Abstract
Benzofuroxane reacts under Beirut reaction conditions with active methylene nitriles to give new 2-aminoquinoxaline-1,4-dioxides. The treatment of known 2-amino-3-cyanoquinoxaline-1,4-dioxide with chloroacetyl chloride afforded corresponding chloroacetamide which is useful for the preparation of various heterocycles bearing a quinoxaline-1,4-dioxide core system.
Published: 14 November 2020
1. Introduction
Quinoxaline-1,4-dioxides, mostly prepared through the Beirut reaction described for the first time by M. Haddadin and C. Issidorides at the American University of Beirut, Lebanon, in 1965, have been recognized as compounds of practical interest, primarily due to the wide spectrum of their biological activity (for reviews see [1,2,3,4,5,6]). Thus, 2-aminoquinoxaline-1,4-dioxides are known to possess leishmanicidal and antiplasmodial activities [7,8], anti-tumor activity [9], and antitubercular effects [10,11]. On the other hand, Tirapazamine (3-amino-1,2,4-benzotriazine-1,4-dioxide) (Figure 1), which has been known for a long time as an anticancer drug, has a very closely related structure. The above reasons prompted us to study the reactions of benzofuroxane with some active methylene nitriles in order to prepare new compounds with 2-aminoquinoxaline-1,4-dioxide core. Such compounds may be useful for the development of new antiprotozoal and anticancer agents.
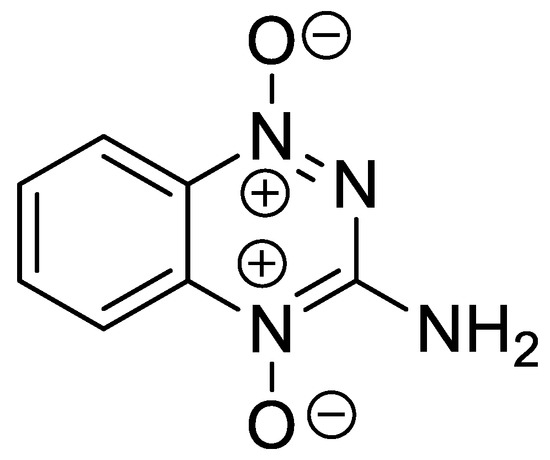
Figure 1.
The structure of Tirapazamine (3-amino-1,2,4-benzotriazine-1,4-dioxide) closely related to 2-aminoquinoxaline-1,4-dioxides.
2. Results and Discussion
First, we reacted benzofuroxane with 2-cyanomethylthiazoles [12], easily available by the Hantzsch reaction of cyanothioacetamide [13,14] with phenacyl bromides (Scheme 1). Hybrid polyheterocycles 2, bearing both thiazole and quinoxaline fragments, were recognized as the reaction products.
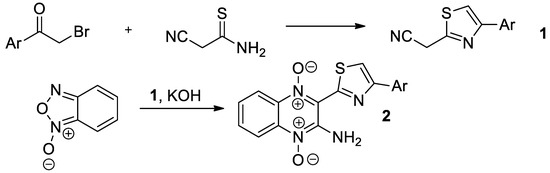
Scheme 1.
The synthetic pathway to 2-aminoquinoxaline-1,4-dioxides 2.
Then, cyanomethylpyrazole 3, prepared by the reaction of malononitrile dimer with hydrazine [15,16], reacted with benzofuroxane to give a product with the suggested structure 4 (Scheme 2). The evidence for the structure of compound 4 rests chiefly on its FT-IR spectrum, while its NMR data cannot be interpreted unambiguously. Thus, the IR spectrum revealed the presence of an amino group and conjugated CN group, while the band corresponding to the non-conjugated CN group was absent. This fact allows one to exclude the possible structure 5 (Scheme 2). The studies on the structure and reactions of compound 4 are in progress.
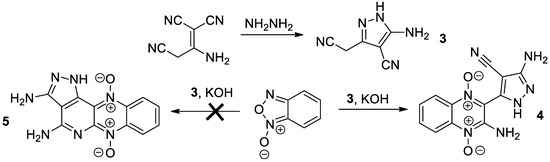
Scheme 2.
The synthetic pathway to 2-aminoquinoxaline-1,4-dioxide 4.
Finally, we prepared 2-amino-3-cyanoquinoxaline-1,4-dioxide 6 by known method [17], which was reacted with chloroacetyl chloride. After short-term heating in AcOH, a bright yellow precipitate of chloroacetamide 7 formed (Scheme 3). It should be noted that the only reported method for the chloroacetylation of 2-aminoquinoxaline-1,4-dioxides was based on the reaction of 6,7-difluoro-2-quinoxalinecarbonitrile 1,4-di-N-oxide with hardly available chloroacetic anhydride [11]. The compound 7 is expected to be a promising reagent for the synthesis of polyheterocyclic ensembles with quinoxaline core fragment.
