Chemical Synthesis and Hemi-Synthesis of Novel Benzimidazole Derivatives Using Microwave-Assisted Process: Chemical Characterization, Bioactivities and Molecular Docking †
Abstract
:1. Introduction
- A chemical synthesis and hemi-synthesis of new benzimidazole derivatives;
- A physicochemical characterization (purification and structural analysis) of the synthesized compounds by 1H NMR spectroscopy and FTIR;
- Evaluation of antioxidant and antimicrobial activities by in-vitro assays;
- Evaluation of docking scores of the synthesized compounds on 4QGH (PDB: Protein data base entry) protein of Staphylococcus aureus thymidylate kinase (TMK).
2. Methods
2.1. Plant Material and Extraction Procedure
2.2. Essential Oil Analysis
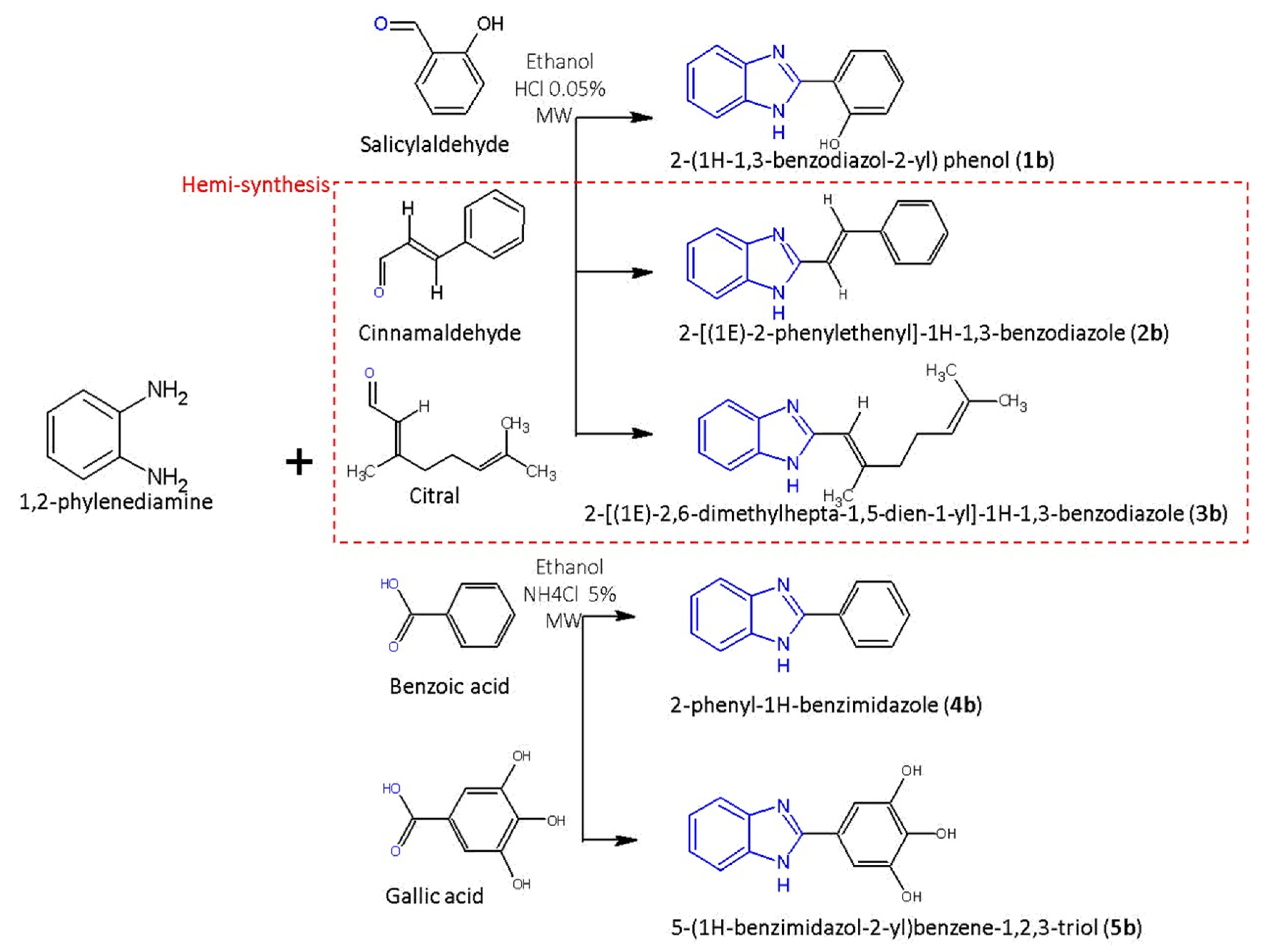
2.3. Synthesis Procedure
2.3.1. Synthesis/Hemi-Synthesis of Three Benzimidazole Aldehyde Derivatives
2.3.2. Synthesis of Benzimidazole Phenolic Acid Derivatives
2.4. Fractionating/Purification
2.5. Structural Analysis
2.6. Bioactivities Properties Evaluation
2.6.1. Antioxidant Activity
2.6.2. Antimicrobial Activity
2.7. Molecular Docking Study
3. Results and Discussion
3.1. GC/MS Profiles of Essential Oils of Cinnamon and Lemongrass
3.2. Separation and Purification of Synthetic Products
3.3. Structural Analysis
3.4. Bioactivity Properties
3.4.1. Antioxidant Activity
3.4.2. Antimicrobial Activity
3.5. Molecular Docking Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Rt (min) | Concentration % | Compound |
|---|---|---|
| 4.3 | 0.02 | Tetrachloroethylene |
| 8.6 | 0.04 | γ-Terpinene |
| 9.4 | 0.03 | Camphene |
| 10.6 | 0.25 | Benzaldehyde |
| 14.6 | 0.05 | D-Limonene |
| 23.1 | 0.05 | 1,2-Chromene |
| 24.6 | 0.92 | Hydrocinnamaldehyde |
| 25.3 | 0.07 | Phenyl 2-Propynyl Ether |
| 34.9 | 90.54 | Cinnamaldehyde |
| 38.2 | 0.61 | α-Cubebene |
| 39.1 | 0.06 | Oxirane |
| 39.4 | 0.05 | α-Copaene |
| 39.8 | 0.07 | (+)-Sativene |
| 40.7 | 0.04 | (−)-Isosativene |
| 41.3 | 0.05 | β-Thujene |
| 43.2 | 0.03 | Benzenamine |
| 43.8 | 2.87 | Coumarin |
| 45.0 | 0.21 | Naphthalene |
| 46.1 | 0.05 | Amide Hydrocinnamique |
| 46.5 | 0.66 | α-Cadinene |
