Data-Efficiency with Comparable Accuracy: Personalized LSTM Neural Network Training for Blood Glucose Prediction in Type 1 Diabetes Management
Abstract
1. Introduction
2. Materials and Methods
2.1. T1D Dataset Utilized for Analysis
2.2. LSTM Model Development
2.3. Individual Versus Aggregate Model Training
2.4. Evaluation and Comparative Analysis
3. Results
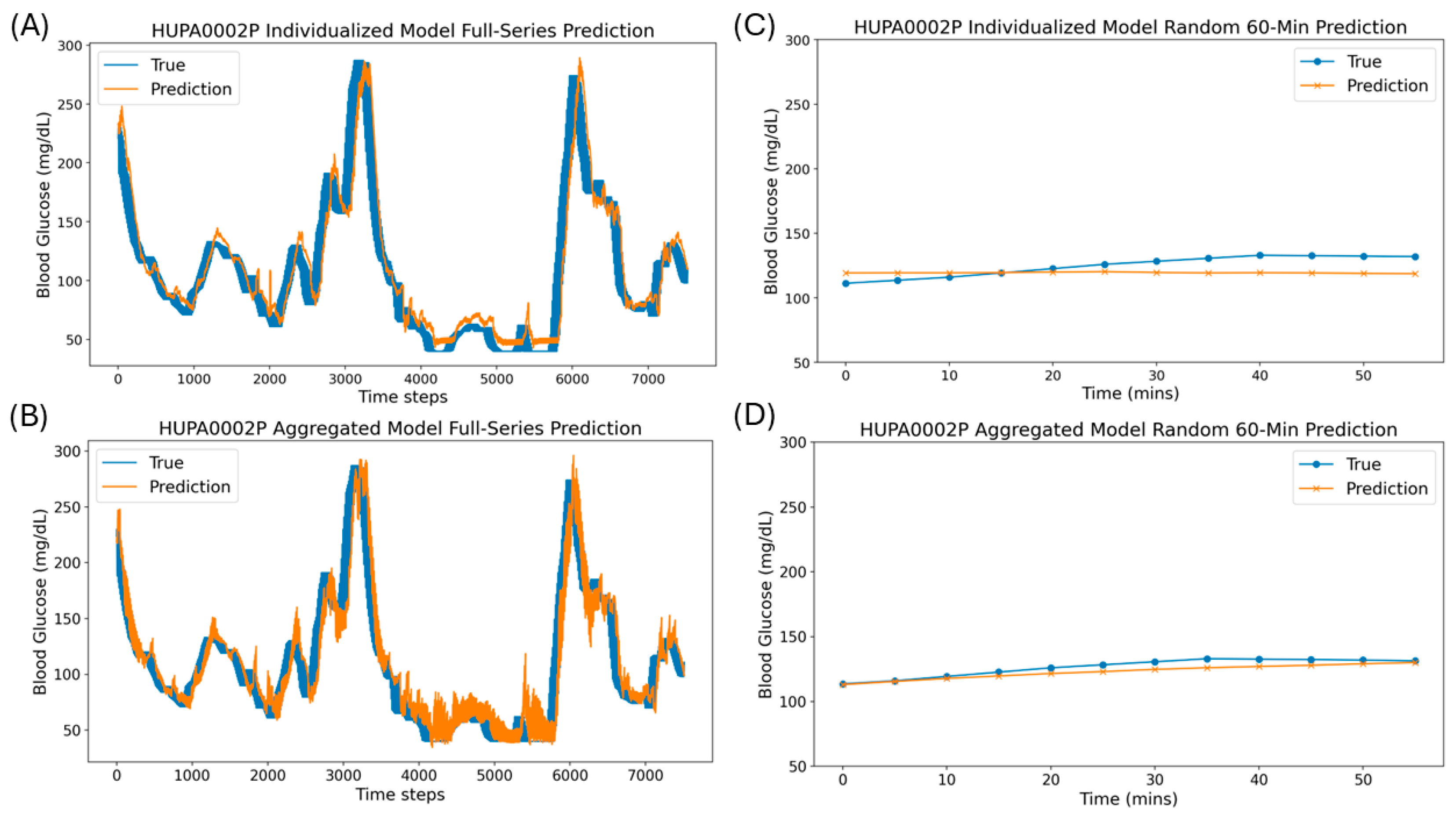
3.1. Single Window and Full-Series Rolling Forecasts Using Individualized and Aggregated Models
3.2. Individualized Models Achieve Comparable Quantitative Accuracy to Aggregated Models
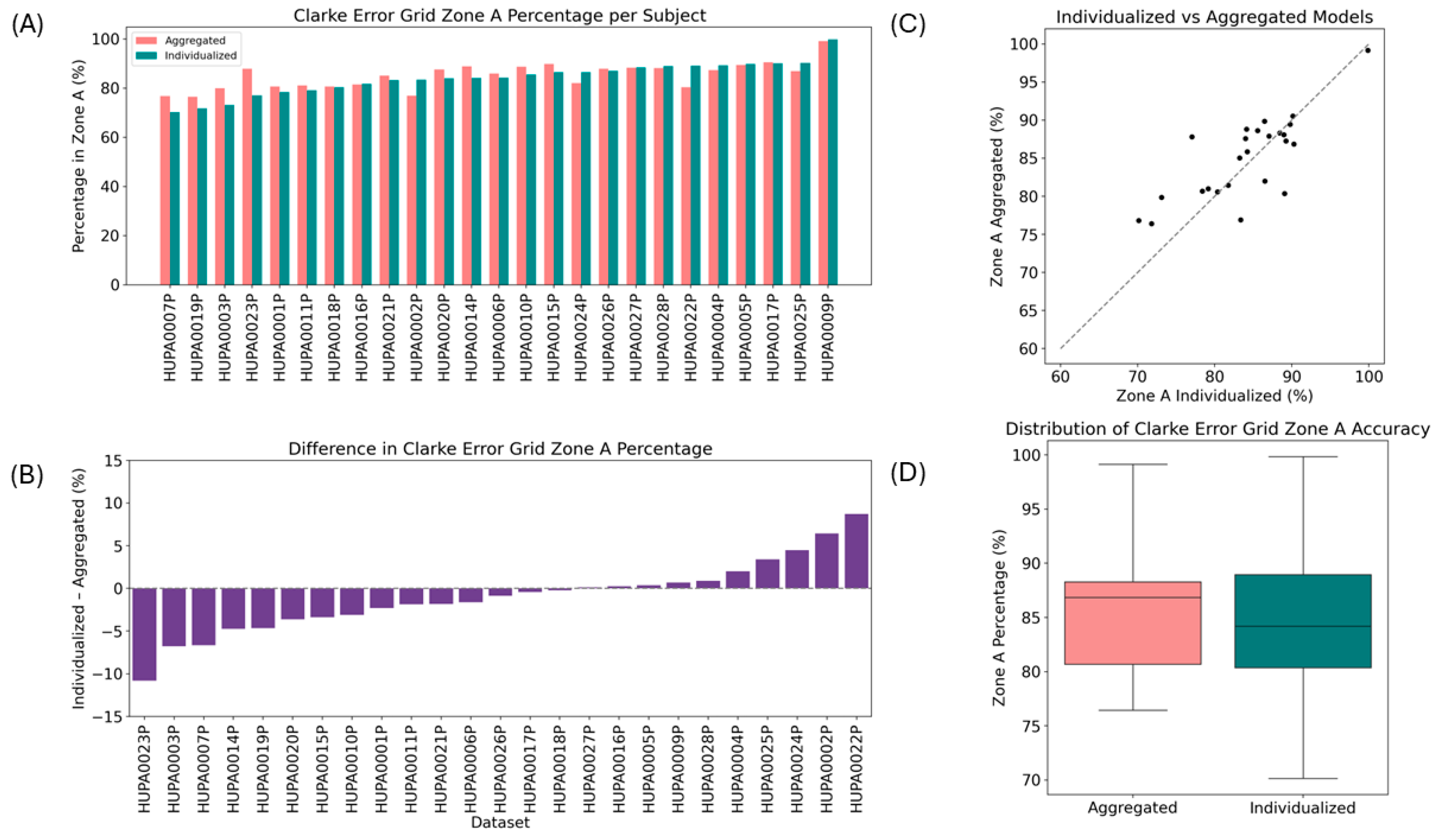
3.3. Clarke Error Grid Analysis of the Individualized and Aggregated Models
3.4. Individualized Models Achieve Comparable Clinical Accuracy to Aggregated Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T1D | Type 1 diabetes |
| CGM | Continuous glucose monitoring |
| LSTM | Long short-term memory |
| RMSE | Root mean squared error |
References
- Mauvais, F.-X.; van Endert, P.M. Type 1 Diabetes: A Guide to Autoimmune Mechanisms for Clinicians. Diabetes Obes. Metab. 2025, 27, 40–56. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Lambadiari, V.; Roden, M.; Dimitriadis, G.D. Insulin Resistance in Type 1 Diabetes: Pathophysiological, Clinical, and Therapeutic Relevance. Endocr. Rev. 2025, 46, 317–348. [Google Scholar] [CrossRef]
- Mao, Y.; Gau, J.-T.; Jiang, N. Obesity, Metabolic Health, and Diabetic Complications in People with Type 1 Diabetes. Endocrinol. Diabetes Metab. 2025, 8, e70017. [Google Scholar] [CrossRef]
- Braffett, B.H.; Bebu, I.; Lorenzi, G.M.; Martin, C.L.; Perkins, B.A.; Gubitosi-Klug, R.; Nathan, D.M.; DCCT/EDIC Research Group. The NIDDK Takes on the Complications of Type 1 Diabetes: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care 2025, 48, 1089–1100. [Google Scholar] [CrossRef]
- Ogle, G.D.; Wang, F.; Haynes, A.; Gregory, G.A.; King, T.W.; Deng, K.; Dabelea, D.; James, S.; Jenkins, A.J.; Li, X.; et al. Global type 1 diabetes prevalence, incidence, and mortality estimates 2025: Results from the International diabetes Federation Atlas, 11th Edition, and the T1D Index Version 3.0. Diabetes Res. Clin. Pract. 2025, 225, 112277. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef]
