Abstract
A series of epoxyisoindolinones were synthesized by microwave-assisted post-IMCR transformation-based domino strategy via the Ugi-4CR/Intramolecular-Diels-Alder (U-4CR/IMDA) sequence under mild, solvent-, catalyst-free ecofriendly conditions, and orthogonal-bifunctional components. Epoxyisoindolinones are a privileged core of high interest in medicinal chemistry mainly for its anticancer activity in several cell lines.
1. Introduction
Multicomponent reactions (RMC) are one of the most efficient tools in modern organic synthesis, since they have all features that contribute to an ideal synthesis: high atom economy and convergence, efficiency, mild conditions, operational simplicity, broad scope, and concomitant step economy compared to other synthetic tools [1,2,3,4,5].
Epoxyisoindolinones are scaffolds present in numerous bioactive molecules (Figure 1). Isoindolinones and epoxyisoindolinones are recognized as privileged cores and are used as building blocks for the design of new pharmacologically active compounds [6,7], and they exhibit a wide range of biological activities such as antibiotic, antiviral and anticancer [8,9,10].
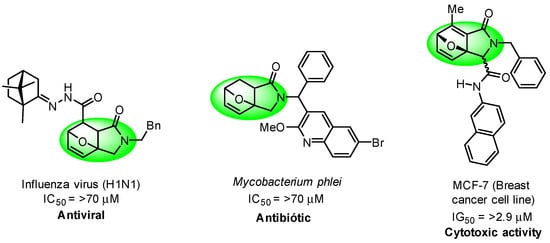
Figure 1.
Some representative biological activities of epoxyisoindolinone.
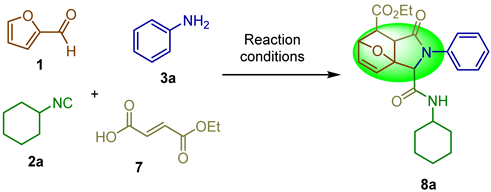
In a previous report, the synthesis of epoxyisoindolinones was performed under drastic conditions, using high temperatures and long reaction times [6]. Our research group is interested in the design and development of green strategies based on I-MCR’s (isocyanide-based multicomponent reactions) toward the synthesis of complex molecules containing several heterocyclic cores. Herein, we describe the microwave-assisted synthesis of epoxyisoindolinones 8a–c from 2-furaldehyde 1, cyclohexyl isocyanide 2a, anilines 3, and fumaric acid monoethyl ester 7 without solvent and a short time of reaction (Scheme 1).
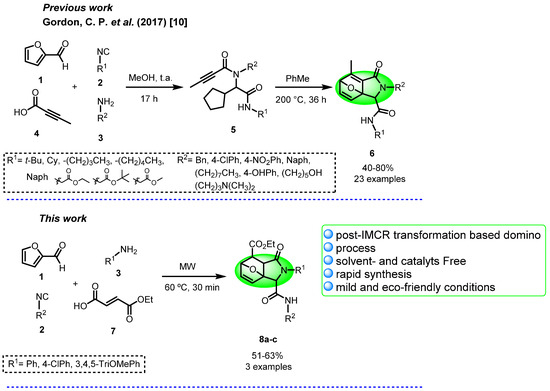
Scheme 1.
Previous report and our work.
2. Results and Discussion
Initially, the U-4CR/IMDA sequence of a domino process for the synthesis of epoxyisoindolinones was performed without solvent at room temperature and conventional stirring using aniline (3a), 2-furaldehyde (1), ciclohexyl isocyanide (2a), and fumaric acid monoethyl ester (7), but only traces of desired epoxyisoindoline were obtained (Table 1, entry 1). In a second attempt, it was decided to replace conventional agitation with ultrasound irradiation (USI) for 2 h at room temperature; the yield was 17% (Table 1, entry 2). With these results, we decided to increase the temperature to 40 °C using USI again, and it was possible to increase the yield to 35% (Table 1, entry 3). Since temperature is key in this one pot process in domino manner, we decided to change the method of assistance by microwave (MW) at 60 °C for 30 min obtaining the desired epoxyisoindolinone in 63% of yield (Table 1, entry 4).

