Abstract
The present study refers to the preparation of isonicotinic acid hydrazide (isoniazid (INH)) cocrystals with two α-hydroxycarboxylic acids. The interaction of glycolic acid (H2ga) or dl-mandelic acid (H2ma) resulted in the formation of cocrystals, or salts of composition, as isoniazid−glycolic acid cocrystal (INH)·(H2ga) (1) and isoniazid−dl-mandelic acid salt cocrystal [HINH]+[Hma]−·(H2ma) (2), when reacted with isoniazid. An N’-(propan-2-ylidene)isonicotinic hydrazide hemihydrate, (pINH)·1/2(H2O) (3), was also prepared by condensation of isoniazid with acetone in the presence of glycolic acid. The prepared compounds were well characterized by elemental analysis and spectroscopic methods, and their three-dimensional molecular structure was determined by single-crystal X-ray crystallography. Hydrogen bonds involving carboxylic acid occur consistently with the pyridine ring N atom of the isoniazid and its derivatives. The remaining hydrogen-bonding sites on the isoniazid backbone vary on the basis of the steric influences of the derivative group. These are contrasted in each of the molecular systems.
1. Introduction
As an important part of supramolecular chemistry, crystal engineering is the subject of continuous research in solid and materials science. Rapid development in this field has revealed the use of a variety of organic components with specific functional groups to create supramolecular arrays through the coordination of metals or non-covalent forces, presenting interesting structures and useful properties. In this context, numerous recent examples of multicomponent crystals are known, the assembly of which is driven by non-covalent interactions, mainly hydrogen bonding with or without charge assistance [1]. Acid-base binary cocrystals are an important technological topic in pharmaceutical science that have attracted scientific and pharmaceutical interest in recent decades because of their potential ability to modify important properties of active pharmaceutical ingredients (APIs), such as solubility, dissolution rate, bioavailability, hygroscopicity, and/or thermal stability [2]. Furthermore, the formation of these multicomponent crystals does not lead to changes in the nature of the API, unlike the situation observed during salt formation, where the API must protonate or be protonated [3].
In this field of research, isonicotinic acid hydrazide (isoniazid (INH)) (Scheme 1) is an important API that, among others, is applied in combination with rifampicin, pyrazinamide, and ethambutol for the treatment of tuberculosis, which are known as fixed-dose combinations [4]. Furthermore, the reaction of INH with ketones is used to modify or improve their molecular efficacy at the biological level [5]. For example, in the compound obtained with 2-propanone, an increase in activity against mycobacterium tuberculosis was observed with respect to INH [6]. From the point of view of crystal engineering, INH is of interest in the design of cocrystals, since it has an N-pyridine, which is well known for forming pyridine-carboxylic acid heterosynthons with carboxylic acids, and for a carbohydrazide group that can form a range of homo- and heterosynthons with other functional groups [7,8]. Therefore, isoniazid can be considered an ideal molecule for studies in the field of pharmaceutical cocrystals.
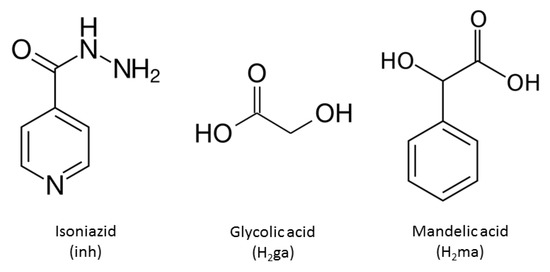
Scheme 1.
Molecular structures of the formers/coformers.
As possible coformers of salts or cocrystals against INH, we considered α-hydroxycarboxylic acids, glycolic (H2ga) and dl-mandelic (H2ma) (Scheme 1) [9,10]. From the point of view of hydrogen bonding, each of them contains three acceptor oxygen atoms and two donor O-H groups. Both participate in many biochemical processes and have widespread applications both in biological systems and in industry. Thus, glycolic acid, a common component of sugarcane juice and other foods, has an important role in photosynthesis and plant respiration, and is a known precursor to oxalate in humans [11]; on the other hand, mandelic acid is a useful precursor to various drugs, for example, homatropine and cyclandelate, which are esters of mandelic acid, and it is also known to have antibacterial properties [12] and has been studied in the preparation of antitumor compounds [13]. Taking into account the previous considerations, the main objective of this work is the design, preparation, and characterization of the physicochemical properties, and the identification of recurrent supramolecular patterns, within a new set of multicomponent pharmaceutical crystals that involve isoniazid with glycolic and dl-mandelic acids as coformers (Scheme 1).