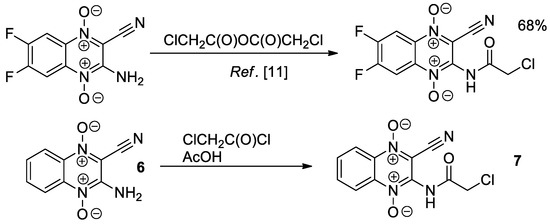
Scheme 3.
Chloroacetylation of 2-amino-3-cyanoquinoxaline-1,4-dioxides.
3. Experimental
3.1. Preparation of Compound 2: General Procedure
Equimolar amounts of thiazol-2-ylacetonitrile 1 and benzofuroxane were dissolved in DMF and treated with the 1.5 eq. of base (KOH or Et3N). The dark colored reaction mixture was left to stand in a freezer for 24–72 h, then diluted with cold alcohol. The precipitated solid was filtered off and recrystallized to produce analytically pure samples of quinoxalines 2.
Chloroacetylation of Compound 6
2-Amino-3-cyanoquinoxaline 1,4-dioxide 6 (0.01 mol) was suspended in glacial AcOH (15 mL) and treated with chloroacetyl chloride (0.011 mol). When the reaction mixture was gently heated to 50 °C, the red color of 6 disappeared and the reaction mass turned bright yellow and then brick orange. The mixture was heated under vigorous stirring until the starting of 6 was fully consumed (TLC (thin-layer chromatography) control). The brick-orange precipitate was filtered off and washed with EtOH to produce pure chloroacetamide 7 at a yield of 90%. The compound is soluble in hot DMF and DMSO, but sparingly soluble in AcOH, EtOH.
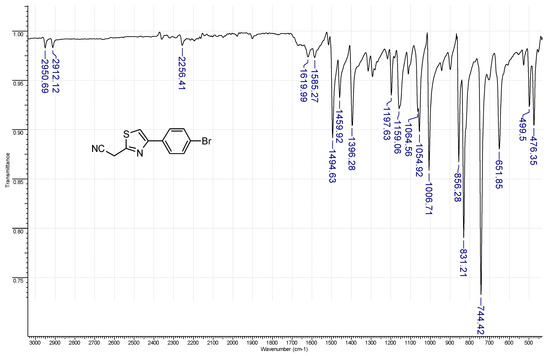
Figure 2.
The FT–IR (ATR—Attenuated total reflectance mode) spectrum of 4-(4-bromophenyl)-2-cyanomethylthiazole 1a.
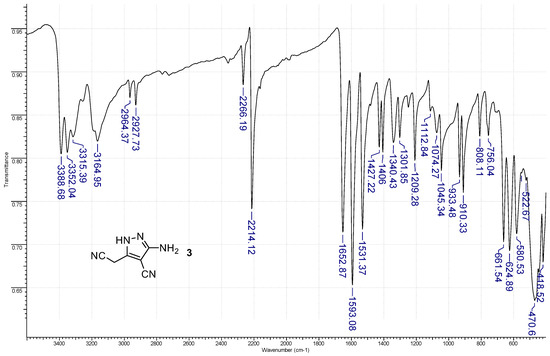
Figure 3.
The FT–IR (ATR mode) spectrum of 3-amino-5-(cyanomethyl)-1H-pyrazole-4-carbonitrile 3.
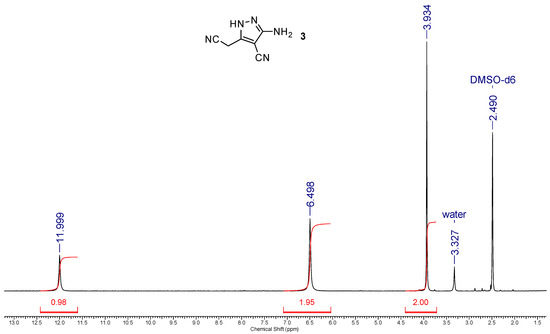
Figure 4.
1H NMR spectrum (400 MHz, DMSO-d6) of 3-amino-5-(cyanomethyl)-1H-pyrazole-4-carbonitrile 3.
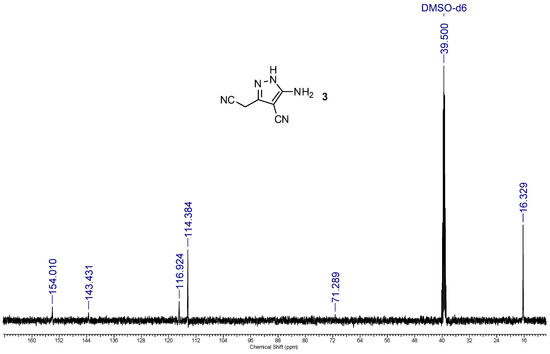
Figure 5.
13C NMR spectrum (101 MHz, DMSO-d6) of 3-amino-5-(cyanomethyl)-1H-pyrazole-4-carbonitrile 3.
Figure 6.
The FT-IR (ATR mode) spectrum of 2-amino-3-cyanoquinoxaline 1,4-dioxide 6.
