Quantitative Assessment of Retention Mechanisms of Nucleosides on a Bare Silica Stationary Phase in Hydrophilic Interaction Liquid Chromatography (HILIC)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
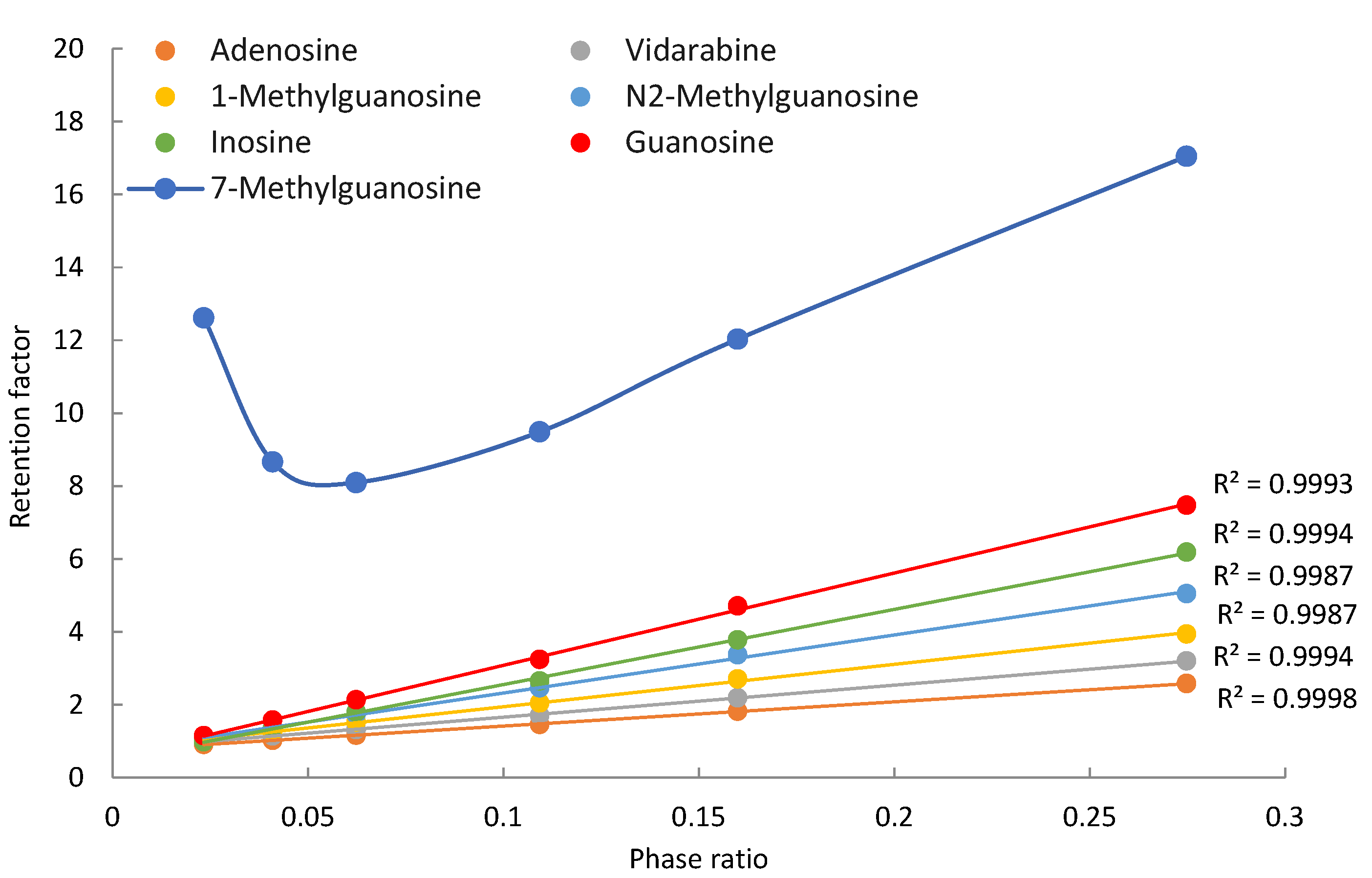
3.1. Purine-Related Nucleosides
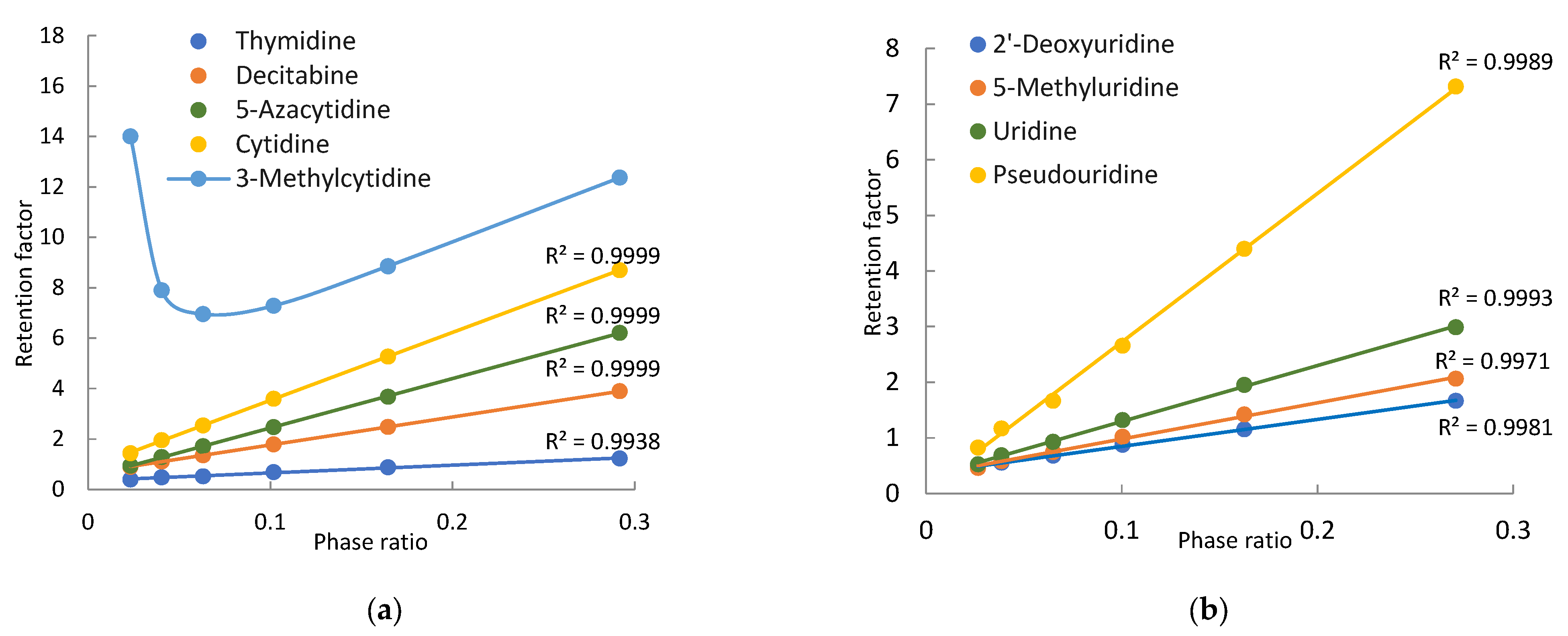
3.2. Pyrimidine-Related Nucleosides
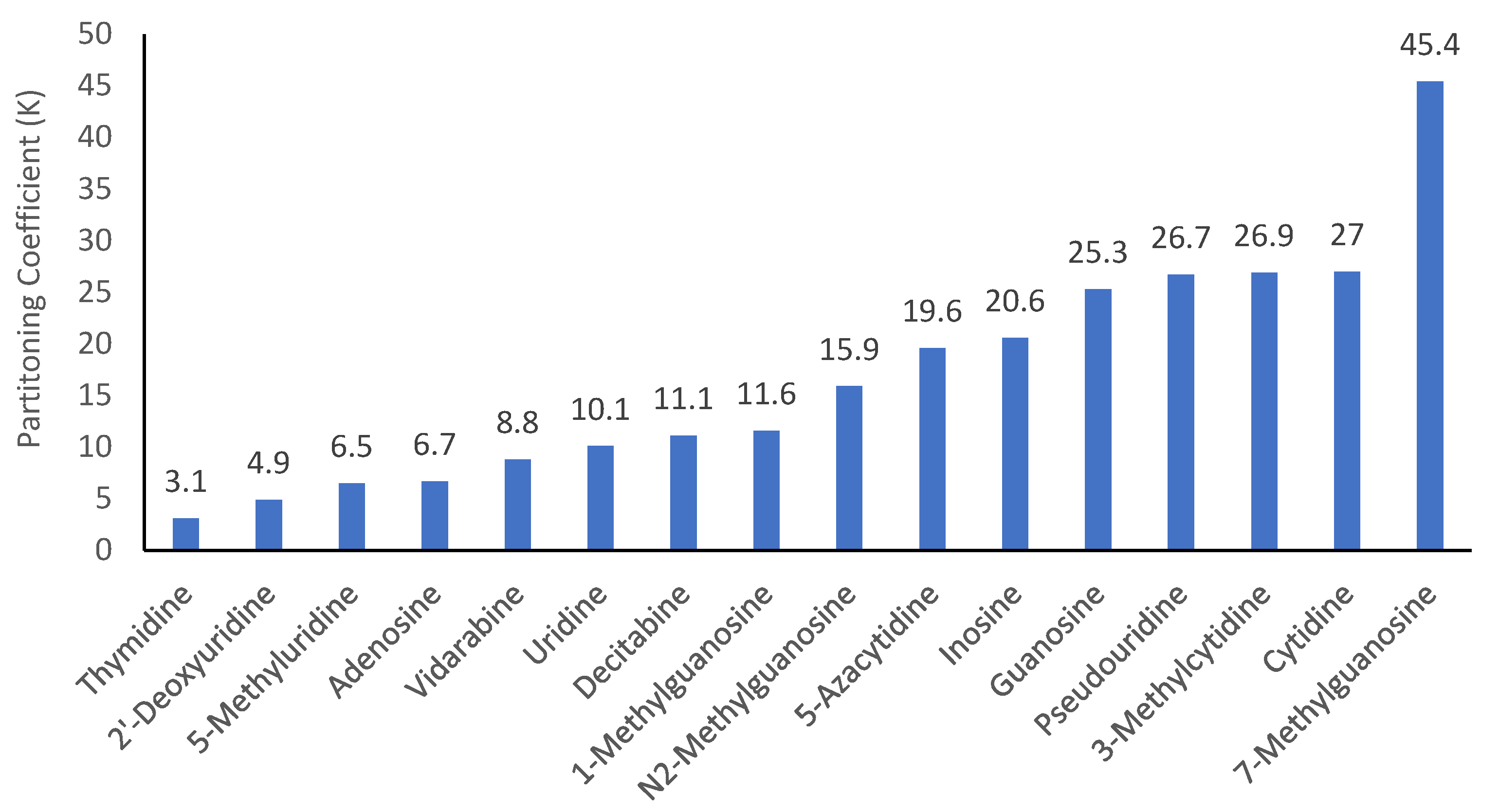
3.3. Polarity of Modified Nucleosides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HILIC | Hydrophilic Interaction Liquid Chromatography |
References
- Zhang, G. Nucleosides/nucleotides biomarkers. In Targeted Biomarker Quantitation, 1st ed.; Weng, N., Jian, W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 389–406. [Google Scholar]
- Berdis, A. Nucleobase-modified nucleosides and nucleotides: Application in biochemistry, synthetic biology, and drug discovery. Front. Chem. 2022, 10, 1051525. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Muramatsu, H.; Welsh, F.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.Y.; Han, H.; Wang, B.; Li, N.; Dong, T.T.X.; Zhang, T.; Tsim, K.W.K. Fast Simultaneous determination of 13 nucleosides and nucleobases in Cordyceps sinensis. Molecules 2015, 20, 21816–21825. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Sanz, A.; Scott-Ward, T.S.; Gill, H.S.; Jacoby, J.C.; Birch, R.E.; Malone-Lee, J.; Taylor, K.M.G.; Peppiatt-Wildman, C.M.; Wildman, S.S.P. Simultaneous quantitation of 12 different nucleotides and nucleosides released from renal eithelium and in human urine samples using ion-pair reversed-phase HPLC. Purinergic Signal. 2012, 8, 741–751. [Google Scholar] [CrossRef]
- Guo, Y. Separation of nucleobases, nucleosides, nucleotides and oligonucleotides by hydrophilic interaction liquid chromatography (HILIC): A state-of-the-art review. J. Chromatogr. A 2024, 1738, 465467. [Google Scholar] [CrossRef]
- Tyeca, E.; Guillarme, D.; Desmet, G. The use of individual retention modeling for gradient optimization in hydrophilic interaction chromatography: Separation of nucleobases and nucleosides. J. Chromatogr. A 2014, 1368, 125–131. [Google Scholar] [CrossRef]
- Mateos-Vivas, M.; Rodriguez-Gonzalo, E.; Carcia-Gomez, D.; Carabias-Martinez, R. Hydrophilic interaction chromatography coupled to tandem mass spectrometry in the presence of hydrophilic ion-paring reagents for the separation of nucleosides and nucleotide mono-, di- and triphosphates. J. Chromatogr. A 2015, 1414, 129–137. [Google Scholar] [CrossRef]