Table 1.
Reaction optimizing conditions.
Using our optimized conditions, we synthesized the series of epoxyisoindolinones shown in Scheme 2. We explored the reaction scope with different anilines (8a–c), as 4-chloroaniline, 3,4,5-trimethoxy aniline, and aniline derivatives (a–c). The products 8a–c were obtained in moderate to good overall yields (51–63%).
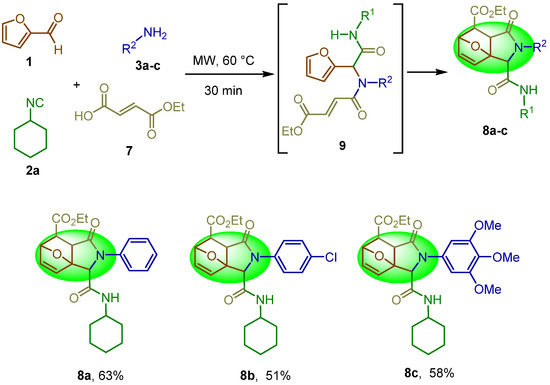
Scheme 2.
Substrate scope.
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
1H and 13C NMR spectra were acquired using Bruker Avance III spectrometers (500 and 125 MHz, respectively). The solvent used for NMR samples was deuterated chloroform (CDCl3). Chemical shifts are reported in parts per million (δ/ppm). The internal reference for 1H NMR spectra is TMS at 0.00 ppm. The internal reference for 13C NMR spectra is CDCl3 at 77.00 ppm. Coupling constants are reported in Hertz. Multiplicities of the signals are reported using the standard abbreviations: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). NMR spectra were analyzed using the MestreNova software version 12.0.0–20080. Microwave-assisted reactions were performed in a closed vial using a monomodal CEM Discover unit. Reaction progress was monitored by thin-layer chromatography (TLC) on precoated silica-gel 60 F254 plates and the spots were visualized under UV light at 254 or 365 nm. Mixtures of hexane and ethyl acetate (EtOAc) were used mobile phase in TLC and for measuring retention factors (Rf). Flash column chromatography was performed using silica gel (230–400 mesh) and mixtures of hexane and EtOAc in different proportions (v/v) as the mobile phase. All reagents were purchased from Sigma-Aldrich and were used without further purification. Chemical names and drawings were obtained using the ChemBioDraw Ultra 16.0.0.82 software package.
3.2. General Procedure
In a MW vial (10 mL) equipped with a magnetic stirring were sequentially added aniline (1.0 equiv.), 2-furaldehyde (1.0 equiv.), ciclohexyl isocyanide (1.0 equiv.), and fumaric acid monoethyl ester (1.0 equiv.), the reaction mixture was MW heated (100 W, 60 °C) for 30 min, and then, the crude was immediately purified by silica gel column chromatography using a mixture of hexanes with ethyl acetate (7/3 V/V) to afford the corresponding Epoxyisoindolinones 8a–c.
3.3. Spectral Data
Ethyl 3-(cyclohexylcarbamoyl)-1-oxo-2-phenyl-1,2,3,6,7,7a-hexahydro-3a,6-epoxyisoindole-7-carboxylate 8a Brown solid; Rf = 0.33 (Hexanes-AcOEt = 7/3 V/V); 1H NMR (500 MHz, CDCl3, 25 °C, TMS): δ 7.50 (d, J = 8.0 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.21 (t, J = 7.4 Hz, 1H), 6.57 (d, J = 5.9 Hz, 0H), 6.37 (dd, J = 5.9, 1.6 Hz, 0H), 6.06 (d, J = 8.2 Hz, 0H), 5.29 (dd, J = 4.8, 1.6 Hz, 0H), 4.74 (s, 0H), 4.14 (q, J = 7.1 Hz, 1H), 3.92–3.79 (m, 0H), 3.56 (dd, J = 4.8, 3.5 Hz, 0H), 3.21 (d, J = 3.5 Hz, 0H), 1.91–1.79 (m, 1H), 1.73–1.53 (m, 1H), 1.40–1.30 (m, 1H), 1.27 (t, J = 7.1 Hz, 2H), 1.18–1.04 (m, 2H) (Figure 2).
