Abstract
(1) Background: Caffeine is one of the most widely consumed psychoactive substances. Its safety profile and short half-life make it an ideal drug model for studying the pharmacokinetics of caffeine. This study aimed to develop a method for determination of caffeine in a small volume of saliva (200 µL). (2) Methods: Solid-phase extraction was employed to isolate caffeine from saliva, followed by quantitative analysis using liquid chromatography coupled with diode-array detection. Chromatographic separation was achieved on a C18 column, using a gradient mobile phase of acetonitrile and 0.1% formic acid. (3) Results: The method was validated for selectivity, linearity, precision, and accuracy. Linearity was established over the range of 10–10,000 ng/mL (R2 = 0.995). The coefficients of variation for intra- and inter-day precision for the three tested caffeine concentrations did not exceed 12.11%. Recovery from spiked saliva samples exceeded 90.53%. The developed method was applied to preliminary studies to follow the pharmacokinetics of caffeine in saliva. The concentration of the substance was studied in the saliva obtained from a volunteer after espresso consumption. (4) Conclusions: The developed method will offer a reliable approach for non-invasive caffeine monitoring in clinical and research applications.
1. Introduction
Caffeine (CAF) (1,3,7-trimethylxanthine), a methylxanthine derivative, is a natural alkaloid and well-known bioactive component of coffee. It is also found in tea, cocoa, energy drinks, and various other beverages, food, and pharmaceuticals (over-the-counter pain and weight-loss medications, numerous prescription drugs) [1,2,3]. Globally, approximately 80% of adults consume caffeinated products daily, though the average intake differs significantly across countries. These variations are influenced by cultural preferences and the types of caffeinated beverages popular in each region [4].
In pure form, CAF is a very bitter tasting, odorless, white powder. It is not acidic but can be protonated as the other methylxanthines, pKa = 10.4 at 40 °C. CAF is a neutral lipophilic molecule that penetrates readily from the blood into the saliva, independent of flow rate and salivary pH [5,6,7].
CAF is rapidly and completely absorbed in humans, with 99 percent being absorbed within 45 min of ingestion. Faster absorption of CAF can be achieved through products like chewing gum, which allow for absorption via the oral mucosa [8]. After CAF is absorbed, approximately 10–35% of CAF is reversibly bound to plasma proteins like albumin, while the rest of the CAF molecules are rapidly distributed to all body tissues and organs, CAF is characterized as an amphiphilic molecule, which makes CAF pass through all biological membranes, including the blood–brain barrier and the placental barrier. Oral administration of caffeine in humans is not subject to a significant first-pass effect [6,9,10,11,12]. After oral administration, peak plasma levels of caffeine are typically observed at a Tmax ranging between 30 and 120 min [13]. A dose of caffeine at 1 mg/kg, roughly equivalent to a standard cup of coffee, results in peak plasma concentrations (Cmax) of 1 to 2 mg/L (5 to 10 μmol/L). Higher doses, between 5 and 8 mg/kg, produce Cmax values of 8 to 10 mg/L (40 to 50 μmol/L) [12]. The average half-life (t1/2) of caffeine in adults typically ranges from 4 to 6 h but can extend from 1.5 to 9.5 h. Factors such as pregnancy may prolong the half-life, while cigarette smoking can reduce it [8,13].
CAF is metabolized by multiple enzymes, but the major metabolite paraxanthine (81.5%) is obtained solely through the CYP1A2 pathway. Other CAF metabolites are metabolized through both the CYP1A2 and CYP2E1 pathways [14,15,16]. Less than 2% of caffeine is excreted unchanged in urine. Its half-life ranges from 3 to 6 h in adults, but is significantly prolonged in caffeine-naive individuals and neonates, with neonates exhibiting an average half-life of 100 h [12]. CAF impacts the body through multiple biochemical pathways and mechanisms, varying with concentration levels. At typical consumption levels, caffeine primarily acts by blocking adenosine receptors. This action enhances alertness, reduces fatigue, and stimulates the central nervous system. Additionally, caffeine inhibits the phosphodiesterase enzyme, which raises cyclic adenosine monophosphate (cAMP) levels. Elevated cAMP promotes fat breakdown, improves cardiac output, and boosts exercise performance. At higher concentrations, caffeine facilitates calcium release from intracellular stores, increasing the strength and endurance of cardiac and skeletal muscles. In rare cases, toxic levels of caffeine may antagonize γ-aminobutyric acid type A (GABA-A) receptors. These mechanisms collectively enhance wakefulness, cognitive performance, and physical stamina. The pharmacological effects of caffeine are typically activated at blood concentrations below 100 µM [6,10,17].
The FDA (Food and Drug Administration) recognizes CAF as generally safe when used in cola beverages and stimulant drugs. CAF contained in over-the-counter analgesics is limited to a dose of 64–65 mg per tablet, though two tablet doses are common, and it isgenerally well-tolerated but may cause mild side effects. While CAF can increase gastric acid production and potentially raise the risk of ulcers, the evidence remains mixed. It is effective in treating migraines but may also be linked to withdrawal headaches. In order to avoid any adverse effects, it is recommended for a healthy adult to limit daily caffeine intake to 400 mg (about 5.7 mg/kg), children should not ingest more than 2.5 mg/kg body weight and infants should only ingest controlled low doses in the range of 5–10 mg/kg/day for medical use. A blood concentration of 80 mg/mL of CAF is reported to be lethal for adults [1,3,4,6,18,19].
