Abstract
Background/Objectives: Understanding prescribing patterns for type 2 diabetes mellitus, a complex condition affecting over 10% of the global adult population, can optimise prescribing practices, guide policymakers in promoting evidence-based medicine, and help tailor first-line treatments to individual characteristics or specific subgroups, improving patient outcomes. This study aimed to identify factors influencing the prescription and non-prescription of metformin, the recommended first-line therapy in Western guidelines, and to evaluate whether these prescribing patterns align with evidence-based recommendations. It also explores factors associated with initial combination therapy, a more recent and controversial approach compared to stepwise therapy. Methods: We conducted a systematic search in PubMed, Scopus, and Web of Science on 25 August 2023, without language or time restrictions, to identify observational analytical studies assessing factors associated with the initiation of metformin or combination therapy in adults with type 2 diabetes mellitus who were naïve to antidiabetic medications. Studies involving pregnant or breastfeeding women were excluded. A narrative synthesis was conducted. Study quality was assessed using the Joanna Briggs Institute critical appraisal checklists (PROSPERO registration number CRD42023438313). Results: Thirty studies were included, evaluating 105 variables, most of which (62%) were assessed in one study. The 25 variables using combination therapy as the outcome were mostly (72%) evaluated also in one study. Initial metformin prescription was strongly and positively associated with younger age, lower glycated haemoglobin levels, higher body mass index, and absence of renal impairment. Initial combination therapy was associated with higher HbA1c levels and a lower burden of comorbidities. Findings also highlighted a discrepancy between clinical practice and evidence-based recommendations. However, concerns were raised regarding both the internal and external validity of the included studies. Conclusions: Our systematic review, which offers insights into real-world clinical practices, indicated that there is a misalignment between clinical practices and evidence-based recommendations, supporting the need for interventions in this field.
1. Introduction
More than 90% of people with diabetes have type 2 diabetes mellitus (T2DM), a chronic and complex condition requiring a multifactorial approach to prevent or delay microvascular and macrovascular complications [1]. With a global prevalence of 10.5% among adults aged 20 to 79 in 2021 [2], T2DM contributed to 11.3% of deaths worldwide [3]. It has led to a 315% increase in healthcare expenditures over 15 years (2007–2021) [2], significantly burdening healthcare systems and society. Given this impact, optimising initial treatment strategies is essential. Prescribing the most appropriate treatment from the beginning can influence long-term outcomes and reduce the overall burden on healthcare systems.
Several classes of antidiabetic drugs (ADs) are currently available, each with distinct profiles of effectiveness and safety [4]. The most commonly used ADs belong to seven drug classes: biguanides, sulfonylureas (SUs), thiazolidinediones (TZDs), dipeptidyl peptidase-4 inhibitors (DPP4i), sodium-glucose transporter-2 inhibitors (SGLT2i), glucagon-like peptidase-1 receptor agonists (GLP1-RA) and insulin [5]. Although metformin is widely endorsed as the preferred first-line therapy [6,7], data from Nicolucci et al. [7] show that approximately one-fifth of patients across 37 countries in six global regions did not receive metformin as their initial treatment. Additionally, prescribing patterns vary considerably, with sulfonylureas and DPP4i commonly used, while newer agents like SGLT2i and GLP1-RA remain underutilised in many regions. Evidence on the use of initial combination therapy is more limited. Current guidelines suggest this option when HbA1c exceeds target levels by 1.5% or more [4], although its benefits and risks remain under debate [8]. In the context of a complex condition with multiple therapeutic options, selecting the most appropriate initial treatment could pose a significant challenge for clinicians.
Variations in healthcare resource allocation and utilisation raise critical questions about quality, equity, and efficiency, with significant implications for health policies [9]. Such variation is particularly evident when multiple treatment options are available, contributing to uncertainty in clinical decision-making, a phenomenon known as “professional uncertainty” [10]. Furthermore, evidence from systematic reviews [11,12] suggests that prescribing decisions are influenced by a range of interconnected factors, including patients’ clinical conditions, patient preferences, physician characteristics, medication costs, and pharmaceutical industry influence. Some variations in clinical practice may reflect legitimate differences in patient needs or preferences; however, any unwarranted prescribing must be identified and requires closer examination.
A systematic review addressing factors influencing first-line choice decisions in T2DM has yet to be found. Such a review addressing this gap would enhance our understanding of prescribing patterns and assess the robustness of existing scientific evidence. Furthermore, identifying key determinants of prescribing behaviour could help reduce unwarranted clinical variation, promote evidence-based practice, and enhance healthcare equity. Given that metformin is the recommended first-line treatment in guidelines [4,13] and that combination therapy, which may include metformin, is an emerging approach, this systematic review aims to identify the key factors influencing the choice of metformin or combination therapy as the first-line treatment for T2DM.
2. Materials and Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14] and was registered in the PROSPERO database in July 2023 under CRD42023438313. The registered protocol encompasses a broader systematic review, part of which is presented here.
2.1. Search Strategy
A search was conducted in Medline (PubMed), Scopus, and Web of Science on 25 August 2023, without restrictions on language or publication date.
The search strategy was based on the PECO-S framework: Population (individuals with T2DM drug-naïve to antidiabetic drugs), Exposure (predictive factors), Comparator (not applicable), Outcome (starting metformin or combination therapy), and Study type (observational analytical studies). Initial combination therapy was defined as the simultaneous initiation of two or more oral antidiabetic agents as the first-line treatment for T2DM. Sequential initiation (e.g., stepwise addition of a second drug after the initial prescription) was not classified as initial combination therapy.
The search strategy combined medical subject headings, free-text terms, and terms in the title/abstract and is available in Supplementary Tables S1–S3. Additional relevant studies were identified through manual reference screening and expert consultation. Grey literature was searched through ProQuest, the Networked Digital Library of These and Dissertations, Eldis, and targeted websites, including those of the World Health Organization (WHO), United Nations, International Diabetes Federation, New York Academy of Medicine, and National Institutes of Health.
2.2. Inclusion and Exclusion Criteria
The inclusion and exclusion criteria were selected based on the PECO-S elements, and all details are shown in Table 1.

Table 1.
Study inclusion and exclusion criteria.
Pregnant and breastfeeding individuals were excluded due to significant differences in treatment approaches compared to the general adult population [15,16].
2.3. Study Selection Process
EndNote 20® was used to manage references and identify duplicates. After that, the Rayyan QCRI [17] tool was employed to support the blinded and independent screening of studies by two reviewers (SMH and MF or JA). Titles and abstracts were screened in the first stage, with any disagreements between researchers leading to studies being transferred to the second stage. In the second stage, the full text was analysed. During the initial screening, studies published in languages other than English, Portuguese, or Spanish were evaluated using an online translation tool. If eligibility criteria were met, the authors were contacted to provide a translated version. A follow-up was sent after 15 days, and if no response was received within 30 days, the study was excluded.
Disagreements were resolved by consensus; a third researcher was consulted if a consensus was not reached. This procedure was conducted for study selection, data extraction, and risk of bias analysis. The agreement proportion between the two reviewers was calculated for each stage.
2.4. Data Extraction
Data were extracted by one reviewer (SMH) and independently checked for accuracy and completeness by a second reviewer (MF or JA). The extraction followed a predefined set of variables, including (1) study identification (title, author(s), publication year, and country), (2) methods (sources and methods of participants selection, inclusion and exclusion criteria, sample size and its characteristics (sex and mean age), period(s) of data collection, follow-up, setting, analysis methods, and potential biases), (3) outcomes analysed and their prevalence, list of variables (factors), and degree of statistical significance associated with the outcomes.
