On the Use of CeCl3.7H2O as a Catalyst for the Synthesis of Hydrazones Derived from Aromatic Aldehydes and Ketones †
Abstract
:1. Introduction
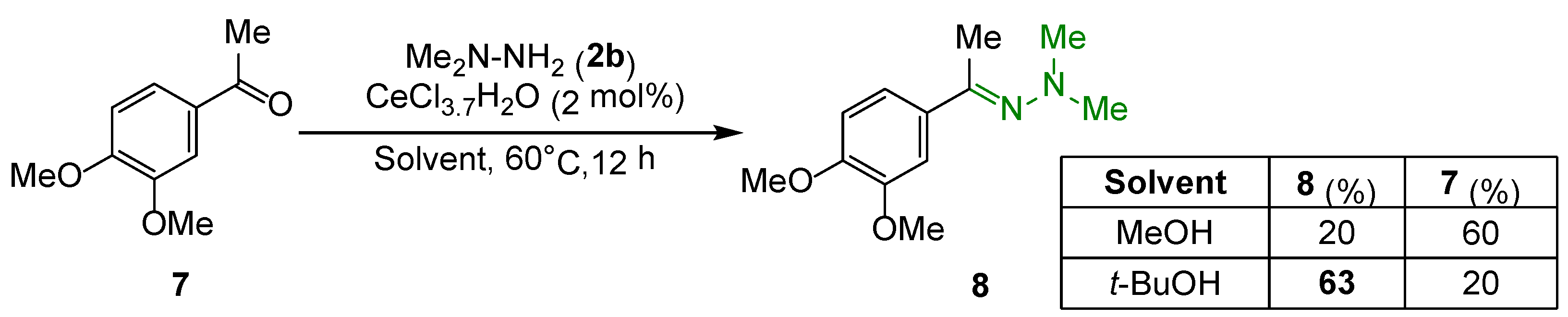
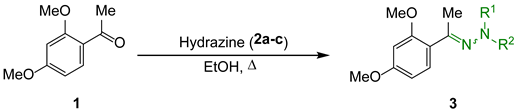
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Details
3.2. General Procedure for the Preparation of N,N-Dimethylhydrazones
3.3. Computational Details
4. Conclusions
Acknowledgments
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Kölmel, D.K.; Kool, E.T. Oximes and hydrazones in bioconjugation: Mechanism and catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Aprahamian, I. Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev. 2014, 43, 1963–1981. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Singh, Y. Glyoxylic hydrazone linkage-based PEG hydrogels for covalent entrapment and controlled delivery of doxorubicin. Biomacromolecules 2019, 20, 2174–2184. [Google Scholar] [CrossRef]
- Tatum, L.A.; Su, X.; Aprahamian, I. Simple hydrazone building blocks for complicated functional materials. Acc. Chem. Res. 2014, 47, 2141–2149. [Google Scholar] [CrossRef]
- Wahbeh, J.; Milkowski, S. The use of hydrazones for biomedical applications. SLAS Technol. Transl. Life Sci. Innov. 2019, 24, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Ali, M.R.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar] [CrossRef]
- De Gracia Retamosa, M.; Matador, E.; Monge, D.; Lassaletta, J.M.; Fernández, R. Hydrazones as singular reagents in asymmetric organocatalysis. Chem. Eur. J. 2016, 22, 13430–13445. [Google Scholar] [CrossRef]
- Groenendaal, B.; Ruijter, E.; Orru, R.V.A. 1-Azadienes in cycloaddition and multicomponent reactions towards N-heterocycles. Chem. Commun. 2008, 5474–5489. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Xia, H.; Wu, J. C(sp2)–H functionalization of aldehyde-derived hydrazones via a radical process. Org. Biomol. Chem. 2018, 16, 1227–1241. [Google Scholar] [CrossRef]
