Thermal Characterization of Biochars Produced in Slow Co-Pyrolysis of Spent Coffee Ground and Concentrated Landfill Leachate Residue †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass and Additive
2.2. Py-Process
2.3. Characterization
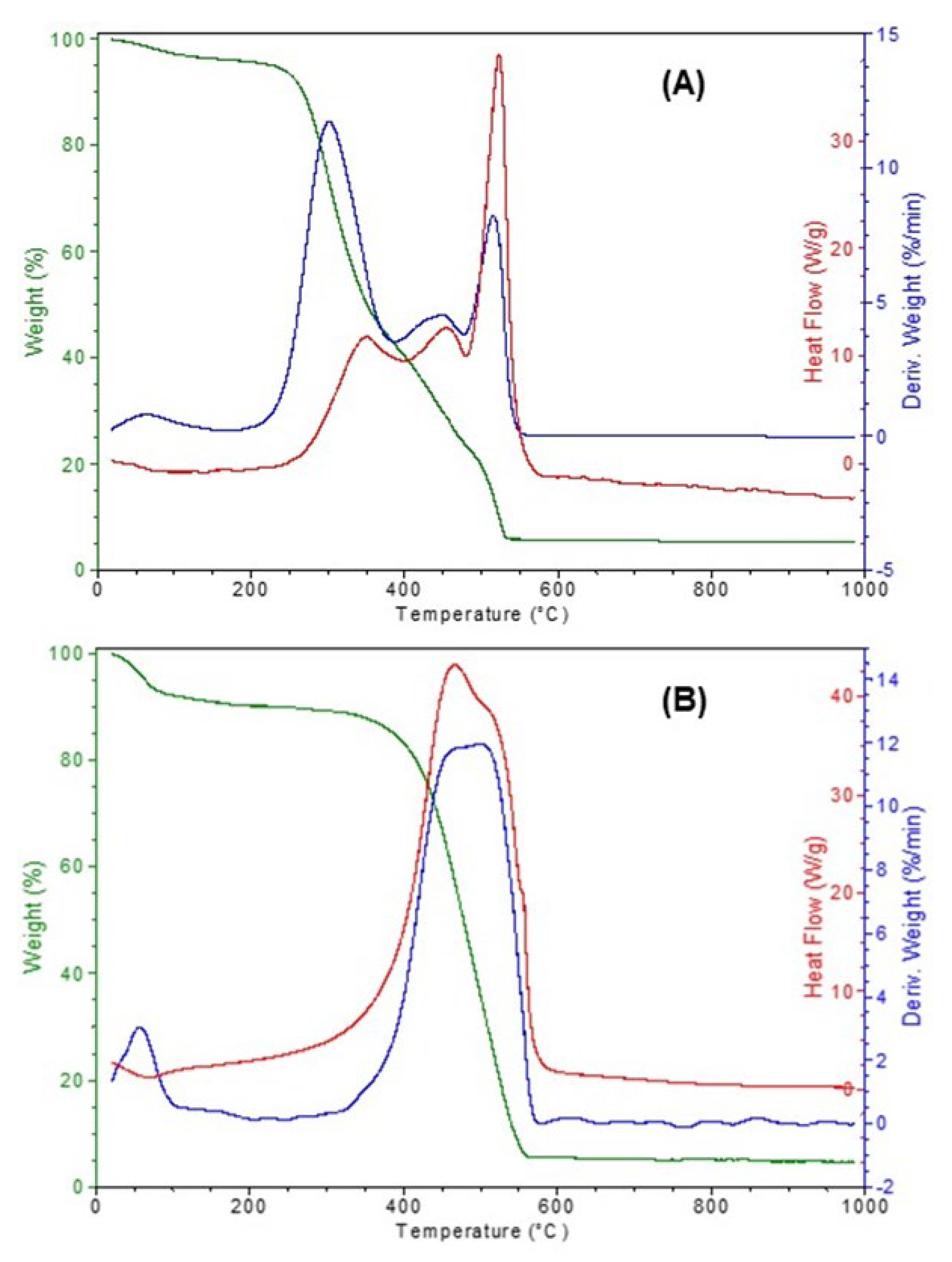
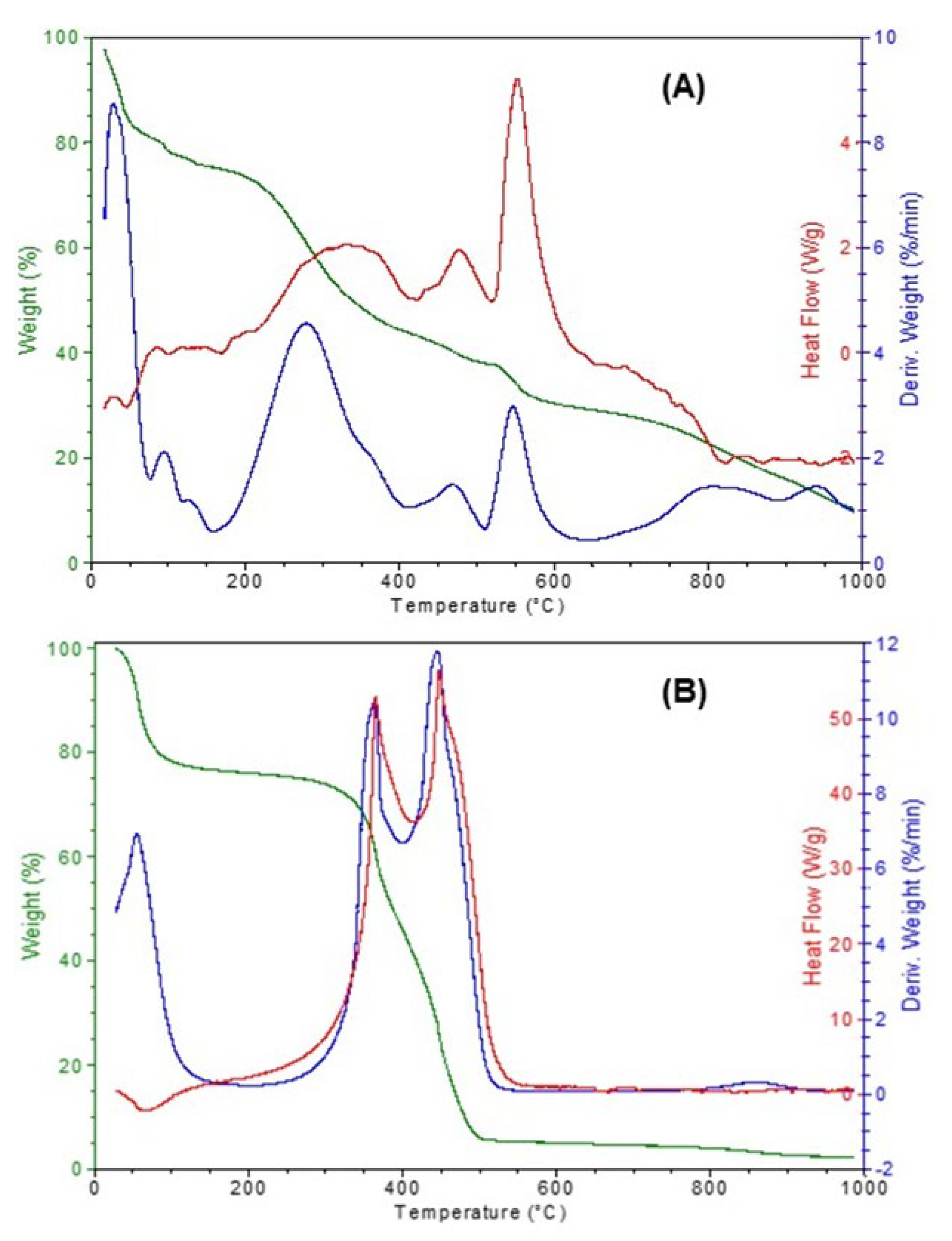
3. Results and Discussion
3.1. Thermal Analysis
3.2. SEM/EDS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Almeida, R.; Porto, R.F.; Quintaes, B.R.; Bila, D.M.; Lavagnolo, M.C.; Campos, J.C. A review on membrane concentrate management from landfill leachate treatment plants: The relevance of resource recovery to close the leachate treatment loop. Waste Manag. Res. 2023, 41, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Gripa, E.; Daflon, S.D.A.; de Almeida, R.; da Fonseca, F.V.; Campos, J.C. Landfill Leachate Treatment by High-presssure Membranes and Advanced Oxidation Techniques with a focus on Ecotoxicity and By-products Management: A Review. Process Saf. Environ. Prot. 2023, 173, 747–764. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Karatas, O.; Rezania, H.; Kobya, M.; Vatanpour, V.; Khataee, A. A review on treatment of membrane concentrates generated from landfill leachate treatment processes. Sep. Purif. Technol. 2021, 259, 118182. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, H.; Su, L.; Zhang, R.; Cao, R.; Wang, L.; Lou, Z. Molecular transformation and composition flow of dissolved organic matter in four typical concentrated leachates from the multi-stage membrane system. J. Environ. Manag. 2022, 310, 114759. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Remmen, K.; Wintgens, T.; Melin, T. Nanofiltration in Landfill Leachate Treatment. In Nanofiltration; Wiley: Hoboken, NJ, USA, 2021; pp. 663–690. [Google Scholar]