2.5. Risk of Bias and Data Analysis
Two independent reviewers (SMH and MF or JA) assessed the quality of studies using the Joanna Briggs Institute (JBI) quality assessment checklist for cross-sectional and cohort studies [18]. Studies were not classified as “high”, “moderate”, or “low” quality due to the lack of a universally accepted categorisation. However, the appraisal informed the interpretation of findings, particularly when assessing the robustness of evidence.
Due to the expected and observed high heterogeneity of studies, a meta-analysis was not feasible. Instead, a narrative synthesis was conducted to explore the factors associated with metformin or combination therapy initiation. No formal methods were used to assess risk of reporting bias, such as small-study effects or publication bias, due to the heterogeneity in comparisons, outcomes, and the absence of statistical synthesis.
Given the nature of the narrative synthesis and the absence of a meta-analysis, the use of the GRADE system was not deemed appropriate, as the primary aim was to identify factors influencing physician prescribing, without the intention of providing direct clinical recommendations.
Data were summarised in cross-tabulated tables, grouped by treatment comparisons (e.g., metformin vs. sulfonylureas). Factors were organised according to categories commonly reported in the literature [11,12], including physician-related, healthcare system-related, patient-related, and disease-related factors. Furthermore, as patient-related factors were expected to constitute the largest group due to their availability in clinical records, these factors were subcategorised into sociodemographic, lifestyle and metabolic, cardiovascular, renal, and other clinical factors that did not fit into the previous categories. Each table included the study references and indicated whether the statistical results were significant, specifying if significance was found in univariate and/or multivariate analyses. The criterion for a statistically significant association between exposure and outcomes was p-value < 0.05, or a 95% confidence interval that did not include 1 in studies where the association was reported through risk measures (relative risk, odds ratio, or hazard ratio).
3. Results
3.1. Study Selection
Figure 1 presents the PRISMA 2020 flow diagram outlining the study selection process and main reasons for exclusion. Initially, 1645 studies were identified, and 4 additional studies were retrieved through citation searching. In the first stage, 1535 studies were excluded during the title and abstract screening. The main reason for exclusion was that studies presented different outcomes, with many studies researching adherence to first-line treatment, investigating the effectiveness and side effects of first-line therapies, or performing cost-effectiveness analyses. In the second screening stage (full-text review), the main reason for exclusion remained outcome-related discrepancies. Several studies evaluated the initiation of different drug classes, such as sulfonylureas, or compared treatment initiation with non-initiation. The proportion of agreement between the independent reviewers from the first and second stages of screening studies was 51.4% and 69.5%, respectively.
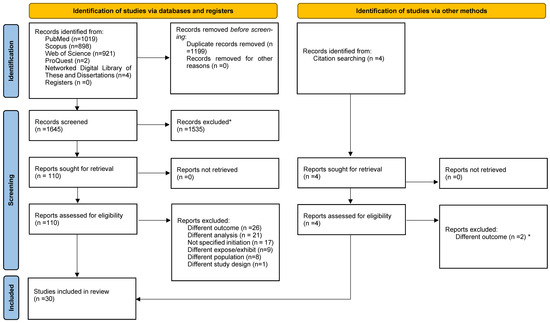
Figure 1.
PRISMA 2020 flow diagram for study inclusion. * Automation tools were not used for the study inclusion/exclusion.
3.2. Study Characteristics
Table 2 and Table 3 summarise the characteristics of the included studies. Of the 30 studies, 9 (30%) were retrospective cohort studies [19,20,21,22,23,24,25,26,27] and 21 (70%) were cross-sectional studies [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. A total of 12 studies (40%) were conducted in Europe, 11 (36.7%) in North America, 6 (20%) in Asia, and 1 (3.3%) in Australia. The number of participants ranged from 415 to 1,136,723, with a median of 27,138.

Table 2.
Characteristics of the retrospective cohort studies which were eligible for inclusion.

Table 3.
Characteristics of the cross-sectional studies which were eligible for inclusion.
Twenty studies employed data from multicentre settings [20,22,23,25,26,28,29,30,31,33,36,37,39,41,42,43,44,45,46,47] and six studies from primary care settings or general practitioners [19,21,24,27,32,40]. All studies relied on secondary data sources, defined as data collected by others for purposes different from the objectives of the research, such as medical records and healthcare billing files [49].
Twenty-eight studies [19,20,21,22,23,24,25,26,28,29,30,31,32,33,34,35,36,37,38,39,41,42,43,44,45,46,47,48] examined factors associated with metformin initiation. Nineteen of these [19,22,23,24,25,28,29,30,31,32,34,35,36,37,38,43,44,45,48] employed statistical models that examined factors linked to metformin initiation in direct comparison to initiation of other antidiabetic drug(s). The sulfonylureas group was the most frequently compared drugs, accounting for 36.7% (n = 11) of these studies [19,22,23,24,25,31,32,34,35,36,43]. A total of 8 studies [19,27,33,39,40,41,43,46] analysed factors influencing the initiation of combination therapy, and 6 studies [19,33,39,41,43,46] assessed both metformin and combination therapy in the same study.
3.3. Data Extracted and Analysed
The 105 variables extracted from the 30 studies are categorised into 4 main groups of factors: physician (Table 4), healthcare system (Table 5), patient (Table 6, Table 7 and Table 8), and disease factors (Table 9). The most extensive group of factors belongs to patient factors, with five subgroups of factors recognised: sociodemographic, lifestyle and metabolic, cardiovascular, renal, and other clinical factors. Sixty-five variables (62%) were evaluated by one study, and eleven (10.5%) were assessed by five or more studies.

Table 4.
Strength of association between physician-related factors and the outcomes.

Table 5.
Strength of association between healthcare system-related factors and the outcomes.

Table 6.
Strength of association between patient-related factors—sociodemographic, lifestyle, and metabolic—and the outcomes.

Table 7.
Strength of association between patient-related cardiovascular and renal factors and the outcomes.

Table 8.
Strength of association between patient-related factors: other clinical factors and the outcomes.

Table 9.
Strength of association between disease-related factors and the outcomes.
It is also important to highlight that one study [45] showed data analysis not as a single block but for different periods and two databases, and another [37] employed two statistical analyses: multivariable logistic regression and multilevel linear model.
3.3.1. Physician-Related Factors
Table 4 shows that physician age presented a statistical association with the prescription profile [28,37], with older physicians more frequently initiating non-metformin therapies compared to metformin. Three studies [28,32,37] reported a non-association between physician sex and initial therapy choice. However, Liu et al. [37], using logistic regression, reported that male physicians were more likely to prescribe non-metformin treatments than their female counterparts. Physician speciality was the most assessed variable in this category, with six studies [28,37,39,42,44,45] showing a statistically significant association. Campbell et al. [44] reported that specialists were more likely than general practitioners (GPs) to prescribe metformin in combination or other antidiabetic agents, rather than metformin monotherapy. Pinto et al. [39] also reported GPs had a lower prevalence of prescribing combination therapy compared to other specialists (4.2% vs. 33.3%, p < 0.001). However, no significant differences were observed between GPs and specialists in the prescription rates of metformin monotherapy or metformin in combination therapy. Another study [37] presented that GPs had a higher chance of prescribing non-metformin than metformin compared to endocrinologists. In contrast, Shin et al. [45] observed that individuals visiting endocrinologists had a lower chance of initiating metformin than other drugs independently of their cardiovascular benefits; the opposite was found when visiting internists. Finally, the two additional variables, years of experience and the medical evidence questionnaire, assessed by Wang et al. [32], presented a non-statistically significant association.