Caffeine can be used therapeutically combined with analgesics like aspirin and paracetamol to enhance pain relief. This enhancement is likely due to caffeine’s ability to promote faster absorption of these drugs by lowering gastric pH, increasing drug uptake rates. CAF has been used to treat a wide variety of clinical diseases, including atopic dermatitis, minimal brain dysfunction in children and apnea in preterm infants since the 1970s [1,3,6,20]. CAF has neuroprotective effects that may help prevent Alzheimer’s and Parkinson’s diseases. In Alzheimer’s, CAF reduces β-amyloid plaques by lowering beta and gamma secretase levels in the hippocampus and inhibiting peptide aggregation through hydrophobic interactions. For Parkinson’s, CAF blocks the adenosine A2A receptor, enhancing dopamine release and potentially reducing motor decline [6]. Many studies indicate that caffeine consumption may contribute to reductions in body weight, Body Mass Index (BMI), and body fat [21].
CAF’s safety, well-documented pharmacokinetics, global prevalence, ability to be measured in multiple biological matrices, well-known metabolites, and short half-life make CAF ideal as a drug model to study the pharmacokinetics using a small volume of saliva. However, inter-individual variability presents a significant challenge, potentially affecting the consistency of results across different subjects [18].
Several studies have shown that CAF concentrations in saliva closely correlate with those in plasma, with a correlation coefficient of 0.87 (p < 0.001) [5] and 0.981 [22]. These findings suggest that saliva can be used as a reliable alternative to blood for monitoring CAF intake, especially when non-invasive collection of the sample is required.
Methods for the determination of CAF include separation techniques like liquid chromatography (LC) [5,14,21,22,23,24,25,26,27,28,29] and gas chromatography (GC) [30] among others. LC methods with UV detection have been successfully applied for the determination of CAF in a wide range of biological samples such as blood [5,22,23,25,27], saliva [5,14,21,22,23,25,26,27,28,29,30], and urine [23,24,27]. Caffeine detection was also performed using MS/MS [23,31].
CAF has generally been isolated from saliva using liquid–liquid extraction (LLE) [5,14,25,32,33], solid-phase extraction (SPE) [28,29], solid phase microextraction (SPME) [27], and precipitation [26]. LLE is common method for isolating caffeine from saliva; however, it typically requires large volumes of toxic solvents, like ethyl acetate [5,14,25,31] or a mixture of chlorophorm and isopropanol [33]. In contrast, SPE reduces both the sample and solvent volumes needed for analysis. An essential advantage of SPE is the large variety of available sorbents, which allows better matching of phase for the analytes [34].
Previously developed methods that used SPE to isolate caffeine required relatively large sample volumes, from 1 to as much as 3 mL of saliva. Such a volume can be cumbersome to collect in elderly people or those suffering from dry mouth. Therefore, the aim of this study was to develop an effective method for the determination of CAF in a small volume (200 μL) of saliva. The developed method is a less invasive diagnostic tool that can also be used for routine caffeine level testing and for pharmacokinetics studies requiring frequent sampling. To this end, we investigated changes in salivary caffeine levels after a volunteer consumed espresso and then estimated within-person variability.
2. Materials and Methods
2.1. Chemicals and Solvents
CAF was purchased from Amara (Krakow, Poland). The internal standard (IS) theophylline was purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol and acetonitrile of HPLC gradient grade were obtained from POCh (Gliwice, Poland). Reagent-grade formic acid (98–100%) and ammonia solution (NH4OH) were purchased from POCh (Gliwice, Poland), and potassium hydrogen phosphate anhydrous (K2HPO4) from (Chempur, Poland). Water was purified by Ultra-Toc/UV system, Hydrolab (Straszyn, Poland). For solid-phase extraction columns EVOLUTE® EXPRESS ABN cartridge (1 mL/30 mg) were purchased from Biotage (Hengoed, UK).
2.2. LC-DAD Conditions
Chromatographic analysis was performed using a Nexera XR UHPLC equipment (Shimadzu, Kyoto, Japan) comprising a CBM-20 Atile control system, LC-30AD pump, CTO-20AC thermostat, SIL-30AC autosampler, SPD-M30A UV-VIS detector with diode array (DAD), and an SPD-M30A high-sensitivity measuring cell (85 mm). Chromatographic separation was performed using Nucleosil column (C18; 125 mm × 4 mm, 5 μm; Knauer, Berlin, Germany) with precolumn, maintained at 35 °C, and at 10 °C for autosampler. Acetonitrile and 0.1% formic acid in purified water for HPLC were used for the mobile phase, at a flow rate of 0.9 mL/min, as a gradient. The total run time of the analysis was 12 min.