- Tow, E.W.; Ersan, M.S.; Kum, S.; Lee, T.; Speth, T.F.; Owen, C.; Bellona, C.; Nadagouda, M.N.; Mikelonis, A.M.; Westerhoff, P.; et al. Managing and treating per- and polyfluoroalkyl substances (PFAS) in membrane concentrates. AWWA Water Sci. 2021, 3, e1233. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassen-Trabelsi, A.; Kallel, A.; Ben Amor, E.; Cherbib, A.; Naoui, S.; Trabelsi, I. Up-Grading Biofuel Production by Co-pyrolysis of Landfill Leachate Concentrate and Sewage Sludge Mixture. Waste Biomass Valorization 2020, 11, 291–301. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Chen, M.; Min, F.; Zhang, S.; Ren, Z.; Yan, Y. Catalytic effects of six inorganic compounds on pyrolysis of three kinds of biomass. Thermochim. Acta 2006, 444, 110–114. [Google Scholar] [CrossRef]
- Turek, M.E.; Freitas, K.S.; Armindo, R.A. Spent coffee grounds as organic amendment modify hydraulic properties in a sandy loam Brazilian soil. Agric. Water Manag. 2019, 222, 313–321. [Google Scholar] [CrossRef]
- Kim, M.-S.; Min, H.-G.; Koo, N.; Park, J.; Lee, S.-H.; Bak, G.-I.; Kim, J.-G. The effectiveness of spent coffee grounds and its biochar on the amelioration of heavy metals-contaminated water and soil using chemical and biological assessments. J. Environ. Manag. 2014, 146, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Mendoza Martinez, C.L.; Saari, J.; Melo, Y.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Evaluation of thermochemical routes for the valorization of solid coffee residues to produce biofuels: A Brazilian case. Renew. Sustain. Energy Rev. 2021, 137, 110585. [Google Scholar] [CrossRef]
- Grossule, V.; Fang, D.; Yue, D.; Lavagnolo, M.C.; Raga, R. Preparation of artificial MSW leachate for treatment studies: Testing on black soldier fly larvae process. Waste Manag. Res. 2022, 40, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lavagnolo, M.C.; Bai, H.; Pivato, A.; Raga, R.; Yue, D. Environmental and economic assessment of leachate concentrate treatment technologies using analytic hierarchy process. Resour. Conserv. Recycl. 2019, 141, 474–480. [Google Scholar] [CrossRef]
