Abstract
Heterocyclic compounds are abundant in natural products and bioactive compounds and play a huge role in the present repertoire of medicinal chemists due to their potential capability to modulate physicochemical properties. As a result, chemists have focused their efforts on the functionalization of heterocycles. Nitrogen-containing fused heterocyclic compounds are important organic molecules. They are found in a variety of natural products, medicinal compounds and functional materials as structural fragments. The imidazo[1,2-a]pyrimidine skeleton is one of them, and it is linked to the pharmacological activity of related drugs. Anticancer activity medicines, anxiolytic drugs and anti-inflammatory activity pharmaceuticals all have this structural pattern. Many of them have biological, antifungal, antimicrobial, antiviral and anxiolytic properties, which are used in medications such as divaplon and fasiplon. This invention of a new approach to manufacturing 2-arylsubstituted imidazo[1,2-a]pyrimidines efficiently piqued our interest, given the powerful bioactivities of molecules with an imidazopyrimidine core. As a result, appropriate methods for manufacturing such molecules remain appealing. In this context, we would like to present a feasible green chemistry approach for the synthesis of 2-phenyl-imidazo[1,2-a]pyrimidines.
1. Introduction
The development of greener protocols for the synthesis of highly functionalized motifs with medicinal value has always been welcome in pharmaceutical science and is an attractive research thrust area [1]. Fused heterocyclic compounds are key structural scaffolds in a broad variety of natural products, drug molecules and functional materials [2]. Numerous imidazo[1,2-a]pyrimidine derivatives are significant as pharmaceuticals since they have been found to have several biological activities, with some remarkable clinical examples being fasiplon and divaplon [3,4]. For those reasons, imidazo[1,2-a]pyrimidines are precious artificial targets. Due to their excessive pharmacologic interests, a widespread variety of synthetic protocols have been defined within the literature over an extended period of time [5].
Recently, the use of catalysed organic chemistry methods has become a very powerful green chemical technology procedure from both economical and synthetic points of view [6,7,8,9]. There is also another route through which to combine economic aspects with environmental ones, that is, the use of green solvents [8,9]. Hence, we now report a green, efficient, and rapid procedure for the synthesis of imidazo[1,2-a]pyrimidine derivatives (Figure 1) obtained by different agents, using supported gold nanoparticles as the catalyst.
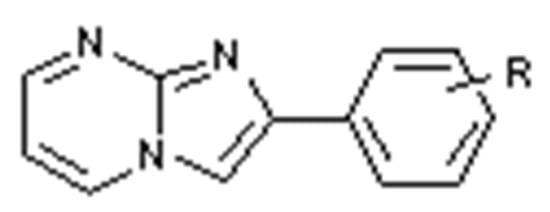
Figure 1.
Structure of imidazo[1,2-a]pyrimidines.
2. Results and Discussion
In conjugation with our recent research on the synthesis of nitrogen heterocycles, we describe here a novel and efficient procedure for the synthesis of four imidazo[1,2-a]pyrimidine derivatives (Table 1). This method is a process that combines two components in a single reaction under green chemistry conditions.

Table 1.
Synthesis of 2-aryl-imidazo[1,2-a]pyrimidine derivatives.
3. Experimental Procedure
Herein, we describe a simple and efficient method of synthesis of imidazo[1,2-a]pyrimidines under green conditions.
General procedure: A mixture of aryl ketone derivatives and 2-aminopyrimidine was stirred under heating of the green solvent and catalyzed using gold nanoparticles. After cooling, the solid obtained was washed several times to give the desired products.
4. Conclusions
We have developed a procedure to efficiently synthesize imidazo[1,2-a]pyrimidines through the reaction between aryl ketones and 2-aminopyrimidine under green conditions. The compound’s structure is confirmed using spectral analysis. The important features of this protocol are mild reaction conditions, an environmentally friendly process, and high yields which reflect the activity of the nanocatalyst we prepared. The environmental friendliness and simplicity of this synthetic strategy offers an attractive alternative to conventional methods.