| 47.9 | 0.90 | δ-Cadinene |
| 48.5 | 0.19 | 1H-3a,7-Methanoazulene |
| 49.2 | 1.13 | 4-Methoxycinnamaldehyde |
| 53.4 | 0.08 | 2,4-Hexadiene |
| 55.9 | 0.36 | γ-Cadinene |
| Rt (Min) | % | Compound |
|---|---|---|
| 4.6 | 0.02 | Tridodecylamine |
| 7.8 | 0.08 | D-Limonene |
| 12.2 | 4.55 | β-Myrcene |
| 14.6 | 0.1 | α-Limonene |
| 15.4 | 0.31 | α-Pinene |
| 16.1 | 0.33 | β-Ocimene |
| 16.4 | 0.06 | Myrcenylacetat |
| 19.3 | 0.05 | Nortricyclene |
| 19.8 | 0.4 | Furan |
| 20.3 | 1.52 | L-Linalool |
| 21.3 | 0.06 | Fenchol |
| 22.5 | 0.23 | Cyclohexene |
| 23.3 | 0.44 | Trans-Chrysanthemal |
| 23.6 | 0.35 | (R)-(+)-Citronellal |
| 24.5 | 0.81 | Cyclopropene |
| 25.8 | 1.29 | 7-Methyl-1-Nonyne |
| 28.4 | 0.11 | O-Mentha-1(7),8-Dien-3-Ol |
| 30.5 | 34.87 | Neral |
| 32.8 | 43.88 | Cis-Citral |
| 33.7 | 0.32 | Geranial |
| 34.2 | 0.22 | Geranyl Vinyl Ether |
| 35.8 | 3.5 | Geraniol |
| 38.4 | 0.24 | Nerol |
| 39.5 | 3.37 | Nerol Acetate |
| 40.2 | 0.65 | Geranic Acid |
| 41.3 | 0.21 | β-Caryophyllene |
| 42.4 | 0.17 | α-Bergamotene |
| 50.1 | 0.06 | Neryl Acetate |
| 51.4 | 0.08 | β-Citronellal |
| 54.1 | 0.14 | Trans-β-Farnesene |
| 70.8 | 0.18 | Farnesyl |
| 72.5 | 0.06 | Trans-Caryophyllene |
| 75.3 | 0.11 | Cyclopropane Carboxamide |
| 76.7 | 0.2 | α-Trans-Sequicyclogeraniol |
| 78.3 | 0.31 | Farnesol |
| 79.6 | 0.14 | 3,7-Nonadien-2-Ol |
| 80.0 | 0.07 | Geranylacetone |
References
- Shah, M.S.; Eppinger, M.; Ahmed, S.; Shah, A.A.; Hameed, A.; Hasan, F. Multidrug-resistant diarrheagenic E. coli pathotypes are associated with ready-to-eat salad and vegetables in Pakistan. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 267–273. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Barros, L.; Boumehira, A.Z.; Bachari, K.; Heleno, S.A.; Alves, M.J.; Ferreira, I.C.F.R. Profiling polyphenol composition by HPLC-DAD-ESI/MSn and the antibacterial activity of infusion preparations obtained from four medicinal plants. Food Funct. 2018, 9, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-casassus, C.; Pouysøgu, L. Natural Products Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Nat. Prod. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Salahuddin; Shaharyar, M.; Mazumder, A. Benzimidazoles: A biologically active compounds. Arab. J. Chem. 2017, 10, S157–S173. [Google Scholar] [CrossRef]