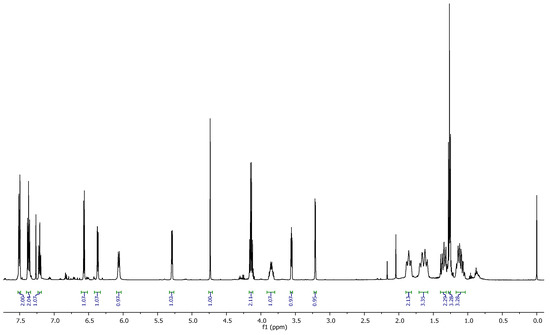
Figure 2.
1H RMN spectrum of compound 8a.
4. Conclusions
We developed a green and efficient strategy methodology for the synthesis of epoxyisoindolinones derivatives through of the post-IMCR transformation-based-domino process with MW-assistance. under mild, solvent-, and catalyst-free ecofriendly conditions.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
F.T.H. thanks CONACYT for a scholarship (825326). R.G.-M. thanks CONACYT-México (CB-2016-285622) and DAIP Universidad de Guanajuato (CIIC 154/2019 and 111/2020) for financial support, the Laboratorio Nacional de Caracterización de Propiedades Fisicoquímícas y Estructura Molecular (CONACYT-México, Project: 123732) for the instrumentation time provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wender, P.A. Toward the ideal synthesis and molecular function through synthesis-informed design. Nat. Prod. Rep. 2014, 31, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Haji, M. Multicomponent reactions: A simple and efficient route to heterocyclic phosphonates. Beilstein J. Org. Chem. 2016, 12, 1269–1301. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A.E.; Manzano-Velázquez, J.C.; Rojas-Lima, S.; Jiménez-Halla, J.O.C.; Gámez-Montaño, R. Synthesis of 2-Tetrazolylmethyl-isoindolin-1-ones via a One-Pot Ugi-Azide/(N-Acylation/exo-Diels–Alder)/Dehydration Process. ACS Omega 2018, 1, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Pharande, S.G.; Corrales-Escobosa, A.R.; Gámez-Montaño, R. Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction. Green Chem. 2017, 19, 1259–1262. [Google Scholar] [CrossRef]
- Spare, L.K.; Falsetta, P.; Gilbert, J.; Harman, D.G.; Baker, M.A.; Li, F.; McCluskey, A.; Clegg, J.K.; Sakoff, J.A.; Aldrich-Wright, J.R.; et al. Cytotoxicity of a Series of Norcantharidin-Inspired Tetrahydroepoxyisoindole Carboxamides. ChemMedChem 2017, 12, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Troelsen, N.S.; Shanina, E.; Gonzalez-Romero, D.; Danková, D.; Jensen, I.S.A.; Śniady, K.J.; Nami, F.; Zhang, H.; Rademacher, C.; Cuenda, A.; et al. The 3F Library: Fluorinated Fsp3-Rich Fragments for Expeditious 19F NMR Based Screening. Angew. Chem. Int. Ed. 2020, 59, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Yuan, L.; Chen, Y.; Wang, L.J.; Wang, C.; Sun, T.M. Synthesis, Crystal and Calculated Structure, and Biological Activity of 2-((6-Bromo-2-methoxyquinolin-3-yl)(phenyl)methyl)-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one. J. Chem. Crystallogr. 2012, 42, 318–322. [Google Scholar] [CrossRef]
- Kovaleva, K.S.; Zubkov, F.I.; Bormotov, N.I.; Novikov, R.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Gatilov, Y.V.; Zarubaev, V.V.; Yarovaya, O.I.; Shishkina, L.N.; et al. Synthesis of D-(+)-camphor-based N-acylhydrazones and their antiviral activity. Med. Chem. Commun. 2018, 9, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Hizartzidis, L.; Gilbert, J.; Gordon, C.P.; Sakoff, J.A.; McCluskey, A. Synthesis and Cytotoxicity of Octahydroepoxyisoindole-7-carboxylic Acids and Norcantharidin–Amide Hybrids as Norcantharidin Analogues. Chem. Med. Chem. 2019, 14, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).