2. Materials and Methods
Glycolic acid, dl-mandelic acid, and isoniazid were purchased from Sigma-Aldrich. Commercially available solvents were used as received without further purification. Compounds were prepared by cocrystallization via solvent-drop grinding: Stoichiometric amounts of INH with H2ga or H2ma were ground with a mortar and pestle for about 5–7 min with the addition of 10 μL of solvent per 50 mg of cocrystal formers. The resulting solutions were left to evaporate slowly under ambient conditions. The single crystals of isoniazid−glycolic acid cocrystal (INH)·(H2ga) (1), isoniazid−dl-mandelic acid salt cocrystal [HINH]+[Hma]−·(H2ma) (2), and N’-(propan-2-ylidene)isonicotinic hydrazide hemihydrate (pINH)·1/2(H2O) (3), suitable for X-ray diffraction studies, were obtained in 2–15 days from ethyl acetate or cyclohexane, water and acetone solutions, respectively. Microanalyses (C, H, and N) were carried out using a Carlo–Erba 1108 elemental analyzer. FT-IR spectra were recorded from potassium bromide pellets over the range 4000–400 cm−1 on a Bruker IFS-66v spectrometer. For X-ray analysis, intensity data were collected at 100 K on a Bruker X8 KappaAPEXII diffractometer. Structures were solved by direct methods followed by difference Fourier calculations, and were refined by a full-matrix least-squares procedure using SHELXLTL. The structures were deposited at the Cambridge Crystallographic Data Center (CCDC) with numbers 2041154-2041156, respectively.
3. Results and Discussion
The three crystals were obtained from the crystallization of the solutions prepared by reacting the isoniazid with glycolic or mandelic acids in a molar ratio of 1:1. Although the X-ray diffraction data were taken at 100 K, solid handling was always done at room temperature.
The cocrystallization processes was carried out considering the pKa of isoniazid and the coformers, the glycolic and dl-mandelic acids. Isoniazid has three pKa values: 1.8 based on hydrazine nitrogen, 3.6 based on pyridine nitrogen, and 10.8 based on the acidic group [14]. This makes the pyridine the more basic of the two, and in the presence of a carboxylic acid group, it is protonated first. The pKa of the glycolic acid molecule is 3.2 [15], giving a value of ΔpKa = pKa(base) − pKa(acid) = 0.4 for the combination of the acid with the pyridine group, and a value of −1.4 of the acid with the hydrazine nitrogen. Regarding mandelic acid, the pKa is 3.4, and the values of ΔpKa are 0.2 and −1.6, respectively. This range has been given by previous researchers as a rule of thumb, where the result cannot be easily predicted. [16]. In general, a ΔpKa > 3 is expected to form a salt, while a ΔpKa < 0 almost certainly results in a neutral cocrystal. It is the narrow region between 0 and 3 that does not allow for accurate predictions [17]. The ΔpKa for the acid and amine combination was −1.4/−1.6, which would predict that no proton transfer should occur, since the value is less than 0. However, in the case of dl-mandelic acid, the crystallographic results confirmed that proton transfer occurs. A caveat to the calculations is that the reported pKa for the two molecules, isoniazid and glycolic acid or dl-mandelic acid, depends on the solvent used and its polarity. While compound (1) was obtained in ethyl acetate (ϵ = 6.2) or cyclohexane (ϵ = 2.02), compound (2) was crystallized from water (ϵ = 80). Furthermore, it should be noted that a comprehensive study of 6465 crystalline compounds containing ionized (A+B−) and non-ionized (AB) acid-base pairs in the Cambridge Structural Data (CSD) was conducted at 1 < ΔpKa < 2 values, and the occurrences of AB and A+B− were practically the same [17].
Structural Description and Supramolecular Analysis
The isoniazid−glycolic acid cocrystal (1) crystallized in the monoclinic P21/n space group with unit cell dimensions of a = 3.8930(3) Å, b = 9.9754(5) Å, c = 23.6410(12) Å, β = 92.480(3)°, and V = 917.22(10) Å3. The asymmetric unit contained one isoniazid molecule and one glycolic acid molecule (Figure 1a). Glycolic acid was hydrogen-bonded to isoniazid pyridine N through O−H···N. The angle between the carboxyl group plane and the pyridyl ring plane was 4.9°. A weak pyridyl−glycolic acid C−H···O hydrogen bond resulted in an (7) ring motif (Figure 1b). This synthon has also been observed in other isoniazid cocrystals with carboxylic acids. Furthermore, each isoniazid molecule was linked by hydrogen bonding to the two other nearest neighbor molecules through N-H···O, as the donor and acceptor, respectively, forming zig-zag chains parallel to the “b” axis, which, in turn, were linked through head-to-tail glycolic acid molecules by two new hydrogen bonds O-H···N and O-H···O, so the sequence … H2ga-INH-H2ga-INH … was established along the “c” axis.
