Abstract
Endometriosis is a chronic inflammatory disorder affecting about 190 million women of reproductive age worldwide. It represents a major health challenge due to its broad impact on physical, reproductive, and psychological well-being and is clinically characterized by pelvic pain, menstrual irregularities, and infertility. This narrative review synthesized current evidence on the relationship between adiposity, metabolic and inflammatory markers, and endometriosis from a biopsychosocial and intersectional perspective. A comprehensive search was conducted in PubMed, Scopus, and Web of Science for peer-reviewed studies published in English over the past decade.: Results pointed out that endometriosis significantly affects inflammatory activity within adipose tissue, especially in visceral adipose tissue. Studies also reported reduced adipocyte size and altered adipose tissue function. The endometriosis cytokine profile exhibited a pattern of systemic and tissue-specific inflammatory activation (i.e., elevated levels of interleukin-6 and monocyte chemoattractant protein-1). Sociodemographic factors (i.e., age, race/ethnicity, socioeconomic status, and educational level) also play a significant role in differences in symptomatology, disease course, and healthcare access. To sum up, endometriosis need to be considered as a multisystem condition related to metabolic, inflammatory, and psychosocial factors. It is necessary to adopt a biopsychosocial and intersectional perspective to improve diagnosis and support more equitable and personalized therapeutic approaches.
1. Introduction
Endometriosis is a chronic inflammatory disorder characterized by severe pelvic pain (i.e., dysmenorrhea, dyspareunia, and chronic pelvic discomfort), abnormal uterine bleeding (i.e., heavy menstrual bleeding and intermenstrual spotting), and infertility, which constitutes one of the most impactful consequences of the condition. Additional symptoms frequently include fatigue, gastrointestinal disturbances consistent with irritable bowel syndrome and urinary symptoms. Endometriosis has a high global prevalence and is estimated to affect approximately 190 million women and girls of reproductive age worldwide, representing a major public health concern [1,2,3,4,5]. The World Health Organization (WHO) acknowledges the significance of endometriosis and its far-reaching impact on sexual and reproductive health, related rights, quality of life, and the overall well-being of affected individuals [1,6,7,8].
There is considerable heterogeneity in clinical presentation, which complicates both diagnosis and treatment. In general, symptoms tend to worsen during menstruation and sexual intercourse; however, the relationship between symptom intensity and disease stage is inconsistent. Many individuals in early stages may report severe pain that is debilitating, whereas some with advanced-stage disease may remain largely asymptomatic. This discordance is associated with delayed diagnosis and variable clinical trajectories, underscoring the need for frameworks that better capture biological heterogeneity and patient-reported burden [2,3,4,5,8,9,10].
In addition to gynecological manifestations, endometriosis is associated with a broad spectrum of psychosocial consequences that require careful consideration. The condition can negatively affect mental health through the interplay between chronic pain, emotional stress, and infertility, contributing to increased prevalence of anxiety and depressive symptoms. Furthermore, endometriosis imposes a substantial socioeconomic burden on patients, families, and healthcare systems due to long-term pharmacological treatment, frequent surgical interventions, and reduced participation in work or education. Direct out-of-pocket costs can also be considerable. Collectively, these factors impair quality of life and social functioning, and these consequences may be further compounded by stigma and by prevailing beliefs that minimize menstrual pain. In many settings, normalization of pelvic pain and limited awareness among the general population and even health professionals remain barriers to timely recognition and appropriate care [1,2,3,4,5,11,12,13,14,15,16].
The etiology of endometriosis remains multifactorial, involving hormonal, genetic, immunological, and environmental factors, and increasing evidence supports a systemic dimension of the disease, in which inflammatory and metabolic signaling may be related to lesion persistence and symptom burden. Ectopic endometrial tissue can persist through estrogen-dependent inflammatory pathways that promote lesion survival, pathological angiogenesis, and fibrosis [17,18]. These mechanisms may intersect with metabolic homeostasis and immune regulation, providing a rationale to examine metabolic and inflammatory markers in relation to endometriosis without presupposing a causal direction [6,7,8].
In this context, it is important to clarify the scope of the present review. This manuscript is not intended as a review of obesity per se, nor does it assume that individuals with endometriosis are typically obese. Indeed, clinical experience and several epidemiological studies suggest that many women with endometriosis are not obese, and associations between BMI and endometriosis have been reported as inverse or heterogeneous across populations and phenotypes. Therefore, the focus here is on adiposity-related biology, including fat distribution, visceral adipose tissue activity, and adipose tissue function, as well as their potential relationship with inflammatory and metabolic markers in endometriosis. Composite indices such as the Lipid Accumulation Product (LAP) and the Visceral Adiposity Index (VAI) have been proposed as proxies of central adiposity and metabolic dysfunction that may capture risk-relevant physiology beyond BMI. However, the current evidence base remains largely observational, and several commonly cited analyses—including NHANES-based studies—rely on self-reported clinician diagnosis of endometriosis and cross-sectional designs. These features limit diagnostic precision and preclude causal inference, and the causal relationship between obesity and endometriosis therefore remains uncertain. Accordingly, throughout this review we use associational language and emphasize that robust prospective cohorts and mechanistic studies are needed to establish temporality and causality [17,18,19,20,21,22].