2.3. Preparation of Quality Control (QC) and Standard Solutions
To prepare the calibrators and the quality control (QC) samples, a stock solution of CAF at a concentration of (1 mg/mL) and an internal standard (IS), theophylline (1 mg/mL) were prepared by dissolving each compound in methanol. Working solutions of the analytes were made by diluting the stock solutions in methanol. The IS working solution was prepared at 100 μg/mL by diluting 100 μL of the stock solution with 900 μL of methanol. To prepare the calibration standard for CAF at 100 μg/mL, 100 μL of the 1 mg/mL CAF stock solution was mixed with 900 μL of methanol. Further dilutions were made to achieve concentrations of 10 μg/mL, 1 μg/mL, 100 ng/mL, and 10 ng/mL by combining 100 μL of the respective solutions with 900 μL of methanol. All solutions were stored at −21 °C.
2.4. Collection of Blank Saliva Samples
Blank saliva for calibration and quality control was collected after a 72 h abstinence from CAF-containing products into 5 mL plastic tubes by spitting. Blank saliva samples were stored at −21 °C.
2.5. Extraction of Caffeine from Saliva Samples
A total of 200 μL of blank saliva was transferred into plastic tubes, diluted with 0.5 mL of 0.1 M HCl solution, and mixed. Next, appropriate amounts of CAF and IS were added, and the samples were vortexed again. Then the samples were loaded onto SPE columns, which had been pre-conditioned with 0.5 mL of methanol and 0.5 mL of 0.1 M HCl. The cartridges were washed with 0.5 mL of 0.1 M HCl, and 0.5 mL of the mixture of methanol and water (10:90, v/v) and dried for 5 min. The analytes were then eluted with 0.5 mL of a mixture of ammonia and methanol (5:95, v/v). The eluents were dried at 37 °C under air stream, and the residues were reconstituted in 100 μL of a solution consisting of acetonitrile and 1% formic acid (1:9, v/v), vortex, and transferred to autosampler vials. Finally, 10 μL of the sample was injected into the chromatographic column.
2.6. Preparation of Calibration and Quality Control Samples
For the calibration samples, 200 μL of blank saliva was spiked with 200 μL of 0.1 M HCl, along with 30 μL of the 100 μg/mL IS working solution and CAF working solutions at concentrations of 10 μg/mL, 1 μg/mL, 100 ng/mL, and 10 ng/mL, corresponding to final CAF concentrations of 10, 50, 100, 800, 2000, 6000, and 10,000 ng/mL.
QC samples were prepared by spiking 200 μL of blank saliva with 200 μL of 0.1 M HCl, along with 30 μL of the IS working solution and CAF working solutions at 10 μg/mL and 100 ng/mL, resulting in final CAF concentrations of 30, 4000, and 8000 ng/mL.
2.7. Validation
The validation procedure followed the European Medicines Agency (EMA) guidelines [35].
2.7.1. Linearity
Linearity was evaluated by preparing four calibration curves (ranging from 10 to 10,000 ng/mL) over four separate days. To meet the acceptance criteria, the coefficient of determination (R2) had to be ≥0.99, and coefficient of variation (CV) at each concentration level had to be ≤15%, except at the lower limit of quantification (LLOQ), where CV could be ≤20%.
2.7.2. Intra- and Inter-Day Precision
The precision of the method was tested at three concentration levels (30, 4000, and 8000 ng/mL). For intra-day precision, each concentration was analyzed in five replicates within a single day. Inter-day precision was determined by analyzing each concentration five times over four consecutive days (n = 20). Method precision was evaluated by calculating the coefficient of variation for both within-day and between-day measurements, with acceptable imprecision set at CV ≤ 15%.
2.7.3. Lower Limit of Quantification (LLOQ)
The lower limit of quantification was defined as the minimum concentration that could be measured with acceptable precision (CV% < 20%) and accuracy (within ±20% of the target concentration). The LLOQ was assessed by analyzing five replicates at the lowest concentration level.
2.7.4. Selectivity
The selectivity of the method was evaluated by ensuring the absence of endogenous interfering substances. To achieve this, blank saliva samples from 10 different donors were processed according to the extraction procedures described in Section 2.5 and analyzed using the chromatographic conditions detailed in Section 2.2. The method was considered selective if no interfering peaks were detected in the chromatograms of these blank samples.
2.7.5. Extraction and Absolute Recovery
Recovery of the method was assessed at three concentration levels (30, 4000, and 8000 ng/mL). Each concentration in a particular study was analyzed six times using separate solutions. Absolute recovery was determined by comparing the peak areas of the extracted analytes with those of six neat standards. Extraction recovery, on the other hand, was evaluated by comparing the peak areas of the extracted analyte with those from ten blank samples that were spiked post-extraction. In both assessments, results were considered acceptable if they exceeded 50% of the average value of either the neat standards or the post-spiked samples at each concentration level.
2.7.6. Stability
CAF stability was assessed at three different concentrations (30, 4000, and 8000 ng/mL). This was performed by evaluating its stability through a freeze–thaw test on spiked saliva samples that had been stored at −21 °C, as well as by examining the stability of CAF extracts in an autosampler at 10 °C for 72 h. For both tests three saliva samples of 800 µL at proper concentration (30, 4000, and 8000 ng/mL) were prepared in plastic tubes, spiked with the appropriate amount of CAF, and mixed. From each sample, 200 µL of saliva was taken, and IS was added, and extraction and chromatography were performed as described in Section 2.2 and Section 2.5. The resulting CAF extracts were stored in the autosampler at 10 °C for 72 h, while the remaining sample volume was returned to −21 °C for storage and re-analyzed over three freeze–thaw cycles on subsequent days, with daily calibration preparation. CAF stability was confirmed if concentration changes were within 15% under these storage conditions.
