Enhanced “Greener” and Sustainable Ultrasonic Extraction of Bioactive Components from Waste Wild Apple (Malus sylvestris (L.) Mill.) Fruit Dust: The Impact of Pretreatment with Natural Deep Eutectic Solvents
Abstract
1. Introduction
- (a)
- Developing newer “green” extraction techniques;
- (b)
- Selecting the appropriate extraction agent.
- (a)
- Development of novel, “greener” and environmentally friendly UAE methodologies using crude herbal powder (waste wild apple fruit dust) and four different solvent systems (distilled water, aqueous 37% (v/v) ethanol, aqueous 38% (v/v) propylene glycol, or aqueous 38% (v/v) glycerol);
- (b)
- Choosing the best solvent system for the UAE procedure using NADES-pretreated herbal powder in order to enhance the UAE efficiency;
- (c)
- UHPLC-DAD-MS/MS identification and comparison of bioactive components in produced extracts;
- (d)
- Comparison of their total phenolic content (TPC) and total flavonoid content (TFC) values, as well as antioxidant activity of produced extracts using DPPH and ABTS assays;
- (e)
- Evaluation and comparison of energy demands and environmental impacts of suggested UAE methodologies.
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. NADES Preparation Procedure
2.4. Pretreatment of Plant Material
2.5. UAE Procedures
2.5.1. UAE Procedure Using Crude Herbal Powder
2.5.2. UAE Procedure Using NADES-Pretreated Herbal Powder
2.6. Qualitative and Quantitative Analyses
2.6.1. UHPLC-DAD-MS/MS Analysis
2.6.2. Determination of TPC and TFC
2.6.3. Antioxidant Activity Assessment
2.6.4. Statistical Analysis
3. Results and Discussion
3.1. UHPLC-DAD-MS Analyses of Extracts
3.2. TPC, TFC, and Antioxidant Activity in Waste Wild Apple UAE
3.3. Differences in Extraction Efficiencies of Applied UAE/Aqueous Alcoholic Systems
3.4. Evaluation and Comparison of the Proposed UAE Methodologies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| ANOVA | Analysis of variance |
| ChCl | Choline chloride |
| DES | Deep eutectic solvent |
| DAP | Dry apple powder |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GAE | Gallic acid equivalent |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| NADES | Natural deep eutectic solvent |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| QE | Quercetin equivalent |
| UAE | Ultrasound-assisted extraction |
| UAE/aEtOH | Extract produced after UAE with aqueous 37% (v/v) ethanol |
| UAE/aGLYC | Extract produced after UAE with aqueous 38% (v/v) glycerol |
| UAE/aPPG | Extract produced after UAE with aqueous 38% (v/v) propylene glycol |
| UAE/water | Extract produced after UAE with distilled water |
References
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Anani, O.A.; Olaniyan, O.T.; Bodunrinde, R.E.; Osemwegie, O.O.; Ubi, B.E. Integrated processes for production of pharmaceutical products from agro-wastes. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 439–461. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Pavlić, B.; Naffati, A.; Hojan, T.; Vladić, J.; Zeković, Z.; Vidović, S. Microwave-assisted extraction of wild apple fruit dust—Production of polyphenol-rich extracts from filter tea factory by-products. J. Food Process Eng. 2017, 40, e12508. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernandez, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.; Fabiano-Tixier, A.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Singh, N.; Panwar, D.; Kumar, G.; Kashyap, P. New horizons for the enhanced recovery of phenolic compounds by integration of natural deep eutectic solvents and microwave-assisted extraction. Food Biosci. 2024, 60, 104375. [Google Scholar] [CrossRef]
- Chien, W.-J.; Saputri, D.S.; Lin, H.-Y. Valorization of Taiwan’s Citrus depressa Hayata peels as a source of nobiletin and tangeretin using simple ultrasonic-assisted extraction. Curr. Res. Food Sci. 2022, 5, 278–287. [Google Scholar] [CrossRef]