3.3.2. Healthcare System-Related Factors
Eleven studies [19,22,23,25,28,29,31,37,43,45,48] demonstrated a statistically significant association between more recent time periods and the prescription profile. However, in two studies, statistical significance varied depending on the database analysed [45] or the specific drug and comparator used [19]. Studies reported that the most recent periods were positively associated with metformin initiation [25,45] and also when compared with sulfonylureas [22,23,31,43], other monotherapies [29,48], other antidiabetic agents (eventually in monotherapy or combination therapy) [28,37], or combination therapy [43]. Additionally, Wang et al. [32] studied how primary care physicians responded to a change in the Canadian Diabetes Association Guidelines, which significantly increased metformin initiation as a first line, except when compared to sulfonylureas.
One cross-sectional study [45] reported that individuals with three or more HbA1c tests within 365 days before the index date had higher odds of initiating any other medicine than metformin, irrespective of their cardiovascular benefits. Additionally, variables such as the number of office visits [31,38], hospitalisations [25,31], emergency visits [31], and the length of stay [28] were statistically significantly associated with lower odds of metformin prescriptions. However, Abdelmoneim et al. [31] and Shin et al. [45] reported no statistically significant association with some of these variables (see Table 5).
Two studies [28,29] reported an association between the health insurance and the initial therapy choice. Regarding the co-payment and cost of drugs, one study [28] indicated that individuals with a co-payment waiver had a lower chance of starting metformin than sulfonylureas, the same that Li [25] found for individuals in the top 10% of prescription drug expenses under Medicare Part D. Medicare is a health programme for individuals aged 65 and over and younger individuals with disabilities, with Medicare Part D being an optional plan that covers drug costs [50]. Shin et al. [45] stated that individuals with more brand-name experience also had a lower chance of starting metformin than drugs independently of their cardiovascular benefits.
3.3.3. Patient-Related Factors: Sociodemographic
Twenty-two studies [19,20,21,22,23,24,25,26,28,29,30,31,32,35,36,37,38,40,41,43,44,45] evaluated the association between age and initial therapy choice. Metformin initiation was associated with younger individuals when compared to sulfonylureas [19,22,23,24,25,31,35,36,43], other antidiabetic agents (including monotherapy (MT) and eventually MT or combination therapy (AHA)) [28,30,32,37,43,44], thiazolidinediones [19], DPP4i [38], and drugs without cardiovascular benefits [45]. Even when age was analysed in interaction with insurance type [48], metformin prescription decreased with advancing age compared to other monotherapies. On the other hand, when metformin is compared with drugs with cardiovascular benefits, the chance of prescribing increases with age [45]. Two studies [19,43] found a significant but inverse association between age and combination therapy. The other two studies [40,41] indicated that younger people were more prevalent in combination therapy than monotherapy. Moreover, one study [19] found no association between age and combination therapy compared to sulfonylureas.
Nineteen studies assessed the association between sex and prescribing patterns [19,22,23,25,28,29,30,31,32,35,37,38,40,41,43,44,45,46,48]. Nine studies [22,25,28,29,30,37,38,40,48] reported a statistically significant association, while six found no such association [23,32,35,41,44,46]. In the remaining four studies [19,43,45,46] several analyses were conducted within each study. This led to the report of statistically significant associations depending on the prescribing profile evaluated (i.e., the same study analysed different prescribing profiles with sex) or the database analysed (i.e., the same study analysed two different databases). The statistical associations found that females were more likely to initiate metformin than men compared to sulfonylureas [22,25], other monotherapies [30,48], other antidiabetic agents [37], or DPP4i [38]. On the other hand, Winkelmayer et al. [28] identified a significant association, though its direction varied between univariate and multivariate analyses, and Desai et al. [29] noted that men had a higher chance of starting metformin than other monotherapies.
Regarding race/ethnicity, black individuals showed a lower chance of starting metformin than sulfonylureas compared to white individuals [19,22,25]. White individuals also showed more chance of starting drugs with cardiovascular benefits than metformin compared to non-white [45]. However, there was no statistically significant association between race/ethnicity and combination therapy compared to metformin or sulfonylureas [19].
Individuals with lower socioeconomic status were associated with more frequent non-metformin prescriptions [29,37] or sulfonylurea prescriptions [22,25] compared to metformin. Conversely, Liu et al. [37] also found the same for a higher socioeconomic status category, and statistical significance was lost in the multilevel linear model.
3.3.4. Patient-Related Factors: Lifestyle and Metabolic
Nine studies [22,23,24,31,35,38,43,45,48] evaluated at least one lifestyle or metabolic variable (Table 6). A higher body mass index (BMI) was significantly associated with increased chance of starting metformin compared to sulfonylureas [22,23,24,35], DPP4i [38], or drugs without cardiovascular benefits [45]. However, when compared with agents that provide cardiovascular benefits, the chance of prescribing metformin initiation decreased with increasing BMI [45]. Liver disease was associated with a decreased chance of starting metformin compared to sulfonylureas [24,31], although no statistically significant association was observed when compared with DPP4i [38]. Individuals with dyslipidaemia had higher odds of initiating combination therapy than metformin [43]. For other variables within this category, either no statistically significant associations were found or the direction of associations was inconsistent.
3.3.5. Patient-Related Factors: Cardiovascular
The impact of twenty-seven cardiovascular variables (the most extensively represented group) was assessed in fourteen studies [22,23,24,25,31,32,34,35,38,40,43,45,47,48]. Hypertension was evaluated with initial therapy choice in five studies [22,31,35,43,45]. Three studies [22,31,35] reported statistically significant results, although the findings were conflicting. Abdelmoneim et al. [31] found that hypertension increased the odds of starting metformin compared to sulfonylureas, while Fujihara et al. [35] reported the opposite. Shin et al. [45] also linked hypertension to a decreased chance of starting metformin compared to drugs with or without cardiovascular benefits. However, in the same study, the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (both antihypertensive agents) increased the odds of initiating metformin. No significant association was found between hypertension and combination therapy compared to metformin [43].
Two studies [22,25] reported that cardiovascular disease was negatively associated with metformin initiation compared to sulfonylureas. Similarly, Shin et al. [45] found the same when comparing metformin to drugs with or without cardiovascular benefits. In contrast, no statistically significant association was found by Wang et al. [32].
Three studies [22,31,43] reported that heart failure was negatively associated with metformin compared to sulfonylureas. Additionally, Wood et al. [43] reported that individuals with heart failure had higher odds of initiating combination therapy rather than metformin. Similarly, cerebrovascular disease was associated with decreased odds of initiating metformin compared to sulfonylureas or combination therapy, although no significant association was found when comparing metformin to non-metformin therapies for either heart failure or cerebrovascular disease [43]. A similar pattern was found for valvular disease, which was associated with lower odds of initiating metformin compared to sulfonylureas [31].