Finally, adiposity and metabolic function are shaped by sociodemographic and structural determinants—including age, race/ethnicity, education, socioeconomic status, neighborhood environment, stress exposure, and healthcare access—that may also influence endometriosis recognition, diagnostic pathways, and management. Evidence indicates that these factors affect not only the lived experience of the disease but also differential access to timely diagnosis and effective care. Despite growing support for biopsychosocial and intersectional perspectives, research and care models frequently examine biomedical, behavioral, and social dimensions in isolation, limiting our understanding of how adiposity-related biology and sociodemographic conditions may jointly play a role in the endometriosis heterogeneity. Therefore, the present review aims to integrate three domains that are often treated separately: (1) endometriosis-related inflammatory and metabolic mechanisms; (2) adiposity-related markers and fat distribution as biologically relevant contexts; and (3) sociodemographic determinants and inequities that may modify vulnerability, clinical course, and care trajectories. This integrative approach is intended to identify evidence gaps, guide future research axes, and support the development of more comprehensive sociosanitary models of care [23,24,25,26,27,28,29].
The novelty of this review lies in its integrative and clinically oriented synthesis of endometriosis through the combined lenses of adiposity-related biology and psychosocial factors. First, rather than treating “obesity” as a binary exposure, we prioritize adiposity-relevant physiology beyond BMI—particularly visceral adipose tissue activity and adipose dysfunction proxies (e.g., VAI and LAP)—and critically appraise the strengths and limitations of the available observational evidence, including the frequent reliance on cross-sectional designs and self-reported clinician diagnosis in population-based datasets (e.g., NHANES), which precludes causal inference. Second, we embed these biological considerations within a biopsychosocial and intersectional framework by examining how sociodemographic and structural determinants (e.g., socioeconomic position, race/ethnicity, healthcare access, and contextual factors) may shape both metabolic risk profiles and endometriosis recognition, diagnostic timing, and management. Finally, building on this integrated perspective, we highlight key evidence gaps and propose priority axes for prospective and mechanistic research, alongside practical recommendations to support more comprehensive and equitable sociosanitary care for women living with endometriosis.
2. Results
This section summarizes current evidence on the biological mechanisms linking adiposity, metabolic dysfunction, and inflammation to the pathophysiology of endometriosis; and determining the influence of sociodemographic factors (i.e., age, race or ethnicity, socioeconomic status, and educational level). Particular attention is given to the differential inflammatory activity of visceral and subcutaneous adipose tissue, alterations in adipocyte morphology and function, the role of adipokines and cytokines in mediating local and systemic immune responses, the metabolic markers of VAT, and the influence of severity and types of endometriosis in the relationship between adiposity and endometriosis. Evidence is also reviewed regarding how these adipose- and inflammation-related mechanisms associated with disease heterogeneity, severity, and clinical outcomes. To conclude this section, the influence of sociodemographic factors is discussed to highlight their potential implications for understanding and managing endometriosis as a systemic and multifactorial condition.
2.1. Differential Inflammatory Responses in Visceral and Subcutaneous Adipose Tissue of Women with Endometriosis
Abobeleira et al. [30] reported smaller adipocytes in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) of endometriosis patients, along with increased serum IL-6 and reduced norepinephrine levels. VAT from endometriosis patients exhibited upregulated browning-related proteins (UCP-1, PGC-1α) and pro-inflammatory markers (IL-6, MCP-1), alongside reduced CD206 expression, indicating M1 macrophage predominance. SAT showed no significant changes in protein expression. Gene expression analysis revealed trends of decreased browning-related genes in VAT and SAT of endometriosis patients, except for DIO2, which was significantly downregulated on SAT.