- Demirkol, O.; Erşatır, M.; Giray, E.S.; Kırıcı, S. Comparison of the effects of green and sustainable extraction methods on the extraction yield and chemical composition of Ruta chalepensis roots. Sustain. Chem. Pharm. 2022, 29, 100750. [Google Scholar] [CrossRef]
- Hernández-Estrada, S.; Anaya-Esparza, L.M.; González-Torres, S.; Hernández-Villaseñor, L.A.; Gómez-Rodríguez, V.M.; Ramírez-Vega, H.; Villagrán, Z.; Ruvalcaba-Gómez, J.M.; Rodríguez-Barajas, N.; Montalvo-González, E. Extraction of soluble phenols and flavonoids from native Mexican pigmented corn kernel powder by ultrasound: Optimization process using response surface methodology. Appl. Sci. 2024, 14, 7869. [Google Scholar] [CrossRef]
- Nikolić, V.G.; Troter, D.Z.; Savić, I.M.; Savić Gajić, I.M.; Zvezdanović, J.B.; Konstantinović, I.B.; Konstantinović, S.S. Design and optimization of “greener” and sustainable ultrasound-assisted extraction of valuable bioactive compounds from common centaury (Centaurium erythraea Rafn) aerial parts: A comparative study using aqueous propylene glycol and ethanol. Ind. Crops Prod. 2023, 192, 116070. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Aurora-Vigo, E.F.; Villagrán, Z.; Rodríguez-Lafitte, E.; Ruvalcaba-Gómez, J.M.; Solano-Cornejo, M.Á.; Zamora-Gasga, V.M.; Montalvo-González, E.; Gómez-Rodríguez, H.; Aceves-Aldrete, C.E.; et al. Design of experiments for optimizing ultrasound-assisted extraction of bioactive compounds from plant-based sources. Molecules 2023, 28, 7752. [Google Scholar] [CrossRef]
- Albarri, R.; Şahin, S. Kinetics, thermodynamics, and mass transfer mechanism of the ultrasound-assisted extraction of bioactive molecules from Moringa oleifera leaves. Biomass Convers. Biorefinery 2023, 13, 7919–7926. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Bragagnolo, F.S.; Strieder, M.M.; Pizani, R.S.; de Souza Mesquita, L.M.; Gonzalez-Miquel, M.; Rostagno, M.A. Revisiting natural deep eutectic solvents (NADES) as extraction media and ready-to-use purposes. TrAC Trends Anal. Chem. 2024, 175, 117726. [Google Scholar] [CrossRef]
- Freitas, D.S.; Cavaco-Paulo, A.; Silva, C. Enhancing insights into the phenomena of deep eutectic solvents. Sustain. Mater. Technol. 2024, 41, e01039. [Google Scholar] [CrossRef]
- González-Campos, J.B.; Pérez-Nava, A.; Valle-Sánchez, M.; Delgado-Rangel, L.H. Deep eutectic solvents applications aligned to 2030 United Nations Agenda for Sustainable Development. Chem. Eng. Process. Process Intensif. 2024, 199, 109751. [Google Scholar] [CrossRef]
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs): Designer solvents for green extraction of anthocyanin. Sustain. Chem. Pharm. 2023, 34, 101168. [Google Scholar] [CrossRef]
- Sadrmousavi-Dizaj, A.; Xu, D.; Zhang, L.; Gao, J.; Ma, Y.; Wang, Y. Removal of phenolic compounds from aqueous solution using hydrophobic deep eutectic solvent: Liquid–liquid phase behavior and intermolecular interactions. J. Mol. Liq. 2024, 408, 125385. [Google Scholar] [CrossRef]
- Sportiello, L.; Favati, F.; Condelli, N.; Di Cairano, M.; Caruso, M.C.; Simonato, B.; Tolve, R.; Galgano, F. Hydrophobic deep eutectic solvents in the food sector: Focus on their use for the extraction of bioactive compounds. Food Chem. 2023, 405, 134703. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Zhang, Y.; Bi, W. Switching from deep eutectic solvents to deep eutectic systems for natural product extraction. Green Chem. Eng. 2024, 6, 36–53. [Google Scholar] [CrossRef]
- Wei, J.; Ge, H.; Zhu, B.; Xu, Y.; Wang, S.; Li, B.; Xu, H. Probing the interaction mechanism of choline chloride-based deep eutectic solvents with flavonoid and polyphenol systems. J. Mol. Liq. 2024, 408, 125310. [Google Scholar] [CrossRef]