- Kamrath, C.; Holl, R.W.; Rosenbauer, J. Elucidating the Underlying Mechanisms of the Marked Increase in Childhood Type 1 Diabetes During the COVID-19 Pandemic—The Diabetes Pandemic. JAMA Netw. Open 2023, 6, e2321231. [Google Scholar] [CrossRef]
- Dib, S.A. Hypoglycemia in type 1 diabetes: A burden to worry about during treatment. Arch. Endocrinol. Metab. 2022, 66, 776–779. [Google Scholar] [CrossRef]
- Kovatchev, B.P. Metrics for glycaemic control—From HbA1c to continuous glucose monitoring. Nat. Rev. Endocrinol. 2017, 13, 425–436. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef]
- Starr, L.; Dutta, S.; Danne, T.; Karpen, S.R.; Hutton, C.; Kowalski, A. The Urgent Need for Breakthrough Therapies and a World Without Type 1 Diabetes. Diabetes Ther. 2025, 16, 1063–1076. [Google Scholar] [CrossRef]
- Boughton, C.K.; Hovorka, R. New closed-loop insulin systems. Diabetologia 2021, 64, 1007–1015. [Google Scholar] [CrossRef]
- Royston, C.; Roman, H.; Boughton, C.K. Closed-loop therapy: Recent advancements and potential predictors of glycemic outcomes. Expert. Opin. Drug Deliv. 2025, 22, 875–892. [Google Scholar] [CrossRef]
- Yu, T.S.; Song, S.; Yea, J.; Jang, K.-I. Diabetes Management in Transition: Market Insights and Technological Advancements in CGM and Insulin Delivery. Adv. Sens. Res. 2024, 3, 2400048. [Google Scholar] [CrossRef]
- Hughes, M.S.; Levy, C.J. The Future of Automated Insulin Delivery Systems. Endocr. Pract. 2025, 31, 1162–1170. [Google Scholar] [CrossRef]
- Man, C.D.; Micheletto, F.; Lv, D.; Breton, M.; Kovatchev, B.; Cobelli, C. The UVA/PADOVA Type 1 Diabetes Simulator: New Features. J. Diabetes Sci. Technol. 2014, 8, 26–34. [Google Scholar] [CrossRef]
- Hovorka, R.; Canonico, V.; Chassin, L.J.; Haueter, U.; Massi-Benedetti, M.; Federici, M.O.; Pieber, T.R.; Schaller, H.C.; Schaupp, L.; Vering, T.; et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol. Meas. 2004, 25, 905. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.T. A Physiologic Model of Glucose Metabolism in Man and Its Use to Design and Assess Improved Insulin Therapies for Diabetes; Massachusetts Institute of Technology: Boston, MA, USA, 1985. [Google Scholar]