2.8. Application of the Analytical Method and Statistical Analysis of the Results
The developed method was used to determine CAF in saliva from a volunteer after consuming an espresso. The study protocol was approved by the ethical committee of the Medical University of Gdansk, Poland (KB/448/2023; 16 October 2023). The volunteer was instructed to abstain from all CAF-containing products for 72 h prior to the experiment. A blank saliva sample was collected from the volunteer by spitting into a plastic tube at time zero, just before consuming the espresso. After drinking a cup of espresso (50 mL), the volunteer rinsed his mouth thoroughly with tap water for 3 min. Saliva samples were then collected at specific intervals by spitting into plastic tubes at 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, and 180 min after consuming the espresso. All samples were centrifuged at 8000 rpm for 5 min, then 200 μL was transferred into a plastic tube, spiked with 200 μL of 0.1 M HCl and 30 μL of the IS (100 μg/mL), and followed by extraction and chromatography analysis, as outlined in Section 2.2 and Section 2.5. This experiment was carried out three times, and the average caffeine concentration for each time point was calculated, which was then plotted as a function of time.
2.9. Statistical Analysis
Statistical evaluation of the obtained results was performed using the Statistica package (13.3). To determine the intra-individual variability of CAF concentrations after the consumption of espresso with a similar compound content, the Wilcoxon test was performed, and the level of significance was set at p < 0.05.
3. Results
3.1. Optimization of the Separation and Extraction Processes
To optimize the LC procedure, both the composition and flow rate of the mobile phase were selected. Various conditions of mobile phase composition and flow rates were systematically studied to identify the optimal parameters. Finally, the following gradient was selected for chromatographic analysis: up to minute 8, the concentration of acetonitrile (B) increased linearly from 10 to 60%. between minutes 8 and 9, the concentration of solvent B increased to 90%. From minute 9 to 9.20, solvent B decreased to a concentration of 10%. It then remained at 10% until minute 12. Total chromatographic analysis time was 12 min and the detection was carried out using DAD, at λ = 275 nm, which was selected on the basis of the absorption spectrum. Theophylline was selected as the internal standard (IS) due to its similar physicochemical properties. The chromatogram for the standard solution, obtained under optimized conditions, is presented in Figure 1.
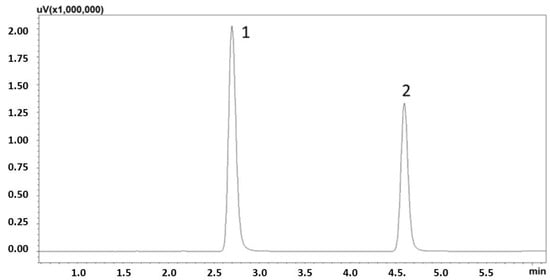
Figure 1.
Chromatogram of the standard solutions obtained by optimized LC. (1) theophylline (IS); (2) caffeine, λ = 275 nm.
The extraction process was optimized through several stages. Initially, a protocol was developed to purify blank saliva samples to maximize the removal of interfering substances from the biological matrix. To achieve this goal, saliva samples were diluted with 0.1 M HCl, shaken, and then loaded onto activated SPE columns. Three types of solid-phase extraction cartridges (Strata-X, Strata-X-CW, and EVOLUTE® EXPRESS ABN) were evaluated using different procedures outlined in Table 1.

Table 1.
Procedures used to examine the efficacy of SPE columns.
For Strata-X, the analyte was extracted using redistilled water, and a vacuum was applied to collect the extract. For Strata-X-CW, after sample loading, the cartridge was washed with a 100 mM K2HPO4 solution followed by methanol. The sorbent was then dried under vacuum for 5 min before eluting the analyte using 5% formic acid in methanol. For the EVOLUTE® EXPRESS ABN, after loading the saliva samples, the cartridge was washed with 0.1 M HCl followed by methanol or 0.1 M HCl and a mixture of methanol and water (10:90, v/v). The sorbent was then dried under vacuum for 5 min, and the analyte was eluted using 5% ammonia in methanol solution. To assess the performance of the SPE sorbents, calibration curves were prepared and linearity assessed, and the procedures were then applied to isolate CAF and IS from CAF-loaded saliva samples so that the final analyte concentrations were 30, 4000, and 8000 ng/mL, respectively. Three curve analysis was performed for each sorbent type.
The results of procedures (1) and (2) showed that the linearity was not sufficient because of the coefficient of determination (R2 < 0.99), while with procedure (3) the linearity was sufficient (R2 > 0.99). However, the results with the procedure (3) showed inconsistencies in extraction efficiency. The chromatographic analysis of the eluates revealed losses of caffeine during the methanol washing stage. Taking into account these observations and the physicochemical properties of caffeine, in procedure (4) a mixture of methanol and water (10:90, v/v) was used in the second washing step, and then the compounds were eluted with 5% ammonia in methanol. The results with the procedure (4) were sufficient. Figure 2 shows chromatograms of a blank saliva sample extract and samples containing different concentrations of CAF and IS using an EVOLUTE® EXPRESS ABN cartridge with procedure (4).