3.3.6. Patient-Related Factors: Renal
Eleven studies [19,22,24,25,31,32,34,38,41,45,48] evaluated renal-related variables. It is unanimously reported that renal impairment, such as elevated serum creatinine, renal disease, chronic renal disease (CKD), renal failure, and diabetes-related nephropathy, was consistently associated with a reduced chance of initiating metformin therapy [19,22,24,25,31,32,34,38,45,48]. For example, Raebel et al. [22] showed that individuals with serum creatinine levels between 1.4 and ≤2 mg/dl, compared to those with levels < 1.4mg/dL (reference group), had a relative risk of 2.21 (95% CI: 2.05–2.39) of starting sulfonylureas instead of metformin. Similarly, Wang et al. [32] found that individuals with renal disease had significantly lower odds (OR 0.14; 95% CI: 0.05–0.40) of starting metformin than other antidiabetic agents.
Regarding combination therapy, Brouwer et al. [19] reported that elevated serum creatinine levels were associated with a decreased chance of beginning combination therapy compared to sulfonylureas. Another study [41] observed that individuals with CKD were less frequently prescribed combination therapy than monotherapies such as sulfonylureas or DPP4i.
3.3.7. Patient-Related Factors: Other Clinical Factors
Twenty-one other clinical variables were assessed with the prescription profile in twelve studies [22,24,25,28,29,31,34,40,41,43,44,45]. Of these, 81% (17) of the variables were examined in only one study. Dementia was negatively associated with metformin initiation compared to sulfonylureas [24]. Wood et al. [43] reported that depression was positively associated with metformin initiation compared to sulfonylureas, which aligned with Raebel et al.’s [22] findings. However, these associations differed in direction from the univariable analysis reported by Abdelmoneim et al. [31]. Wood et al. [43] also reported that depression was positively associated with metformin initiation compared with combination therapy, and statistical significance was not found comparing non-metformin monotherapies with metformin. Juste et al. [40] reported that the prevalence of neuropsychiatric medication was higher among monotherapy initiators than among combination therapy initiators.
Regarding medication use, Desai et al. [29] reported that the chances of starting metformin rather than other monotherapies decreased for each additional prescription. However, Li [25] found no statistical association, and Winkelmayer et al. [28] observed that the odds of initiating metformin increased with a higher number of therapeutic class prescriptions compared to other antidiabetic agents. However, this pattern shifted in the univariable analysis, where individuals taking ≥9 medications had reduced odds of initiating metformin compared to those taking none. Two studies [40,41] observed that the prevalence of other types of medication use was higher among monotherapy initiators than among combination therapy initiators.
Wood et al. [43] presented that individuals with one to three comorbidities had a lower chance of starting sulfonylureas than metformin compared to those with no comorbidities. No statistically significant association was found when comparing four or more comorbidities to zero. Additionally, individuals with one to six comorbidities had lower odds of initiating non-metformin monotherapy compared to metformin, though this was not statistically significant when comparing seven or more comorbidities to zero. Nevertheless, compared to zero, one or more comorbidities reduced the odds of initiating combination therapy rather than metformin. Similarly, Campbell et al. [44] also reported that individuals with one or more comorbidities were less likely to start metformin in combination therapy or other drug therapies instead of metformin alone compared to those with no comorbidities. Additionally, Juste et al. [40] noted that individuals who initiated combination therapy had a lower comorbidity score compared to those initiating monotherapy.
3.3.8. Disease-Related Factors
Data were extracted from fourteen studies [19,22,23,24,27,32,33,34,35,36,37,38,44,45] with ten variables collected. HbA1c was the most studied variable, being addressed in ten studies [19,22,23,24,27,33,35,36,38,44]. Five studies [19,22,23,24,35] stated that higher HbA1c levels were statistically associated with the initial sulfonylureas therapy instead of metformin, but one study did not find statistical significance [36]. Campbell et al. [44] also indicated that individuals with a higher HbA1c level were more likely to start non-metformin and combination therapy than metformin alone. On the other hand, Morita et al. [38] reported the opposite when comparing metformin to DPP4i, and no statistically significant association was noted when comparing metformin to thiazolidinediones [19]. Combination therapy was statistically associated with higher levels of HbA1c compared to metformin, sulfonylureas [19], or monotherapy [27].
Raebel et al. [22] also assessed fasting and random glucose levels and reported that both levels were higher among those who initiated sulfonylureas than those who initiated metformin in univariable analysis. Vashisht et al. [34] identified glucose and diabetes without complications as variables statistically associated with pioglitazone (TZD) choice instead of metformin. However, the study did not specify how glucose was measured, nor whether the association with pioglitazone was positive or negative.
Shorter diabetes duration [23,35] and earlier treatment initiation [23] were statistically associated with metformin initiation compared to sulfonylureas. Ouchi et al. [27] also found that combination therapy (89.62 days, SD 279.1) was prescribed significantly earlier (p < 0.001) than monotherapy (190.7 days, SD 366.2).
3.4. Quality Assessment
The quality assessment results for observational cohort studies are presented in Table 10. None of the studies ensured the similarity between compared groups (exposed vs. unexposed). It was also not possible to confirm the exposure measured similarly between groups and their validity and reliability, as all studies relied on secondary data without detailing the method of exposure measurement. None of the studies identified potential confounders or strategies to address them. Five studies [19,22,23,25,27] raised concerns regarding outcome validity and reliability, as the data sources might have reflected treatment adherence (e.g., pharmacy dispensing records) rather than prescriptions. Follow-up loss was either ignored [23] or avoided through inclusion/exclusion criteria [19,20,21,22,24,25,26,27] without strategies being expected to address it. Although five studies [19,22,23,24,25] used multivariable analysis (appropriate statistical analysis considered), none reported on the assumptions underlying the statistical models. Additionally, one study [27] did not report the statistical methods used.

Table 10.
Quality assessment and risk of bias of cohort studies included.
Table 11 presents the results of quality assessment for observational cross-sectional studies. Eight studies [33,34,35,36,38,42,44,46] did not provide clear inclusion criteria, and eight studies [29,30,34,35,36,39,46,47] lacked information to infer the health status of the sample. It was also not possible to confirm the exposure measured validity and reliability. However, four studies [29,30,39,46] reported exposures without a gold standard measurement (e.g., age, sex, type of insurance), suggesting no risk of bias. Only two studies [32,44] identified potential confounding factors, but the associated statistical models also included these variables as outcomes of interest, raising concerns about whether they were exclusively used for confounding control. Therefore, only two other studies [42,47] addressed confounding through matching or stratification. Twelve studies [28,29,30,31,32,33,37,40,41,43,44,45] relied on pharmacy dispensing data or lacked clarity on data sources, undermining outcome validity and reliability. None used appropriate statistical analyses due either to the absence of multivariable analysis [33,36,38,39,40,41,42,46,47], unreported statistical model assumptions [28,29,30,31,32,35,37,43,44,45,48], or omission of p-value results [34]. Overall, the quality assessment underscores multiple issues across the studies, pointing to a high risk of bias.

Table 11.
Quality assessment and risk of bias of cross-sectional studies included.
4. Discussion
This systematic review identified 30 studies exploring factors influencing first-line treatment decisions in T2DM, focusing on metformin and combination therapy. Although clinical decision-making can be inherently complex, the identification of key factors serves two main purposes: first, it enables the alignment of clinical practice with evidence-based guidelines by addressing gaps in knowledge and practice patterns; second, it aids in tailoring treatment decisions to individual characteristics, thereby improving the quality of care and patient outcomes [51].
