Abstract
In this study, Bi2O3 nanoparticles were successfully synthesized through a facile hydrothermal procedure. The structure of the Bi2O3 nanoparticles was analyzed by Fourier transfer infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). The synthesized Bi2O3 nanoparticles have unique properties, such as high activity, high purity, and high surface area. Hence, we have reported the Bi2O3 nanoparticles as an efficient, cost-effective, and mild catalyst for the synthesis of acridinedione derivatives via a one-pot four-component reaction. In addition, the effect of the morphology of the Bi2O3 nanostructure was investigated on catalytic performance. Therefore, Bi2O3 nanoparticles were prepared and applied as a heterogeneous catalyst in the synthesis of acridinedione derivatives. The present approach offers several advantages, such as excellent yields within short times, green catalyst, and ease of recovery.
1. Introduction
Nowadays, scientists are using multicomponent reactions to synthesis complex organic compounds due to their high efficiency. These reactions have attracted sizeable attention due to their many applications in various fields, such as medicine, agriculture, and intermediates []. The different multicomponent reactions, like Mannich, Biginelli, Strecker, Hantzsch, and acridinedione derivative synthesis, are significant organic transformations for the synthesis of pharmaceutical compounds []. Acridinedione compounds are a class of heterocyclic compounds due to their unique properties, such as anticancer, antimicrobial, antibacterial, and their fluorescence properties, their use in various fields, including pharmaceutical, biological, and laser dyes. Different methods have been used to synthesize acridinedione derivatives, which usually suffer from hazard solvents, expensive reagents, and high reaction times. Heterogeneous catalysts have a crucial role in determining the conditions of reactions [,]. They are known as compounds or substances that speed up a chemical reaction without changing it. The advantages of heterogeneous catalysts are high activity, high surface area, long lifetimes, thermal stability, selectivity, and non-toxicity [].
As mentioned, the acridinedione derivatives are one of the attractive reactions in chemistry that have been synthesized with various catalysts, such as nano-ferrite, TiO2 carbon nanotube, SiO2, Rh (III), and Amberlyst-15. In addition, bismuth oxides have shown excellent catalytic properties. They have potential applications in solar cells, gas sensors, and piezoelectric-optical materials. The other attractive features of bismuth oxides included non-toxicity, ionic conductivity, a high refractive index, and remarkable conductivity, making it possible for them to be used as efficient catalysts to promote the synthesis of acridinedione derivatives []. Besides these properties, the various morphologies of Bi2O3 nanoparticles, including nanowire, nanotube, nanoflake, nanofiber, and nanobelt, have been successfully synthesized. The synthesis of bismuth oxide nanoparticles has been reported with various protocols such as sol-gel, hydrothermal, and precipitated. These nanoparticles have extraordinary properties, such as high activity, high purity, and high surface area [].
In this paper, we report the synthesis of Bi2O3 nanoparticles as a heterogeneous recyclable catalyst for the preparation of acridinedione derivatives through four multicomponent condensation reactions, as shown in Scheme 1.
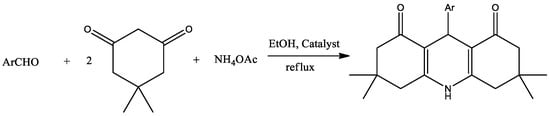
Scheme 1.
Synthesis of acridinedione derivatives catalyzed by Bi2O3 nanoparticles.
2. Experimental
2.1. General
All reagents were purchased from the Fluka and Merck companies and used without further purification. Thin-layer chromatography (TLC) was used for the purity determination of substrates, products, and reaction monitoring over a silica gel 60 F254 aluminum sheet. Melting points were measured in open capillary tubes with an Electrothermal 9100 melting point apparatus. The FTIR spectra were measured with a Shimadzu IR-100 spectrometer. A MIRA3 TESCAN-XMU was used for FE-SEM images.
2.2. Preparation of Bi2O3 Nanoparticles
2.2.1. Method (A)
We added 20 mg of Bi2O3 to 100 mL of H2O and raised the pH to 11 using a 1.0 M NaOH solution. While increasing the pH, a white powder was obtained. The powder was filtered and washed with water and acetone. After drying the powder in an oven at 50 °C, it was calcined at 400 °C at the rate of 10 °C/min and remained there for 30 min. After calcination, a yellow powder was obtained.