- He, C.; Zhang, L.; Zhao, X.; Xin, J.; Li, C.; Li, C.; Zhang, X. Pretreatment of multiple lignocellulosic batches with recoverable and fermentable deep eutectic solvent (FDES) for co-production of sugar, lignin and lipid. Renew. Energy 2024, 229, 120745. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Dai, Y.; Jin, R.; Verpoorte, R.; Lam, W.; Cheng, Y.-C.; Xiao, Y.; Xu, J.; Zhang, L.; Qin, X.-M.; Chen, S. Natural deep eutectic characteristics of honey improve the bioactivity and safety of traditional medicines. J. Ethnopharmacol. 2020, 250, 112460. [Google Scholar] [CrossRef]
- Troter, D.; Todorović, Z.; Đokić-Stojanović, D.; Đordević, B.; Todorović, V.; Konstantinović, S.; Veljković, V. The physicochemical and thermodynamic properties of the choline chloride-based deep eutectic solvents. J. Serbian Chem. Soc. 2017, 82, 1039–1052. [Google Scholar] [CrossRef]
- Kočović, A.; Jeremić, J.; Bradić, J.; Sovrlić, M.; Tomović, J.; Vasiljević, P.; Anđić, M.; Draginić, N.; Grujović, M.; Mladenović, K.; et al. Phytochemical analysis, antioxidant, antimicrobial, and cytotoxic activity of different extracts of Xanthoparmelia stenophylla lichen from Stara Planina, Serbia. Plants 2022, 11, 1624. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.U.; Tabassum, F.; Khursheed, A.; Zaman, Q.U.; Bukhari, N.A.; Alfagham, A.; Hatamleh, A.A.; Chen, Y. Phytochemical profiling, antioxidant, anti-inflammatory, thrombolytic, hemolytic activity in vitro and in silico potential of Portulacaria afra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef]
- Masike, K.; Mhlongo, M.I.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, F.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Gong, X.; Zhou, X.; Zhao, Y.; Li, M. An UHPLC–MS/MS method for simultaneous quantification of gallic acid and protocatechuic acid in rat plasma after oral administration of Polygonum capitatum extract and its application to pharmacokinetics. J. Ethnopharmacol. 2015, 162, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Silva, C.T.; Pozo-Bayón, M.A.; Osorio, C. Targeted metabolomic analysis of polyphenols with antioxidant activity in sour guava (Psidium friedrichsthalianum Nied.) fruit. Molecules 2017, 22, 11. [Google Scholar] [CrossRef]
- Clifford, M.N.; Zheng, W.; Kuhnert, N. Profiling the chlorogenic acids of aster by HPLC–MSn. Phytochem. Anal. 2006, 17, 384–393. [Google Scholar] [CrossRef]
- Hernández, M.; Ventura, J.; Castro, C.; Boone, V.; Rojas, R.; Ascacio-Valdés, J.; Martínez-Ávila, G. UPLC-ESI-QTOF-MS2-based identification and antioxidant activity assessment of phenolic compounds from red corn cob (Zea mays L.). Molecules 2018, 23, 1425. [Google Scholar] [CrossRef]
- Petkovska, A.; Gjamovski, V.; Stanoeva, J.P.; Stefova, M. Characterization of the polyphenolic profiles of peel, flesh and leaves of Malus domestica cultivars using UHPLC-DAD-HESI-MSn. Nat. Prod. Commun. 2017, 12, 35–42. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D.; Verardo, G.; Gorassini, A.; Stocchi, V.; Sestili, P. Activity of Vitis vinifera tendrils extract against phytopathogenic fungi. Nat. Prod. Commun. 2015, 10, 1037–1042. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lee, S.H. Protocatechuic acid protects hepatocytes against hydrogen peroxide-induced oxidative stress. Curr. Res. Food Sci. 2022, 5, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-cancer effects of green tea epigallocatchin-3-gallate and coffee chlorogenic acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Cao, L.; Li, R.; Fang, X.; Miao, Y. Protective effect of chlorogenic acid on the focal cerebral ischemia reperfusion rat models. Saudi Pharm. J. 2017, 25, 556–563. [Google Scholar] [CrossRef]
- Jang, S.A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.H.; Koo, H.J.; Kang, S.C. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef]
