put on a glass Petri plate with a lid (Figure 1).

Table 1.
Composition of Mouth Dissolving Film.

Figure 1.
(a) Picture of Mouth Dissolving Film (batch A4); (b) Picture of MDF cut in desired shape and size.
4. Determination of λmax
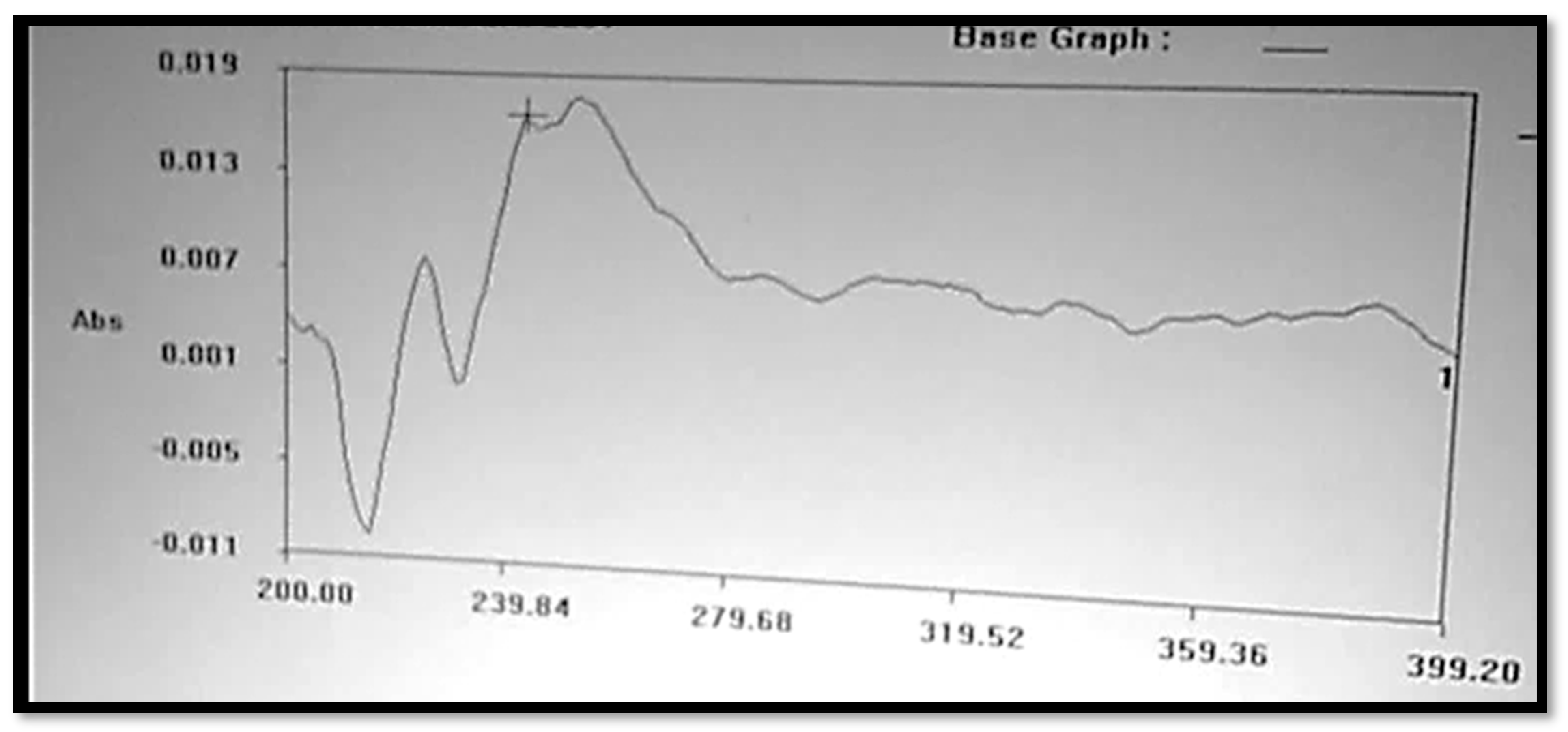
The Clopidogrel Hydrogen Sulphate solution was prepared in a 6.8 buffer solution, or pH 6.8, which is shown in Figure 2, and had a wavelength of maximum absorbance (max) that was consistent with the available literature. At 240 nm, the maximum concentration of Clopidogrel Hydrogen Sulphate was noted.
