Tafamidis Drug Delivery Systems Based on Chitosan/Polyvinyl Alcohol Matrix †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Solution Preparation
2.3. In Vitro Drug Release Study
2.4. Chromatographic Conditions
3. Results and Discussion
3.1. Tafamidis Concentration in the Prepared Solutions
3.2. In Vitro Drug Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Aguanno, V.; Ralli, M.; Artico, M.; Russo, F.Y.; Scarpa, A.; Fiore, M.; Tirassa, P.; Severini, C.; de Vincentiis, M.; Greco, A. Systemic Amyloidosis: A Contemporary Overview. Clin. Rev. Allergy Immunol. 2020, 59, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Shams, P.; Ahmed, I. Cardiac Amyloidosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK580521/ (accessed on 10 July 2023).
- Griffin, J.M.; Rosenblum, H.; Maurer, M.S. Pathophysiology and Therapeutic Approaches to Cardiac Amyloidosis. Circ. Res. 2021, 128, 1554–1575. [Google Scholar] [CrossRef]
- Mallus, M.T.; Rizzello, V. Treatment of amyloidosis: Present and future. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2023, 25 (Suppl. B), B99–B103. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Kasi, A.; Khan, B. Bradyarrhythmias in Cardiac Amyloidosis and Role of Pacemaker. Curr. Probl. Cardiol. 2023, 48, 101912. [Google Scholar] [CrossRef]
- Andreeva, S.E.; Snetkov, P.P.; Vakhrushev, Y.A.; Piankov, I.A.; Yaznevich, O.O.; Bortsova, M.A.; Morozkina, S.N.; Kajava, A.V.; Kostareva, A.A.; Uspenskaya, M.V. Molecular basis of amyloid deposition in myocardium: Not only ATTR and AL. Case report. Transl. Med. 2022, 9, 26–35. [Google Scholar] [CrossRef]
- Snetkov, P.; Morozkina, S.; Olekhnovich, R.; Vu, T.H.N.; Tyanutova, M.; Uspenskaya, M. Curcumin/Usnic Acid-Loaded Electrospun Nanofibers Based on Hyaluronic Acid. Materials 2020, 13, 3476. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.N.; Morozkina, S.N.; Uspenskaya, M.V.; Olekhnovich, R.O. Fabrication of Polyvinyl Alcohol Nanofibers for the Delivery of Biologically Active Molecules. In Proceedings of the IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 7–9 December 2022; pp. 344–349. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; Diflunisal; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548132/ (accessed on 3 January 2018).
- Griffin, J.M.; Rosenthal, J.L.; Grodin, J.L.; Maurer, M.S.; Grogan, M.; Cheng, R.K. ATTR Amyloidosis: Current and Emerging Management Strategies. JACC CardioOncol. 2021, 3, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Zahran, F.; Cabañas, A.; Cheda, J.A.R.; Renuncio, J.A.R.; Pando, C. Dissolution rate enhancement of the anti-inflammatory drug diflunisal by coprecipitation with a biocompatible polymer using carbon dioxide as a supercritical fluid antisolvent. J. Supercrit. Fluids 2014, 88, 56–65. [Google Scholar] [CrossRef]
- VYNDAQEL—Tafamidis Meglumine Capsule, Liquid Filled; VYNDAMAX—Tafamidis Capsule, Liquid Filled. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1b4121ee-a733-4456-a917-be2603477839 (accessed on 30 June 2023).
- Snetkov, P.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Diflunisal Targeted Delivery Systems: A Review. Materials 2021, 14, 6687. [Google Scholar] [CrossRef] [PubMed]
- Hong Nhung, V.T. Nonwoven Materials Based on Polyvinyl Alcohol and Chitosan as Mangiferin Carriers. Ph.D. Thesis, ITMO University, Saint Petersburg, Russia, 2023. Available online: https://dissovet.itmo.ru/dissertation/?number=911530; http://fppo.ifmo.ru/?page1=16&page2=86&number_file=DB2D4CC18085DCB24973352DE3BDF705 (accessed on 30 June 2023).