2.2.2. Method (B)
We added 20 mg of Bi2O3 to a solution of 40 mL of H2O and 40 mL of ethanol and raised the pH to 11 using a 1.0 M NaOH solution. After a few hours of vigorous stirring, the solution was poured into a Teflon-lined stainless autoclave and heated at 180 °C for 24 h. Then the products were filtered and washed with water and acetone, and then dried at 80 °C and a white powder was obtained.
2.3. General Procedure for the Preparation of Acridinediones Derivatives
We mixed 1.0 mmol of ammonium acetate, 2.0 mmol of dimedone, 1.0 mmol of aromatic aldehyde, 3.0 mL of ethanol as a solvent, and 20.0 mg of Bi2O3 nanoparticle as a catalyst in a round bottom flask. They were stirred in reflux conditions for an appropriate time. After completing the reaction (monitored by TLC), the reaction mixture was filtered and recrystallized by ethanol to afford the pure product.
2.4. Spectral Data
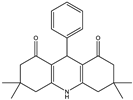
3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (1a): mp: 274–276 °C, IR (KBr: /cm−1): 3278, 3213, 2964, 1636, 1603, 1477, 1366, 1251, 1165 [].
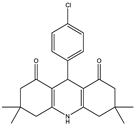
3,3,6,6-Tetramethyl-9-(4-chlorophenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (2a): mp: 297–299 °C, IR (KBr: /cm−1): 2976, 2902, 1648, 1606, 1490, 1366, 1220, 1149 [].
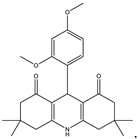
3,3,6,6-Tetramethyl-9-(2,4-dimethoxyphenyl)-1,2,3,4,5,6,7,8,9,10-decahydroacridine-1,8-dione (3a): mp: 265–267 °C, IR (KBr: /cm−1): 3190, 3065, 2954, 1636, 1602, 1480, 1395, 1363, 1292, 1261, 1217, 1144, 1124, 1041, 928, 825 [].
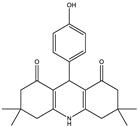
9-(4-hydroxyphenol)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a): mp: 272–274 °C, IR (KBr: /cm−1): 3273, 2963, 1645, 1394 [].
3,3,6,6-Tetramethyl-9-(3-nitrophenyl)-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (5a): mp: 296–298 °C, IR (KBr: /cm−1): 3273, 3185, 3064, 2959, 1646, 1601, 1525, 1345 [].
9-(4-nitrophenol)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (6a): mp: 272–274 °C, IR (KBr: /cm−1): 3192, 2961, 1637, 1597 [].
3. Results and Discussion
FE-SEM images and FTIR spectrum were used for the characterization of the Bi2O3 nanoparticles. In Figure 1, the FTIR spectrum, in the range of 400–4000 cm−1 to investigate the chemical bonding of the Bi2O3 nanoparticles, is shown. The 558 and 446 cm−1 peaks are related to Bi-O stretching vibration modes, the 2364 cm−1 peak is related to the CO2 of the instrument, and the rest of the FTIR transmittance looks flat. The flat FTIR transmittance is evidence of the complete preparation of the Bi2O3 nanoparticles [].
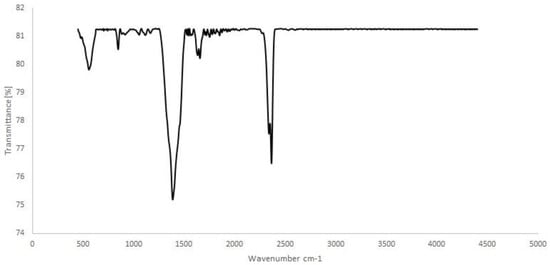
Figure 1.
The FTIR spectra of Bi2O3 nanoparticles.
The morphology of the synthesized Bi2O3 nanoparticles was investigated using FE-SEM and is shown in Figure 2.

Figure 2.
The FE-SEM images of Bi2O3 nanoparticles. (a) Method A (1) (b) Method B (500 nm).
We applied Bi2O3 nanoparticles as a catalyst in the synthesis of acridinedione derivatives through multicomponent reactions to indicate the Bi2O3 nanoparticles’ merits in organic synthesis. For this, we used six different aromatic aldehydes in optimum conditions, and the intended products were obtained in excellent yields. The results are shown in Table 1.

Table 1.