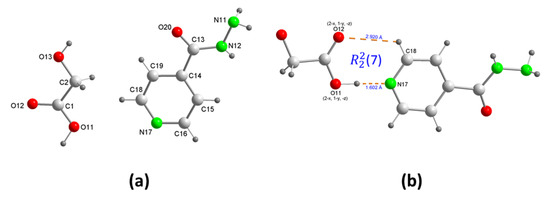
Figure 1.
(a) Asymmetrical unit of isoniazid−glycolic acid (INH)·(H2ga) cocrystal (1) showing the atom-numbering scheme, and (b) detail of (7) ring motif.
The isoniazid−dl-mandelic acid salt cocrystal ([HINH]+[Hma]−(H2ma)) (2) was formed by cocrystallization, using a 1:1 ratio of racemic dl-mandelic acid and achiral isoniazid by mechanochemistry and liquid-assisted grinding, in the presence of water. This resulted in the formation of a salt that crystallizes in the monoclinic P21/c space group with unit cell dimensions of a = 5.6049(5) Å, b = 24.388(3) Å, c = 15.1471(16) Å, β = 92.025(7)°, and V = 2069.2(4) Å3. The asymmetric unit of (2) contained one isoniazide-ammonium cation [HINH]+, one dl-mandelate anion, and one dl-mandelic acid molecule of solvation. Figure 2a shows the crystal structure of (2). In the unusual, protonated isoniazid cation, the dl-mandelate anion transferred its proton to the hydrazine nitrogen, giving rise to a robust hydrogen bond N+–H···O− that was supported by N–H···O− and C–H···O− hydrogen bonds, resulting in (7) and (7) rings, respectively (Figure 2b). The molecule of neutral dl-mandelic acid of the crystallization was hydrogen bonded to the N pyridine of the isoniazide-ammonium cation through O–H···N. The angle between the plane of the carboxyl group and the plane of the pyridyl ring was 9.23°. A weak hydrogen bond, C–H···O, and pyridyl-mandelic acid, resulted in a (7) ring motif (Figure 2b).
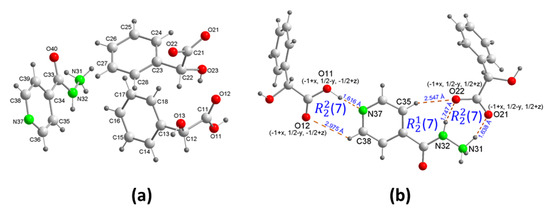
Figure 2.
(a) Asymmetrical unit of isoniazid−dl-mandelic acid salt cocrystal [HINH]+[Hma]−(H2ma) (2) showing the atom-numbering scheme, and (b) details of the ring motifs.
From a crystal engineering viewpoint, INH is well known to form pyridine–carboxylic acid heterosynthons with carboxylic acids. According to a recent study, 39 structures of INH cocrystals have been identified with coformers containing COOH groups, representing approximately 40% of all INH structures deposited in the CSD, where the acid-pyridine synthon is the most recurrent, and is present in approximately 87% of structures [3].
N’-(propan-2-ylidene)isonicotinhydrazide hemihydrate (3), was prepared by adapting the crystallization method described in the literature [18]. The product was obtained by the cocrystallization of isoniazid and glycolic acid from acetone at room temperature. Slow evaporation of the solvent under ambient conditions produced single crystals of the product (3).
The process consisted of a one-pot synthesis, with covalent modification occurring in situ, where INH is reacted with acetone, or in general with molecules containing ketone or aldehyde functional groups (RC=O), so that the NH2 group of the carbohydrazide moiety undergoes a condensation reaction and replaces the two H atoms with alkyl groups to form isonicotinichydrazides (Scheme 2). This technique is similar to what has been called “covalence-assisted supramolecular synthesis”, with the difference being that, in this case, a hemihydrate was obtained instead of a cocrystal with a glycolic acid molecule as a coformer, as expected by the authors of [19].
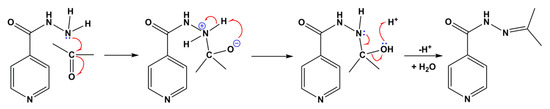
Scheme 2.