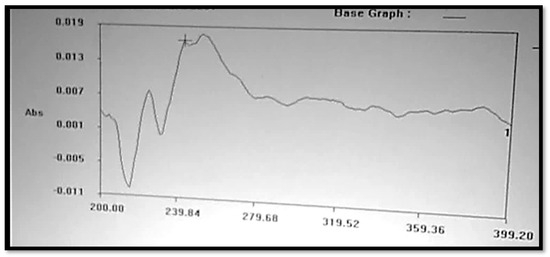
Figure 2.
UV spectrum of Clopidogrel Hydrogen Sulphate.
5. Preparation of Calibration Curve [5]
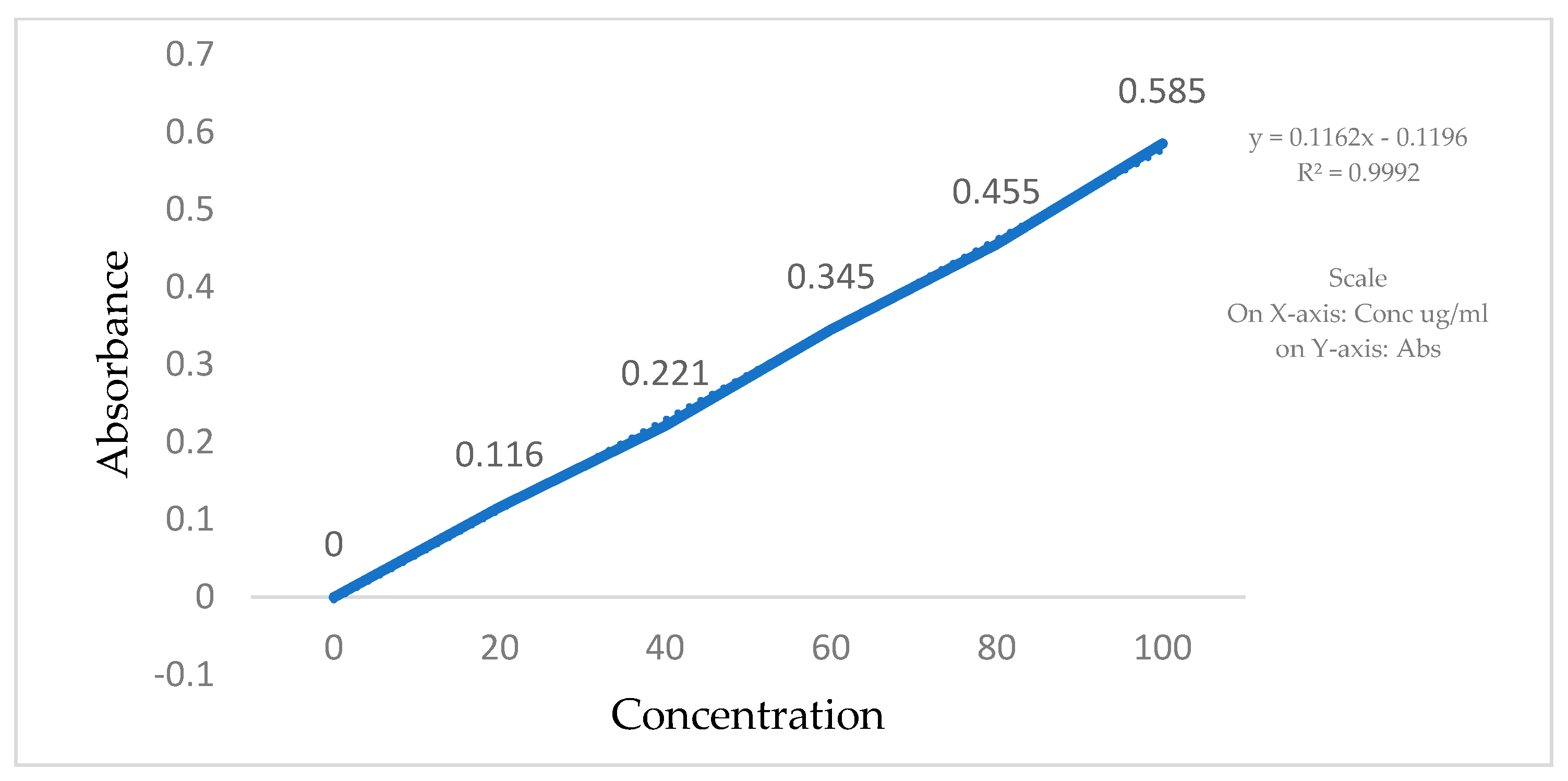
The calibration curves for Clopidogrel Hydrogen Sulphate were made in a phosphate buffer, pH 6.8, using a UV visible spectrophotometer. Stock solutions of Clopidogrel Hydrogen Sulphate were prepared by dissolving 2 mg of the drug in 10 mL of the phosphate buffer (pH 6.8). In total, 200 µg/mL of a Clopidogrel Hydrogen Sulphate standard stock solution was prepared in order to make the following dilutions: 20, 40, 60, 80, and 100 µg/mL. Absorbance of all the solutions was measured using a UV-VIS spectrophotometer in comparison to a blank at 240 nm (Table 2, Figure 3).