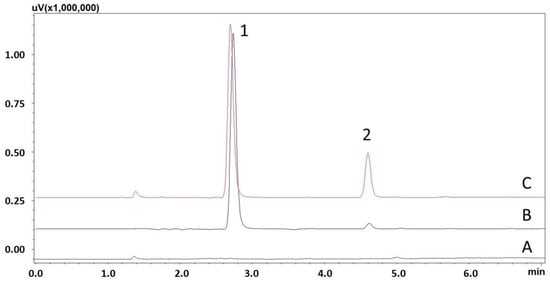
Figure 2.
Chromatograms of saliva extracts using EVOLUTE® EXPRESS ABN sorbent: A—blank sample; B—spiked with caffeine (2) (800 ng/mL) and IS (1) (15,000 ng/mL); C—spiked with caffeine (2) (6000 ng/mL) and IS (1) (15,000 ng/mL).
3.2. Method Validation
The method’s linearity was evaluated within the range of 10–10,000 ng/mL, and the results confirmed its compliance with the predefined acceptance criterion (R2 = 0.995 ± 0.0051, Table 2). Additional information on the average calibration curve, concentration range, and their range of variation is presented in Figure S1 and Table S1. The lower limit of quantification for CAF was determined to be 10 ng/mL. Figure 3 shows the chromatograms of blank saliva samples and samples spiked with 10 ng/mL caffeine, which is an LLOQ for the method. As shown in Table 3, the validation parameters indicated that the method demonstrated a high level of precision, with CV ranging from 1.52% to 12.11% for both intra- and inter-day precision.

Table 2.
Calibration curve parameters for the developed method.
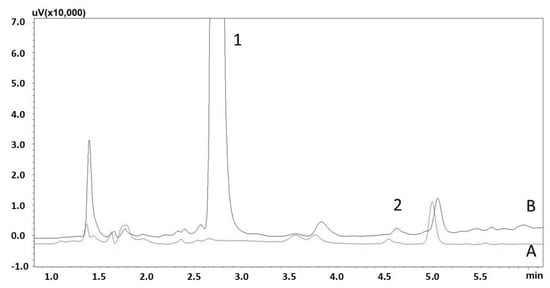
Figure 3.
Chromatograms of saliva extracts A—blank sample; B—spiked with caffeine (2) (10 ng/mL) and IS (1) (15,000 ng/mL).

Table 3.
Validation parameters for the developed method. Stability of CAF at three QC concentrations after storage at 10 °C and −21 °C, expressed as % of loss.
The selectivity was assessed by analyzing 10 blank saliva samples, revealing no interfering peaks from matrix components near the retention times of the analyte and internal standard. Consequently, the method was considered selective. Chromatograms showing extracts from 10 blind saliva samples, based on which we concluded the selectivity of the method, are presented in Figure S2.
The extraction recovery was evaluated at three QC concentrations (30, 4000, and 8000 ng/mL). The recovery rates ranged between 90.53% and 97.37%. The absolute recovery values ranged from 83.77% to 90.58% (Table 3), which met the acceptance criteria (>50%) for all concentrations tested. The stability study of CAF showed that it is stable under the tested storage conditions, with minimal loss of concentration over the evaluated time periods. Detailed test results are presented in Table 3.
3.3. Application of the Method and Statistical Analysis of the Results
To determine within-person variability, saliva samples were collected three times from healthy volunteers at appropriate intervals for a period of three hours after consuming a cup of espresso, with a caffeine content between 167 and 180 mg/cup. The developed method successfully quantified CAF in all samples. The determined salivary CAF concentration ranged between 1427.5 and 5543.0 ng/mL. A representative chromatogram of the saliva extract, taken 45 min after espresso consumption, is shown in Figure 4.
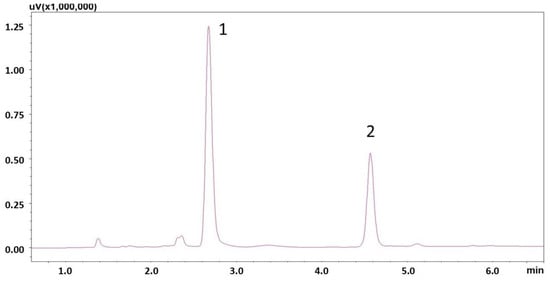
Figure 4.
Chromatogram of saliva extract of a volunteer after 45 min of espresso consumption (CAF concentration—2683 ng/mL), (1) IS (2) caffeine.
The changes in salivary CAF concentration profiles over time, with data presented as mean ± standard deviation (n = 3) Figure 5. The detailed concentration values are summarized in Table S2. The Wilcoxon test showed no statistically significant differences in salivary caffeine concentrations at the same time points, in samples taken on different days. It was also found that the highest caffeine concentration in saliva was reached 75 min after espresso consumption.