Endometriosis significantly affects inflammatory activity within adipose tissue, particularly in VAT. VAT from women with endometriosis exhibited markedly elevated levels of pro-inflammatory markers, including a 3.5-fold increase in interleukin-6 (IL-6) and a 4.5-fold increase in monocyte chemoattractant protein-1 (MCP-1), both associated with macrophage activation and local inflammation. Galectin-3 expression also showed a tendency to increase in VAT, although this change did not reach statistical significance. In contrast, CD206, a marker of anti-inflammatory M2 macrophages, was significantly reduced in the VAT of endometriosis patients, suggesting a shift toward a pro-inflammatory M1 macrophage phenotype. Consistent with these tissue findings, serum IL-6 concentrations were significantly higher in women with endometriosis than in controls, indicating the presence of systemic inflammation. No significant differences were detected in SAT for IL-6, MCP-1, Galectin-3, or CD206, although there was a mild trend toward higher IL-6 and Galectin-3 and lower CD206 expression in the endometriosis group. These findings point out that endometriosis is associated with localized inflammation in VAT, driven by upregulation of pro-inflammatory cytokines and macrophage activity, whereas SAT remains relatively unaffected. This VAT-specific inflammatory response may be related to adipose tissue dysfunction and play a role in the pathophysiological progression of endometriosis [30].
Moreover, inflammation exerts a profound influence on adipose tissue physiology in endometriosis, particularly in regions adjacent to endometriotic lesions. Histological and molecular analyses reveal that these perilesional adipose areas display marked fibrosis and angiogenesis compared with tissues distant from the lesions or from women without the disease. A pronounced infiltration of macrophages -predominantly of the pro-inflammatory M1 subtype- was observed in these regions, contrasting with the predominance of anti-inflammatory M2 macrophages typically found in the peritoneal fluid of endometriosis patients. Moreover, adipose tissue adjacent to endometriotic lesions exhibited elevated expression of fatty acid-binding protein 4 (FABP4), vascular endothelial growth factor (VEGF), and pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β, indicating a state of immune and metabolic dysregulation. These findings support the notion that perilesional adipose tissue is an active participant in the local inflammatory microenvironment of endometriosis [31] (See Table 1 for further details).

Table 1.
Adipose Tissue Dysregulation in Endometriosis: Inflammatory and Metabolic Evidence.
Reduced Adipocyte Size and Altered Adipose Tissue Function in Women with Endometriosis
Adipocytes in both visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) of women with endometriosis were significantly smaller than those observed in control women, with VAT showing the smallest adipocyte size overall. Higher BMI was associated with an increase in SAT adipocyte size in both control and endometriosis groups; however, even under conditions of elevated BMI, women with endometriosis continued to exhibit smaller adipocytes compared to controls. In control women, VAT adipocyte size did not vary significantly with BMI, whereas in patients with endometriosis, VAT adipocyte size increased with higher BMI but remained consistently smaller than in the control group [30] (See Table 1 for further details).
2.2. Adipokine Dysregulation in Women with Endometriosis
Adipokines, bioactive molecules secreted by adipose tissue, have emerged as key modulators of the inflammatory and endocrine pathways implicated in endometriosis. Among them, leptin has been the most extensively studied, showing consistently elevated concentrations in both the serum and peritoneal fluid of affected women. Leptin enhances the proliferation, migration, and invasion of endometrial stromal cells through activation of the JAK2/STAT3 signaling cascade, which upregulates matrix metalloproteinase-2 (MMP-2) and promotes extracellular matrix degradation. Moreover, leptin stimulates angiogenesis by increasing vascular endothelial growth factor A (VEGF-A) expression, thereby facilitating lesion vascularization and progression. Other adipokines, such as chemerin and follistatin, are also associated with the pro-inflammatory and proliferative milieu. Chemerin and its receptor (CMKLR1) are overexpressed in ovarian endometriomas, with elevated levels detected in peritoneal fluid, suggesting a role in local immune activation and inflammation. Follistatin, elevated in both serum and ovarian tissue, inhibits activin A -a cytokine that promotes cell invasion and inflammatory signaling-thereby influencing tissue remodeling. In addition, the apelin receptor (APLNR) has been identified as a potential regulatory gene in endometriosis, although its precise functional role remains under research. These findings related to adipokines reinforce the link between visceral adipose activity, angiogenesis, and chronic inflammation, supporting their contribution to endometriosis pathophysiology [32].
By contrast, adiponectin appears to exert a protective influence against endometriosis progression. As an anti-inflammatory and anti-proliferative adipokine, adiponectin helps maintain immune balance and suppresses the growth of endometrial stromal cells. Experimental studies have shown that recombinant adiponectin inhibits the proliferation and viability of both endometriotic and normal endometrial stromal cells, suggesting its capacity to restrict lesion growth. Clinically, women with endometriosis exhibit significantly lower serum and peritoneal fluid adiponectin levels, particularly in advanced stages of the disease, suggesting that reduced adiponectin bioavailability may diminish this protective effect. The decrease in adiponectin might further amplify inflammatory and metabolic disturbances, exacerbating disease severity and persistence. The divergent profiles of leptin and adiponectin highlight the dual endocrine function of adipose tissue in endometriosis, where an imbalance favoring pro-inflammatory adipokines over anti-inflammatory ones might contribute to sustaining and exacerbating the disorder [32].