- Zanello, P.R.; Koishi, A.C.; Rezende Júnior, C.O.; Oliveira, L.A.; Pereira, A.A.; de Almeida, M.V.; Duarte dos Santos, C.N.; Bordignon, J. Quinic acid derivatives inhibit dengue virus replication in vitro. Virol. J. 2015, 12, 223. [Google Scholar] [CrossRef]
- Ma, J.-N.; Ma, C.-M. Antifungal inhibitory activities of caffeic and quinic acid derivatives. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 635–641. [Google Scholar] [CrossRef]
- Omi, N.; Shiba, H.; Nishimura, E.; Tsukamoto, S.; Maruki-Uchida, H.; Oda, M.; Morita, M. Effects of enzymatically modified isoquercitrin in supplementary protein powder on athlete body composition: A randomized, placebo-controlled, double-blind trial. J. Int. Soc. Sports Nutr. 2019, 16, 39. [Google Scholar] [CrossRef]
- Owczarek-Januszkiewicz, A.; Magiera, A.; Olszewska, M.A. Enzymatically modified isoquercitrin: Production, metabolism, bioavailability, toxicity, pharmacology, and related molecular mechanisms. Int. J. Mol. Sci. 2022, 23, 14784. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxidative Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin improves anxiety and reserpine-induced depression in rats. Molecules 2022, 27, 7313. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Badwaik, H.; Choudhary, R.; Sakure, K.; Agrawal, Y.O.; Sharma, C.; Ojha, S.; Goyal, S.N. Therapeutic potential and pharmaceutical development of a multitargeted flavonoid phloretin. Nutrients 2022, 14, 3638. [Google Scholar] [CrossRef]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Ramniwas, S.; Vashishth, K.; Varol, M.; Jaswal, V.S.; Haque, S.; Sak, K. Phloretin, as a potent anticancer compound: From chemistry to cellular interactions. Molecules 2022, 27, 8819. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Pitaya: A potential plant resource of citramalic acid. CyTA J. Food 2020, 18, 249–256. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Mayer, H.K. A novel basis for monitoring the coffee roasting process: Isomerization reactions of 3-caffeoylquinic and 4-caffeoylquinic acids. LWT 2021, 137, 112343. [Google Scholar] [CrossRef]
- Vasyliev, G.; Lyudmyla, K.; Hladun, K.; Skiba, M.; Vorobyova, V. Valorization of tomato pomace: Extraction of value-added components by deep eutectic solvents and their application in the formulation of cosmetic emulsions. Biomass Convers. Biorefinery 2022, 12, 95–111. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press LLC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Yaws, C.L. Chemical Properties Handbook: Physical, Thermodynamic, Environmental, Transport, Safety, and Health Related Properties for Organic and Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Zengin, G.; Cádiz-Gurrea, M.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Carretero, A.S.; Momotko, M.; Yildiztugay, E.; Karatas, R.; Jugreet, S.; Mahomoodally, M.F.; et al. Selectivity tuning by natural deep eutectic solvents (NADESs) for extraction of bioactive compounds from Cytinus hypocistis—Studies of antioxidative, enzyme-inhibitive properties and LC-MS profiles. Molecules 2022, 27, 5788. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarraguça, M. A comprehensive review on deep eutectic solvents and its use to extract bioactive compounds of pharmaceutical interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef]
- Yi, L.; Wang, J.; Liu, J.; Luo, H.; Wu, X.; Li, W.Y. Extraction mechanism of phenolic compounds by a choline chloride/glycerol solvent: DFT and molecular dynamics studies. Phys. Chem. Chem. Phys. 2024, 26, 29140–29149. [Google Scholar] [CrossRef]
- Jiménez-Ortega, L.A.; Kumar-Patra, J.; Kerry, R.G.; Das, G.; Mota-Morales, J.D.; Heredia, J.B. Synergistic antioxidant activity in deep eutectic solvents: Extracting and enhancing natural products. ACS Food Sci. Technol. 2024, 4, 2776–2798. [Google Scholar] [CrossRef]