Mechanism for the formation of N’-(propan-2-ylidene)isonicotinichydrazide from isoniazid.
Compound 3 crystallizes in the orthorhombic, Aba2 space group and unit cell dimensions a = 18.8351(5) Å, b = 12.6568(4) Å, c = 8.0435(3) Å, and V = 1917.51(11) Å3. The asymmetric unit of 3 contains one N’-(propan-2-ylidene)isonicotinichydrazide molecule and half a molecule of water. Figure 3a shows the crystal structure of pINH.
Symmetry positions: a, 1/2 − x, y, −1/2 + z; b, 1/2 − x, y, 1/2 + z
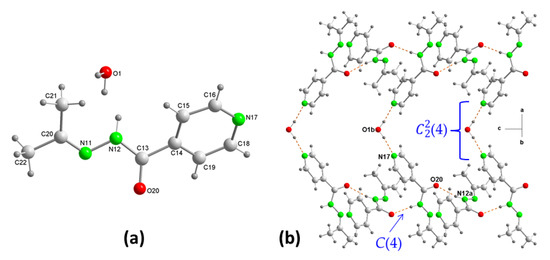
Figure 3.
(a) Asymmetrical unit of N’-(propan-2-ylidene)isonicotinic hydrazide ((pINH)·1/2(H2O)) (3) showing the atom-numbering scheme, and (b) hydrogen-bonding (dashed lines) motifs.
The distances and angles within the pINH were as expected. The crystal structure of (3) showed a substantial change in the pattern and packing of hydrogen bonds with respect to the structures of (1) and (2). The replacement of the two hydrazine hydrogen atoms by the propylidene group removed most of the functionality of the hydrogen bonds of isoniazid. Likewise, the presence of half a molecule of water in the crystalline structure, instead of a COOH group of a carboxylic acid, when this was a coformer in a cocrystal with the modified isoniazid, gave rise to a different packing. The crystal structure consists of a 1D network of C(4) chains formed by homomeric hydrogen bonding of the amide group, i.e., N12–H12A···O20, linking symmetrically related pINH molecules to form chains along the “c” axis (Figure 3b). Such chains have previously been observed in N’-(propan-2-ylidene)isonicotinichydrazide cocrystals with carboxylic acids [18,19]. Furthermore, the characteristic carboxylic acid-pyridine heterosynton of (7) graph-set motif [18,19] that was formed between the COOH group and the N’-(propan-2-ylidene)isonicotinichydrazide molecule in the carboxylic acid cocrystals with the modified isoniazid, in (3) was replaced by a water-pyridine hydrogen bond. Thus, each water molecule acted as a bridge joining two chains of pINH molecules to form a 2D structure parallel to the “ac” plane, where a graphic set (4) was present (Figure 3b).
Author Contributions
Conceptualization, R.A.-V., I.G.-S., R.T.-I. and A.C.; methodology, R.A.-V., I.G.-S., R.T.-I. and A.C.; software, A.C.; validation, A.C., I.G.-S.; formal analysis, R.A.-V., I.G.-S., R.T.-I. and A.C.; investigation, R.A.-V., I.G.-S., R.T.-I.; resources, X.X.; data curation, X.X.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and I.G.-S.; visualization, R.A.-V., I.G.-S., R.T.-I. and A.C.; supervision, A.C. and I.G.-S.; project administration, A.C. and A.F.; funding acquisition, A.C. and I.G.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Financial support from the Network of Excellence “Metallic Ions in Biological Systems” CTQ2017-90802-REDT [Ministerio de Economía y Competitividad (Spain) and European Regional Development Fund (EU)], and the Xunta de Galicia (Spain) [Rede de Excelencia MetalBIO ED431D 2017/01].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du, M.; Zhang, Z.-H.; Wang, X.-G.; Wu, H.-F.; Wang, Q. Flexible Building Blocks of N,N′-Bis(picolinoyl)hydrazine for Hydrogen-Bonding Directed Cocrystallization: Structural Diversity, Concomitant Polymorphs, and Synthon Prediction. Cryst. Growth Des. 2006, 6, 1867–1875. [Google Scholar] [CrossRef]
- Wouters, J.; Quere, L. (Eds.) Pharmaceutical Salts and Co-Crystals; RSC Drug Discovery Series No. 16; RSC Publisher: Cambridge, UK, 2011. [Google Scholar]
- Diniz, R.; Souza, M.S.; Carvalho, P.S.; Da Silva, C.C.; D’Vries, R.F.; Ellena, J.A. Novel Isoniazid cocrystals with aromatic carboxylic acids: Crystal engineering, spectroscopy and thermochemical investigations. J. Mol. Struct. 2018, 1153, 58–68. [Google Scholar] [CrossRef]
