Study of Diels–Alder Reactions of Purpurogallin Tetraacetate with Various Dienophiles †
Abstract
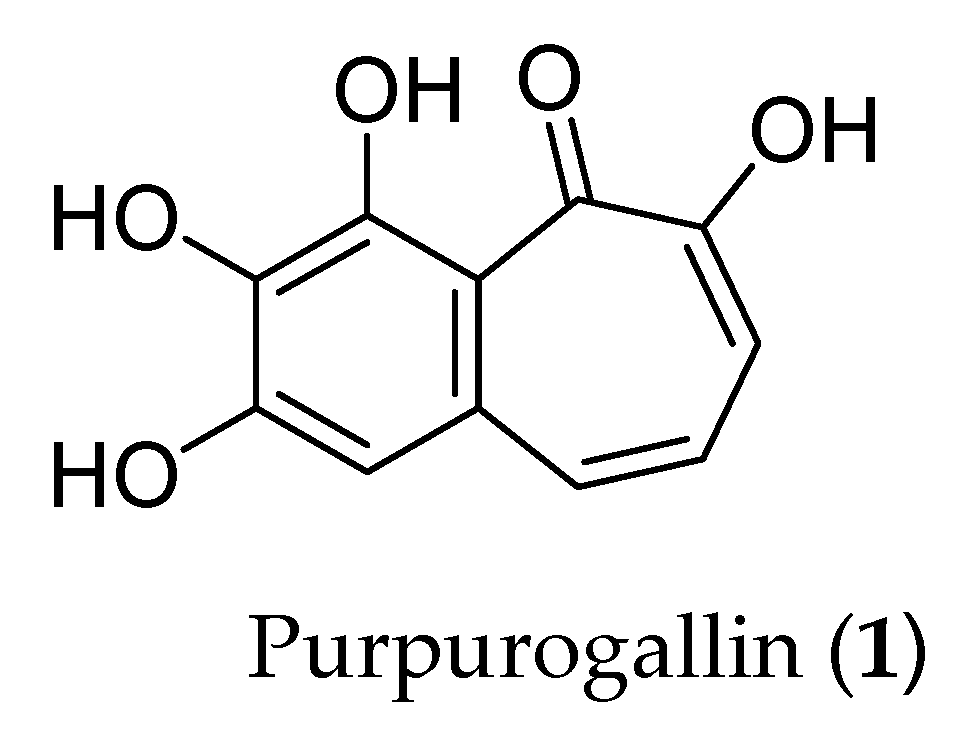
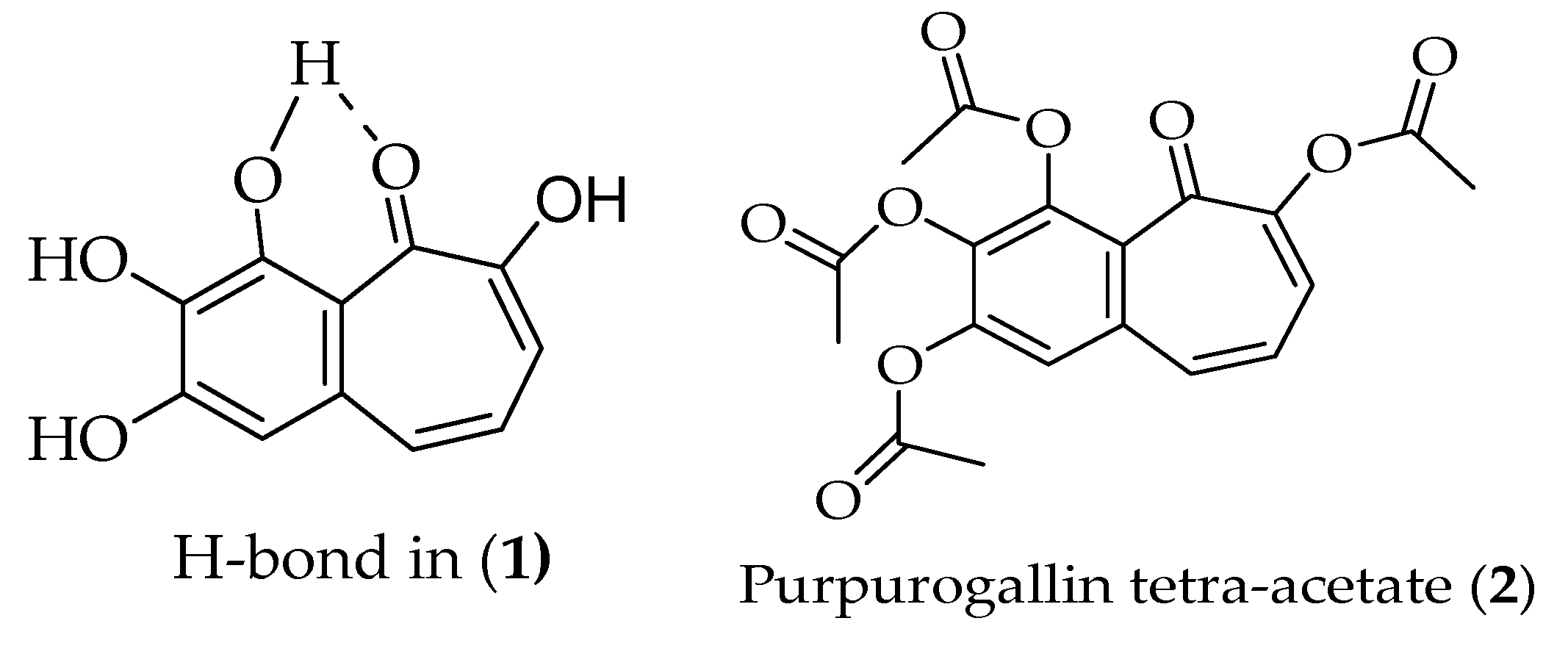
:1. Introduction