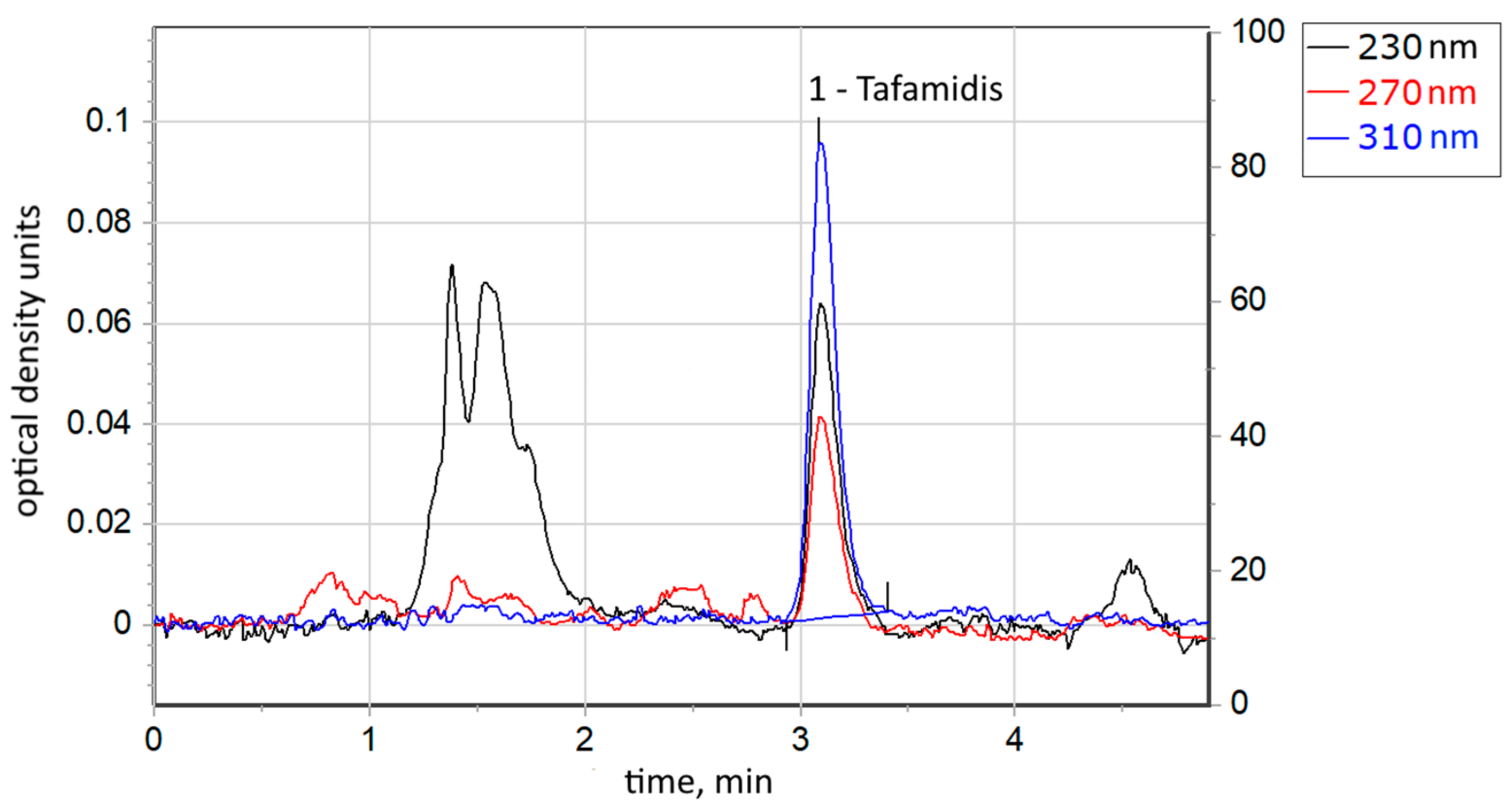

| TAF-01 | TAF-02 | |
|---|---|---|
| Tafamidis concentration, wt.% | 0.10 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snetkov, P.; Generalova, Y.; Vu, T.H.N.; Morozkina, S.; Uspenskaya, M. Tafamidis Drug Delivery Systems Based on Chitosan/Polyvinyl Alcohol Matrix. Eng. Proc. 2023, 56, 260. https://doi.org/10.3390/ASEC2023-15905
Snetkov P, Generalova Y, Vu THN, Morozkina S, Uspenskaya M. Tafamidis Drug Delivery Systems Based on Chitosan/Polyvinyl Alcohol Matrix. Engineering Proceedings. 2023; 56(1):260. https://doi.org/10.3390/ASEC2023-15905
Chicago/Turabian StyleSnetkov, Petr, Yuliya Generalova, Thi Hong Nhung Vu, Svetlana Morozkina, and Mayya Uspenskaya. 2023. "Tafamidis Drug Delivery Systems Based on Chitosan/Polyvinyl Alcohol Matrix" Engineering Proceedings 56, no. 1: 260. https://doi.org/10.3390/ASEC2023-15905
APA StyleSnetkov, P., Generalova, Y., Vu, T. H. N., Morozkina, S., & Uspenskaya, M. (2023). Tafamidis Drug Delivery Systems Based on Chitosan/Polyvinyl Alcohol Matrix. Engineering Proceedings, 56(1), 260. https://doi.org/10.3390/ASEC2023-15905