2.3. Key Cytokines Linking Systemic and Adipose Tissue Inflammation in Endometriosis
Inflammation in adipose tissue near endometriotic lesions appear to contribute to fibrosis, angiogenesis, and the maintenance of a pro-inflammatory environment that may promote disease progression. In addition, elevated levels of several cytokines have been identified, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-8 (IL-8) in women with endometriosis. These cytokines are increased both in the peritoneal cavity and in adipose tissues adjacent to endometriotic lesions. Their presence reflects an ongoing inflammatory response that supports lesion survival and chronicity. These findings suggest that cytokine overexpression is a key feature of the immune and metabolic dysregulation associated with endometriosis [31].
The cytokine profile associated with endometriosis reveals a pattern of both systemic and tissue-specific inflammatory activation. Serum interleukin-6 (IL-6) levels were significantly higher in women with endometriosis compared with controls, consistent with a state of chronic systemic inflammation. Within VAT, IL-6 expression was also upregulated, contributing to localized inflammatory signaling and potentially promoting adipose tissue browning. Monocyte chemoattractant protein-1 (MCP-1) levels were likewise elevated in VAT, reflecting increased macrophage recruitment and activation within this depot. In contrast, serum transforming growth factor-beta (TGF-β) concentrations did not differ significantly between groups; however, TGF-β has been implicated in the survival and proliferation of ectopic endometrial cells and exhibits mechanistic overlap with cancer-related metabolic dysregulation. These cytokines appear to play complementary roles in mediating both systemic and localized inflammation, contributing to adipose tissue dysfunction and to the pathophysiological progression of the condition [30].
2.4. Metabolic Markers of Visceral Adiposity: Lipid Accumulation Product (LAP) and Visceral Adiposity Index (VAI)
In women with endometriosis, the evaluation of adiposity and metabolic risk has been limited by the constraints of traditional anthropometric measures, such as the Body Mass Index (BMI). BMI does not differentiate between lean mass and fat mass, nor does it provide information about the distribution of adipose tissue. As a result, it frequently underestimates excess body fat and fails to assess the accumulation of VAT, which plays a more critical role in metabolic dysfunction and inflammation. Considering the complex nature of endometriosis and its characterization as a condition with systemic inflammatory and metabolic components, relying solely on BMI is insufficient to meaningfully elucidate its underlying pathophysiological mechanisms. Indeed, current scientific evidence highlights the limitations of BMI in terms of its diagnostic sensitivity and specificity for obesity-related metabolic disturbances, emphasizing the requirement for more reliable and biologically relevant metrics of visceral adiposity [33].
In this context, the Visceral Adiposity Index (VAI) offers a more comprehensive and clinically meaningful assessment of adipose tissue function. The VAI integrates waist circumference, BMI, triglyceride levels, and high-density lipoprotein cholesterol (HDL-C) into a composite index that comprises visceral fat activity and metabolic impairment. Unlike BMI, VAI accounts for sex-specific differences in fat distribution, making it particularly suitable for studying female reproductive disorders such as endometriosis. Elevated VAI values have been associated with insulin resistance, dyslipidemia, and systemic inflammation processes that reflect the metabolic and immunological alterations observed in endometriosis. Furthermore, the VAI’s simplicity, cost-effectiveness, and accessibility enhance its clinical utility for identifying women at increased risk of metabolic dysfunction and endometriosis-related comorbidities. These findings position the VAI as a valuable tool for both research and clinical screening, supporting individualized prevention and management strategies in women with endometriosis [33].
Zhang et al. [33] found a positive linear association between VAI and increased risk endometriosis. This relationship remained significant even after adjusting for several demographic, lifestyle, and reproductive factors. Additionally, subgroup analyses showed that this association was not significantly influenced by factors such as age, education level, marital status, or oral contraceptive use. Nevertheless, the study concluded that additional large-scale prospective research is required to confirm these findings [33].
To further examine the metabolic implications of adipose tissue dysfunction in endometriosis, recent evidence has explored the relationship between two composite metabolic indices -the lipid accumulation product (LAP) and VAI- and the presence of endometriosis. In a separate NHANES-based analysis [17], using data from the National Health and Nutrition Examination Survey (NHANES, 1999–2006), both markers were found to be significantly associated with endometriosis. Women in the highest quartiles of LAP and VAI exhibited 56% and 54% higher odds of having endometriosis, respectively, even after controlling for a comprehensive set of relevant covariates. Subgroup analyses indicated that these associations were more pronounced among women aged 35 years or older, those with a history of pregnancy, users of exogenous female hormones, and individuals without chronic kidney disease. These findings suggest that LAP and VAI, which are noninvasive and cost-effective measures integrating anthropometric and biochemical parameters, might serve as potential tools for early risk stratification and metabolic assessment in women at higher risk for endometriosis. However, the cross-sectional nature of the study and dependence on self-reported diagnostic information limit causal inference, and the generalizability of the findings beyond the U.S. populations. Nevertheless, the observed associations highlight the relevance of considering metabolic dysfunction and visceral adiposity as integral components of endometriosis pathophysiology, warranting further prospective and mechanistic research to confirm their clinical relevance [17].