- Miller, A.C.; Foti, N.J.; Fox, E. Learning Insulin-Glucose Dynamics in the Wild. Proc. Mach. Learn. Res. 2020, 126, 1–25. [Google Scholar]
- Nemat, H.; Khadem, H.; Elliott, J.; Benaissa, M. Data-driven blood glucose level prediction in type 1 diabetes: A comprehensive comparative analysis. Sci. Rep. 2024, 14, 21863. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Z.; Sun, X.; Liu, H.; Li, H.; Lu, J.; Zhou, J.; Cinar, A. Deep Reinforcement Learning for Automated Insulin Delivery Systems: Algorithms, Applications, and Prospects. AI 2025, 6, 87. [Google Scholar] [CrossRef]
- Zhang, M.; Flores, K.B.; Tran, H.T. Deep learning and regression approaches to forecasting blood glucose levels for type 1 diabetes. Biomed. Signal Process. Control 2021, 69, 102923. [Google Scholar] [CrossRef]
- Prendin, F.; Pavan, J.; Cappon, G.; Del Favero, S.; Sparacino, G.; Facchinetti, A. The importance of interpreting machine learning models for blood glucose prediction in diabetes: An analysis using SHAP. Sci. Rep. 2023, 13, 16865. [Google Scholar] [CrossRef] [PubMed]
- Rabby, M.F.; Tu, Y.; Hossen, M.I.; Lee, I.; Maida, A.S.; Hei, X. Stacked LSTM based deep recurrent neural network with kalman smoothing for blood glucose prediction. BMC Med. Inform. Decis. Mak. 2021, 21, 101. [Google Scholar] [CrossRef]
- Sun, Q.; Jankovic, M.V.; Bally, L.; Mougiakakou, S.G. Predicting Blood Glucose with an LSTM and Bi-LSTM Based Deep Neural Network. In Proceedings of the 14th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 20–21 November 2018. [Google Scholar]
- Neumann, A.; Zghal, Y.; Cremona, M.A.; Hajji, A.; Morin, M.; Rekik, M. A data-driven personalized approach to predict blood glucose levels in type-1 diabetes patients exercising in free-living conditions. Comput. Biol. Med. 2025, 190, 110015. [Google Scholar] [CrossRef]
- Carvalho, C.F.; Liang, Z. Glucose Prediction with Long Short-Term Memory (LSTM) Models in Three Distinct Populations. Eng. Proc. 2024, 82, 87. [Google Scholar]