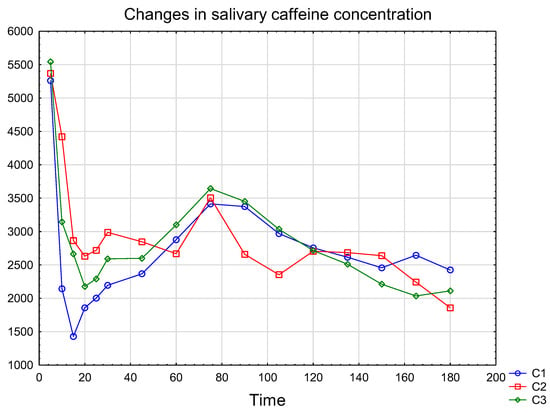
Figure 5.
Mean salivary CAF concentration over 3 h after consuming a cup of espresso by a 39-year-old male volunteer (n = 3). C1, C2, C3—salivary CAF concentration (ng/mL) measured on different days.
4. Discussion
The aim of this study was to determine the CAF in small volume of saliva (200 µL) using SPE and LC with DAD detection. Previous studies have proven that there is a strong correlation between the concentration of CAF in saliva and plasma [5,22]. Therefore, we decided to determine CAF in saliva, which allows for non-invasive sampling. We selected theophylline as the IS. In order to confirm or exclude the presence of theophylline in saliva and its potential impact on test results, in addition to analyzing blind samples, we determined the average surface area of the analyzed samples loaded with IS (Table S3).
Therefore, we decided to determine CAF in saliva, which allows for non-invasive sampling. Before proceeding to the chromatographic analysis, saliva requires a suitable procedure for the isolation of the compound. LLE is the most widely used method for isolating CAF from saliva. The use of SPE in this developed method will reduce the use of large amounts of toxic volatile solvents, whose use is required during LLE. As well as allowing the use of a small sample volume. The SPE was performed by EVOLUTE® EXPRESS ABN columns (30 mg/1 mL), which allowed for efficient isolation of CAF and IS from the biological matrix. To our knowledge, this study is the first one that uses only (200 μL) of salvia for SPE. In the study by Napierała and Florek [28], SPE was employed to isolate CAF from saliva using DSC-18 columns from SupelcoVisiprep™. For the extraction, 1 mL of saliva was collected from each patient. Leodori et al. [29] conducted a study to explore salivary CAF as a potential biomarker for Parkinson’s disease. They used an Oasis HLB 96-well plate (30 mg/well) for the SPE, with 500 µL of saliva. The use of a small volume of saliva will allow the determination of CAF even in patients suffering from dry mouth, having difficulty spitting out a large volume of saliva, as well as in children.
The developed method has a high precision of determination, as evidenced by low CV values below 15%. It also showed accuracy, in which recoveries exceeded 90.53%. In addition, it proved the stability of CAF in saliva (storage at −21 °C) and the stability of its extracts in autosampler (10 °C) for 72 h. The developed method demonstrated strong linearity over a wide concentration range (10–10,000 ng/mL; R2 = 0.995) with LLOQ of 10 ng/mL. Previous studies using SPE for CAF extraction combined with HPLC demonstrated linearity ranges of 50–2000 ng/mL (R2 = 0.9991) and 75–25,000 ng/mL, with LOQs of 40 ng/mL and 250 ng/mL, respectively [28,29]. The range used in this study was carefully selected based on multiple factors: firstly, CAF’s pharmacokinetics and physicochemical properties; secondly, the maximum daily dose of 400 mg and maximum single dose of 200 mg for healthy adults [18,19]; thirdly, the previous studies. Liguori et al. reported average peak salivary CAF levels of 9.7 ± 1.2 µg/mL for coffee, 9.8 ± 0.9 µg/mL for cola, and 7.8 ± 0.6 µg/mL for capsules after administering 400 mg of caffeine via these forms [30]. Finally, it was confirmed the used range which corresponded to levels found in saliva after consuming a cup of espresso in this study.
The developed method was applied to measure CAF concentrations over three hours following the consumption of an espresso (175 ± 7.7 mg of CAF in the cup), with samples collected at various time points. The initial two measurements revealed CAF concentrations that remained in the oral cavity after ingestion, even after thoroughly rinsing the mouth with water for several minutes. This finding is particularly significant for future studies involving CAF release into saliva after ingestion, as it sheds light on the time CAF remains in the mouth before it is absorbed, enters the bloodstream, and is secreted into saliva again. The Tmax was observed at 75 min, with a medium Cmax about 3522 ng/mL, consistent with findings reported in previous studies [12,13]. Other studies revealed that administering a 400 mg caffeine dose to 13 participants yielded salivary Tmax values of 42 min for coffee, 39 min for sugar-free cola, and 67 min for capsules [30]. Which may confirm that the absorption of CAF is strongly influenced by the presence of other food components in the stomach [8]. Furthermore, we determined within-person variability by statistically assessing CAF concentrations after saliva collection at the respective time points, on three different days. Saliva samples were collected from the same individual, after drinking a specific volume of espresso, with similar CAF content. Each time, the volunteer was required to abstain from caffeinated foods and beverages for 72 h before drinking the espresso. Statistical evaluation showed little within-person variability, and the determined concentrations were not statistically significantly different from one another. This allows us to conclude that the procedure developed for the preparation of the test subject and the method itself for the determination of CAF in saliva is reliable and valid. And also, that the developed method will have a great potential application in pharmacokinetic studies of CAF where frequent samples at multiple time points are necessary. To our knowledge, this will be the first study that used SPE for isolating CAF from saliva in order to study CAF’s pharmacokinetics.