Author Contributions
Z.K. and A.B.; methodology; Z.K. validation; D.B. writing—original draft preparation, N.C.-B. and R.B. data collection. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the Directorate General for Scientific Research and Technological Development (DGRSDT), the University of Tlemcen and the University of Ain Témouchent for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahire, V.N.; Patil, G.P.; Deore, A.B.; Chavan, P.G.; Jirimali, H.D.; Mahulikar, P.P. Sulfonated chitosan-encapsulated HAp@ Fe3O4: An efficient and recyclable magnetic nanocatalyst for rapid eco-friendly synthesis of 2-amino-4-substituted-1,4-dihydrobenzo [4,5] imidazo [1,2-a] pyrimidine-3-carbonitriles. Res. Chem. Intermediat. 2018, 44, 5801–5815. [Google Scholar] [CrossRef]
- Wu, J.; Luo, H.; Wang, T.; Sun, H.; Zhang, Q.; Chai, Y. Diverse synthesis of pyrimido [1,2-a] benzimidazoles and imidazo [2,1-b] benzothiazoles via CuI-catalyzed decarboxylic multicomponent reactions of heterocyclic azoles, aldehydes and alkynecarboxylic acids. Tetrahedron 2019, 75, 1052–1063. [Google Scholar] [CrossRef]
- Rao, C.; Mai, S.; Song, Q. Cu-catalyzed synthesis of 3-formyl imidazo [1,2-a] pyridines and Imidazo [1,2-a] pyrimidines by employing ethyl tertiary amines as carbon sources. Org. Lett. 2017, 19, 4726–4729. [Google Scholar] [CrossRef] [PubMed]
- Yarie, M.; Zolfigol, M.A.; Baghery, S.; Khoshnood, A.; Alonso, D.A.; Kalhor, M.; Bayat, Y.; Asgari, A. Design, synthesis, and application of 1 H-imidazol-3-ium trinitromethanide {[HIMI]C(NO2)3} as a recyclable nanostructured ionic liquid (NIL) catalyst for the synthesis of imidazo [1,2-a] pyrimidine-3-carbonitriles. J. Iran. Chem. Soc. 2018, 15, 2259–2270. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, M.; Ding, M. Efficient Synthesis of New Tetracyclic Benzofuro [3,2-d]-imidazo [1,2-a] pyrimidine-2,5-(1H,3H)-diones. Chin. J. Chem. 2010, 28, 309–312. [Google Scholar] [CrossRef]
- Hou, Z.W.; Mao, Z.Y.; Malcamu, Y.Y.; Lu, X.; Xu, H.C. Electrochemical Synthesis of Imidazo-Fused N-Heteroaromatic Compounds through a C−N Bond-Forming Radical Cascade. Angew. Chem. 2018, 130, 1652–1655. [Google Scholar] [CrossRef]
- Hamidinasab, M.; Mobinikhaledi, A. Organoacid-decorated NiFe2O4 nanoparticles: An efficient catalyst for green synthesis of 2H-indazolo [2,1-b] phthalazine-triones and pyrimido [1,2-a] benzimidazoles. Chem. Sel. 2019, 4, 17–23. [Google Scholar]
- Hu, L.; Zhan, Z.; Lei, M.; Hu, L. Facile and green method for the synthesis of 4-amino-1, 2-dihydrobenzo [4,5] imidazo [1,2-a] pyrimidine-3-carbonitriles catalysed by ammonium acetate. J. Chem. Res. 2012, 36, 738–739. [Google Scholar] [CrossRef]
- Fekri, L.Z.; Nikpassand, M.; Khakshoor, S.N. Green, effective and chromatography free synthesis of benzoimidazo [1,2-a] pyrimidine and tetrahydrobenzo [4,5] imidazo [1,2-d] quinazolin-1 (2H)-one and their pyrazolyl moiety using Fe3O4@ SiO2@ L-proline reusable catalyst in aqueous media. J. Org. Chem. 2019, 894, 18–27. [Google Scholar] [CrossRef]
- Atif, H.Y.S.; Wagare, D.S.; Ahmed, A.Z.; Durrani, A.N. Ultrasound promoted one-pot synthesis of 2-arylimidazo[1,2-a]pyrimidines in glycerol. Rasayan J. Chem. 2021, 14, 2645–2651. [Google Scholar] [CrossRef]
- Xie, Y.Y. Organic Reactions in Ionic Liquids: Ionic Liquid-Accelerated One-Pot Synthesis of 2-Arylimidazo [1,2-a] pyrimidines. Synth. Commun. 2005, 35, 1741–1746. [Google Scholar] [CrossRef]
- Rao, R.N.; Balamurali, M.M.; Maiti, B.; Thakuria, R.; Chanda, K. Efficient access to imidazo[1,2-a] pyridines/pyrazines/pyrimidines via catalyst-free annulation reaction under microwave irradiation in green solvent. ACS Comb. Sci. 2018, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).