- Chikhale, H.; Nerkar, A. Review on In-Silico techniques an approach to Drug discovery Current. Trends Pharm. Pharm. Chem. 2020, 2, 24–32. [Google Scholar]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. In silico Methods for Drug Design and Discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Rassem, H.H.A.; Nour, A.H.; Yunus, R.M. Techniques for Extraction of Essential Oils from Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Ziani, B.E.C.; Calhelha, R.C.; Barreira, J.C.M.; Barros, L.; Hazzit, M.; Ferreira, I.C.F.R. Bioactive properties of medicinal plants from the Algerian flora: Selecting the species with the highest potential in view of application purposes. Ind. Crops Prod. 2015, 77. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Carocho, M.; Abreu, R.M.V.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Talhi, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profiling, biological activities and in silico studies of Acacia tortilis (Forssk.) Hayne ssp. raddiana extracts. Food Biosci. 2020, 36, 100616. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. The Role of Phenolic Compounds in the Fight against Cancer—A Review. Anticancer. Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Agents Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
| Synthetized Molecule | Antioxidant Activity EC5O (µg/mL) | |||
|---|---|---|---|---|
| DPPH Test | Ferric Ion Reducing Power | β-Carotene | TBARS | |
| 1b | 53 ± 1 | 54 ± 4 | 192 ± 7 | 134 ± 2 |
| 2b | 139 ± 4 | 101 ± 7 | 181 ± 5 | 156 ± 52 |
| 3b | 220 ± 15 | 102 ± 22 | 220 ± 11 | 134 ± 2 |
| 4b | 767 ± 6 | 544 ± 4 | 872 ± 37 | 1554 ± 25 |
| 5b | 78 ± 5 | 96 ± 8 | 94 ± 3 | 101 ± 7 |
| BHT | 23 ± 3 | 30 ± 6 | 48 ± 5 | 76 ± 1 |
| Trolox | 51 ± 4 | 44 ± 4 | 63 ± 2 | 84 ± 6 |
| Synthetized Molecule | E. coli | S. aureus | P. aeruginosa | S. typhi | C. albicans | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 1b | 0.3125 | 2.5 | ˂0.3125 | 1.25 | 0.625 | 5 | 0.3125 | 5 | ˂0.3125 | 2.5 |