- Oritz-Villanueva, E.; Navarro-Reig, M.; Jaumot, J.; Tauler, R. Chemometric evaluation of hydrophilic interaction liquid chromatography stationary phases: Resolving complex mixtures of metabolites. Anal. Methods 2017, 5, 774–785. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Hong, X.; Zhang, X.; Pan, T.; Pan, C.; Zheng, S.; Guo, C. Simultaneous determination of methylated nucleosides by HILIC-MS/MS revealed their alternations in urine from breast cancer patients. Metabolites 2022, 12, 973. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Hong, X.; Wang, M.; Fang, Z.; Cao, X.; Jiang, K.; Guo, C. Determination of adenosine and its modifications in urine and plasma from breast cancer patients by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2022, 1209, 123428. [Google Scholar] [CrossRef]
- Cao, X.; Wang, M.; Huang, Y.; Zhang, M.; Zheng, F.; Zhang, G.; Su, J.; Yuan, Y.; Guo, C. Determination of demethylated nucleosides in serum from colorectal cancer patients by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2024, 1232, 123973. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J. Chromatogr. A 2015, 1074, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, Y.; Ikegami, T.; Takubo, H.; Ikegami, Y.; Miyamoto, M.; Tanaka, N. Chromatographic characterization of hydrophilic interaction liquid chromatography stationary phases: Hydrophilicity, charge effects, structural selectivity, and separation efficiency. J. Chromatogr. A 2011, 1218, 5903–5919. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y. Recent progress in the fundamental understanding of hydrophilic interaction chromatography. Analyst 2015, 140, 6452–6466. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Janas, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef]
- Jandera, P.; Hajek, T.; Sromov, Z. Mobile phase effects in reversed-phase and hydrophilic interaction liquid chromatography revisited. J. Chromatogr. A 2018, 1543, 48–57. [Google Scholar] [CrossRef]
- Khrisanfova, A.; Smagina, M.; Maksimov, G.; Tsizin, G.; Shpigun, O.; Chernobrovkina, A. Evaluating independent effect of mobile phase components on retention mechanism of ionizable analytes in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2025, 1758, 466201. [Google Scholar] [CrossRef]
- Gritt, F.; Holtzel, A.; Tallarek, U.; Guiochon, G. The relative importance of the adsorption and partitioning mechanisms in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2015, 1376, 112–125. [Google Scholar] [CrossRef]
- Guo, Y.; Fattal, B. Relative significance of hydrophilic partitioning and surface adsorption to the retention of polar compounds in hydrophilic interaction chromatography. Anal. Chim. Acta 2021, 1184, 339025. [Google Scholar] [CrossRef]
- Guo, Y.; Baran, D.; Ryan, L. Quantitative assessment of retention mechanisms of ionized compounds in hydrophilic interaction chromatography. Anal. Chem. 2025, 97, 4057–4065. [Google Scholar] [CrossRef]
