Ecotoxicity of Mixtures of IL and Lithium Salt †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Section
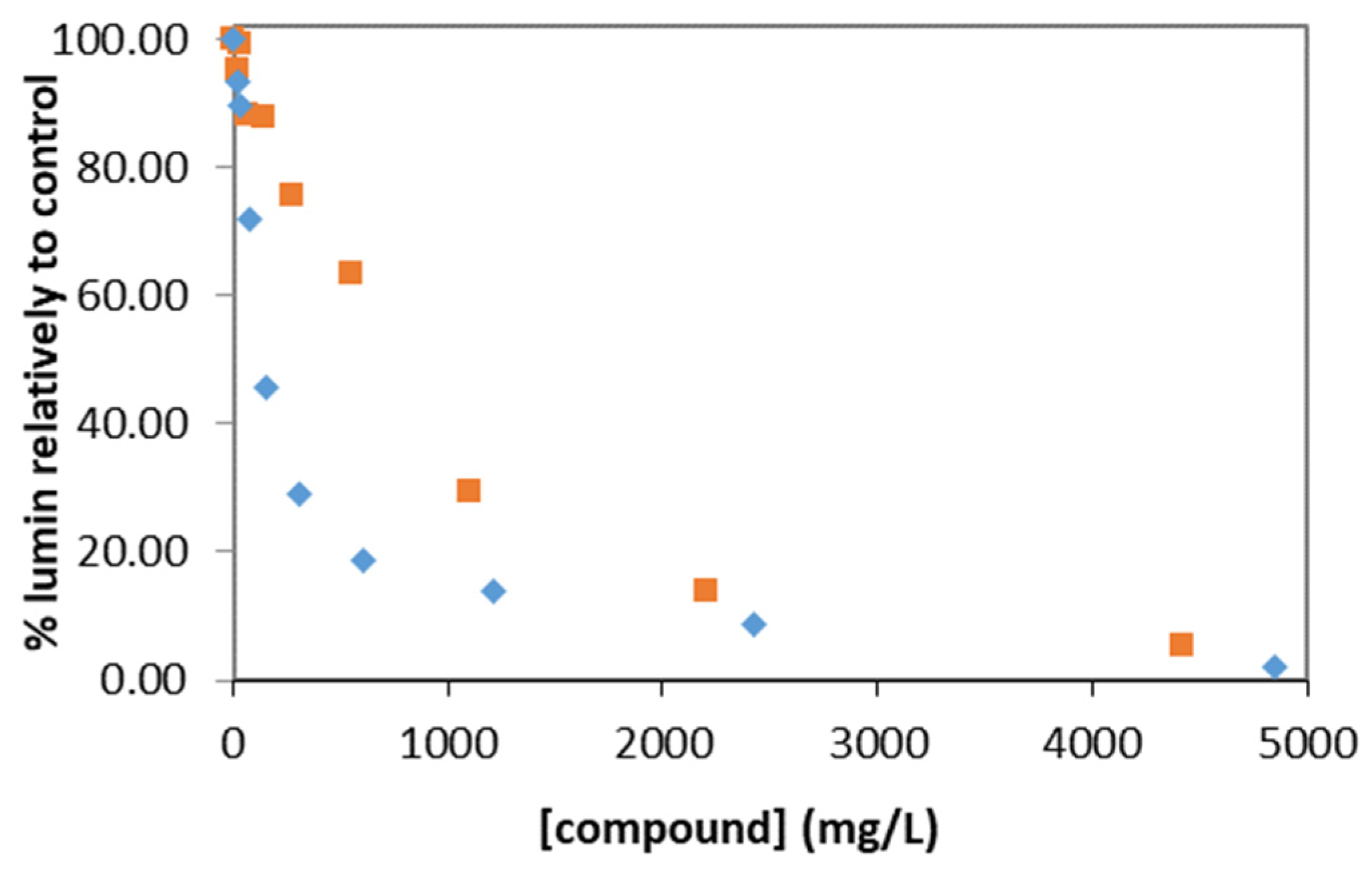
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salgado, J.; Parajó, J.J.; Villanueva, M.; Rodríguez, J.R.; Cabeza, O.; Varela, L.M. Liquid range of ionic liquid–Metal salt mixtures for electrochemical applications. J. Chem. Thermodyn. 2019, 134, 164–174. [Google Scholar] [CrossRef]
- Lui, M.Y.; Crowhurst, L.; Hallett, J.P.; Hunt, P.A.; Niedermeyer, H.; Welton, T. Salts dissolved in salts: Ionic liquid mixtures. Chem. Sci. 2011, 2, 1491–1496. [Google Scholar] [CrossRef]
- Peric, B.; Sierra, J.; Martí, E.; Cruañas, R.; Garau, M.A.; Arning, J.; Bottin-Weber, U.; Stolte, S. (Eco)toxicity and biodegradability of selected protic and aprotic ionic liquids. J. Hazard. Mater. 2013, 261, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Jeschke, S.; Jankowski, P.; Abdelhamid, M.; Brousse, T.; Le Bideau, J.; Johansson, P.; Matic, A. Charge storage mechanism of α-MnO2 in protic and aprotic ionic liquid electrolytes. J. Power Sources 2020, 460, 228111. [Google Scholar] [CrossRef]
- Salgado, J.; Villanueva, M.; Parajó, J.J.; Fernández, J. Long-term thermal stability of five imidazolium ionic liquids. J. Chem. Thermodyn. 2013, 65, 184–190. [Google Scholar] [CrossRef]
- Sánchez, P.B.; González, B.; Salgado, J.; José Parajó, J.; Domínguez, Á. Physical properties of seven deep eutectic solvents based on L-proline or betaine. J. Chem. Thermodyn. 2019, 131, 517–523. [Google Scholar] [CrossRef]
- Yang, H.; Luo, X.-F.; Matsumoto, K.; Chang, J.-K.; Hagiwara, R. Physicochemical and electrochemical properties of the (fluorosulfonyl)(trifluoromethylsulfonyl)amide ionic liquid for Na secondary batteries. J. Power Sources 2020, 470, 228406. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lim, D.-H.; Scheers, J.; Wilken, S.; Johansson, P.; Ahn, J.-H.; Matic, A.; Jacobsson, P. Properties of N-butyl-N-methyl-pyrrolidinium Bis(trifluoromethanesulfonyl) Imide Based Electrolytes as a Function of Lithium Bis(trifluoromethanesulfonyl) Imide Doping. J. Korean Electrochem. Soc. 2011, 14, 92–97. [Google Scholar] [CrossRef]
- Union OJ of the E (2007) Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).