- Mohamady, S.; Kralt, B.; Samwel, S.K.; Taylor, S.D. Efficient one-pot, two-component modular synthesis of 3,5-disubstituted pyrazoles. ACS Omega 2018, 3, 15566–15574. [Google Scholar] [CrossRef]
- Chuang, S.-C.; Gandeepan, P.; Cheng, C.-H. Synthesis of isoquinolines via Rh(III)-catalyzed C–H activation using hydrazone as a new oxidizing directing group. Org. Lett. 2013, 15, 5750–5753. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, H.F.; Gülgeze, B.; Aubé, J. Copper-catalyzed oxaziridine-mediated oxidation of C–H bonds. J. Org. Chem. 2012, 77, 7005–7022. [Google Scholar] [CrossRef] [PubMed]
- Dabb, S.L.; Messerle, B.A. Rh(I) and Ir(I) catalysed intermolecular hydroamination with substituted hydrazines. Dalt. Trans. 2008, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Ghabbour, H.A.; El-Husseiny, W.M.; Maarouf, A.R.; Mohamed, M.A.; Abdel-Aziz, A.A.-M. Lewis acid-promoted direct synthesis of N-unsubstituted hydrazones via the reaction of hydrazine with acetophenone and isatin derivatives. Russ. J. Gen. Chem. 2016, 86, 2837–2844. [Google Scholar] [CrossRef]
- dos Santos Filho, J.M. Mild, Stereoselective, and highly efficient synthesis of n-acylhydrazones mediated by CeCl3·7H2O in a broad range of solvents. Eur. J. Org. Chem. 2014, 2014, 6411–6417. [Google Scholar] [CrossRef]
- Fonzo, S.; Vargas, D.F.; Kaufman, T.S. A ruthenium-catalyzed C–H activation strategy as an efficient shortcut in the total synthesis of 6,8-dimethoxy-1,3-dimethylisoquinoline. Synthesis 2019, 51, 3908–3914. [Google Scholar] [CrossRef]
- Cortés, I.; Borini Etichetti, C.M.; Girardini, J.E.; Kaufman, T.S.; Bracca, A.B.J. Total synthesis and cytotoxic activity of 6,8-dimethoxy-1,3-dimethylisoquinoline isolated from Ancistrocladus tectorius: A 6π-azaelectrocyclization approach. Synthesis 2019, 51, 433–440. [Google Scholar] [CrossRef]