2.5. Adiposity and Endometriosis Severity and Typology
The study of Byun et al. [34] revealed an inverse relationship between adiposity and endometriosis, showing that women with the condition generally exhibited lower adiposity measures than those without it. This trend was consistent across multiple indicators of body fat and visceral adiposity. When stratified by disease stage, women with stage I or IV endometriosis presented lower adiposity measures than those with stage II or III, suggesting a non-linear association between fat accumulation and disease severity [34].
Endometriosis severity was classified according to the revised American Society for Reproductive Medicine (rASRM) staging system, which categorizes the disease into four stages: stage I (minimal, scores 1–5), stage II (mild, scores 6–15), stage III (moderate, scores 16–40), and stage IV (severe, scores > 40)—based on lesion size, location, and adhesions. Staging was determined intraoperatively through laparoscopy or laparotomy using a standardized scoring algorithm. The study also analyzed endometriosis typology using operative phenotyping, distinguishing superficial peritoneal endometriosis (SE), ovarian endometrioma (OE), and deep infiltrating endometriosis (DIE), with an additional category for combined OE and DIE [34].
Different adiposity patterns emerged across these types. Women with ovarian endometrioma (OE) and/or deep infiltrating endometriosis (DIE) had the lowest adiposity indicators (i.e., BMI, skinfold thickness, and body circumferences) while those with superficial endometriosis (SE) showed comparatively higher measures. These findings highlight a possible link between adipose tissue burden and distribution and the severity and clinical presentation of endometriosis [34].
In sum, the association between adiposity and endometriosis appears complex and multifactorial, likely involving biological and metabolic pathways. Although consistent patterns were observed, confidence intervals were wide and overlapping, warranting cautious interpretation. It remains unclear whether reduced adiposity acts as a predisposing factor for endometriosis or results from the disease’s metabolic and inflammatory alterations. Future studies should assess adiposity measures prior to disease onset and investigate how variations in the anatomical distribution of fat are associated with the development and progression of endometriosis [34].
Studies of Zhang et al. [33] and Byun et al. [34] address the relationship between adiposity and endometriosis from complementary perspectives, which explains why their findings are not contradictory. Zhang et al. [33] focuses on the association between the visceral adiposity index (VAI) and the risk of developing endometriosis, identifying a positive relationship between elevated VAI and an increased risk of the disease, with factors such as age, educational level, or contraceptive use not significantly influencing this association. By contrast, Byun et al. [34] examines anthropometric and body composition measures in relation to the severity and typology of endometriosis, observing that women with endometriosis tend to have lower adiposity compared with women without the disease, and that adiposity patterns vary according to the stage and type of endometriosis. These differences in focus and in the variables examined explain why the results are not contradictory but rather reflect distinct aspects of the relationship between adiposity and endometriosis.
2.6. Integrating Biopsychosocial and Intersectional Perspectives into Adiposity–Endometriosis Research
The studies reviewed indicate that, although there is evidence of relevant sociodemographic differences in the prevalence, clinical expression, and severity of endometriosis, these factors are often not examined in depth. Several works focus almost exclusively on biological, metabolic, and molecular aspects, without adequately integrating variables such as age, race or ethnicity, socioeconomic status, or educational attainment [30,31,32,35] (See Figure 1 for more details).
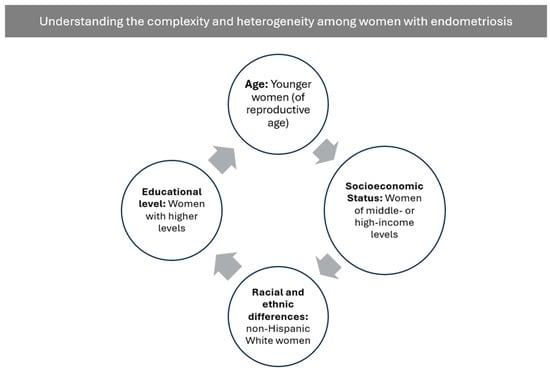
Figure 1.
Overview of Psychosocial Factors in Endometriosis. Note: Figure developed from the data reported in the analyzed studies.