2. Materials and Methods
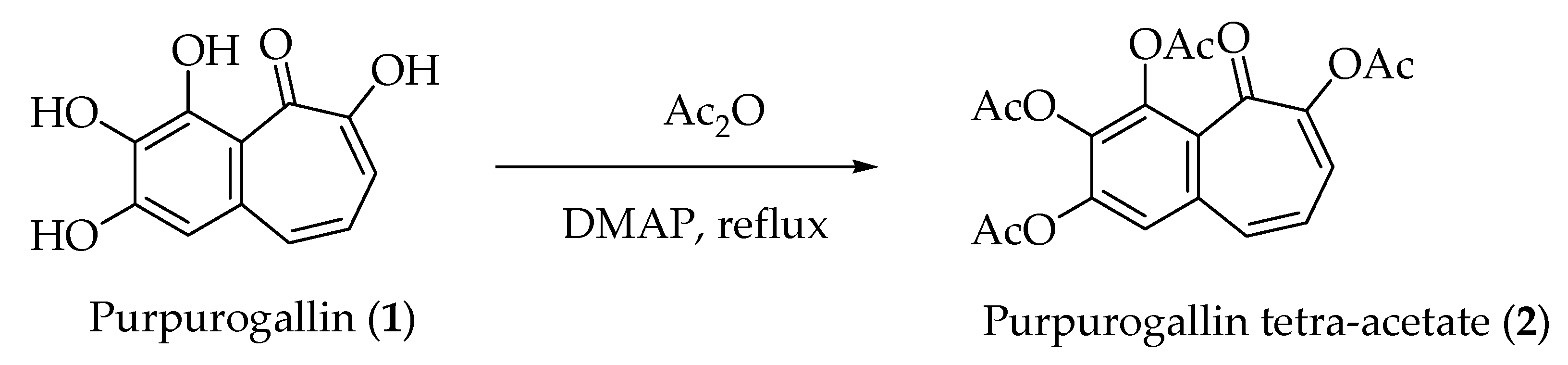
2.1. Synthesis of Purpurogallin Tetraacetate (2)
2.2. General Experimental Procedure for [4+2] Cycloaddition
- Products 4a-d.