Table 2.
Standard calibration curve of Clopidogrel Hydrogen Sulphate in phosphate buffer (pH 6.8).
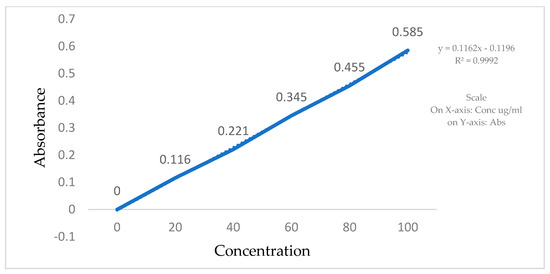
Figure 3.
Standard calibration curve of Clopidogrel Hydrogen Sulphate in phosphate buffer.
6. Drug–Polymer Compatibility Study [6]
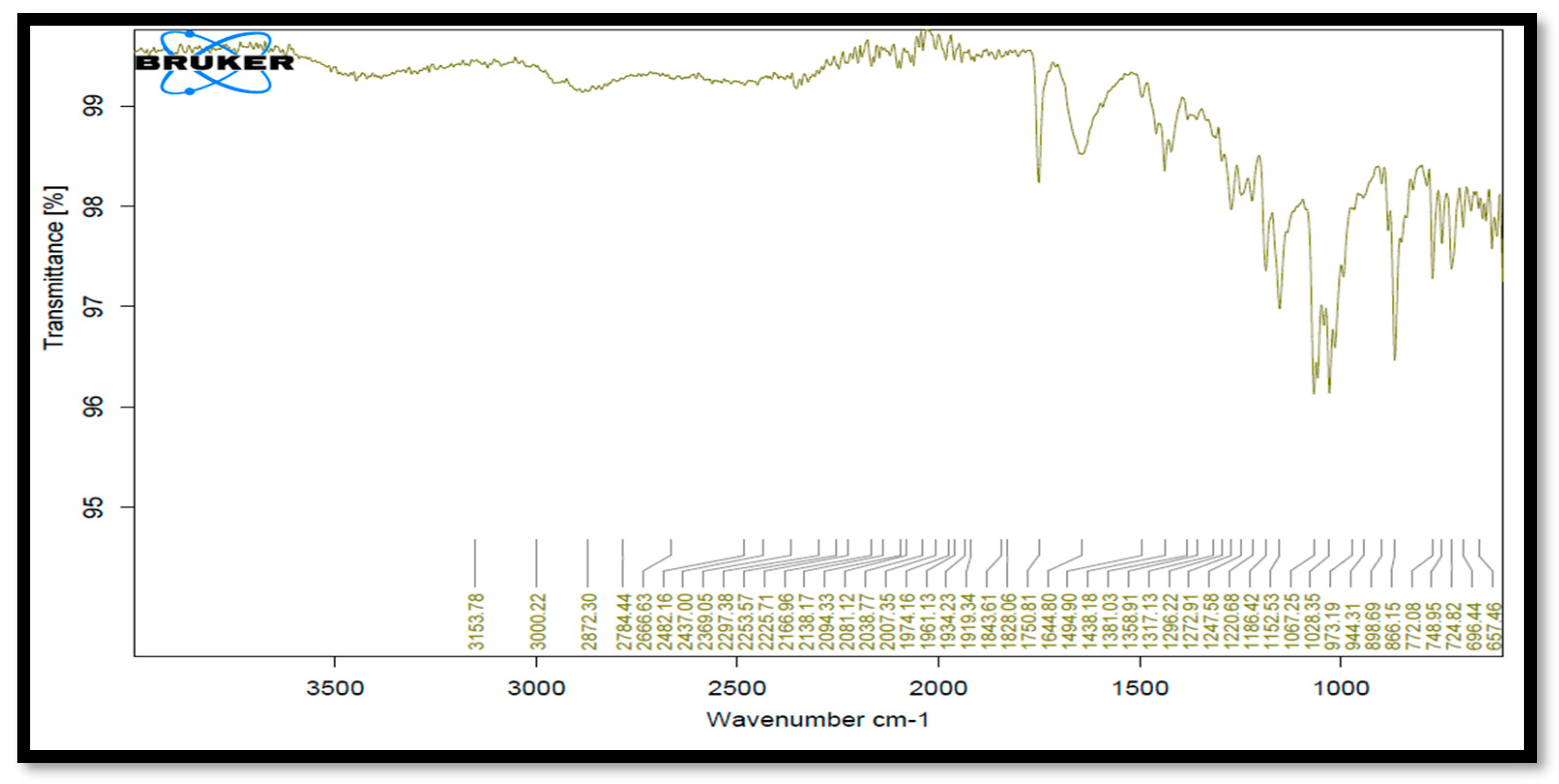
After keeping the drug, the excipient sample was in a stability chamber at a temperature of 40 °C and 75% relative humidity. Drug and HPMC E5 and PVP compatibility was discovered. None of these excipients interfered with any of the peaks, according to IR data. It demonstrated that each excipient was acceptable for use with the medication (Table 3, Figure 4).

Table 3.
Interpretation of IR Spectra of Physical mixture.
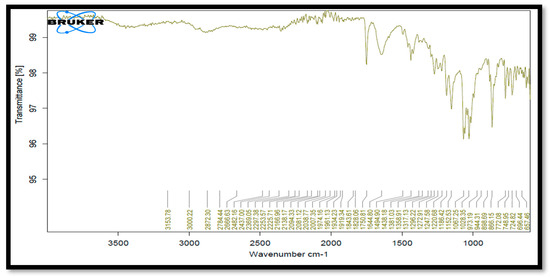
Figure 4.
FTIR spectra of Clopidogrel Hydrogen Sulphate with HPMC E5 and PVP.
7. Result and Discussion
7.1. Thickness
The film’s thickness was measured at three different places using micrometer screw gauges and the average of those three readings was calculated.
7.2. Weight Variation
One-square-inch sections were cut at three different positions. On an electronic scale, the weight of each strip was recorded, and weight variation was calculated.
7.3. Folding Endurance
The exact value of folding endurance (a measure of fragility) was determined by how many times the strip could be folded at the same location without breaking. For prepared films, a 2 × 2 cm2 strip was cut uniformly and folded repeatedly on the same spot and the folding endurance was measured.
7.4. Percentage Drug Content
For determination of Clopidogrel Hydrogen Sulphate content, the complete strip (full Petri plate) as well as film of known sizes (4 × 5 cm2) were dissolved in the phosphate buffer, 0.2 M (pH 6.8). The amount of the drug present was determined by measuring the absorbance at 240 nm (UVVIS spectrophotometer, systronics2201, Ahmedabad, India). The drug content was determined by plotting a standard calibration curve of the drug in the phosphate buffer (pH 6.8).
7.5. Surface pH Determination
The film to be tested was placed in a Petri dish and was moistened with 0.5 mL of distilled water and kept for 30 s. By using a pH meter, the pH was noted after bringing the electrode into contact with the surface of the formulation and allowing equilibration for 1 min. The average of three determinations for each formulation was determined.