- Iseman, M.D. Tuberculosis therapy: Past, present and future. Eur. Respir. J. 2002, 20, 87S–94S. [Google Scholar] [CrossRef] [PubMed]
- Hearn, M.J.; Cynamon, M.H.; Chen, M.F.; Coppins, R.; Davis, J.; Kang, H.J.-O.; Noble, A.; Tu-Sekine, B.; Terrot, M.S.; Trombino, D. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur. J. Med. Chem. 2009, 44, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Lemmerer, A. Covalent assistance to supramolecular synthesis: Modifying the drug functionality of the antituberculosis API isoniazidin situ during co-crystallization with GRAS and API compounds. CrystEngComm 2012, 14, 2465–2478. [Google Scholar] [CrossRef]
- Sarcevica, I.; Orola, L.; Veidis, M.V.; Podjava, A.; Belyakov, S. Crystal and Molecular Structure and Stability of Isoniazid Cocrystals with Selected Carboxylic Acids. Cryst. Growth Des. 2013, 13, 1082–1090. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B. Novel solid forms of the anti-tuberculosis drug, Isoniazid: Ternary and polymorphic cocrystals. CrystEngComm 2013, 15, 5877. [Google Scholar] [CrossRef]
- Castiñeiras, A.; García-Santos, I.; Gonzalez-Perez, J.M.; Bauzá, A.; Zaręba, J.K.; Niclós-Gutiérrez, J.; Torres, R.; Vilchez, E.; Frontera, A. Multicomponent Supramolecular Assemblies of Melamine and α-Hydroxycarboxylic Acids: Understanding the Hydrogen Bonding Patterns and Their Physicochemical Consequences. Cryst. Growth Des. 2018, 18, 6786–6800. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Castiñeiras, A.; Frontera, A.; Garcia-Santos, I.; González-Pérez, J.M.; Niclos-Gutierrez, J.; Rodríguez-González, I.; Vílchez-Rodríguez, E.; Zaręba, J.K. Recurrent motifs in pharmaceutical cocrystals involving glycolic acid: X-ray characterization, Hirshfeld surface analysis and DFT calculations. CrystEngComm 2020, 22, 6674–6689. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Hou, S.-Y.; Cao, Z.-X.; Wan, H.-L.; Ng, S.-W. Syntheses, crystal structures and biological relevance of glicolato and S-lactato molybdates. J. Inorg. Biochem. 2004, 98, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Tumanov, N.A.; Payen, R.; Springuel, G.; Norberg, B.; Robeyns, K.; Le Duff, C.S.; Wouters, J.; Leyssens, T. Cocrystallization out of the blue: Dl-mandelic acid/ethyl-dl-mandelate cocrystal. J. Mol. Struct. 2017, 1127, 397–402. [Google Scholar] [CrossRef]
- Brunner, H.; Maiterth, F.; Treittinger, B. Synthesis and antitumor activity of water-soluble 2-benzyl-1,2-diaminobutane-α-oxycarboxylatoplatinum(II) complexes. Inorganica Chim. Acta 1992, 198, 79–84. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, R.A. Voltammetric Behavior and Determination of Isoniazid Using PEDOT Electrode in Presence of Surface Active Agents. Int. J. Electrochem. Sci. 2011, 6, 5097–5113. [Google Scholar]
- Banerjee, S.; Bhanja, S.K.; Chattopadhyay, P.K. Quantum chemical predictions of aqueous pKa values for OH groups of some a-hydroxycarboxylic acids based on ab initio and DFT calculations. Comput. Theor. Chem. 2018, 1125, 29–38. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, A.G.P.; Park, A. The Salt−Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Lemmerer, A.; Bernstein, J.; Kahlenberg, V. One-pot covalent and supramolecular synthesis of pharmaceutical co-crystals using the API isoniazid: A potential supramolecular reagent. CrystEngComm 2010, 12, 2856–2864. [Google Scholar] [CrossRef]
- Madeley, L.G.; Levendis, D.C.; Lemmerer, A. Covalent-assisted supramolecular synthesis: The effect of hydrogen bonding in cocrystals of 4-tert-butylbenzoic acid with isoniazid and its derivatized forms. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 200–207. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).