- Rancati, S.; Bosoni, P.; Schiaffini, R.; Deodati, A.; Mongini, P.A.; Sacchi, L.; Toffanin, C.; Bellazzi, R. Exploration of Foundational Models for Blood Glucose Forecasting in Type-1 Diabetes Pediatric Patients. Diabetology 2024, 5, 584–599. [Google Scholar] [CrossRef]
- Hidalgo, J.I.; Alvarado, J.; Botella, M.; Aramendi, A.; Velasco, J.M.; Garnica, O. HUPA-UCM diabetes dataset. Data Brief 2024, 55, 110559. [Google Scholar] [CrossRef]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating Clinical Accuracy of Systems for Self-Monitoring of Blood Glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef]
- Krouwer, J.S. Validation of the Diabetes Technology Society Error Grid. J. Diabetes Sci. Technol. 2025, 19, 853–854. [Google Scholar] [CrossRef]
- Tyler, N.S.; Jacobs, P.G. Artificial Intelligence in Decision Support Systems for Type 1 Diabetes. Sensors 2020, 20, 3214. [Google Scholar] [CrossRef]
- Lindholm, M.; Palmborg, L. Efficient use of data for LSTM mortality forecasting. Eur. Actuar. J. 2022, 12, 749–778. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Lara-Abelenda, F.J.; Chushig-Muzo, D.; Peiro-Corbacho, P.; Wägner, A.M.; Granja, C.; Soguero-Ruiz, C. Personalized glucose forecasting for people with type 1 diabetes using large language models. Comput. Methods Programs Biomed. 2025, 265, 108737. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, I.; Chatzigiannakis, I.; Rodríguez, J.-V.; Maranghi, M.; Gentili, M.; Zamora-Izquierdo, M.-Á. Utility of Big Data in Predicting Short-Term Blood Glucose Levels in Type 1 Diabetes Mellitus Through Machine Learning Techniques. Sensors 2019, 19, 4482. [Google Scholar] [CrossRef]
- Iacono, F.; Magni, L.; Toffanin, C. Personalized LSTM-based alarm systems for hypoglycemia and hyperglycemia prevention. Biomed. Signal Process. Control 2023, 86, 105167. [Google Scholar] [CrossRef]
- Shen, Y.; Kleinberg, S. Personalized Blood Glucose Forecasting from Limited CGM Data Using Incrementally Retrained LSTM. IEEE Trans. Biomed. Eng. 2024, 72, 1266–1277. [Google Scholar] [CrossRef]