- Pellera, F.; Regkouzas, P.; Manolikaki, I.; Diamadopoulos, E. Biochar production from waste biomass: Characterization and evaluation for agronomic and environmental applications. Detritus 2021, 17, 15–29. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Zhang, M.; Zhu, X.; Min, F.; Tan, Z. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, R.; Lanero, F.; Lavagnolo, M.C.; Sgarbossa, P.; Bertani, R.; Vianna, M.M.; Campos, J.C. Thermal Characterization of Biochars Produced in Slow Co-Pyrolysis of Spent Coffee Ground and Concentrated Landfill Leachate Residue. Eng. Proc. 2023, 37, 12. https://doi.org/10.3390/ECP2023-14614
de Almeida R, Lanero F, Lavagnolo MC, Sgarbossa P, Bertani R, Vianna MM, Campos JC. Thermal Characterization of Biochars Produced in Slow Co-Pyrolysis of Spent Coffee Ground and Concentrated Landfill Leachate Residue. Engineering Proceedings. 2023; 37(1):12. https://doi.org/10.3390/ECP2023-14614
Chicago/Turabian Stylede Almeida, Ronei, Francesco Lanero, Maria Cristina Lavagnolo, Paolo Sgarbossa, Roberta Bertani, Marcelo Mendes Vianna, and Juacyara Carbonelli Campos. 2023. "Thermal Characterization of Biochars Produced in Slow Co-Pyrolysis of Spent Coffee Ground and Concentrated Landfill Leachate Residue" Engineering Proceedings 37, no. 1: 12. https://doi.org/10.3390/ECP2023-14614
APA Stylede Almeida, R., Lanero, F., Lavagnolo, M. C., Sgarbossa, P., Bertani, R., Vianna, M. M., & Campos, J. C. (2023). Thermal Characterization of Biochars Produced in Slow Co-Pyrolysis of Spent Coffee Ground and Concentrated Landfill Leachate Residue. Engineering Proceedings, 37(1), 12. https://doi.org/10.3390/ECP2023-14614