- Sombutsuwan, P.; Durand, E.; Aryusuk, K. Effect of acidity/alkalinity of deep eutectic solvents on the extraction profiles of phenolics and biomolecules in defatted rice bran extract. PeerJ Anal. Chem. 2024, 6, e29. [Google Scholar] [CrossRef]
- Voroshylova, I.V.; Ferreira, E.S.; Cordeiro, M.N. Influence of deep eutectic solvent composition on micelle properties: A molecular dynamics study. Molecules 2025, 30, 574. [Google Scholar] [CrossRef]
- Dimitriu, L.; Constantinescu-Aruxandei, D.; Preda, D.; Nichițean, A.L.; Nicolae, C.A.; Faraon, V.A.; Ghiurea, M.; Ganciarov, M.; Băbeanu, N.E.; Oancea, F. Honey and its biomimetic deep eutectic solvent modulate the antioxidant activity of polyphenols. Antioxidants 2022, 11, 2194. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, M.X.; Li, S.Y.; Su, J.; Wei, L.Y.; Yuan, Y.T.; Shu, P.H.; Tang, K. A green deep eutectic solvent-based aqueous two-phase system combined with chemometrics for flavonoids extracting and detecting in honey. Food Chem. X 2024, 24, 101932. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Luo, L.; Fan, W.; Qin, J.; Guo, S.; Xiao, H.; Tang, Z. Study on process optimization and antioxidant activity of polysaccharide from Bletilla striata extracted via deep eutectic solvents. Molecules 2023, 28, 5538. [Google Scholar] [CrossRef]
| NADES Name | Constituent Used for NADES Preparation | Molar Ratio | MNADES, g∙mol−1 | ρ, g cm−3 (20 °C) | η, mPa∙s (20 °C) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| Honey-mimicking | Glucose | Fructose | Sucrose | Water | 0.178:0.178:0.0146:1.72 | 99.9585 | 1.31 | 8990 |
| Reline | Choline chloride | Urea | - | - | 1:2 | 86.58 | 1.1829 | 3195 |
| Oxaline | Choline chloride | Oxalic acid· 2H2O | - | - | 1:1 | 132.845 | 1.120 | 330 |
| Glyceline | Choline chloride | Glycerol | - | - | 1:2 | 107.936 | 1.1951 | 490 |
| Experiment No. | Type of Herbal Powder | NADES Used for Pretreatment | Extracting Solvent |
|---|---|---|---|
| 1 | Crude | None | Distilled water |
| 2 | Crude | None | Aqueous 37% (v/v) ethanol |
| 3 | Crude | None | Aqueous 38% (v/v) glycerol |
| 4 | Crude | None | Aqueous 38% (v/v) propylene glycol |
| 5 | Pretreated | Honey-mimicking | Aqueous 38% (v/v) propylene glycol |
| 6 | Pretreated | Reline | Aqueous 38% (v/v) propylene glycol |
| 7 | Pretreated | Oxaline | Aqueous 38% (v/v) propylene glycol |
| 8 | Pretreated | Glyceline | Aqueous 38% (v/v) propylene glycol |
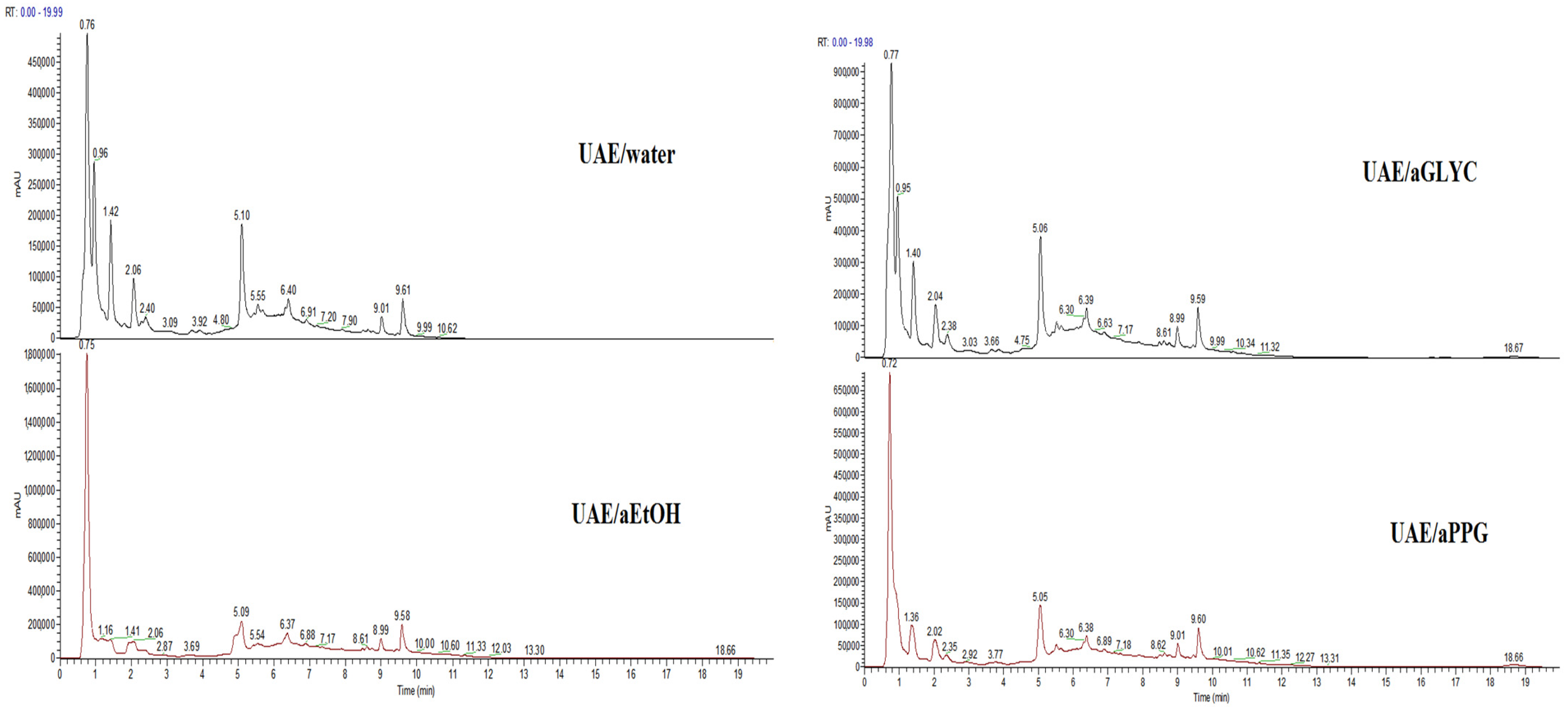
| Peak No. | tR, min | λmax, nm | Molecular Ion [M − H]− m/z | MS/MS Fragment Ions | UAE/ Water | UAE/ aEtOH | UAE/ aGLYC | UAE/ aPPG | Assignment | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.76 | - | 191 | 173, 129, 117, 85(100%), 73, 57 | + | + | + | + | Quinic acid | [33] |
| 2 | 0.78 | - | 195 | 129(100%) | + | + | + | + | - | - |
| 3 | 0.88 | - | 133 | 115(100%), 87 | + | + | + | + | Malic acid | Standard |
| 4 | 0.95 | - | 191 | 173, 111(100%), 99 | + | + | + | + | Citric acid | Standard |
| 5 | 1.04 | - | 179 | 161, 143, 131, 119, 113, 89(100%) | + | + | + | + | Glucose | MB:KO000804 |
| 6 | 2.14 | 220 261 297 | 153 | 109(100%) | + | + | + | + | Protocatechuic acid | [34] |
| 7 | 2.30 | 327 290sh | 353 | 191(100%), 179, 173 | + | + | + | + | Neochlorogenic acid | [35] |
| 8 | 3.60 | 329 290sh | 515 | 455, 425, 353(100%), 191, 179 | + | - | - | - | Dicaffeoyllquinic acid, isomer | [35] |
| 9 | 3.90 | 305 286sh | 337 | 267, 249(100%), 163 | + | + | + | + | 3-O-p-cumaroyl-quinic acid | [35] |
| 10 | 4.60 | - | 515 | 455, 425, 353(100%), 191 | + | + | + | + | Dicaffeoyllquinic acid, isomer | [35] |
| 11 | 5.00 | 325 303sh | 353 | 191(100%) | - | + | - | - | Chlorogenic acid, cis isomer | [35] |
| 12 | 5.10 | 325 303sh | 353 | 191(100%) | + | + | + | + | Chlorogenic acid | Standard |
| 13 | 5.40 | 278 | 577 | - | + | + | + | + | Procyanidin B2 | [36] |