| 2b | 0.3125 | 10 | 0.3125 | 5 | 1.25 | >10 | 0.3125 | 5 | 0.625 | 2.5 |
| 3b | 0.3125 | 5 | 0.3125 | 2.5 | 2.5 | 5 | 0.3125 | 5 | 0.3125 | 5 |
| 4b | 2.5 | 2.5 | 1.25 | 1.25 | >10 | >10 | 2.5 | >10 | 5 | >10 |
| 5b | 0.625 | 2.5 | 0.156 | 0.625 | 2.5 | 5 | 0.3125 | 5 | 0.3125 | 5 |
| Antibiotics | ||||||||||
| Gentamicine 10Ug | ˂0.078 | ˂0.078 | 0.156 | ˂0.156 | nt | |||||
| Ceftazidime 30Ug | ˂0.156 | 0.156 | 0.156 | ˂0.156 | nt | |||||
| Nystatine | nt | nt | nt | nt | ˂0.078 | |||||
| Protein | Interacting Residue | Binding Energy, ΔG (Kcal/Mol) | Inhibition Constant, Ki (µm) |
|---|---|---|---|
| thymidylate kinase TMK (4QGH) | 1b | −8.3 | 0.812 |
| 5b | −9.4 | 0.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sameut, A.; Zanndouche, S.Y.; Boumaza, C.; Dikes, C.; Ziani, B.E.C. Chemical Synthesis and Hemi-Synthesis of Novel Benzimidazole Derivatives Using Microwave-Assisted Process: Chemical Characterization, Bioactivities and Molecular Docking. Chem. Proc. 2021, 3, 71. https://doi.org/10.3390/ecsoc-24-08306
Sameut A, Zanndouche SY, Boumaza C, Dikes C, Ziani BEC. Chemical Synthesis and Hemi-Synthesis of Novel Benzimidazole Derivatives Using Microwave-Assisted Process: Chemical Characterization, Bioactivities and Molecular Docking. Chemistry Proceedings. 2021; 3(1):71. https://doi.org/10.3390/ecsoc-24-08306
Chicago/Turabian StyleSameut, Asmaâ, Sarah Yasmine Zanndouche, Chaimaa Boumaza, Chaima Dikes, and Borhane Eddine Cherif Ziani. 2021. "Chemical Synthesis and Hemi-Synthesis of Novel Benzimidazole Derivatives Using Microwave-Assisted Process: Chemical Characterization, Bioactivities and Molecular Docking" Chemistry Proceedings 3, no. 1: 71. https://doi.org/10.3390/ecsoc-24-08306
APA StyleSameut, A., Zanndouche, S. Y., Boumaza, C., Dikes, C., & Ziani, B. E. C. (2021). Chemical Synthesis and Hemi-Synthesis of Novel Benzimidazole Derivatives Using Microwave-Assisted Process: Chemical Characterization, Bioactivities and Molecular Docking. Chemistry Proceedings, 3(1), 71. https://doi.org/10.3390/ecsoc-24-08306






