Abstract
Due to the presence of two acrylonitrile fragments, 3,5-di(α-cyanostyryl)-1,2,4-thiadiazoles are prone to react under Radziszewski reaction conditions (oxidative hydrolysis of nitriles to amides) with the simultaneous epoxidation and formation of epoxyamides. We found that the reaction proceeds in a non-selective way and yields a mixture of products of regioisomeric oxidation. Only in one case we succeeded to isolate the product of double epoxidation. The structure of the epoxyamides products was confirmed by IR and NMR spectroscopy data.
1. Introduction
In previous paper, we reported the synthesis of substituted 1,2,4-thiadiazoles by the oxidative dimerization of arylmethylene cyanothioacetamides upon treatment with DMSO-HCl system [1]. These oxidation products have highly reactive acrylonitrile moieties, so they can be further transformed to yield a plethora of new heterocyclic products.
The Radziszewski reaction is widely used in organic practice to prepare primary carboxamides from nitriles under mild oxidative conditions. It is known [2,3,4,5,6,7,8,9] that acrylonitriles react under Radziszewski conditions with a simultaneous epoxidation of C=С double bond. Therefore, due to the presence of two reactive C=C-C≡N fragments, 3,5-di(α-cyanostyryl)-1,2,4-thiadiazoles appeared to be suitable substrates for the synthesis of new epoxyamides. The aim of our study was to determine the structure of oxidation products as well as their optimal reaction conditions. The resulting amides may be of interest as reagents for heterocyclic and supramolecular synthesis.
2. Results and Discussion
The starting 3,5-di(α-cyanostyryl)-1,2,4-thiadiazoles 2 were prepared by oxidative dimerization of arylmethylene cyanothioacetamides 1 in DMSO-HCl system [1] (Scheme 1).
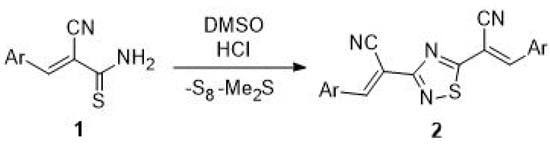
Scheme 1.
Synthesis 3,5-di(α-cyanostyryl)-1,2,4-thiadiazoles 2.
The functionalized 1,2,4-thiadiazoles 2 were oxidized under the Radziszewski reaction conditions (H2O2, KOH) with the involvement of one of the acrylonitrile fragments and the formation of epoxyamides 3a and 3b in moderate yields (up to 50%) (Scheme 2). We found that the oxidation is non-regioselective and yields a mixture of regioisomeric epoxides. Only in one case it was possible to isolate the product of double epoxidation 4. The resulting amides are of interest as reagents for heterocyclic and supramolecular synthesis. The structure of the prepared compounds is confirmed by spectral data.
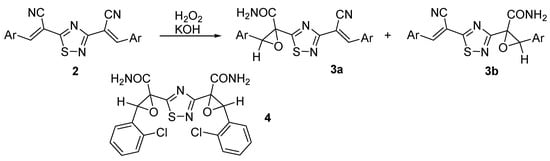
Scheme 2.
Оxidation of 3,5-di(α-cyanostyryl)-1,2,4-thiadiazoles 2.
Compounds 3а and 3b are white or yellowish powders insoluble in water or EtOH. The reaction proceeds very slowly at room temperature. The use of larger amounts of KOH favors the oxidation rate, but generally causes a decrease in yields of epoxyamides 3a and 3b, probably due to the partial hydrolysis of both precursors and reaction products.
The structure of the synthesized compounds was confirmed by spectral data. Thus, the IR spectra of the compounds revealed characteristic absorption bands corresponding to stretching vibrations of N–H bonds, amide C=O and the conjugated C≡N group. It was concluded that only one acrylonitrile fragment is involved in the Radziszewski reaction. The 1H NMR spectrum of epoxyamides is characterized by the presence of two narrow singlets of protons of the oxirane ring in the range of 4.72–4.81 ppm. The signal of the amide group and signals of the aromatic substituent are resolved as a multiplet at 7.35–7.71 ppm. The signals of the CH protons of the acrylonitrile fragment are found in the range of 8.14–8.42 ppm.
3. Experimental Section
3.1. Method for the Synthesis of Thiadiazoles 2
Arylmethylidene cyanothioacetamide 1 (1.5 mmol) was placed in a beaker, and acetone or EtOH (3–5 mL) and DMSO (0.6 mL, 8.45 mmol) were added. For complete dissolution of thioamide, the mixture was gently heated. To a solution formed, conc. HCl (0.5 mL, 4.7 mmol) was added dropwise with constant stirring. The reaction mixture turned red for a moment, then discolored and became turbid (formation of colloidal sulfur). Within a few seconds, the product precipitated (Caution! Dimethyl sulfide has extensively evolved!). The suspension was diluted with EtOH (5 mL), maintained for 24 h at 20 °C, and the solid product was filtered off. To remove the traces of elemental sulfur, the prepared thiadiazoles were purified by recrystallization from acetone, AcOH or by re-precipitation from DMSO with aqueous EtOH.