- Heckenbach, M.E.; Romero, F.N.; Green, M.D.; Halden, R.U. Meta-analysis of ionic liquid literature and toxicology. Chemosphere 2016, 150, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.J.; Macário, I.P.E.; De Gaetano, Y.; Dupont, L.; Salgado, J.; Pereira, J.L.; Gonçalves, F.J.M.; Mohamadou, A.; Ventura, S.P.M. Glycine-betaine-derived ionic liquids: Synthesis, characterization and ecotoxicological evaluation. Ecotoxicol. Environ. Saf. 2019, 184, 109580. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; Gonçalves, A.M.M.; Sintra, T.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Designing ionic liquids: The chemical structure role in the toxicity. Ecotoxicology 2013, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liao, Y.; Zhang, Z. Toxicity of Ionic Liquids. Clean Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.M.; Gonçalves, F.; Coutinho, J.A.P. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Stolte, S.; Matzke, M.; Jürgen, A.; Böschen, A.; Pitner, W.-R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007, 9, 1170–1179. [Google Scholar] [CrossRef]
- Passino, D.R.M.; Smith, S.B. Acute bioassays and hazard evaluation of representative contaminants detected in great lakes fish. Environ. Toxicol. Chem. 1987, 6, 901–907. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Hidalgo, J.M.; Collado-González, M.; Díaz Baños, F.G.; Víllora, G. Assessing chemical toxicity of ionic liquids on Vibrio fischeri: Correlation with structure and composition. Chemosphere 2016, 155, 405–414. [Google Scholar] [CrossRef]
| Name Molecular Mass (g·mol−1) | Abbreviation | Chemical Structure | Purity Provenance |
|---|---|---|---|
| CAS Number | |||
| Ethylammonium Nitrate 108.10 | EAN 22113-86-6 |  | >0.97 Iolitec |
| Ethylimidazolium nitrate 159.14 | EIm NO3 501693-38-5 | 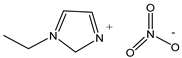 | >0.98 Iolitec |
| butylmethylpyrrolidinium bis(trifluoromethylsulfonyl)imide 422.41 | C4C1pyrr TFSI 223437-11-4 | 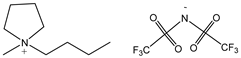 | >0.99 Merck |
| Lithium Nitrate 68.95 | Li NO3 7790-69-4 | 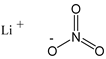 | >0.999 Merck |
| Lithium bis(trifluoromethylsulfonyl)imide 287.09 | LiTFSI 90076-65-6 | 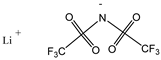 | >0.99 Acros organics |
| Mixture | EAN + Li NO3 | C4C1pyrr TFSI + Li TFSI | EIm NO3 + LiNO3 |
|---|---|---|---|
| Molalitysaturation | 2.000 | 1.500 | 2.000 |
| χmolar sal | 0.178 | 0.388 | 0.241 |