5. Conclusions
In this study, a simple and efficient method for isolating CAF from saliva using solid-phase extraction was successfully developed and optimized. Among the tested procedures, the most effective involved diluting the sample with 0.1 M HCl, followed by washing the sorbents with 0.1 M HCl and a methanol-water mixture (1:9, v/v), and eluting with 5% ammonia in methanol after 5 min of drying. This method requires small volume saliva of (200 µL) and LC with DAD were used for quantitative analysis. The developed method demonstrated good linearity in the range of 10–10,000 ng/mL (R2 > 0.995 ± 0.0051), high precision, with a maximum coefficient of variation of 12.11%, and good accuracy with recovery ≥ 90.53%. For the stability of CAF in saliva after freeze–thaw cycles at −21 °C and the stability of CAF extracts stored at 10 °C for 72 h in an autosampler, the results showed that CAF remained stable under the storage conditions tested. Furthermore, the developed method was successfully applied to determine CAF concentrations in a volunteer’s saliva. Given its efficiency with small sample volumes, this method will offer a reliable, non-invasive diagnostic tool for pharmacological studies that require frequent sampling, such as pharmacokinetics studies. This eliminates the need for uncomfortable interval blood draws, making it more convenient for volunteers or patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica6040040/s1. Figure S1: Calibration plot for determination of caffeine showing the normal range of variation for each concentration level using vertical bars of variation; Figure S2: Chromatograms of extracts from 10 blind saliva samples, on the basis of which the selectivity of the method was determined. The arrows indicate the retention times corresponding to theophylline (1) and caffeine (2); Table S1: Results of the analysis of 4 series of measurements of caffeine calibration curves in saliva; Table S2: Volunteer data showing CAF concentrations in saliva at various time points (n = 3). C1; C2, C3—salivary CAF concentration (ng/mL) measured on different days; Table S3: Mean value of the IS peak areas obtained in samples
Author Contributions
Conceptualization, E.D.; methodology, E.D.; validation, S.A.; formal analysis, S.A.; investigation, S.A.; data curation, E.D.; writing—original draft preparation, S.A.; writing—review and editing, E.D.; supervision, E.D.; project administration, E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAF | caffeine |
| GABA-A | γ-aminobutyric acid type A |
| FDA | Food and Drug Administration |
| LC | liquid chromatography |
| GC | gas chromatography |
| LLE | liquid–liquid extraction |
| SPE | solid-phase extraction |
| SPME | solid phase microextraction |
| QC | quality control |
| IS | internal standard |
| EMA | European Medicines Agency |
| CV | coefficient of variation |
| LLOQ | lower limit of quantification |
| DAD | diode array detector |
| R2 | determination coefficient |
References
- Oliphant, E.A.; Hanning, S.M.; McKinlay, C.J.D.; Alsweiler, J.M. Caffeine for apnea and prevention of neurodevelopmental impairment in preterm infants: Systematic review and meta-analysis. J. Perinatol. 2024, 44, 785–801. [Google Scholar] [CrossRef]
- Belayneh, A.; Molla, F. The Effect of Coffee on Pharmacokinetic Properties of Drugs: A Review. Biomed. Res. Int. 2020, 2020, 7909703. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Lei, X.; Dong, W. Caffeine and bronchopulmonary dysplasia: Clinical benefits and the mechanisms involved. Pediatr. Pulmonol. 2022, 57, 1392–1400. [Google Scholar] [CrossRef]
- Lipton, R.B.; Diener, H.C.; Robbins, M.S.; Garas, S.Y.; Patel, K. Caffeine in the management of patients with headache. J. Headache Pain 2017, 18, 107. [Google Scholar] [CrossRef]
- Dobson, N.R.; Liu, X.; Rhein, L.M.; Darnall, R.A.; Corwin, M.J.; McEntire, B.L.; Ward, R.M.; James, L.P.; Sherwin, C.M.T.; Heeren, T.C.; et al. Salivary caffeine concentrations are comparable to plasma concentrations in preterm infants receiving extended caffeine therapy. Br. J. Clin. Pharmacol. 2016, 82, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Shiva, S.; Manikantan, S.; Ramakrishna, S. Pharmacology of caffeine and its effects on the human body. Eur. J. Med. Chem. Rep. 2024, 10, 100138. [Google Scholar] [CrossRef]
- da Costa Silva, R.G.; Augusto, F. Sol–gel molecular imprinted ormosil for solid-phase extraction of methylxanthines. J. Chromatogr. A 2006, 1114, 216–223. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Military Nutrition Research. Caffeine for the Sustainment of Mental Task Performance; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Benitez, J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin. Pharmacokinet. 2000, 39, 127–153. [Google Scholar] [CrossRef]
- White, J.R.; Padowski, J.M.; Zhong, Y.; Chen, G.; Luo, S.; Lazarus, P.; Layton, M.E.; McPherson, S. Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin. Toxicol. 2016, 54, 308–312. [Google Scholar] [CrossRef]