3.2. Method for the Synthesis of Compounds 3a and 3b
A portion of 10% aqueous KOH solution (1.5 mmol) was added to a mixture of the corresponding thiadiazole (3 mmol), thoroughly ground to a fine powder, and 32% H2O2 (d = 1.1 g/mL) (2.7 mL, 0.03 mol) in EtOH. The reaction mixture was heated under vigorous stirring until an exothermic reaction started. After the reaction was complete and the evolution of oxygen ceased, the crystalline solid was filtered off and washed with cold aq. EtOH to produce epoxyamides 3.
3.3. Method for the Synthesis of Compound 4
A 50 mL beaker was charged with the starting 1,2,4-thiadiazole (1 mmol), previously thoroughly ground to a powdery state, and 10 mL of EtOH. The mixture was heated to 30 °С. A solution of 10% KOH (d = 1.0904 g/cm3) (0.07 mL, 0.1 mmol) was added drop by drop. The reaction mixture turned dark to a cherry-red color. Then, 0.95 mL (0.01 mol) of 32% H2O2 solution (p = 1.112 g/cm3) was added drop by drop. The mixture was heated upon vigorous stirring until a violent exothermic reaction started. The reaction was accompanied by the evolution of oxygen, the reaction mixture turned clear, and the formation of a white fine-crystalline precipitate was observed. The mixture was stirred for another 30 min until the bright-yellow color of the starting thiadiazole completely disappeared. Then, the solid was precipitated with water, filtered, and washed with alcohol and water. A white powder weighing 0.22 g (50%) was obtained. IR spectrum, v, cm−1: 3415, 3249 (NH), 1679 (C=O). NMR spectrum 1H (400 MHz, DMSO-d6), δ, ppm: 4.73 s (1H, CHoxirane); 4.82 s (1H, CHoxirane); 7.48 m (2H, H Ar); 7.50 m (2H, H Ar); 7.58 m (2H, H Ar); 7.60 m (2H, H Ar); 7.55 br s (2H, CONH2); 7.84 br s (2H, CONH2). C20H14Cl2N4O4S Calculated, %: C, 50.33; H, 2.96; Cl, 14.86; N, 11.74; O, 13.41; S, 6.72.
Author Contributions
Conceptualization, V.V.D.; methodology, P.G.D., A.G.L. and V.V.D.; synthesis, P.G.D. and A.G.L.; resources, V.V.D.; writing—original draft preparation, P.G.D. and A.G.L.; writing—review and editing, V.V.D.; interpretation of spectral data, P.G.D., A.G.L. and V.V.D.; supervision, V.V.D.; funding acquisition, V.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by North Caucasus Federal University (interdisciplinary project “Synthesis and antidote activity against the herbicide 2,4-D heterocyclic derivatives of methylene active nitriles”) within the program of strategic academic leadership PRIORITET-2030.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dotsenko, V.V.; Krivokolysko, S.G. Oxidation of thioamides with the DMSO–HCl system: A convenient and efficient method for the synthesis of 1,2,4-thiadiazoles, isothiazolo[5,4-b]pyridines, and heterocyclic disulfides. Chem. Heterocycl. Compd. 2013, 49, 636–644. [Google Scholar] [CrossRef]
- Murray, J.V.; Cloke, J.B. The Formation of Glycidamides by the Action of Hydrogen Peroxide on α,β-Ethylenic Nitriles. J. Am. Chem. Soc. 1934, 56, 2749–2751. [Google Scholar] [CrossRef]
- Payne, G.B. Reactions of hydrogen peroxide. VIII. Oxidation of isopropylidenemalononitrile and ethyl isopropylidenecyanoacetate. J. Org. Chem. 1961, 26, 663–668. [Google Scholar] [CrossRef]
- Igarashi, M.; Midorikawa, H. Syntheses of alpha-keto amides and acids from ethyl alkylidenecyanoacetate. Bull. Chem. Soc. Jpn. 1961, 34, 1543–1544. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Litvinov, V.P.; Gutov, A.V. The Radziszewski oxidation of (E)-3-aryl-2-(thiazol-2-yl)acrylonitriles: A convenient diastereoselective synthesis of (2S,3S)-3-aryl-2-(thiazol-2-yl) oxirane-2-carboxamides. Dokl. Chem. 2007, 412, 29–32. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Litvinov, V.P. Oxidation of 2-(thiazol-2-yl)acrylonitrile derivatives with an H2O2-KOH system: Convenient route to new oxirane-2-carboxamides. Russ. Chem. Bull. 2005, 54, 2394–2397. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Litvinov, V.P. The Radziszewski oxidation of cycloalkylidene-α-(thiazol-2-yl) acetonitriles: A new approach toward spirooxiranes. J. Heterocycl. Chem. 2011, 48, 162–167. [Google Scholar] [CrossRef]
- Chen, M.; Liang, Y.; Dong, T.; Liang, W.; Liu, Y.; Zhang, Y.; Huang, X.; Kong, L.; Wng, Z.; Peng, B. Z-Selective α-arylation of α,β-unsaturated nitriles via [3,3]-sigmatropic rearrangement. Angew. Chem. Int. Ed. 2021, 60, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, S.; Liu, Y.A.; Liao, X. Nickel-catalyzed cyanation of aryl halides and hydrocyanation of alkynes via C–CN bond cleavage and cyano transfer. ACS Catal. 2019, 10, 1397–1405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).