| 14 | 5.60 | 325 303sh | 353 | 191(100%), 179, 173, 135 | + | + | + | + | Cryptochlorogenic acid, isomer | [35] |
| 15 | 6.45 | 313 296sh | 337 | 191, 173(100%), 163 | + | + | + | + | 4-O-p-coumaroyl-quinic acid | [37] |
| 16 | 6.73 | - | 563 | 517(100%) | + | + | + | + | Apigenin-penthosyl-hexoside | [38] |
| 17 | 8.15 | - | 447 | 285(100%) | - | + | + | + | Kaempferol glucoside/galactoside | [39] |
| 18 | 8.27 | - | 515 | 469, 435, 353(100%), 273, 167 | - | + | - | - | Dicaffeoyllquinic acid, isomer | [35] |
| 19 | 8.57 | 300 | 515 | 469(100%), 353 | + | + | + | + | Dicaffeoyllquinic acid, isomer | [35] |
| 20 | 8.69 | 258 356 | 463 | 301(100%) | + | + | + | + | Hyperoside | Standard |
| 21 | 8.87 | 258 356 | 463 | 301(100%) | + | + | + | + | Isoquercitrin | Standard |
| 22 | 8.86 | 258 355 | 609 | - | + | + | + | + | Rutin | Standard |
| 23 | 9.10 | 287 | 567 | 273(100%) | + | + | + | + | Phloretin pentosyl hexoside | [39] |
| 24 | 9.53 | 354 258 | 433 | 301(100%) | + | + | + | + | Quercetin pentoside | [39] |
| 25 | 9.65 | 287 | 435 | 273(100%) | + | + | + | + | Phloridzin | MB:BML0059328.4.2022 |
| 26 | 9.69 | - | 359 | - | + | + | + | + | Rosmarinic acid | Standard |
| 27 | 9.72 | 287 | 481 | 435(100%) | + | + | + | + | Derivative of phloridzin, tent. | |
| 28 | 9.90 | 258 352 | 447 | 301(100%)/300, 285/284 | - | + | + | + | Quercitrin | [40] |
| 29 | 10.88 | 257 372 | 301 | - | + | + | + | + | Quercetin | Standard |
| 30 | 11.49 | 268 317 | 593 | 447, 307, 285(100%) | + | + | + | + | Tiliroside | Standard |
| 31 | 11.63 | - | 273 | 167, 123 | - | + | + | + | Phloretin | [39] |
| 32 | 12.00 | - | 285 | - | - | + | - | - | Kaempferol | Standard |
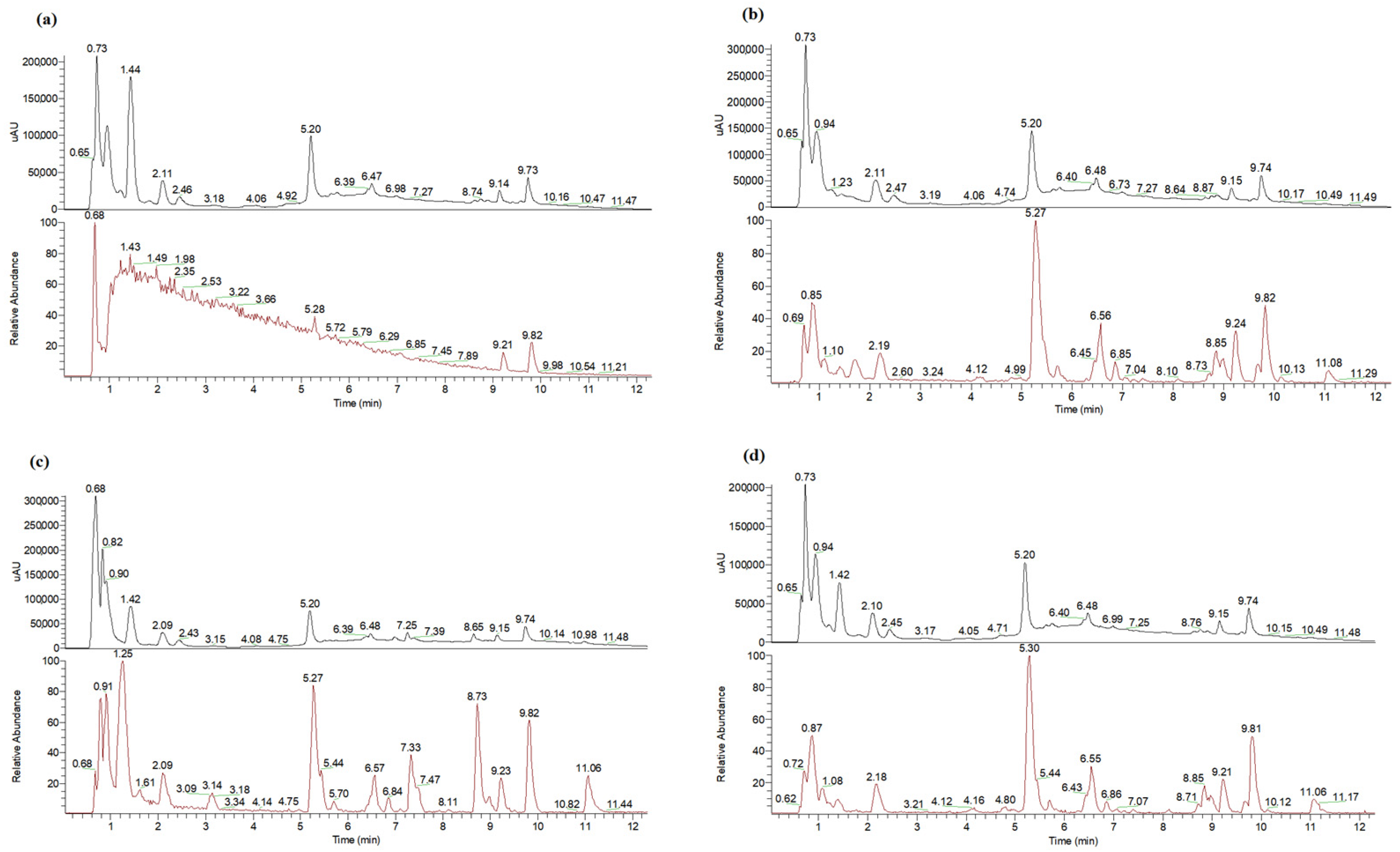
| Peak No. | tR, min | λmax, nm | Molecular Ion [M − H]− m/z | MS/MS Fragment Ions | NADES Used for Pretreatment | Assignment | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Honey | Reline | Oxaline | Glyceline | |||||||
| 1 | 0.76 | - | 191 | 173, 129, 117, 85(100%), 73, 57 | + | - | - | - | Quinic acid | [33] |
| 2 | 0.88 | - | 133 | 115(100%), 87 | + | + | + | + | Malic acid | Standard |
| 3 | 0.95 | - | 191 | 173, 111(100%), 99 | + | + | + | + | Citric acid | Standard |
| 4 | 1.04 | - | 179 | 161, 143, 131, 119, 113, 89(100%) | + | - | - | - | Hexose (glucose or galactose) | MB:KO000804 |
| 5 | 1.09 | - | 147 | 129(100%), 115, 87, 75 | - | + | + | + | Citramalic acid | PubChem:1081 |
| 6 | 1.25 | - | 191 | 173, 115(100%), 99, 71 | - | + | + | + | n.i. | |
| 7 | 1.55 | - | 179 | 161, 143(100%), 131, 119, 113, 89 | + | + | - | - | Hexose (glucose or galactose | MB:KO000804 |
| 8 | 1.60 | 285 | 249 | 173, 111(100%) | + | - | + | + | n.i. citric or quinic acid derivative | |
| 9 | 1.71 | - | 345 | 309, 266, 234, 192(100%) | - | + | - | - | n.i. | |
| 10 | 2.20 | 220 261 297 | 153 | 109(100%) | + | + | + | + | Protocatechuic acid | [34] |
| 11 | 2.46 | 327 290sh | 353 | 191(100%), 179, 173 | + | + | + | + | Neochlorogenic acid | [35] |
| 12 | 3.14 | 270 | 219 | 111(100%) | - | - | + | - | Ethyl citrate | [41] |
| 13 | 3.90 | 329 290sh | 515 | 455, 425, 353(100%), 191, 179 | + | + | - | + | Dicaffeoyllquinic acid, isomer | [35] |
| 14 | 4.10 | 305 286sh | 337 | 267, 249(100%), 163 | + | + | + | + | 3-O-p-cumaroyl-quinic acid | [35] |
| 15 | 4.90 | - | 515 | 455, 425, 353(100%), 191 | + | + | + | + | Dicaffeoyllquinic acid, isomer | [35] |
| 16 | 5.00 | 325 303sh | 353 | 191(100%) | + | + | + | + | Chlorogenic acid, cis isomer | [35] |
| 17 | 5.27 | 325 303sh | 353 | 191(100%) | + | + | + | + | Chlorogenic acid | Standard |
| 18 | 5.51 | 278 | 577 | - | + | + | - | + | Procyanidin B2 | [36] |
| 19 | 5.70 | 325 303sh | 353 | 191, 179, 173(100%), 135 | + | + | + | + | Cryptochlorogenic acid | [35] |
| 20 | 6.55 | 313 296sh | 337 | 191, 173(100%), 163 | + | + | + | + | 4-O-p-coumaroyl-quinic acid | [37] |