3. Result and Discussion
4. Theoretical Studies
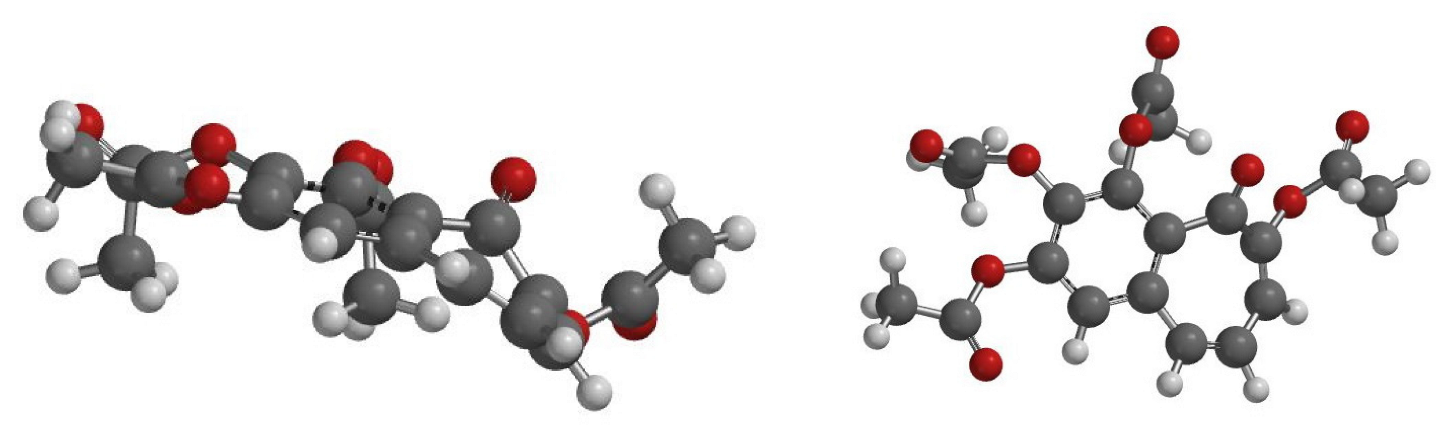
5. Stereochemistry
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abou-Karam, M.; Shier, W.T. Inhibition of oncogene product enzyme activity as an approach to cancer chemoprevention. Tyrosine-specific protein kinase inhibition by purpurogallin fromQuercus sp. Nutgall. Phytotherapy Res. 1999, 13, 337–340. [Google Scholar] [CrossRef]
- Wu, T.-W.; Zeng, L.-H.; Wu, J.; Fung, K.-P.; Weisel, R.D.; Hempel, A.; Camerman, N. Molecular structure and antioxidant specificity of purpurogallin in three types of human cardiovascular cells. Biochem. Pharmacol. 1996, 52, 1073–1080. [Google Scholar] [CrossRef]
- Prasad, K.; Laxdal, V.A. Evaluation of hydroxyl radical-scavenging property of purpurogallin using high pressure liquid chromatography. Mol. Cell. Biochem. 1994, 135, 153–158. [Google Scholar] [CrossRef]
- Sugiyama, H.; Fung, K.; Wu, T. Purpurogallin as an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. Life Sci. 1993, 53, PL39–PL43. [Google Scholar] [CrossRef]
- Lambert, J.D.; Chen, D.; Wang, C.Y.; Ai, N.; Sang, S.; Ho, C.-T.; Welsh, W.J.; Yang, C.S. Benzotropolone inhibitors of estradiol methylation: Kinetics and in silico modeling studies. Bioorganic Med. Chem. 2005, 13, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.-P.; Wu, T.-W.; Lui, C.-P. Purpurogallin Inhibits DNA Synthesis of Murine Fibrosarcoma L-929 and Human U-87 MG Glioblastoma Cells in vitro. Chemotherapy. 1996, 42, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Doulias, P.-T.; Nousis, L.; Zhu, B.-Z.; Frei, B.; Galaris, D. Protection by tropolones against H2O2-induced DNA damage and apoptosis in cultured Jurkat cells. Free. Radic. Res. 2005, 39, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, T.H.; Gil Kim, C.; Kim, G.