The prevalence of the two initial therapies analysed varied widely across studies, with the greatest variation observed in metformin initiation. For instance, Morita et al. [38] reported that 7.1% of their sample started metformin monotherapy, while Campbell et al. [44] found that 89% of participants initiated metformin monotherapy. This discrepancy may be attributed to differences in national clinical guidelines: Canadian guidelines recommend metformin as the first-line treatment unless contraindicated [52], while Japanese guidelines do not specify a preferred drug for initiation [53]. However, it is important to consider access to medicines and their relationship with cost and insurance coverage. Although newer oral agents have increased medication-related costs [54], in Japan, certain social security systems help reduce the burden of out-of-pocket expenses for individuals with low income [55]. Conversely, a 2016 report by Innovative Medicines Canada indicated that only 37% of new medicines received public reimbursement in Canada, and 90% of those included in public drug plans were subject to reimbursement restrictions [56]. These differences in access and coverage may contribute to the variation observed across studies, a notion supported by three studies [25,28,29] that analysed the impact of those factors on prescription patterns.
One hundred and five variables were evaluated as potential predictive factors influencing initial therapy decisions, with only 9.5% (10) showing no association with initial therapy choice. Among these, 25 variables were assessed with combination therapy, with 18 (72%) evaluated in just one study. Notably, only 11 of the 105 variables were assessed in five or more studies, indicating limited replication and, consequently, reduced robustness for most findings.
Age and sex were the most frequently assessed variables, reflecting the accessibility of demographic data. On the other hand, physician-related factors were the group with the least variables evaluated, perhaps due to challenges in extracting this information from secondary data sources. In contrast, the subgroup of cardiovascular factors included the highest number of assessed variables, which may reflect researchers’ interest in studying these factors and the emphasis of guidelines on cardiovascular diseases in individuals with T2DM [57,58]. Among the 21 variables categorised as other clinical factors, several raised concerns regarding their clinical relevance, suggesting that data availability may have driven their inclusion.
Interestingly, while physician age was associated with initial therapy choice, years of experience were not, yielding contradictory results since age is typically linked to years of experience. Moreover, a survey study [59] found that years of experience influenced the factors considered when selecting first-line treatment, and a chart review study [60] revealed that more experienced physicians were less likely to follow guidelines. These findings highlight the complexity of physician-related factors and their interplay with clinical decision-making. Additionally, regarding associations found with physician specialties, it is important to consider that access to these specialties is strongly influenced by the patient’s health status and the severity of the disease [61]. Healthcare utilisation is also significantly affected by the patient’s socioeconomic status [62], and both socioeconomic status and health insurance were associated with prescription profile.
Patient-related factors, particularly age, also play a significant role in influencing therapy decisions, with metformin consistently linked to a younger age. A survey study [63] also reported that reasons cited by physicians to avoid initial dual therapy were often associated with patient age. Additionally, metformin initiation was negatively associated with several cardiovascular conditions and healthcare utilisation, indicating a tendency to avoid metformin in individuals with poorer health status. This trend is surprising given its well-established safety profile [64] and its benefits for various conditions [65].
Concerns about these findings are heightened when comparing metformin to sulfonylureas, which are associated with an increased risk of hypoglycaemia [66]. Guidelines recommend a conservative approach to sulfonylureas, particularly in older individuals [4,67,68], and the scientific literature highlights their potential harm in those at high risk for cardiovascular disease [69]. The preference for sulfonylureas over metformin at high HbA1c levels is also not supported by guideline recommendations [4] or the scientific literature [70,71]. This observed preference for sulfonylureas, despite the presence of factors that would favour metformin use, underscores a misalignment between clinical practice and established guideline recommendations. However, this finding has already been reported by Giorda et al. [72], who, in their cross-sectional observational study, found that 70.6% of their sample presented characteristics that increased the risk of inappropriate sulfonylurea prescription. This tendency may be partially explained by the fact that sulfonylureas are the oldest class of oral antidiabetic agents, which may contribute to a sense of familiarity and perceived reliability among some physicians [73].
On the other hand, renal-related factors present a valid reason for avoiding metformin, as it should not be used in individuals with an estimated glomerular filtration rate < 30 mL/min per 1.73 m2 [74]. This may explain why studies report that physicians are cautious about prescribing it to individuals with renal problems. Similarly, for BMI, the findings also align with guideline recommendations [4,67].
The positive association between high HbA1c levels and combination therapy is consistent with recommendations, particularly the consensus report from the ADA and EASD [4], which advocates for considering initial combination therapy in individuals with elevated HbA1c at diagnosis. Additionally, the tendency to avoid combination therapy in individuals with a high number of comorbidities aligns with Ismail-Beigi et al. [75], who highlighted the importance of less intensive treatment for those with multiple or severe comorbidities.
While these findings are noteworthy, it is important to recognise the significant methodological limitations and weaknesses that affect their validity and reliability. There is a lack of clear definitions for the medications included in combination therapy and those used as the reference category for comparison. Many studies simply refer to “combination therapy” and “other antidiabetic drugs,” leading to ambiguity. Moreover, different reference categories were used for comparison with metformin or combination therapy. For instance, one study compared metformin alone or in combination with other antidiabetic medications that did not include metformin [32], while another compared metformin alone to other antihyperglycemic agents, including metformin used in combination therapy [44]. Adding to this are the inconsistent definitions of independent variables and the variation in the timing of their collection across studies. These inconsistencies not only hinder comparisons between studies but also undermine the external validity of the findings, limiting their generalisability.
The lack of efforts to ensure the similarity of groups and to identify and address potential confounder factors brought possible biases. For example, Zhang et al. [20] divided their sample into individuals <65 years and ≥65 years and reported twenty-five statistically significant differences between the two groups in twenty-nine variables analysed. This aligns with the scientific literature that has shown that the prevalence of cardiovascular disease increases with age [76], CKD is more common among older individuals [77], and multimorbidity is also more prevalent in older adults, strongly associated with increased healthcare utilisation and costs [78,79]. This information, along with the quality assessment results, raises concerns about the internal validity of the studies and suggests a high risk of bias in the findings.
Despite these internal and external validity concerns, some observed clinical practices deviate from established guidelines and the scientific literature. This misalignment highlights an urgent need to bridge the gap between clinical practice and evidence-based recommendations. Furthermore, more robust studies are essential, particularly those that emphasise external validation and minimise the risk of bias. These studies should facilitate direct comparisons across research, providing a more reliable basis for developing evidence-based recommendations grounded in high-quality data.
Strengths and Limitations
As far as we know, this is the first systematic review to map all factors driving physicians to choose metformin or combination therapy as first-line treatment. This review offers an exhaustive overview of the scientific literature, as no time or language restrictions were imposed, and grey literature was also included. Furthermore, compared to Mahmoud et al. [80], who conducted a meta-analysis of factors influencing antidiabetic drug prescribing for T2DM, including initiation therapy, this review expands the scope by incorporating nineteen additional studies.
All studies assessing the outcomes of interest were included, regardless of the reference group used for comparison. This inclusive approach enabled a broader range of comparisons but also introduced heterogeneity into the analysis. Additionally, due to the diversity in study designs, outcome measures, and treatment comparisons, it was not possible to assess the potential for reporting bias using standard tools such as funnel plots. This limits our ability to determine whether the published literature may overrepresent statistically significant findings. Moreover, the weak evidence in the main findings due to the low quality of the studies, which integrated this systematic review, should not be overlooked.