| 21 | 7.33 | 328 297 | 411 | 353, 191(100%), 179, 173, 161, 135 | - | + | + | - | Chlorogenic acid derivative | [35] |
| 22 | 7.40 | - | 483 | 437(100%), 305 | - | + | - | + | n.i. | |
| 23 | 8.13 | - | 619 | 583(100%), 289 | - | + | + | + | n.i. | |
| 24 | 8.37 | - | 447 | 285(100%) | + | + | + | + | Kaempferol glucoside/galactoside | [39] |
| 25 | 8.67 | - | 515 | 469, 435, 353(100%), 273, 167 | + | + | + | + | Dicaffeoyllquinic acid, isomer | [35] |
| 26 | 8.74 | 327 302 | 381 | 191, 179(100%), 173, 161, 135 | - | - | + | - | Quinic acid derivative | [35] |
| 27 | 8.86 | 258 356 | 463 | 301(100%) | + | + | + | + | Hyperoside | Standard |
| 28 | 8.90 | 258 356 | 463 | 301(100%) | + | + | + | + | Isoquercitrin | Standard |
| 29 | 9.09 | 258 355 | 609 | - | + | + | + | + | Rutin | Standard |
| 30 | 9.21 | 287 | 567 | 273(100%) | + | + | + | + | Phloretin pentosylhexoside | [39] |
| 31 | 9.23 | 354 258 | 433 | 301(100%) | + | + | + | + | Quercetin pentoside | [39] |
| 32 | 9.68 | 354 258 | 433 | 301(100%) | + | + | - | + | Quercetin pentoside | [39] |
| 33 | 9.81 | 287 | 435 | 273(100%) | + | + | + | + | Phloridzin | MB:BML0059328.4.2022 |
| 34 | 9.82 | 287 | 481 | 435(100%) | + | + | + | + | Derivative of phloridzin, tent. | |
| 35 | 9.89 | - | 359 | - | + | + | + | + | Rosmarinic acid | Standard |
| 36 | 9.90 | 258 352 | 447 | 301(100%)/300, 285/284 | + | + | + | + | Quercitrin | [40] |
| 37 | 11.08 | 257 372 | 301 | 179(100%), 175, 165, 151 | + | + | + | + | Quercetin | Standard |
| 38 | 11.58 | 268 317 | 593 | 447, 307, 285(100%) | + | + | + | + | Tiliroside | Standard |
| 39 | 11.72 | - | 273 | 167, 123 | + | + | + | + | Phloretin | [39] |
| 40 | 12.00 | - | 285 | - | - | - | + | - | Kaempferol | Standard |
| Extract from Experiment No. | NADES Used for Pretreatment | Extracting Solvent | TPC (mg GAE/g DAP) | TFC (mg QE/g DAP) | TFC/TPC Ratio (%) | DPPH (IC50 µg/g DAP) | ABTS (IC50 µg/g DAP) |
|---|---|---|---|---|---|---|---|
| 1 | None | Distilled water | 161.615 ± 0.385 a | 35.528 ± 0.735 a | 0.22 ± 0.004 c | 291.067 ± 9.025 g | 596.251 ± 24.372 f |
| 2 | None | Aqueous 37% (v/v) ethanol | 292.641 ± 0.588 c | 37.009 ± 0.321 a | 0.1265 ± 0.00084 a | 142.301 ± 3.564 d,e | 258.491 ± 8.392 e |
| 3 | None | Aqueous 38% (v/v) glycerol | 290.974 ± 0.588 c | 54.324 ± 0.893 b | 0.1867 ± 0.0027 b | 152.716 ± 4.07 e,f | 216.734 ± 8.024 d |
| 4 | None | Aqueous 38% (v/v) propylene glycol | 252.513 ± 0.222 b | 62.657 ± 0.160 c | 0.2481 ± 0.00042 d | 154.682 ± 4.006 e,f | 188.134 ± 5.859 d |
| 5 | Honey | Aqueous 38% (v/v) propylene glycol | 802.922 ± 1.444 d | 243.285 ± 1.352 d | 0.303 ± 0.00114 e | 171.420 ± 14.915 f | 276.536 ± 21.912 e |
| 6 | Reline | Aqueous 38% (v/v) propylene glycol | 975.655 ± 1.426 g | 590.271 ± 1.864 g | 0.605 ± 0.001 h | 27.915 ± 1.429 b | 33.824 ± 3.362 a,b |
| 7 | Oxaline | Aqueous 38% (v/v) propylene glycol | 894.421 ± 0.792 f | 451.682 ± 0.723 f | 0.505 ± 0.00036 g | 49.861 ± 3.742 c | 53.834 ± 4.213 b |
| 8 | Glyceline | Aqueous 38% (v/v) propylene glycol | 824.463 ± 0.815 e | 324.838 ± 1.567 e | 0.394 ± 0.0015 f | 131.412 ± 9.845 d | 137.627 ± 11.963 c |
| Ascorbic acid | 5.409 ± 0.576 a | 5.502 ± 0.626 a | |||||
| Trolox | 6.702 ± 0.689 a | 8.310 ± 0.876 a | |||||
| Property/Solvent | Glycerol | Ethanol | Propylene Glycol | Water | Reference | |
|---|---|---|---|---|---|---|
| Molar Mass, g·mol−1 | 92.09 | 46.07 | 76.09 | 18 | ||
| ρ, g·cm−3 | 20 °C | 1.2611 | 0.7893 | 1.0361 | 0.99821 | [58] |
| 25 °C | 1.257 | 0.787 | 1.033 | [59] | ||
| η, mPa·s | 20 °C | 1.203 | 1.002 | [58] | ||
| 25 °C | 934 | 1.074 | 40.4 | 0.89 | [58] | |
| 50 °C | 152 | 0.694 | 11.3 | 0.547 | [58] | |
| Partition coefficient (log P) a | –1.76 | –0.31 | -0.92 | [59] | ||
| –0.30 | [58] | |||||