Regarding age, some studies report meaningful differences between clinical groups. Abobeleira et al. [30] report that women with endometriosis were younger (mean 37.63 years) than those in the control group (mean 45.07 years), a difference interpreted as a methodological limitation due to the potential influence of age on adipose tissue metabolism. Epidemiological evidence aligns with the fact that endometriosis primarily affects women of reproductive age (22–49 years), which may partly explain these group differences. Complementing this, Hong et al. [14] describe that the relationship between obesity and disease severity varies by age: women aged 27–36 and 37–45 years with advanced disease show a lower BMI than those with mild disease, whereas this pattern does not appear in younger women aged 18–27 years. These findings are consistent with Ma et al. [17], who report that endometriosis prevalence is substantially higher in women aged ≥35 years (9.89%) compared with those under 35 (3.77%), and that associations between visceral adiposity indices (LAP and VAI) and endometriosis are stronger in this older age group.
Racial and ethnic differences are also reflected across several studies. According to Ma et al. [17], non-Hispanic White women show the highest prevalence of endometriosis (10.13%), followed by non-Hispanic Black women (6.07%), women from other racial groups (4.33%), and—with the lowest prevalence—Mexican American women (2.41%). A similar pattern appears when examining the distribution of cases: 84.17% of diagnosed women were non-Hispanic White, compared with 7.72% non-Hispanic Black, 5.70% other racial groups, and only 2.41% Mexican American women. Comparable results have been reported in Byun et al. [34] and Zhang et al. [33], where non-Hispanic White women also show greater representation in more severe stages, including stage IV disease.
Socioeconomic factors present a complex relationship with endometriosis. Ma et al. [17] report higher prevalence among women with middle- or high-income levels (PIR > 1; 7.61%) compared with those with lower income (PIR ≤ 1; 4.25%), a difference attributed to factors such as access to healthcare or greater likelihood of receiving a formal diagnosis. However, findings are not uniform across studies. Zhang et al. [33] did not observe significant differences in socioeconomic status—measured through the poverty-to-income ratio—between women with and without endometriosis (p = 0.593), nor relevant interactions between socioeconomic status and the association between visceral adiposity and disease risk. In contrast, Byun et al. [34] point out that women with more severe forms of the disease—such as stage IV endometriosis or deep infiltrating endometriosis—are more likely to be above 180% of the poverty line, suggesting distinct sociodemographic patterns depending on disease subtype and severity.
Educational attainment shows similar patterns. Ma et al. [17] report that endometriosis prevalence is markedly higher among women with education beyond high school (8.02%) compared with those with a high school education (4.39%) or below (1.60%). Zhang et al. [33] also observe significant differences in educational distribution between women with and without the disease, with secondary/GED education more frequent among those with endometriosis (31.74% vs. 21.73%). In terms of severity, Byun et al. [34] show that women with advanced stages (III–IV) and severe subtypes (deep infiltrating endometriosis or ovarian endometrioma) exhibit proportionally higher rates of university education—for example, 46.4% in stage IV and more than 52% in DIE and ovarian endometrioma cases. Although these studies do not establish causality, they suggest that educational attainment may influence the likelihood of seeking healthcare and therefore receiving a diagnosis.
Other sociodemographic variables also appear associated with endometriosis in some studies. Zhang et al. [33] report a higher proportion of married or cohabiting women among those diagnosed with endometriosis (74.88% vs. 67.40% in the non-endometriosis group), whereas Byun et al. [34] find that more severe disease forms are associated with a greater likelihood of never having been pregnant, as well as differences related to place of residence. Additionally, Ma et al. [17] identify variations in the use of female hormones, pregnancy history, use of steroids, and the presence of metabolic and cardiovascular comorbidities between women with and without endometriosis.
Although these findings suggest that sociodemographic factors play a meaningful role in the prevalence, clinical expression, subtype, and severity of endometriosis, the studies seldom explore the potential causes of these differences or the mechanisms underlying them. These limitations highlight the need to integrate biopsychosocial and intersectional approaches to better understand how age, race or ethnicity, education, socioeconomic status, and other determinants combine to shape the experience and manifestation of the disease.
3. Discussion
The discussion is structured into three parts: first, the analysis of the main findings of this narrative review; and second their clinical relevance and practical applications, with the aim of addressing the identified research gaps and contributing to more comprehensive social and health care for women with endometriosis. In third place, the main contributions and novelty of the present review are discussed.