- Jordan, N.Y.; Mimpen, J.Y.; van den Bogaard, W.J.M.; Flesch, F.M.; van de Meent, M.H.M.; Torano, J.S. Analysis of caffeine and paraxanthine in human saliva with ultra-high-performance liquid chromatography for CYP1A2 phenotyping. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 995–996, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Georga, K.A.; Samanidou, V.F.; Papadoyannis, I.N. Use of novel solid-phase extraction sorbent materials for high-performance liquid chromatography quantitation of caffeine metabolism products methylxanthines and methyluric acids in samples of biological origin. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Georga, K.A.; Samanidou, V.F.; Papadoyannis, I.N. Improved micro-method for HPLC analysis of caffeine and its demethylated metabolites in human biological fluids after SPE. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 1523–1537. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.-L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W. The Ambiguous Role of Caffeine in Migraine Headache: From Trigger to Treatment. Nutrients 2020, 12, 2259. [Google Scholar] [CrossRef]
- Tabrizi, R.; Saneei, P.; Lankarani, K.B.; Akbari, M.; Kolahdooz, F.; Esmaillzadeh, A.; Nadi-Ravandi, S.; Mazoochi, M.; Asemi, Z. The effects of caffeine intake on weight loss: A systematic review and dos-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2688–2696. [Google Scholar] [CrossRef]
- Zylber-Katz, E.; Granit, L.; Levy, M. Relationship between caffeine concentrations in plasma and saliva. Clin. Pharmacol. Ther. 1984, 36, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Ptolemy, A.S.; Tzioumis, E.; Thomke, A.; Rifai, S.; Kellogg, M. Quantification of theobromine and caffeine in saliva, plasma and urine via liquid chromatography-tandem mass spectrometry: A single analytical protocol applicable to cocoa intervention studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bendriss, E.K.; Markoglou, N.; Wainer, I.W. Liquid chromatographic method for the simultaneous determination of caffeine and fourteen caffeine metabolites in urine. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 331–338. [Google Scholar] [CrossRef]
- Perera, V.; Gross, A.S.; Xu, H.; McLachlan, A.J. Pharmacokinetics of caffeine in plasma and saliva, and the influence of caffeine abstinence on CYP1A2 metrics. J. Pharm. Pharmacol. 2011, 63, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Sager, M.; Jedamzik, P.; Merdivan, S.; Grimm, M.; Schneider, F.; Kromrey, M.L.; Hasan, M.; Oswald, S.; Kühn, J.; Koziolek, M.; et al. Low dose caffeine as a salivary tracer for the determination of gastric water emptying in fed and fasted state: A MRI validation study. Eur. J. Pharm. Biopharm. 2018, 127, 443–452. [Google Scholar] [CrossRef]
- Ponce-Rodríguez, H.D.; García-Robles, A.A.; Sáenz-González, P.; Verdú-Andrés, J.; Campíns-Falcó, P. On-line in-tube solid phase microextraction coupled to capillary liquid chromatography-diode array detection for the analysis of caffeine and its metabolites in small amounts of biological samples. J. Pharm. Biomed. Anal. 2020, 178, 112914. [Google Scholar] [CrossRef]
- Napierała, M.; Florek, E. Qualitative and quantitative determination of caffeine in saliva by high performance liquid chromatography. Prz. Lek. 2016, 73, 777–780. [Google Scholar]
- Leodori, G.; De Bartolo, M.I.; Belvisi, D.; Ciogli, A.; Fabbrini, A.; Costanzo, M.; Manetto, S.; Conte, A.; Villani, C.; Fabbrini, G.; et al. Salivary caffeine in Parkinson’s disease. Sci. Rep. 2021, 11, 9823. [Google Scholar] [CrossRef]
- Liguori, A.; Hughes, J.R.; Grass, J.A. Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol. Biochem. Behav. 1997, 58, 721–726. [Google Scholar] [CrossRef]
- Tzakri, T.; Rehenbrock, L.; Senekowitsch, S.; Rump, A.; Schick, P.; Krause, J.; Kromrey, M.-L.; Grimm, M.; Weitschies, W. Determination of gastric water emptying in fasted and fed state conditions using a compression-coated tablet and salivary caffeine kinetics. Pharmaceutics 2023, 15, 2584. [Google Scholar] [CrossRef]
- Perera, V.; Gross, A.S.; McLachlan, A.J. Caffeine and paraxanthine HPLC assay for CYP1A2 phenotype assessment using saliva and plasma. Biomed. Chromatogr. 2010, 24, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Begas, E.; Kouvaras, E.; Tsakalof, A.K.; Bounitsi, M.; Asprodini, E.K. Development and validation of a reversed-phase HPLC method for CYP1A2 phenotyping by use of a caffeine metabolite ratio in saliva. Biomed. Chromatogr. 2015, 29, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Plonka, J. Methods of biological fluids sample preparation—Biogenic amines, methylxanthines, water-soluble vitamins. Biomed. Chromatogr. 2015, 29, 1–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en (accessed on 19 August 2025).[Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).