| Dipole moment | 2.56 | 1.69 | 2.25 | 1.8546 | [58] | |
| 4.21 | 1.69 | 3.63 | [59] | |||
| Dielectric constant b | 46.53 | 25.3 | 27.5 | 80.2 | [58] | |
| pKa a | 14.15 | 15.5 | 14.8 | 14 | [58] | |
| Experiment No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Extracting solvent | Distilled water | Aqueous 37% (v/v) ethanol | Aqueous 38% (v/v) glycerol | Aqueous 38% (v/v) propylene glycol | Aqueous 38% (v/v) propylene glycol | Aqueous 38% (v/v) propylene glycol | Aqueous 38% (v/v) propylene glycol | Aqueous 38% (v/v) propylene glycol |
| NADES used for pretreatment | None | None | None | None | Honey | Reline | Oxaline | Glyceline |
| Mass of water (g) per g p.m. | 15 | 9.43 | 9.28 | 9.28 | 9.28 | 9.28 | 9.28 | 9.28 |
| Mass of organic solvent (g) per g p.m. | 0 | 4.38 | 7.19 | 5.91 | 5.91 | 5.91 | 5.91 | 5.91 |
| Mass of solvent system (g) per g p.m. | 15 | 13.81 | 16.47 | 15.19 | 15.19 | 15.19 | 15.19 | 15.19 |
| Specific heat capacity (kW·h/kg·K) | 0.0150 | 0.0163 | 0.0137 | 0.0148 | 0.0148 | 0.0148 | 0.0148 | 0.0148 |
| TPC (mg GAE/g DE) | 161.615 | 292.641 | 290.974 | 252.513 | 802.922 | 975.655 | 894.421 | 824.463 |
| TPC per kW·h (mg GAE/g DE/kW·h) | 2154.87 | 3901.88 | 3879.65 | 3366.84 | 10705.63 | 13008.73 | 11925.61 | 10992.84 |
| TPC per kW·h/K (mg GAE/g DE/kW·h/K) | 0.485 | 0.878 | 0.873 | 0.758 | 2.41 | 2.93 | 2.685 | 2.475 |
| TPC per kW·h·K (mg GAE/g DE/kW·h·K) | 53,842.04 | 97,493.35 | 96,937.99 | 84,124.71 | 267,493.46 | 325,039.46 | 297,976.36 | 274,669.85 |
| TFC (mg QE/g DE) | 35.528 | 37.009 | 54.324 | 62.657 | 243.285 | 590.271 | 451.682 | 324.838 |
| TFC per kW·h (mg QE/g DE/kW·h) | 473.71 | 493.45 | 724.32 | 835.43 | 3243.8 | 7870.28 | 6022.43 | 4331.17 |
| TFC per kW·h/K (mg QE/g DE/kW·h/K) | 0.107 | 0.111 | 0.163 | 0.188 | 0.73 | 1.77 | 1.36 | 0.98 |
| TFC per kW·h·K (mg QE/g DE/kW·h·K) | 11,836.15 | 12,329.5 | 18,098.04 | 20,874.18 | 81,050.4 | 196,648.8 | 150,477.9 | 108,219.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dončić, S.V.; Troter, D.Z.; Sovrlić, M.M.; Zdravković, N.D.; Kočović, A.G.; Milosavljević, M.N.; Stepovic, M.; Mrkalić, E.M.; Zvezdanović, J.B.; Ilić, D.P.; et al. Enhanced “Greener” and Sustainable Ultrasonic Extraction of Bioactive Components from Waste Wild Apple (Malus sylvestris (L.) Mill.) Fruit Dust: The Impact of Pretreatment with Natural Deep Eutectic Solvents. Analytica 2025, 6, 38. https://doi.org/10.3390/analytica6040038
Dončić SV, Troter DZ, Sovrlić MM, Zdravković ND, Kočović AG, Milosavljević MN, Stepovic M, Mrkalić EM, Zvezdanović JB, Ilić DP, et al. Enhanced “Greener” and Sustainable Ultrasonic Extraction of Bioactive Components from Waste Wild Apple (Malus sylvestris (L.) Mill.) Fruit Dust: The Impact of Pretreatment with Natural Deep Eutectic Solvents. Analytica. 2025; 6(4):38. https://doi.org/10.3390/analytica6040038
Chicago/Turabian StyleDončić, Slađana V., Dragan Z. Troter, Miroslav M. Sovrlić, Nebojša D. Zdravković, Aleksandar G. Kočović, Miloš N. Milosavljević, Milos Stepovic, Emina M. Mrkalić, Jelena B. Zvezdanović, Dušica P. Ilić, and et al. 2025. "Enhanced “Greener” and Sustainable Ultrasonic Extraction of Bioactive Components from Waste Wild Apple (Malus sylvestris (L.) Mill.) Fruit Dust: The Impact of Pretreatment with Natural Deep Eutectic Solvents" Analytica 6, no. 4: 38. https://doi.org/10.3390/analytica6040038
APA StyleDončić, S. V., Troter, D. Z., Sovrlić, M. M., Zdravković, N. D., Kočović, A. G., Milosavljević, M. N., Stepovic, M., Mrkalić, E. M., Zvezdanović, J. B., Ilić, D. P., & Konstantinović, S. S. (2025). Enhanced “Greener” and Sustainable Ultrasonic Extraction of Bioactive Components from Waste Wild Apple (Malus sylvestris (L.) Mill.) Fruit Dust: The Impact of Pretreatment with Natural Deep Eutectic Solvents. Analytica, 6(4), 38. https://doi.org/10.3390/analytica6040038