3.1. Adipose Tissue Dysregulation in Endometriosis: Inflammatory and Metabolic Insights Within a Biopsychosocial–Intersectional Framework
Differential inflammatory responses between visceral and subcutaneous adipose tissues in women with endometriosis have been reported. In line with the patterns described in the Section 2, clear depot-specific differences were observed. On purpose, IL-6 protein levels were markedly elevated in visceral adipose tissue (VAT), whereas subcutaneous adipose tissue (SAT) showed only a mild, non-significant increase and a significant reduction in IL-6 mRNA expression. MCP-1 was significantly upregulated in VAT, reflecting enhanced macrophage recruitment, while SAT remained unchanged. CD206, a marker of anti-inflammatory M2 macrophages, was markedly decreased in VAT, indicating a shift toward a pro-inflammatory phenotype, with only a non-significant downward trend in SAT. Galectin-3 expression tended to increase in VAT but showed no variation in SAT. These findings highlight tissue-specific inflammatory regulation, with VAT exhibiting a stronger pro-inflammatory signature than SAT in the context of endometriosis, supporting the notion that VAT browning and inflammation are associated with adipose tissue dysfunction and altered lipid metabolism [30,35,36,37,38].
Overall, these depot-specific alterations indicate that inflammatory activation in endometriosis is more prominent in VAT than in SAT, consistent with the greater metabolic and immunologic activity of VAT. The distinct upregulation of pro-inflammatory mediators and the reduction in anti-inflammatory markers in VAT suggest selective activation of immune and macrophage-related pathways that may be linked to local tissue dysfunction and shape the systemic inflammatory milieu associated with endometriosis. This pattern also aligns with the idea that VAT inflammation might act as a potential driver of systemic inflammatory signaling and interact with reproductive and metabolic pathways, thereby contributing to disease progression [30]. Its anatomical proximity to endometriotic lesions may further amplify these inflammatory processes. Understanding these depot-specific alterations is clinically relevant, as it highlights VAT as a potential therapeutic target within metabolic and anti-inflammatory treatment strategies [30].
From a broader pathophysiological perspective, adipose tissue inflammation may be related to the progression of endometriosis through multiple interconnected mechanisms. The secretion of IL-6, TNF-α, IL-1β, and IL-8 sustains chronic inflammation and promotes tissue remodeling, while increased VEGF supports angiogenesis and lesion survival. Accumulation of M1 macrophages amplifies inflammatory signaling, creating a persistent feedback loop that maintains lesion activity. FABP4 overexpression appears to link lipid metabolism to inflammatory and angiogenic pathways, reinforcing the metabolic–immune connection. Additionally, reductions in adipocyte size reflect underlying adipose tissue dysfunction and a shift toward enhanced browning and thermogenic activity [30]. These processes establish a self-sustaining inflammatory network between endometriotic lesions and adjacent adipose depots, contributing to fibrosis, angiogenesis, and chronic progression [31,38]. These observations reinforce the bidirectional interaction between endometriotic lesions and adipose tissue, where metabolic and inflammatory signaling mutually amplify one another [38].
Consistent with these mechanisms, cytokine activity detected in the peritoneal cavity and in perilesional adipose tissue supports the establishment of a persistent pro-inflammatory microenvironment that promotes the maintenance and progression of endometriotic lesions. Elevated IL-6, TNF-α, IL-1β, and IL-8 serve as key mediators of chronic inflammation. IL-6 and TNF-α support immune cell recruitment and angiogenic signaling, while IL-1β and IL-8 further amplify inflammatory cascades and vascular remodeling. Together, these findings support local-to-systemic immunometabolic interactions in endometriosis [31,38].
Adipose tissue-driven inflammation may also be relevant for comorbid conditions that frequently overlap with endometriosis, particularly gastrointestinal and immune-mediated disorders. Beyond symptom-level overlap, there is epidemiological evidence of co-diagnosis between endometriosis and inflammatory bowel disease (IBD), which can complicate diagnostic pathways and may reflect shared inflammatory mechanisms [39]. From a mechanistic standpoint, adipose tissue—especially visceral depots—acts as an active immunometabolic organ and can sustain cytokine and chemokine signaling, immune-cell recruitment, and barrier-related inflammatory cascades that are central to IBD pathophysiology [40]. In parallel, endometriosis has been associated with a higher prevalence of several autoimmune diseases in population-based studies, supporting the hypothesis that systemic immune dysregulation may represent a shared susceptibility domain [41,42,43]. Given that adipose dysfunction may modulate immune activation and chronic low-grade inflammation, integrating adiposity-related biology into comorbidity-focused research may help clarify whether immunometabolic profiles contribute to endometriosis heterogeneity and its broader multisystem burden.
In parallel, metabolic markers of visceral adiposity also appear relevant. The lipid accumulation product (LAP) emerges as a significant indicator in endometriosis, with women in the highest quartile showing approximately 56% higher odds of developing the condition compared with those in the lowest quartile. As an index integrating waist circumference and triglyceride levels, LAP better reflects visceral fat accumulation than BMI. Because visceral adiposity is closely tied to inflammation, insulin resistance, and oxidative stress, LAP may function as a proxy for metabolic dysfunction linked to endometriosis. Its association is particularly pronounced among women aged ≥ 35 years, those with pregnancy history, and individuals undergoing hormonal therapy. Clinically, LAP represents a simple, non-invasive tool that may facilitate early identification of women at increased risk [17,44]. Given its associations with VAT-related inflammation, LAP—and to a lesser extent VAI—may support metabolic screening strategies to identify patients with adiposity-driven inflammatory risk profiles [17,44].