Synthesis of acridinedione derivatives catalyzed by Bi2O3 nanoparticles.
As shown in Table 1, in comparison with method (B), the Bi2O3 nanoparticles synthesized by method (A) have more yield for acridinedione derivative synthesis. This result indicates that the morphology of Bi2O3 nanoparticles is one of the important agents for catalytic activity.
4. Conclusions
We synthesized two different morphologies of Bi2O3 nanoparticles and used them as a catalyst in organic reactions and acridinedione derivative synthesis. A short reaction time, high yield, use of non-toxic solvent, and a mild condition reaction are the advantages of using Bi2O3 nanoparticles. The comparison of the results from the two morphologies demonstrates that the catalyst’s morphology is among the most critical catalytic activity agents.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, D. Multicomponent Reactions in Organic Synthesis Using Copper-Based Nanocatalysts. ChemistrySelect 2016, 1, 1959–1980. [Google Scholar] [CrossRef]
- Domling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Li, P.; He, M.; Wu, Q.; Ye, L.; Mu, Y. Facile synthesis of acridine derivatives by ZnCl2-promoted intramolecular cyclization of o-arylaminophenyl Schiff bases. Org. Lett. 2014, 16, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Gensicka-Kowalewska, M.; Cholewiński, G.; Dzierzbicka, K. Recent developments in the synthesis and biological activity of acridine/acridone analogues. RSC Adv. 2017, 7, 15776–15804. [Google Scholar] [CrossRef]
- Ramos, L.P.; Cordeiro, C.S.; Cesar-Oliveira, M.A.F.; Wypych, F.; Nakagaki, S. Applications of heterogeneous catalysts in the production of biodiesel by esterification and transesterification. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 255–276. [Google Scholar]
- Li, W. Facile synthesis of monodisperse Bi2O3 nanoparticles. Mater. Chem. Phys. 2006, 99, 174–180. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zhang, L.; Hu, J.S.; Huang, X.H. Facile hydrothermal synthesis and improved photocatalytic activities of Zn2+ doped Bi2MoO6 nanosheets. RSC Adv. 2016, 6, 32349–32357. [Google Scholar] [CrossRef]
- Eyvazzadeh-Keihan, R.; Bahrami, N.; Taheri-Ledari, R.; Maleki, A. Highly facilitated synthesis of phenyl (tetramethyl) acridinedione pharmaceuticals by a magnetized nanoscale catalytic system, constructed of GO, Fe3O4 and creatine. Diam. Relat. Mater. 2020, 102, 107661. [Google Scholar] [CrossRef]
- Shi, D.Q.; Ni, S.N.; Shi, J.W.; Dou, G.L.; Li, X.Y.; Wang, X.S. An efficient synthesis of polyhydroacridine derivatives by the three-component reaction of aldehydes, amines and dimedone in ionic liquid. J. Heterocycl. Chem. 2008, 45, 653–660. [Google Scholar] [CrossRef]
- Chavan, P.N.; Pansare, D.N.; Shelke, R.N. Eco-friendly, ultrasound-assisted, and facile synthesis of one-pot multicomponent reaction of acridine-1, 8 (2H, 5H)-diones in an aqueous solvent. J. Chin. Chem. Soc. 2019, 66, 822–828. [Google Scholar] [CrossRef]
- Javid, A.; Khojastehnezhad, A.; Heravi, M.; Bamoharram, F.F. Silica-supported preyssler nanoparticles catalyzed simple and efficient one-pot synthesis of 1, 8-dioxodecahydroacridines in aqueous media. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2012, 42, 14–17. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, W. α-Bi2O3 nanorods: Synthesis, characterization and UV-photocatalytic. Activity. Mater. Res. Express 2017, 4, 035039. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Gilanizadeh, M. Microwave-promoted three-component Hantzsch synthesis of acridinediones under green conditions. Curr. Chem. Lett. 2020, 9, 71–78. [Google Scholar] [CrossRef]
- Patil, D.; Chandam, D.; Mulik, A.; Patil, P.; Jagadale, S.; Kant, R.; Gupta, V.; Deshmukh, M. Novel Brønsted acidic ionic liquid ([CMIM][CF 3 COO]) prompted multicomponent hantzsch reaction for the eco-friendly synthesis of acridinediones: An efficient and recyclable catalyst. Catal. Lett. 2014, 144, 949–958. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).