Author Contributions
Conceptualization, V.V.D.; methodology, V.V.D.; formal analysis, K.V.K., A.A.R., A.M.S.; investigation, K.V.K., A.A.R., A.M.S.; writing—original draft preparation, V.V.D.; writing—review and editing, V.V.D.; supervision, V.V.D.; funding acquisition, V.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RFBR and Krasnodar region according to the research project No. 19-43-230007.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lima, L.M.; do Amaral, D.N. Beirut Reaction and its Application in the Synthesis of Quinoxaline-N, N′-Dioxides Bioactive Compounds. Rev. Virtual Quim. 2013, 5, 1075–1100. [Google Scholar] [CrossRef]
- Haddadin, M.J.; Issidorides, C.H. Application of benzofurazan oxide to the synthesis of heteroaromatic N-oxides. Heterocycles 1976, 4, 767–816. [Google Scholar]
- Hamama, W.S.; Waly, S.M.; Said, S.B.; Zoorob, H.H. Highlights on the chemistry of 2-amino-3-cyano-quinoxaline 1,4-dioxides and their derivatives. Synth. Commun. 2020, 50, 1737–1757. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Zhukova, N.A. Progress in quinoxaline synthesis (Part 2). In Progress in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 25, pp. 1–45. [Google Scholar]
- Mamedov, V.A. Synthesis of Quinoxalines. In Quinoxalines; Springer: Cham, Switzerland, 2016; pp. 5–133. [Google Scholar]
- González, M.; Cerecetto, H.; Monge, A. Quinoxaline 1,4-dioxide and phenazine 5,10-dioxide. In Bioactive Heterocycles V; Chemistry and Biology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 179–211. [Google Scholar]
- Barea, C.; Pabón, A.; Pérez-Silanes, S.; Galiano, S.; Gonzalez, G.; Monge, A.; Deharo, E.; Aldana, I. New amide derivatives of quinoxaline 1,4-di-N-oxide with leishmanicidal and antiplasmodial activities. Molecules 2013, 18, 4718–4727. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Castillo, D.; Zimic, M.; Quiliano, M.; Galiano, S.; Pérez-Silanes, S.; Monge, A.; Deharo, E.; Aldana, I. New salicylamide and sulfonamide derivatives of quinoxaline 1,4-di-N-oxide with antileishmanial and antimalarial activities. Bioorg. Med. Chem. Lett. 2011, 21, 4498–4502. [Google Scholar] [CrossRef]
- Monge, A.; Martinez-Crespo, F.J.; Lopez de Cerain, A.; Palop, J.A.; Narro, S.; Senador, V.; Marin, A.; Sainz, Y.; Gonzalez, M. Hypoxia-selective agents derived from 2-quinoxalinecarbonitrile 1,4-di-N-oxides. 2. J. Med. Chem. 1995, 38, 4488–4494. [Google Scholar] [CrossRef] [PubMed]
- Ancizu, S.; Moreno, E.; Torres, E.; Burguete, A.; Pérez-Silanes, S.; Benítez, D.; Villar, R.; Solano, B.; Marín, A.; Aldana, I.; et al. Heterocyclic-2-carboxylic acid (3-cyano-1,4-di-N-oxidequinoxalin-2-yl)amide derivatives as hits for the development of neglected disease drugs. Molecules 2009, 14, 2256–2272. [Google Scholar] [CrossRef] [PubMed]
- Sainz, Y.; Montoya, M.E.; Martínez-Crespo, F.J.; Ortega, M.A.; de Cerain, A.L.; Monge, A. New quinoxaline 1,4-di-N-oxides for treatment of tuberculosis. Arzneimittelforschung 1999, 49, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.; Gewald, K. Zur Chemie des 4-Phenyl-thiazoly-(2)-acetonitrils. J. Prakt. Chem. 1974, 316, 684–692. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Dyachenko, I.V.; Nenajdenko, V.G. Cyanothioacetamide: A polyfunctional reagent with broad synthetic utility. Russ. Chem. Rev. 2018, 87, 1. [Google Scholar] [CrossRef]
- Litvinov, V.P. Cyanoacetamides and their thio-and selenocarbonyl analogues as promising reagents for fine organic synthesis. Russ. Chem. Rev. 1999, 68, 737–763. [Google Scholar] [CrossRef]
- Taylor, E.C.; Hartke, K.S. The Reaction of Malononitrile with Hydrazine. J. Am. Chem. Soc. 1959, 81, 2452–2455. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Semenova, A.M. Heterocyclization reactions using malononitrile dimer (2-aminopropene-1,1,3-tricarbonitrile). Chem. Heterocycl. Compd. 2018, 54, 989–1019. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Galiano, S.; Pérez-Silanes, S.; Gonzalez, G.; Deyssard, C.; Monge, A.; Deharo, E.; Aldana, I. Antiplasmodial and leishmanicidal activities of 2-cyano-3-(4-phenylpiperazine-1-carboxamido) quinoxaline 1,4-dioxide derivatives. Molecules 2012, 17, 9451–9461. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).