-Y.; Kim, C.M.; Kim, N.D.; Kim, B.W.; Hwang, H.J.; Choi, Y.H. Purpurogallin exerts anti-inflammatory effects in lipopolysaccharide-stimulated BV2 microglial cells through the inactivation of the NF-κB and MAPK signaling pathways. Int. J. Mol. Med. 2013, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Ku, S.K.; Lee, I.C.; Bae, J.S. Anti-inflammatory functions of purpurogallin in LPS-activated human endothelial cells. BMB Rep. 2012, 45, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Arpin, N.; Favre-Bonvin, J.; Steglich, W. Le fomentariol: Nouvelle benzotropolone isolée de Fomes fomentarius. Phytochemistry 1974, 13, 1949–1952. [Google Scholar] [CrossRef]
- Mesa-Siverio, D.; Estevez-Braun, A.; Ravelo, A.G.; Murquia, J.R.; Rodriguez-Afonso, A. Novel DNA-Damaging Tropolone Derivatives from Goupia glabra. Eur. J. Org. Chem. 2003, 2003, 4243–4247. [Google Scholar] [CrossRef]
- Klostermeyer, D.; Knops, L.; Sindlinger, T.; Polborn, K.; Steglich, W. Novel Benzotropolone and 2H-Furo[3,2-b]benzopyran-2-one Pigments fromTricholoma aurantium (Agaricales). Eur. J. Org. Chem. 2000, 2000, 603–609. [Google Scholar] [CrossRef]
- Dib, S.; France, E.N.U.; Villemin, D.; Mostefa-Kara, B.; Hamhami, A.; BAR, N.; Dekhici, M.; Cheikh, N. On the green catalytic synthesis of purpurogallin. Rev. Roum. du Chim. 2021, 65, 1153–1157. [Google Scholar] [CrossRef]
- Steglich, W.; Vorbrüggen, H. 4- Dialkylaminopyridines as Highly Active Acylation Catalysts. Angew. Chem. Int. Ed. 1978, 17, 569. [Google Scholar] [CrossRef]
- Karas, L.J.; Campbell, A.T.; Alabugin, I.V.; Wu, J.I. Antiaromaticity Gain Activates Tropone and Nonbenzenoid Aromatics as Normal-Electron-Demand Diels–Alder Dienes. Org. Lett. 2020, 22, 7083–7087. [Google Scholar] [CrossRef] [PubMed]
- Spartan software of Wavefunction, Inc. Spartan’20; Spartan of Wavefunction, Inc.: Irvine, CA, USA, 2021; Available online: https://www.wavefun.com (accessed on 10 October 2022).
- Chamberlin, A.C.; Cramer, C.J.; Truhlar, D.G. Performance of SM8 on a Test to Predict Small-Molecule Solvation Free Energies. J. Phys. Chem. B 2008, 112, 8651–8655. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. A Universal Approach to Solvation Modeling. Accounts Chem. Res. 2008, 41, 760–768. [Google Scholar] [CrossRef] [PubMed]