- Vargas, D.F.; Romero, B.S.; Larghi, E.L.; Kaufman, T.S. Rhodium(III)-Catalyzed C–H Activation-Based First Total synthesis of 6-O-methyl anciscochine, an alkaloid isolated from Ancistrocladus tectorius. Synthesis 2020, 52, 119–126. [Google Scholar] [CrossRef]
- Cortés, I.; Kaufman, T.S.; Bracca, A.B.J. A convenient and eco-friendly cerium(III) chloride-catalysed synthesis of methoxime derivatives of aromatic aldehydes and ketones. R. Soc. Open Sci. 2018, 5, 180279. [Google Scholar] [CrossRef]
- Cadav, J.S.; Subba Reddy, B.V.; Reddy, M.S.K.; Sabitha, G. A facile cleavage of ketone N,N-dimethylhydrazones by CeCl3·7H2O-SiO2. Synlett 2001, 1134–1136. [Google Scholar] [CrossRef]
- Gergely, J.; Morgan, J.B.; Overman, L.E. Stereocontrolled synthesis of functionalized cis-cyclopentapyrazolidines by 1,3-dipolar cycloaddition reactions of azomethine imines. J. Org. Chem. 2006, 71, 9144–9152. [Google Scholar] [CrossRef]
- Vivona, N.; Frenna, V.; Buscemi, S.; Ruccia, M. Heterocyclic rearrangements. N,N-diphenylhydrazones, oximes and O-methyloximes of 3-benzoyl-5-phenyl-1,2,4-oxadiazole. J. Heterocyclic Chem. 1985, 22, 97–99. [Google Scholar] [CrossRef]
- Benassi, R.; Taddei, F. Theoretical study of conformational changes in simple hydrazones. J. Chem. Soc. Perkin Trans. 2 1985, 1629–1632. [Google Scholar] [CrossRef]
- Ros, A.; López-Rodríguez, R.; Estepa, B.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Hydrazone as the directing group for Ir-catalyzed arene diborylations and sequential functionalizations. J. Am. Chem. Soc. 2012, 134, 4573–4576. [Google Scholar] [CrossRef]

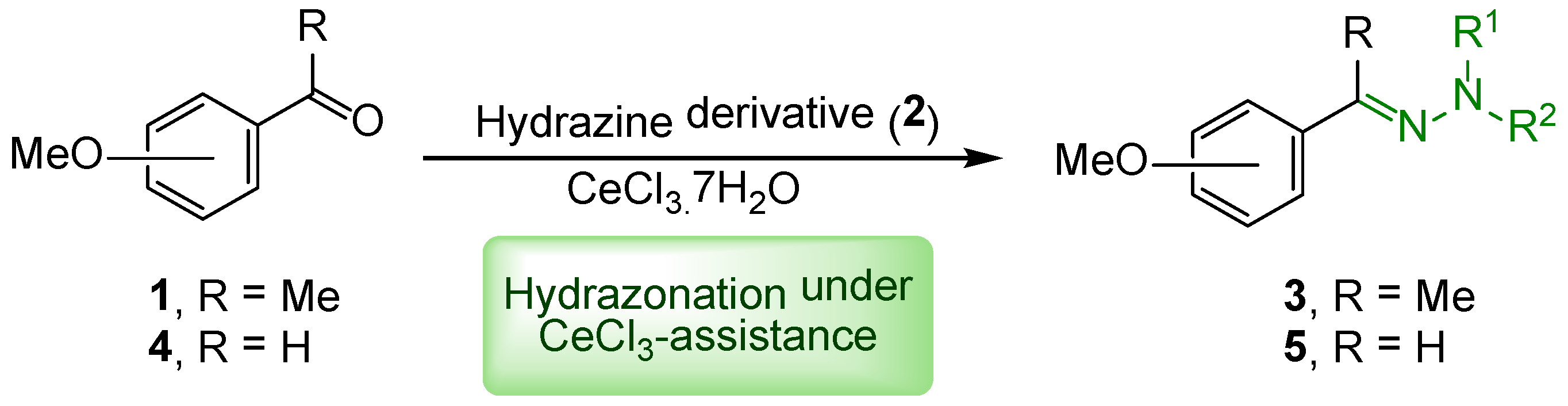
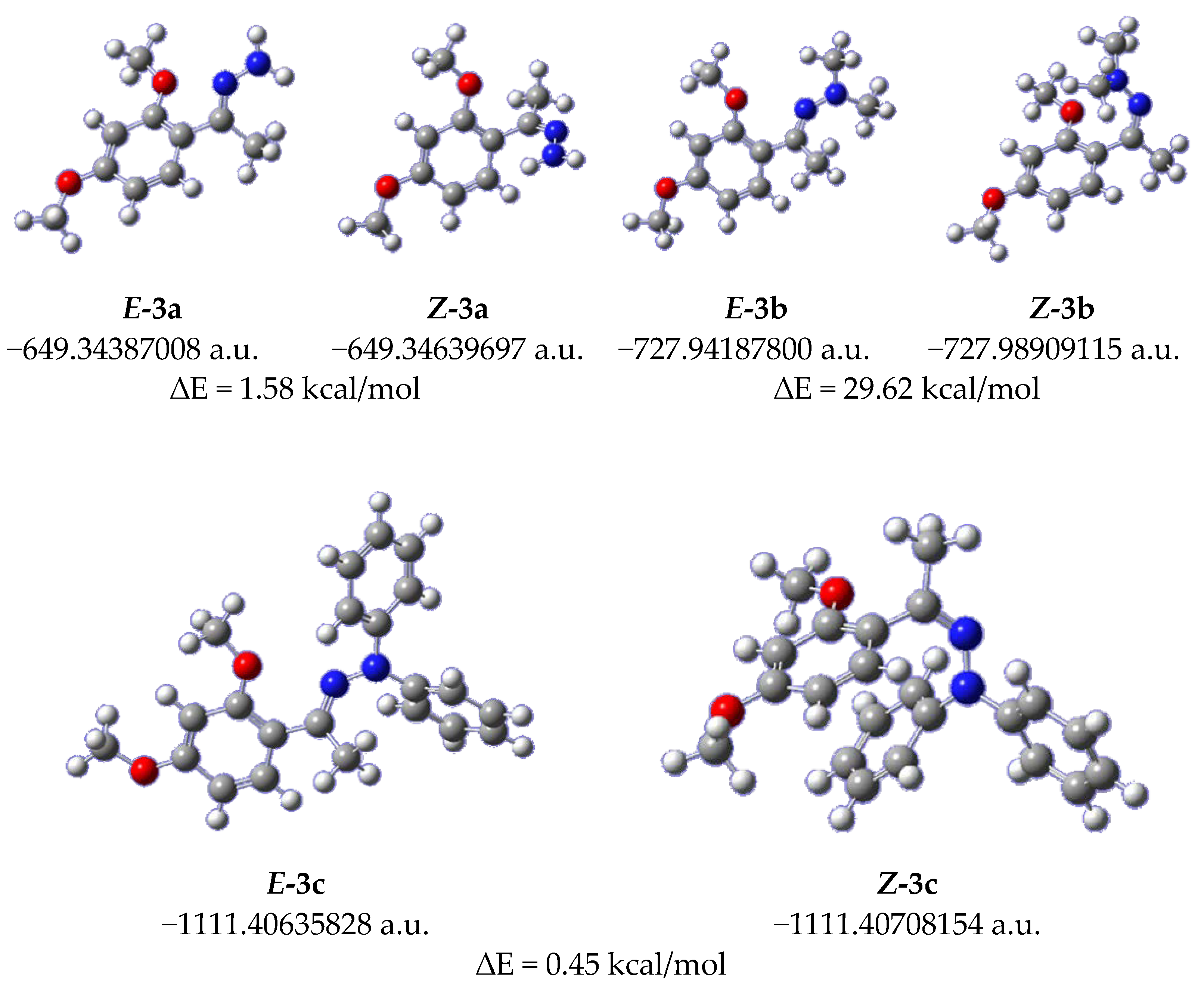
| Entry | R1 | R2 | Hydrazine (Equiv.) | Additive (Equiv.) | t (h) | Yield (%)a |