- Guo, Y.; Bhalodia, N.; Fattal, B.; Serris, I. Evaluating the adsorbed water layer on the polar stationary phases in hydrophilic interaction chromatography. Separations 2019, 6, 6020019. [Google Scholar] [CrossRef]
- Guo, Y.; Baran, D.; Ryan, L. Insights into the selectivity of polar stationary phases based on quantitative retention mechanism assessment in hydrophilic interaction chromatography. J. Chromatogr. A 2024, 1726, 464973. [Google Scholar] [CrossRef]
- Jones, E.L.; Mlotkowski, A.J.; Hebert, S.P.; Schlegel, H.B.; Chow, C.S. Calculations of pKa values for a series of naturally occurring modified nucleobases. J. Phys. Chem. A 2022, 126, 1518–1529. [Google Scholar] [CrossRef]
- Soares, J.X.; Santos, A.; Fernandes, C.; Pinto, M.M.M. Liquid chromatography on the different methods for the determination of lipophilicity: An essential analytical tool in medicinal chemistry. Chemosensors 2022, 10, 340. [Google Scholar] [CrossRef]
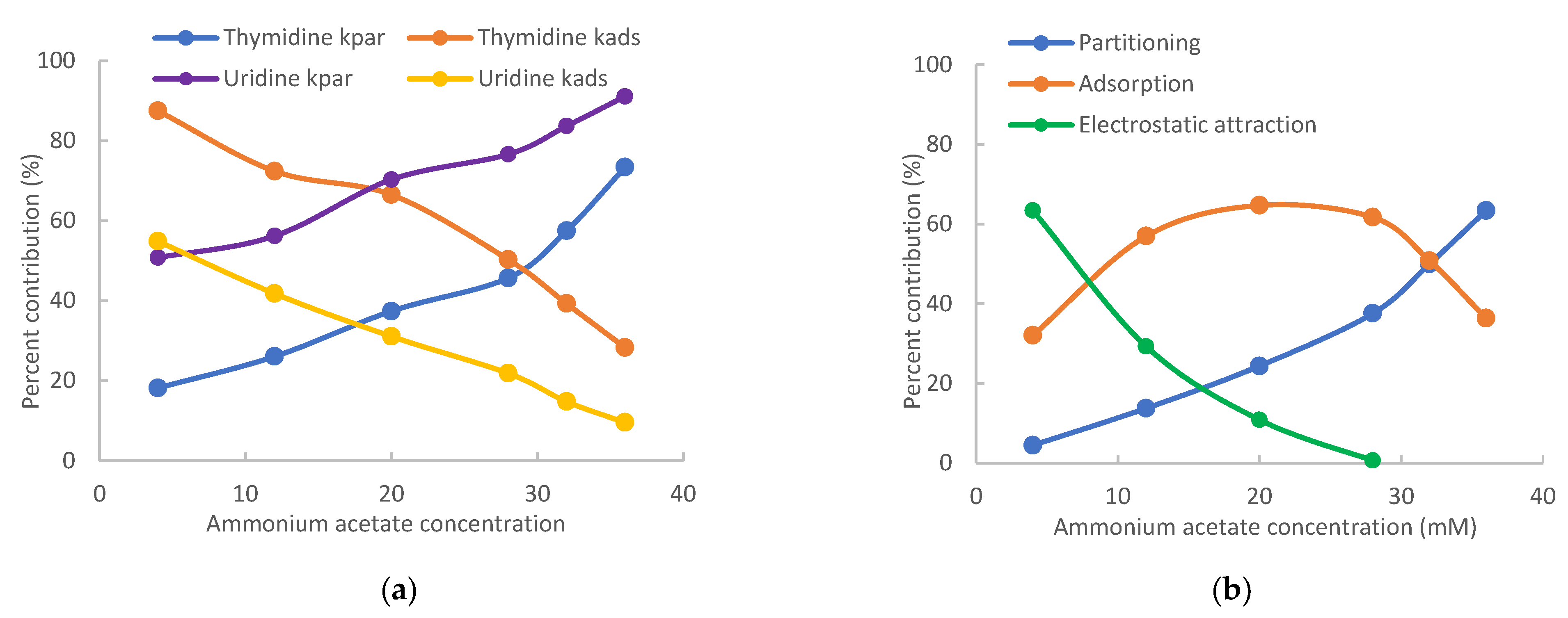

| Nucleoside | 4 mM Ammonium Acetate | 36 mM Ammonium Acetate | ||
|---|---|---|---|---|
| Partitioning (kpar) | Adsorption (kads) | Partitioning (kpar) | Adsorption (kads) | |
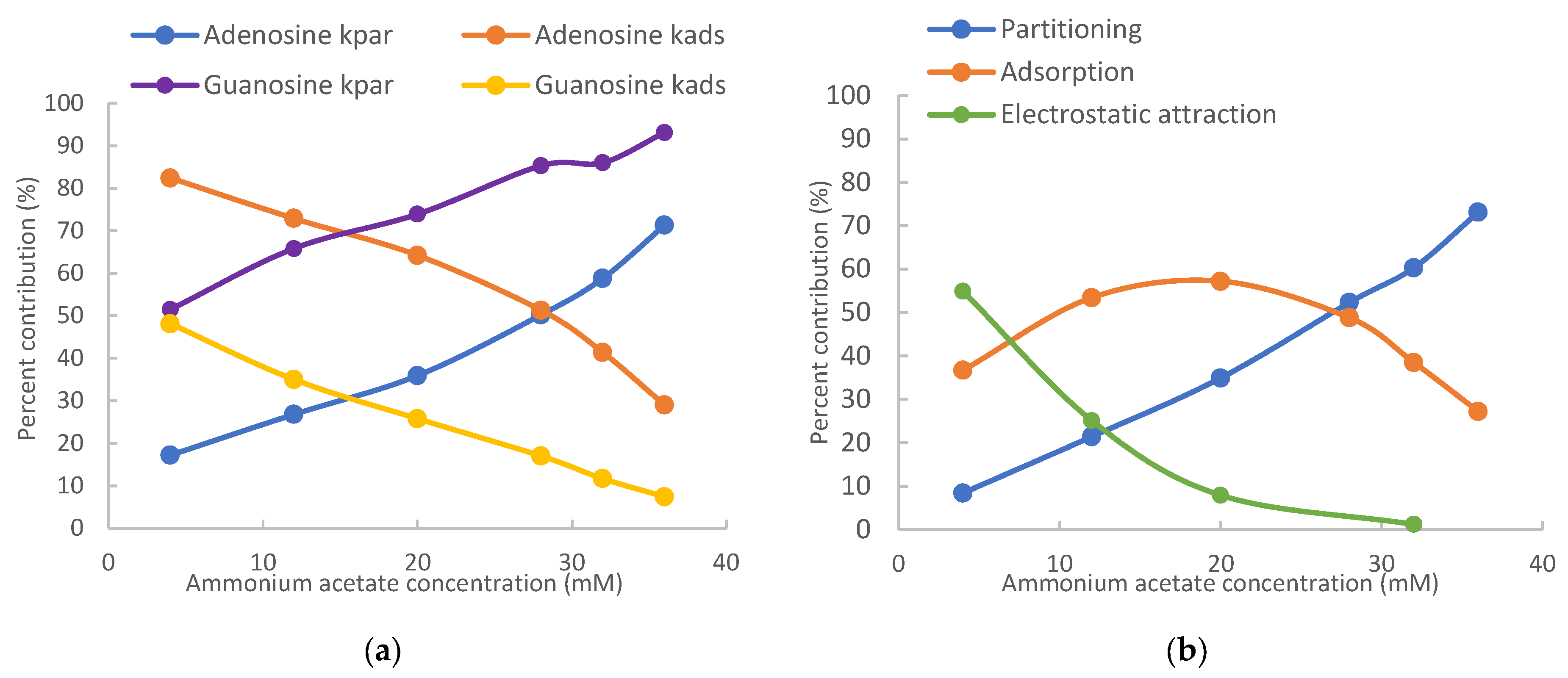