5. Conclusions
This systematic review identified several factors associated with metformin and combination therapy as first-line therapies in T2DM, revealing clinical practices not aligned with evidence-based medicine. However, few studies have focused on combination therapy, assessing physician-related factors, and even fewer have compared metformin with newer drugs, such as SGLT2i. Additionally, the studies included in this review exhibited low certainty evidence. Therefore, a robust methodology is needed to bring scientific evidence. As a result, further research is required to address these weaknesses and potential biases, provide stronger evidence, and ultimately support the findings reported in this review.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6100114/s1, Table S1: Search query on PubMed (Medline); Table S2: Search query on Scopus; Table S3: Search on Web of Science.
Author Contributions
Conceptualisation, H.S.-M. and M.M.-S.; methodology, H.S.-M., F.M., Â.J., P.S. and M.M.-S.; validation, F.M. and Â.J.; formal analysis, H.S.-M., F.M. and Â.J.; investigation, H.S.-M., F.M. and Â.J.; data curation, H.S.-M., F.M. and Â.J.; writing—original draft preparation, H.S.-M.; writing—review and editing, all authors; supervision, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article. No new data have been created for this review.
Acknowledgments
We would like to thank Mandy Ryan and Michael Abbott, who supervised SMH at the Health Economics Research Unit at the University of Aberdeen, for their help in developing this study, which is integrated into her thesis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE | Angiotensin-converting enzyme |
| ADA | American Diabetes Association |
| ADs | Antidiabetic drugs |
| AHA | Other oral antihyperglycemic agents |
| ARBs | Angiotensin receptor blockers |
| BMI | Body mass index |
| Cat. | Categorical |
| CKD | Chronic kidney disease |
| Cont. | Continuous |
| COPD | Chronic obstructive pulmonary disease |
| CT | Combination therapy |
| DCSI | Diabetes complication severity index |
| DPP4i | Dipeptidyl peptidase-4 inhibitors |
| EASD | European Association for the Study of Diabetes |
| GLP1-RA | Glucagon-like peptidase-1 receptor agonists |
| Glip | Glipizide |
| GPs | General practitioners |
| HbA1c | Glycated haemoglobin |
| HDL | High-density lipoprotein |
| IHD | Ischemic heart disease |
| JBI | Joanna Briggs Institute |
| LDL | Low-density lipoprotein |
| Metf. | Metformin |
| MT | Monotherapy |
| Non-Metf | Non-metformin |
| PECO-S | Population, Exposure, Comparator, Outcomes, Study type |
| Pio | Pioglitazone |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SGLT2i | Sodium-glucose transporter-2 inhibitors |
| SU | Sulfonylureas |
| TZDs | Thiazolidinediones |
| W/CVB | Drugs without cardiovascular benefits |
| WCVB | Drugs with cardiovascular benefits |
| WHO | World Health Organization |
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Engler, C.; Leo, M.; Pfeifer, B.; Juchum, M.; Chen-Koenig, D.; Poelzl, K.; Schoenherr, H.; Vill, D.; Oberdanner, J.; Eisendle, E.; et al. Long-term trends in the prescription of antidiabetic drugs: Real-world evidence from the Diabetes Registry Tyrol 2012-2018. BMJ Open Diabetes Res. Care 2020, 8, e001279. [Google Scholar] [CrossRef]
- Heintjes, E.M.; Overbeek, J.A.; Hall, G.C.; Prieto-Alhambra, D.; Lapi, F.; Hammar, N.; Bezemer, I.D. Factors Associated with Type 2 Diabetes Mellitus Treatment Choice Across Four European Countries. Clin. Ther. 2017, 39, 2296–2310.e14. [Google Scholar] [CrossRef]
- Nicolucci, A.; Charbonnel, B.; Gomes, M.B.; Khunti, K.; Kosiborod, M.; Shestakova, M.V.; Shimomura, I.; Watada, H.; Chen, H.; Cid-Ruzafa, J.; et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second-line therapy: Results from the global DISCOVER study programme. Diabetes Obes. Metab. 2019, 21, 2474–2485. [Google Scholar] [CrossRef]
- Cahn, A.; Cefalu, W.T. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care 2016, 39, S137–S145. [Google Scholar] [CrossRef]
- Corallo, A.N.; Croxford, R.; Goodman, D.C.; Bryan, E.L.; Srivastava, D.; Stukel, T.A. A systematic review of medical practice variation in OECD countries. Health Policy 2014, 114, 5–14. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Reames, B.N.; McCulloch, P.; Carr, A.J.; Campbell, W.B.; Wennberg, J.E. Understanding of regional variation in the use of surgery. Lancet 2013, 382, 1121–1129. [Google Scholar] [CrossRef]
- Davari, M.; Khorasani, E.; Tigabu, B.M. Factors Influencing Prescribing Decisions of Physicians: A Review. Ethiop. J. Health Sci. 2018, 28, 795–804. [Google Scholar] [CrossRef]
- Rodrigues, A.T.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Raets, L.; Ingelbrecht, A.; Benhalima, K. Management of type 2 diabetes in pregnancy: A narrative review. Front. Endocrinol. 2023, 14, 1193271. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S254–S266. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis; Epub ahead of print; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Brouwer, E.S.; West, S.L.; Kluckman, M.; Wallace, D.; Masica, A.L.; Ewen, E.; Kudyakov, R.; Cheng, D.; Bowen, J.; Fleming, N.S. Initial and subsequent therapy for newly diagnosed type 2 diabetes patients treated in primary care using data from a vendor-based electronic health record. Pharmacoepidemiol. Drug Saf. 2012, 21, 920–928. [Google Scholar] [CrossRef]
- Zhang, Q.; Rajagopalan, S.; Marrett, E.; Davies, M.J.; Radican, L.; Engel, S.S. Time to treatment initiation with oral antihyperglycaemic therapy in US patients with newly diagnosed type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 149–154. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Alexander, C.M.; Davies, M.J.; Zhao, C.; Mavros, P. Factors associated with initiation of antihyperglycaemic medication in UK patients with newly diagnosed type 2 diabetes. BMC Endocr. Disord. 2012, 12, 1. [Google Scholar] [CrossRef]
- Raebel, M.A.; Xu, S.; Goodrich, G.K.; Schroeder, E.B.; Schmittdiel, J.A.; Segal, J.B.; O’Connor, P.J.; Nichols, G.A.; Lawrence, J.M.; Kirchner, H.L.; et al. Initial Antihyperglycemic Drug Therapy Among 241 327 Adults With Newly Identified Diabetes From 2005 Through 2010: A Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM) Study. Ann. Pharmacother. 2013, 47, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Geier, A.S.; Wellmann, I.; Wellmann, J.; Kajüter, H.; Heidinger, O.; Hempel, G.; Hense, H.W. Patterns and determinants of new first-line antihyperglycaemic drug use in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 106, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.K. Examining Drug Utilisation Patterns and Optimal Treatment Pathways of Antidiabetic Medications; The University of Manchester: Manchester, UK, 2014; Available online: https://pure.manchester.ac.uk/ws/portalfiles/portal/54566935/FULL_TEXT.PDF (accessed on 25 August 2023).