7.6. Disintegration Time
For disintegration time, the film as per the dimension (2 × 2 cm2) required for dose delivery was placed in 10 mL of the phosphate buffer. Disintegration time was noted as per time required by the film to break (Table 4).

Table 4.
Evaluation of Clopidogrel Hydrogen Sulphate MDF.
7.7. In Vitro Dissolution Study
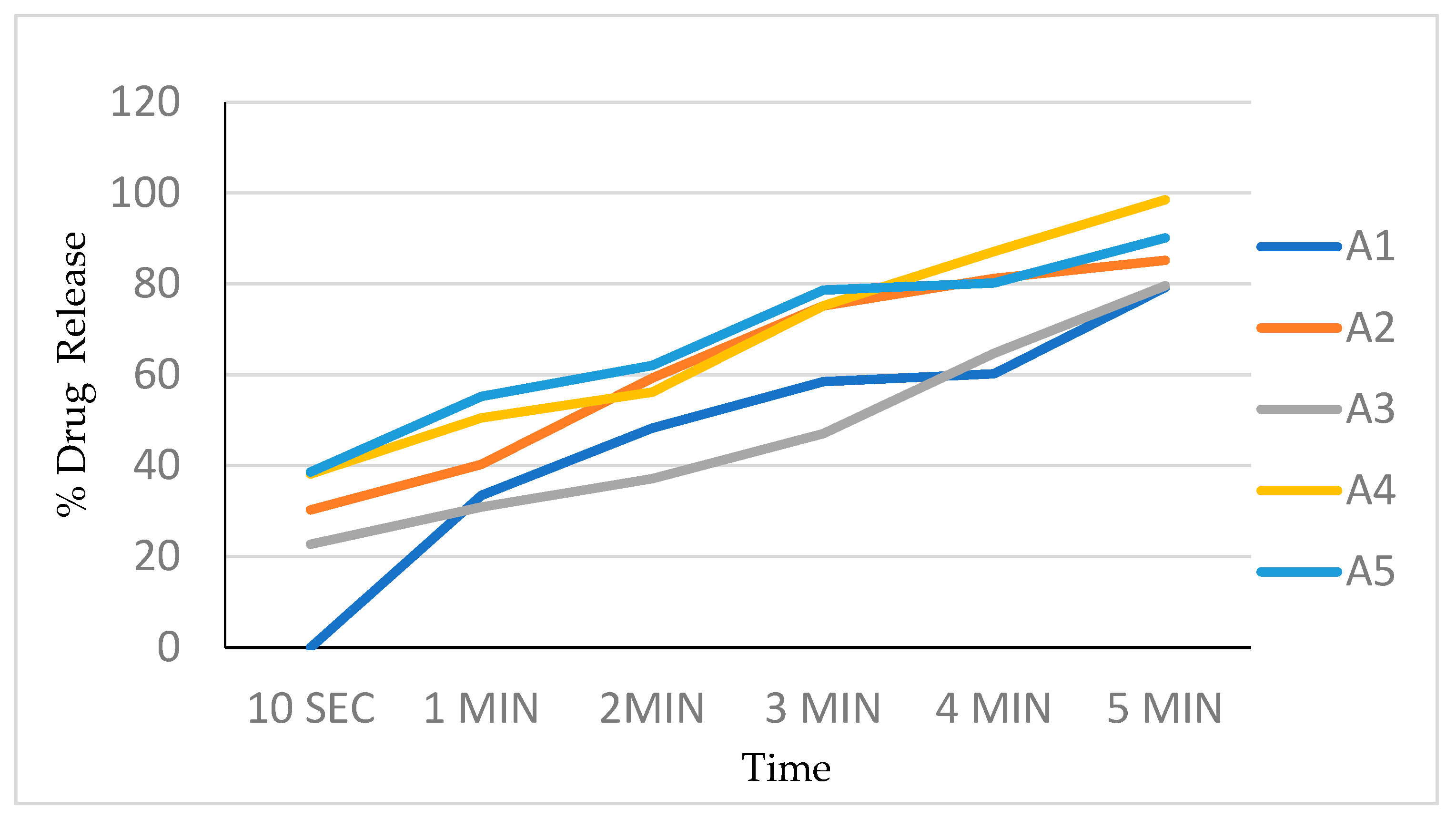
The in vitro dissolution studies were conducted using a 150 mL glass beaker with 125 mL of the phosphate buffer as a dissolution medium. Film (2 × 2 cm2) was placed on one side of the beaker using double-sided tape. The medium was stirred at a speed of 200 rpm using a magnetic stirrer bar. Additionally, 5 mL samples were withdrawn at 10, 20, 30, 40, 50, 60, 80, 100, and 120 s time intervals and replaced with 5 mL of a fresh dissolution medium every time. The samples were analyzed by measuring UV absorbance at 240 nm. The dissolution experiments were conducted in triplicate. Percentages of the drug dissolved at different time intervals are noted (Table 5, Figure 5).