| Adenosine | 0.156 (17.2%) * | 0.748 (82.4%) | 1.840 (71.3%) | 0.748 (29.0%) |
| Vidarabine | 0.205 (20.4%) | 0.781 (77.5%) | 2.409 (75.3%) | 0.781 (24.4%) |
| Inosine | 0.482 (48.9%) | 0.488 (49.6%) | 5.671 (91.6%) | 0.488 (7.9%) |
| Guanosine | 0.591 (51.5%) | 0.552 (48.1%) | 6.955 (93.1%) | 0.552 (7.4%) |
| 1-Methylguanosine | 0.271 (26.8%) | 0.783 (77.2%) | 3.195 (81.1%) | 0.783 (19.9%) |
| 7-Methylguanosine | 1.059 (8.4%) | 4.631 (36.7%) | 12.466 (73.1%) | 4.631 (27.2%) |
| N2-Methylguanosine | 0.372 (35.2%) | 0.728 (68.9%) | 4.374 (86.6%) | 0.728 (14.4%) |
| Nucleoside | 4 mM Ammonium Acetate | 36 mM Ammonium Acetate | ||
|---|---|---|---|---|
| Partitioning (kpar) | Adsorption (kads) | Partitioning (kpar) | Adsorption (kads) | |
| Cytidine | 0.629 (43.8%) | 0.838 (58.3%) | 7.859 (90.4%) | 0.838 (9.6%) |
| 3-Methylcytidine | 0.628 (4.5%) | 4.499 (32.1%) | 7.845 (63.4%) | 4.499 (36.4%) |
| 5-Azacytidine | 0.458 (48.4%) | 0.480 (50.7%) | 5.719 (92.0%) | 0.480 (7.7%) |
| Decitabine | 0.259 (28.9%) | 0.651 (72.5%) | 3.243 (83.3%) | 0.651 (16.7%) |
| Thymidine | 0.072 (18.2%) | 0.347 (87.5%) | 0.901 (73.4%) | 0.347 (28.3%) |
| Uridine | 0.266 (50.8%) | 0.288 (54.9%) | 2.722 (91.1%) | 0.288 (9.6%) |
| 2′-Deoxyuridine | 0.129 (27.9%) | 0.362 (78.6%) | 1.314 (78.9%) | 0.362 (21.7%) |
| 5-Methyluridine | 0.173 (37.6%) | 0.328 (71.4%) | 1.764 (85.5%) | 0.328 (15.9%) |