- Li, Y. Factors Associated with Initiation of Glucose-Lowering Agents Among Medicare Beneficiaries with Newly Diagnosed Type 2 Diabetes, 2007–2017; Epub ahead of print; University of Pittsburgh: Pittsburgh, PA, USA, 2019. [Google Scholar] [CrossRef]
- Carrillo Balam, G.G. Glucose-Lowering Medication Initiation in People with Newly Diagnosed Type 2 Diabetes in Scotland: A Mixed-Methods Study; The University of Edinburgh: Edinburgh, UK, 2020. [Google Scholar] [CrossRef]
- Ouchi, D.; Giner-Soriano, M.; Vilaplana-Carnerero, C.; Monfa, R.; Torres, F.; Morros, R. Longitudinal treatment patterns in patients recently diagnosed with type 2 diabetes mellitus in Catalonia. Diabetes Res. Clin. Pract. 2023, 202, 110777. [Google Scholar] [CrossRef] [PubMed]
- Winkelmayer, W.C.; Stedman, M.R.; Pogantsch, M.; Wieninger, P.; Bucsics, A.; Asslaber, M.; Bauer, R.; Burkhardt, T.; Schautzer, A.; Brookhart, M.A. Guideline-conformity of initiation with oral hypoglycemic treatment for patients with newly therapy-dependent type 2 diabetes mellitus in Austria. Pharmacoepidemiol. Drug Saf. 2011, 20, 57–65. [Google Scholar] [CrossRef]
- Desai, N.R.; Shrank, W.H.; Fischer, M.A.; Avorn, J.; Liberman, J.N.; Schneeweiss, S.; Pakes, J.; Brennan, T.A.; Choudhry, N.K. Patterns of medication initiation in newly diagnosed diabetes mellitus: Quality and cost implications. Am. J. Med. 2012, 125, e1–e302. [Google Scholar] [CrossRef]
- Grimes, R.T.; Bennett, K.; Tilson, L.; Usher, C.; Smith, S.M.; Henman, M.C. Initial therapy, persistence and regimen change in a cohort of newly treated type 2 diabetes patients. Br. J. Clin. Pharmacol. 2014, 79, 1000–1009. [Google Scholar] [CrossRef]
- Abdelmoneim, A.S.; Eurich, D.T.; Gamble, J.M.; Simpson, S.H. Use patterns of antidiabetic regimens by patients with type2 diabetes. Can. J. Diabetes 2013, 37, 394–400. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Eguale, T.; Tamblyn, R. Guidelines adherence in the treatment of patients with newly diagnosed type 2 diabetes: A historical cohort comparing the use of metformin in Quebec pre and post-Canadian Diabetes Association guidelines. BMC Health Serv. Res. 2013, 13, 442. [Google Scholar] [CrossRef]
- Mitchell, B.D.; Eby, E.L.; Lage, M.J. Glycemic control and the first use of oral antidiabetic agents among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2013, 29, 1587–1597. [Google Scholar] [CrossRef]
- Vashisht, R.; Jung, K.; Shah, N. Learning Effective Treatment Pathways for Type-2 Diabetes from a clinical data warehouse. AMIA Annu. Symp. Proc. 2016, 2016, 2036–2042. [Google Scholar]
- Fujihara, K.; Igarashi, R.; Matsunaga, S.; Matsubayashi, Y.; Yamada, T.; Yokoyama, H.; Tanaka, S.; Shimano, H.; Maegawa, H.; Yamazaki, K.; et al. Comparison of baseline characteristics and clinical course in Japanese patients with type 2 diabetes among whom different types of oral hypoglycemic agents were chosen by diabetes specialists as initial monotherapy (JDDM 42). Medicine 2017, 96, e6122. [Google Scholar] [CrossRef]
- Tanabe, M.; Motonaga, R.; Terawaki, Y.; Nomiyama, T.; Yanase, T. Prescription of oral hypoglycemic agents for patients with type 2 diabetes mellitus: A retrospective cohort study using a Japanese hospital database. J. Diabetes Investig. 2017, 8, 227–234. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, S.T.; Chang, C.H.; Chuang, L.M.; Lai, M.S. Prescription trends and the selection of initial oral antidiabetic agents for patients with newly diagnosed type 2 diabetes: A nationwide study. Public Health 2017, 152, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Murayama, H.; Odawara, M.; Bauer, M. Treatment patterns of drug-naive patients with type 2 diabetes mellitus: A retrospective cohort study using a Japanese hospital database. Diabetol. Metab. Syndr. 2019, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Rodrigues, A.P.; Nunes, B. Initial Therapeutic Choices for Type 2 Diabetes in the Portuguese Sentinel Practice Network. Acta Med. Port. 2019, 32, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Juste, A.M.; Menditto, E.; Orlando, V.; Monetti, V.M.; Miguel, A.G.; Rubio, F.G.; Aza-Pascual-Salcedo, M.M.; Cahir, C.; Torres, A.P.; Riccardi, G. Treatment patterns of diabetes in Italy: A population-based study. Front. Pharmacol. 2019, 10, 870. [Google Scholar] [CrossRef]
- Moreno-Juste, A.; Poblador-Plou, B.; Aza-Pascual-Salcedo, M.; González-Rubio, F.; Malo, S.; López, J.L.; Pico-Soler, V.; Labrador, E.G.; Mucherino, S.; Orlando, V.; et al. Initial therapy, regimen change, and persistence in a spanish cohort of newly treated type 2 diabetes patients: A retrospective, observational study using real-world data. Int. J. Environ. Res. Public Health 2020, 17, 3742. [Google Scholar] [CrossRef]
- Yabe, D.; Higashiyama, H.; Kadowaki, T.; Origasa, H.; Shimomura, I.; Watada, H.; Tobe, K.; Iglay, K.; Tokita, S.; Seino, Y. Real-world Observational Study on Patient Outcomes in Diabetes (RESPOND): Study design and baseline characteristics of patients with type 2 diabetes newly initiating oral antidiabetic drug monotherapy in Japan. BMJ Open Diabetes Res. Care 2020, 8, e001361. [Google Scholar] [CrossRef]
- Wood, S.J.; Magliano, D.J.; Bell, J.S.; Shaw, J.E.; Keen, C.S.; Ilomäki, J. Pharmacological treatment initiation for type 2 diabetes in Australia: Are the guidelines being followed? Diabet. Med. 2020, 37, 1367–1373. [Google Scholar] [CrossRef]
- Campbell, D.J.T.; Campbell, D.B.; Ogundeji, Y.; Au, F.; Beall, R.; Ronksley, P.E.; Quinn, A.E.; Manns, B.J.; Hemmelgarn, B.R.; Tonelli, M.; et al. First-line pharmacotherapy for incident type 2 diabetes: Prescription patterns, adherence and associated costs. Diabet. Med. 2021, 38, e14622. [Google Scholar] [CrossRef]
- Shin, H.J.; Schneeweiss, S.; Glynn, R.J.; Patorno, E. Trends in First-Line Glucose-Lowering Drug Use in Adults With Type 2 Diabetes in Light of Emerging Evidence for SGLT-2i and GLP-1RA. Diabetes Care 2021, 44, 1774–1782. [Google Scholar] [CrossRef]
- Bonora, E.; Cataudella, S.; Marchesini, G.; Miccoli, R.; Vaccaro, O.; Fadini, G.P.; Martini, N.; Rossi, E. Initial treatment of diabetes in Italy. A nationwide population-based study from of the ARNO Diabetes Observatory. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2661–2668. [Google Scholar] [CrossRef]
- Barth, S.D.; Kostev, K.; Krensel, M.; Mathey, E.; Rathmann, W. Do Glucagonlike Peptide-1 Receptor Agonist and Sodium-glucose Co-transporter 2 Inhibitor Prescriptions in Germany Reflect Recommendations for Type 2 Diabetes with Cardiovascular Disease of the ADA/EASD Consensus Report? Exp. Clin. Endocrinol. Diabetes 2022, 131, 153–161. [Google Scholar] [CrossRef]
- Bouchi, R.; Sugiyama, T.; Goto, A.; Imai, K.; Ihana-Sugiyama, N.; Ohsugi, M.; Yamauchi, T.; Kadowaki, T.; Ueki, K. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J. Diabetes Investig. 2022, 13, 280–291. [Google Scholar] [CrossRef]
- Hulley, S.B.; Cummings, S.R.; Browner, W.S.; Grady, D.G.; Newman, T.B. Designing Clinical Research; Epub ahead of print; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Centers for Medicare & Medicaid Services. What’s Medicare? Available online: https://www.medicare.gov/ (accessed on 7 December 2024).