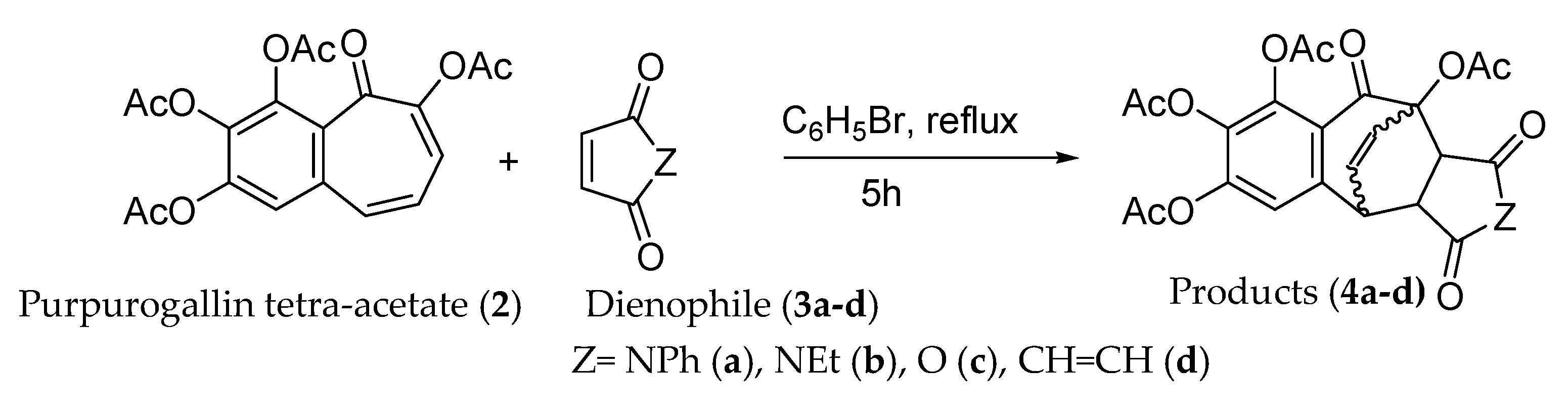
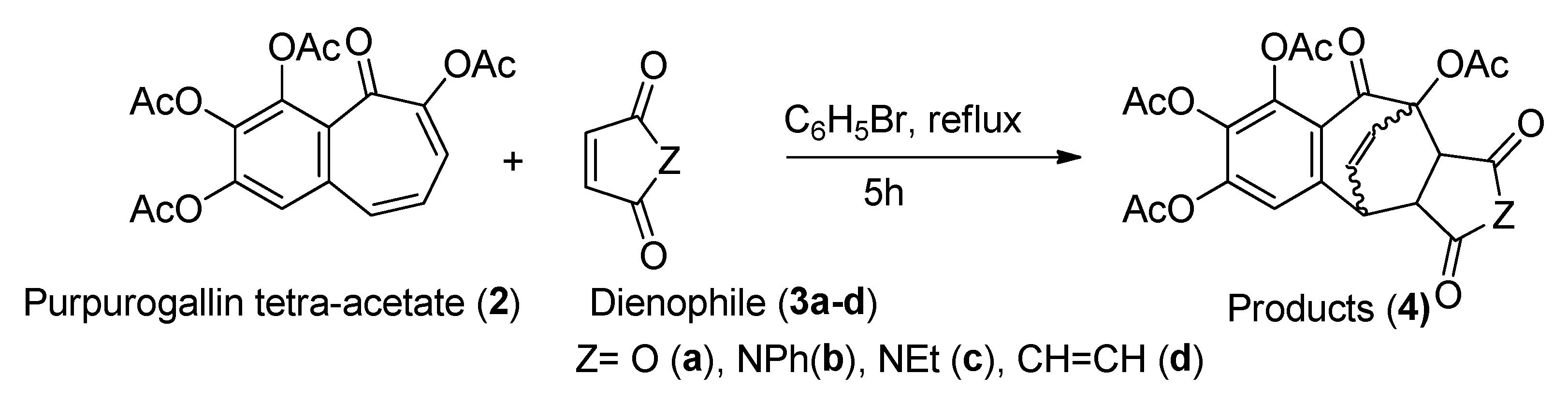
| Entry | Dienophile | Product (3a-d) | Yield |
|---|---|---|---|
| a | N-Phenylmaleimide |  | 39% |
| b | N-Ethylmaleimide | 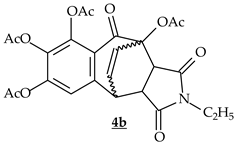 | 36% |
| c | Maleic Anhydrid | 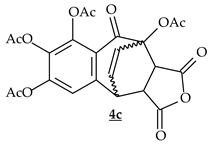 | 58% |
| d | Benzoquinone |  | 41% |
| Product | HOMO (eV) | LUMO (eV) | m Debye | HOMO (eV) | LUMO (eV) | m Debye | |
|---|---|---|---|---|---|---|---|
| In Vacuum | With Solvent: Bromobenzene | ||||||
| Dienes (Donor) | Purpurogallin (1) | −5.54 | −1.89 | 2.71 | −5.59 | −1.86 | 3.36 |
| Tetraacetylpurpurogallin | −6.43 | −1.97 | 11.07 | −6.10 | −1.69 | 12.84 | |
| Tetramethylpurpurogallin | −5.66 | −1.44 | 2.52 | −5.65 | −1.48 | 2.88 | |
| Dienophiles (Acceptor) | Maleic anhydride 3c | −8.18 | −3.75 | 3.64 | −7.99 | −2.88 | 4.15 |
| N-Phenyl maleimide 3a | −6.50 | −2.74 | 0.91 | −6.46 | −2.50 | 1.31 | |
| N-Ethyl maleimide 3b | −7.37 | −2.58 | 0.63 | −7.27 | −2.33 | 0.85 | |
| Benzoquinone 3d | −7.36 | −3.54 | 0 | −7.11 | −3.22 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dib, S.; Mostefa-Kara, B.; Villemin, D.; Bar, N. Study of Diels–Alder Reactions of Purpurogallin Tetraacetate with Various Dienophiles. Chem. Proc. 2022, 8, 109. https://doi.org/10.3390/ecsoc-25-11707
Dib S, Mostefa-Kara B, Villemin D, Bar N. Study of Diels–Alder Reactions of Purpurogallin Tetraacetate with Various Dienophiles. Chemistry Proceedings. 2022; 8(1):109. https://doi.org/10.3390/ecsoc-25-11707
Chicago/Turabian StyleDib, Salima, Bachir Mostefa-Kara, Didier Villemin, and Nathalie Bar. 2022. "Study of Diels–Alder Reactions of Purpurogallin Tetraacetate with Various Dienophiles" Chemistry Proceedings 8, no. 1: 109. https://doi.org/10.3390/ecsoc-25-11707
APA StyleDib, S., Mostefa-Kara, B., Villemin, D., & Bar, N. (2022). Study of Diels–Alder Reactions of Purpurogallin Tetraacetate with Various Dienophiles. Chemistry Proceedings, 8(1), 109. https://doi.org/10.3390/ecsoc-25-11707