Table 5.
In vitro drug dissolution study of Clopidogrel Hydrogen Sulphate.
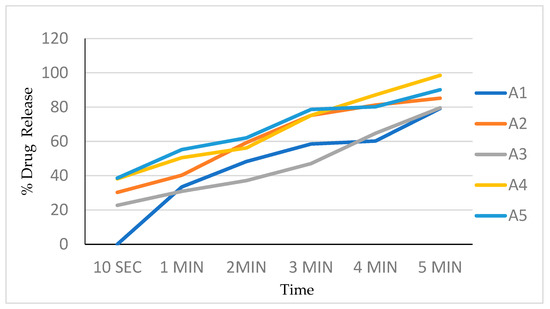
Figure 5.
Drug release profile of batch A1 to A5.
8. Conclusions
According to the results of the current study, it is possible to create Clopidogrel Hydrogen Sulphate Mouth Dissolving Films using the Solvent Casting Process, which will increase therapeutic efficacy while increasing bioavailability and patient compliance. All films produced with PVP as the plasticizer and HPMC E5 as the film-forming polymer can be used as films. The enhanced formulation A4 has the quickest disintegration time of 22.33 ± 2.081, the drug content being 96.33 ± 1.15. There was a maximum folding endurance of 173.6 ± 0.22, and a rapid in vitro drug release of 96.00% in 5 min. The manufactured films seem like a good alternative to conventional marketed formula.
Author Contributions
Conceptualization, A.C.; methodology, A.C. and B.P.; validation, A.C. and B.P.; investigation, A.C.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and S.T.; supervision, S.T. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We would like to convey our obligation to the principal of P.S.G.V.P. Mandal’s College of Pharmacy, Shahada, District Nandurbar, for furnishing all the essential facilities for the completion of research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ankita, C.; Sandip, T.; Sunil, P. Mouth dissolving film: An innovative and effective drug delivery system. Int. J. Res. Pharm. Allied Sci. 2023, 2, 78–79. [Google Scholar]
- Hema, C.; Samita, G.; Permender, R.; Kumar, V. Development and Optimization of Fast Dissolving Oro dispersible Films of Granisetron HCl using Box-Behnken Statistical Design. Bull. Fac. Pharm. 2013, 51, 193–201. [Google Scholar]
- Hiroyoshi, S.; Kazumi, T.; Misao, N.; Katsuhiko, M.; Tadao, T.; Hirotaka, Y. Preparation of a fast-dissolving oral thin film containing dexamethasone: A possible application to anti emesis during cancer chemotherapy. Eur. J. Pharm. Biophar. 2009, 73, 361–365. [Google Scholar]
- Kulkarni, K.; Dixit, M.; Gunashekara, K.; Shahnawaz, A.; Singh, N.; Kulkarni, A. Formulation and Evaluation of Mouth Dissolving Film Containing Rofecoxib. Int. Res. J. Pharm. 2011, 2, 273–278. [Google Scholar]
- Rajat, P.; Ravi, S.; Gajanan, D. Formulation and Evaluation of Mouth Dissolving Film of Prochlorperazine Maleate. J. Drug Deliv. Ther. 2019, 9, 110–115. [Google Scholar]
- Bhalekar, M.; Madgulkar, A.; Shaikh, S. Formulation and Evaluation of Chitosan- Based Mucoadhesive Buccal Patch of prochlorperazine Maleate. Int. J. Pharm. Pharm. Res. 2018, 12, 61–73. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).