- Morieri, M.L.; Longato, E.; Di Camillo, B.; Sparacino, G.; Avogaro, A.; Fadini, G.P. Management of type 2 diabetes with a treat-to-benefit approach improved long-term cardiovascular outcomes under routine care. Cardiovasc. Diabetol. 2022, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee; Cheng, A.Y.Y. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. diabetes 2018, 42 (Suppl. 1), S1–S325. [Google Scholar] [CrossRef]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. J. Diabetes Investig. 2020, 11, 1020–1076. [Google Scholar] [CrossRef] [PubMed]
- Mardetko, N.; Nabergoj Makovec, U.; Locatelli, I.; Janez, A.; Kos, M. Uptake of new antidiabetic medicines in 11 European countries. BMC Endocr. Disord. 2021, 21, 127. [Google Scholar] [CrossRef]
- Funakoshi, M.; Nishioka, D.; Haruguchi, S.; Yonemura, S.; Takebe, T.; Nonaka, M.; Iwashita, S. Diabetes control in public assistance recipients and free/low-cost medical care program beneficiaries in Japan: A retrospective cross-sectional study. BMJ Public Health 2024, 2, e000686. [Google Scholar] [CrossRef]
- Glennie, J.L.; Kovacs Burns, K.; Oh, P. Bringing patient centricity to diabetes medication access in Canada. Clinicoecon. Outcomes Res. 2016, 8, 599–611. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Bashier, A.; Bin Hussain, A.; Abdelgadir, E.; Alawadi, F.; Sabbour, H.; Chilton, R. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol. Metab. Syndr. 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Allyhiani, M.; Kurdi, A.; Abdulaziz, A.; Faqeh, S.; Alhajjaji, A.; Alansari, S.; Althaqafi, A.; Alzaman, N.; Ali, M. Prescribing patterns of antidiabetics in type 2 diabetes and factors affecting them. Saudi Pharm. J. 2022, 30, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Halm, E.A.; Atlas, S.J.; Borowsky, L.H.; Benzer, T.I.; Metlay, J.P.; Chang, Y.C.; Singer, D.E. Understanding physician adherence with a pneumonia practice guideline: Effects of patient, system, and physician factors. Arch. Intern. Med. 2000, 160, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Grustam, A.; Jovic Vranes, A.; Soldatovic, I.; Stojicic, P.; Jovanovic Andersen, Z. Factors Associated with Utilization of Primary and Specialist Healthcare Services by Elderly Cardiovascular Patients in the Republic of Serbia: A Cross-Sectional Study from the National Health Survey 2013. Int. J. Environ. Res. Public Health 2020, 17, 2602. [Google Scholar] [CrossRef]
- Qi, M.; Santos, H.; Pinheiro, P.; McGuinness, D.L.; Bennett, K.P. Demographic and socioeconomic determinants of access to care: A subgroup disparity analysis using new equity-focused measurements. PLoS ONE 2023, 18, e0290692. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, Q.; Tang, J.; Fan, C.P.S.; Li, Z.; Apecechea, M.; Hegar, R.; Shankar, R.; Kurtyka, K.M.; Engel, S.S. Why physicians do not initiate dual therapy as recommended by AACE guidelines: A survey of clinicians in the United States. Diabetes Res. Clin. Pract. 2015, 108, 456–465. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012, 35, 731–737. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Confederat, L.; Constantin, S.; Lupaşcu, F.; Pânzariu, A.; Hăncianu, M.; Profire, L. Hypoglycemia induced by antidiabetic sulfonylureas. Rev. Med. Chir. Soc. Med. Nat. Iasi 2015, 119, 579–584. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Type 2 Diabetes in Adults: Management. NICE Guideline [NG28]; NICE: London, UK, 2015; Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 10 September 2024).
- Direção-Geral da Saúde. Abordagem Terapêutica Farmacológica na Diabetes Mellitus Tipo 2 no Adulto. Norma DGS No.052/2011 Updated 27 April 2015; pp. 1–28. Available online: https://www.mgfamiliar.net/wp-content/uploads/NOC_Diabetes_2015-3.pdf (accessed on 12 September 2024).
- Riddle, M.C. Modern Sulfonylureas: Dangerous or Wrongly Accused? Diabetes Care 2017, 40, 629–631. [Google Scholar] [CrossRef]
- Hirst, J.A.; Farmer, A.J.; Dyar, A.; Lung, T.W.C.; Stevens, R.J. Estimating the effect of sulfonylurea on HbA1c in diabetes: A systematic review and meta-analysis. Diabetologia 2013, 56, 973–984. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009, 52, 17–30. [Google Scholar] [CrossRef]
- Giorda, C.B.; Orsi, E.; De Cosmo, S.; Bossi, A.C.; Guerzoni, C.; Cercone, S.; Gilio, B.; Cavalot, F. Prescription of Sulphonylureas among Patients with Type 2 Diabetes Mellitus in Italy: Results from the Retrospective, Observational Multicentre Cross-Sectional SUSCIPE (Sulphonyl_UreaS_Correct_Internal_Prescription_Evaluation) Study. Diabetes Ther. 2020, 11, 2105–2119. [Google Scholar] [CrossRef] [PubMed]
- Thulé, P.M.; Umpierrez, G. Sulfonylureas: A new look at old therapy. Curr. Diab. Rep. 2014, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Lalau, J.-D.; Kajbaf, F.; Bennis, Y.; Hurtel-Lemaire, A.-S.; Belpaire, F.; De Broe, M.E. Metformin Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease Stages 3A, 3B, or 4. Diabetes Care 2018, 41, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Beigi, F.; Moghissi, E.; Tiktin, M.; Hirsch, I.B.; Inzucchi, S.E.; Genuth, S. Individualizing glycemic targets in type 2 diabetes mellitus: Implications of recent clinical trials. Ann. Intern. Med. 2011, 154, 554–559. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Hazra, N.C.; Rudisill, C.; Gulliford, M.C. Determinants of health care costs in the senior elderly: Age, comorbidity, impairment, or proximity to death? Eur. J. Health Econ. 2018, 19, 831–842. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; de Oliveira Rezende, A.T.; Delpino, F.M.; Mendonça, C.R.; Noll, M.; Nunes, B.P.; de Oliviera, C.; Silveira, E.A. Association between multimorbidity and hospitalization in older adults: Systematic review and meta-analysis. Age Ageing 2022, 51, afac155. [Google Scholar] [CrossRef]
- Mahmoud, F.; Mullen, A.; Sainsbury, C.; Rushworth, G.F.; Yasin, H.; Abutheraa, N.; Mueller, T.; Kurdi, A. Meta-analysis of factors associated with antidiabetic drug prescribing for type 2 diabetes mellitus. Eur. J. Clin. Investig. 2023, 53, e13997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).