|---|---|---|---|---|---|---|
| 1 | H | H | H2NNH2.HCl (2.0) | NaOAc (2.5) | 24 | 74 |
| 2 | H | H | H2NNH2.HCl (2.0) | NaOAc (2.5), CeCl3.7H2O (0.05) | 12 | 20 |
| 3 | Me | Me | Me2NNH2 (2.5) | AcOH (1.5) | 24 | 63 |
| 4 | Me | Me | Me2NNH2 (2.5) | CeCl3.7H2O (0.05) | 12 | 15 |
| 5 | Ph | Ph | Ph2NNH2.HCl (2.0) | NaOAc (2.5) | 24 | 80 |
| 6 | Ph | Ph | Ph2NNH2.HCl (2.0) | NaOAc (2.5), CeCl3.7H2O (0.05) | 12 | 58 |
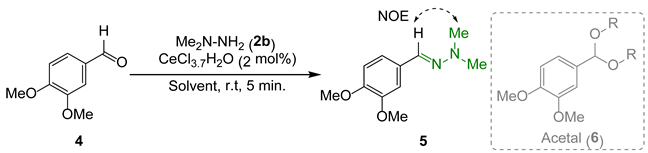
| Entry | Solvent | 5 (%) | 4 (%) | Acetal (%) |
|---|---|---|---|---|
| 1 | MeOH | 79 | 6 | traces |
| 2 | EtOH | 71 | 4 | 22 |
| 3 | i-PrOH | 81 | 16 | traces |
| 4 | t-BuOH | 97 | 0 | 0 |
| 5 | THF | 14 | 63 | 0 |
| 6 | MeCN | 31 | 53 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, D.F.; Romero, B.S.; Kaufman, T.S.; Larghi, E.L. On the Use of CeCl3.7H2O as a Catalyst for the Synthesis of Hydrazones Derived from Aromatic Aldehydes and Ketones. Chem. Proc. 2021, 3, 82. https://doi.org/10.3390/ecsoc-24-08095
Vargas DF, Romero BS, Kaufman TS, Larghi EL. On the Use of CeCl3.7H2O as a Catalyst for the Synthesis of Hydrazones Derived from Aromatic Aldehydes and Ketones. Chemistry Proceedings. 2021; 3(1):82. https://doi.org/10.3390/ecsoc-24-08095
Chicago/Turabian StyleVargas, Didier Farley, Brenda S. Romero, Teodoro Saul Kaufman, and Enrique Leandro Larghi. 2021. "On the Use of CeCl3.7H2O as a Catalyst for the Synthesis of Hydrazones Derived from Aromatic Aldehydes and Ketones" Chemistry Proceedings 3, no. 1: 82. https://doi.org/10.3390/ecsoc-24-08095
APA StyleVargas, D. F., Romero, B. S., Kaufman, T. S., & Larghi, E. L. (2021). On the Use of CeCl3.7H2O as a Catalyst for the Synthesis of Hydrazones Derived from Aromatic Aldehydes and Ketones. Chemistry Proceedings, 3(1), 82. https://doi.org/10.3390/ecsoc-24-08095