| Mm/g mol−1 | 123.01 | 604.31 | 181.08 |
| IL | Time/min | EC50 (Lower Limit; Upper Limit)/mg·L−1 | EC20 (Lower Limit; Upper Limit)/mg·L−1 | EC10 (Lower Limit; Upper Limit)/mg·L−1 |
|---|---|---|---|---|
| EAN | 5 | 12,582 (8186; 16,977) | 4314 (1548; 7081) | 2304 (248; 4361) |
| 15 | 10,665 (6650; 14,680) | 3236 (951; 5522) | 1609 (56; 3163) | |
| 30 | 9711 (6561; 12,860) | 3012 (1264; 4761) | 1517 (332; 2703) | |
| EAN + Li NO3 2 m | 5 | 13,911 (12,469; 15,232) | 8892 (7412; 10,373) | 6842 (5316; 8368) |
| 15 | 11,210 (9613; 12,808) | 7495 (5603; 9386) | 5920 (3841; 8000) | |
| 30 | 9706 (7233; 12,179) | 6145 (3301; 8988) | 4701 (1744; 7658) | |
| EIm NO3 | 5 | 612 (395; 828) | 195 (79; 312) | 100 (21; 179) |
| 15 | 573 (372; 774) | 194 (79; 310) | 103 (22; 184) | |
| 30 | 597 (408; 785) | 223 (105; 342) | 127 (37; 214) | |
| EIm NO3 + Li NO3 2 m | 5 | 1178 (691; 1665) | 423 (119; 727) | 232 (9; 455) |
| 15 | 1114 (644; 1583) | 435 (118; 753) | 251 (6; 496) | |
| 30 | 1073 (626; 1520) | 442 (125; 759) | 263 (10; 515) | |
| C4C1pyrr TFSI | 5 | 1463 (1162; 1765) | 684 (441; 926) | 438 (225; 650) |
| 15 | 964 (791; 1137) | 416 (286; 545) | 254 (146; 362) | |
| 30 | 714 (577; 851) | 289 (192; 386) | 170 (93; 247) | |
| C4C1pyrr TFSI + Li TFSI 1.5 m | 5 | 453 (360; 547) | 89 (56; 123) | 35 (17; 51) |
| 15 | 208 (142; 274) | 51 (23; 79) | 23 (6; 39) | |
| 30 | 149 (108; 189) | 44 (23; 64) | 21 (8; 35) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parajó, J.J.J.; Vallet, P.; Fernádez-Míguez, L.; Villanueva, M.; Salgado, J. Ecotoxicity of Mixtures of IL and Lithium Salt. Chem. Proc. 2021, 3, 84. https://doi.org/10.3390/ecsoc-24-08361
Parajó JJJ, Vallet P, Fernádez-Míguez L, Villanueva M, Salgado J. Ecotoxicity of Mixtures of IL and Lithium Salt. Chemistry Proceedings. 2021; 3(1):84. https://doi.org/10.3390/ecsoc-24-08361
Chicago/Turabian StyleParajó, Juan José José, Pablo Vallet, Lois Fernádez-Míguez, María Villanueva, and Josefa Salgado. 2021. "Ecotoxicity of Mixtures of IL and Lithium Salt" Chemistry Proceedings 3, no. 1: 84. https://doi.org/10.3390/ecsoc-24-08361
APA StyleParajó, J. J. J., Vallet, P., Fernádez-Míguez, L., Villanueva, M., & Salgado, J. (2021). Ecotoxicity of Mixtures of IL and Lithium Salt. Chemistry Proceedings, 3(1), 84. https://doi.org/10.3390/ecsoc-24-08361