- Adadi, A. A survey on data-efficient algorithms in big data era. J. Big Data 2021, 8, 24. [Google Scholar] [CrossRef]
- Ahmed, B.M.; Ali, M.E.; Masud, M.M.; Naznin, M. Recent trends and techniques of blood glucose level prediction for diabetes control. Smart Health 2024, 32, 100457. [Google Scholar] [CrossRef]
- Zhu, T.; Li, K.; Herrero, P.; Georgiou, P. Personalized Blood Glucose Prediction for Type 1 Diabetes Using Evidential Deep Learning and Meta-Learning. IEEE Trans. Biomed. Eng. 2022, 70, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.; Park, S.-W.; Kim, N.; Jin, S.-M.; Park, S.-M. A personalized blood glucose level prediction model with a fine-tuning hanstrategy: A proof-of-concept study. Comput. Methods Programs Biomed. 2021, 211, 106424. [Google Scholar] [CrossRef]
- Del Giudice, L.L.; Piersanti, A.; Göbl, C.; Burattini, L.; Tura, A.; Morettini, M. Availability of Open Dynamic Glycemic Data in the Field of Diabetes Research: A Scoping Review. J. Diabetes Sci. Technol. 2025, 19322968251316896. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manchanda, E.; Zeng, J.; Lo, C.H. Data-Efficiency with Comparable Accuracy: Personalized LSTM Neural Network Training for Blood Glucose Prediction in Type 1 Diabetes Management. Diabetology 2025, 6, 115. https://doi.org/10.3390/diabetology6100115
Manchanda E, Zeng J, Lo CH. Data-Efficiency with Comparable Accuracy: Personalized LSTM Neural Network Training for Blood Glucose Prediction in Type 1 Diabetes Management. Diabetology. 2025; 6(10):115. https://doi.org/10.3390/diabetology6100115
Chicago/Turabian StyleManchanda, Esha, Jialiu Zeng, and Chih Hung Lo. 2025. "Data-Efficiency with Comparable Accuracy: Personalized LSTM Neural Network Training for Blood Glucose Prediction in Type 1 Diabetes Management" Diabetology 6, no. 10: 115. https://doi.org/10.3390/diabetology6100115
APA StyleManchanda, E., Zeng, J., & Lo, C. H. (2025). Data-Efficiency with Comparable Accuracy: Personalized LSTM Neural Network Training for Blood Glucose Prediction in Type 1 Diabetes Management. Diabetology, 6(10), 115. https://doi.org/10.3390/diabetology6100115