| Pseudouridine | 0.707 (84.4%) | 0.054 (6.5%) | 7.226 (98.8%) | 0.054 (0.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleiner, D.; Muscatiello, D.; Gutierrez, Z.; Asare, V.; Guo, Y. Quantitative Assessment of Retention Mechanisms of Nucleosides on a Bare Silica Stationary Phase in Hydrophilic Interaction Liquid Chromatography (HILIC). Analytica 2025, 6, 39. https://doi.org/10.3390/analytica6040039
Kleiner D, Muscatiello D, Gutierrez Z, Asare V, Guo Y. Quantitative Assessment of Retention Mechanisms of Nucleosides on a Bare Silica Stationary Phase in Hydrophilic Interaction Liquid Chromatography (HILIC). Analytica. 2025; 6(4):39. https://doi.org/10.3390/analytica6040039
Chicago/Turabian StyleKleiner, David, David Muscatiello, Zugeily Gutierrez, Vanessa Asare, and Yong Guo. 2025. "Quantitative Assessment of Retention Mechanisms of Nucleosides on a Bare Silica Stationary Phase in Hydrophilic Interaction Liquid Chromatography (HILIC)" Analytica 6, no. 4: 39. https://doi.org/10.3390/analytica6040039
APA StyleKleiner, D., Muscatiello, D., Gutierrez, Z., Asare, V., & Guo, Y. (2025). Quantitative Assessment of Retention Mechanisms of Nucleosides on a Bare Silica Stationary Phase in Hydrophilic Interaction Liquid Chromatography (HILIC). Analytica, 6(4), 39. https://doi.org/10.3390/analytica6040039