Regarding adiposity patterns, epidemiological studies consistently report an inverse association between endometriosis and BMI, with affected women tending to have lower BMI and reduced adipose mass (especially below the waist). This lean phenotype may influence estrogen regulation, which is highly relevant given the estrogen-dependent nature of the disease [36]. Several mechanisms have been proposed: obesity-related anovulation and oligomenorrhea may reduce retrograde menstruation; chronic pain and emotional distress may reduce appetite; and adipokines may influence immune regulation, inflammation, and angiogenesis. Notably, associations between fat distribution and endometriosis risk appear age-dependent: inverse relationships between waist-to-hip and waist-to-thigh ratios and endometriosis risk are seen in women under 30 but not in older women. These findings emphasize a complex interplay among fat distribution, estrogen levels, and inflammatory pathways, and reinforce the need to consider sociodemographic factors when interpreting these associations [36,38,44].
Adipose tissue may further influence clinically relevant long-term outcomes through pathways implicated in carcinogenesis, including altered steroid hormone bioavailability, insulin resistance, and chronic inflammation. At the population level, increased adiposity is a well-established risk factor for endometrial cancer, and adipose tissue–organ crosstalk (including adipokine and inflammatory mediator signaling) has been proposed as a key biological substrate linking metabolic dysfunction with tumour development and progression [45]. In addition, emerging evidence suggests that not only overall adiposity but also adipose tissue distribution (e.g., visceral versus subcutaneous compartments) may be associated with endometrial cancer characteristics and prognosis [46]. Importantly, endometriosis and adenomyosis have been associated with increased risks of certain gynecological malignancies (including endometrial pathology and ovarian cancer subtypes), although causality and effect modification remain under investigation [7,47,48,49]. In this context, evaluating adiposity-related markers beyond BMI may be particularly informative for clarifying whether metabolic phenotypes and fat distribution contribute to cancer-related risk stratification among women with endometriosis/adenomyosis and for identifying evidence gaps that require prospective study designs.
Moreover, fertility represents a key clinical endpoint where endometriosis and adiposity-related biology may converge. Endometriosis is a well-recognized contributor to infertility, and current clinical guidance emphasizes the heterogeneity of infertility mechanisms and the need for individualized management strategies [7,8]. In parallel, excess adiposity and adipose dysfunction have been associated with impaired ovulatory function, altered endocrine signaling, and reduced endometrial receptivity, with downstream effects on natural conception and assisted reproduction outcomes [50,51]. From an integrative standpoint, this suggests that inflammatory and metabolic profiles linked to adipose tissue—captured by markers beyond BMI—may be relevant when interpreting fertility trajectories in endometriosis, while also underscoring the importance of prospective studies that can disentangle temporality, treatment effects, and confounding.
Taken together, these observations highlight the need to further examine the temporal and anatomical dimensions of the adiposity–endometriosis link. Future research should evaluate adiposity measures before diagnosis, and ideally before disease onset, and should consider anatomical distinctions between VAT and SAT to clarify metabolic and inflammatory interactions underlying endometriosis and to support earlier detection of metabolic phenotypes associated with the disease [34]. Further research is needed to explore the systemic impact of adipose tissue dysfunction in endometriosis.
Furthermore, lifestyle interventions are also discussed in the reviewed literature as a resource for prevention and management, including dietary modifications, weight management, and regular physical activity, which may support lipid metabolism, reduce systemic inflammation, and improve insulin sensitivity and immune modulation [17]. Among lifestyle approaches, diet has been proposed as a complementary, non-pharmacological strategy that may be relevant to both endometriosis-related symptom management and adipose tissue-related inflammatory and metabolic profiles. The available evidence remains heterogeneous and does not support diet as a curative intervention; however, dietary strategies are increasingly discussed as adjunctive measures within comprehensive, patient-centred care models, particularly in the context of chronic inflammatory conditions and metabolic health. In line with this perspective, a recent review [52] summarises the clinical relevance of nutrition in estrogen-influenced gynecological disorders and highlights emerging therapeutic considerations, supporting the rationale for including diet within a broader, complementary framework while emphasising the need for more robust and clinically informative research.
Despite the relevance of these biological and metabolic interactions, several methodological limitations characterize the reviewed literature. Many studies relied on small sample sizes, used cross-sectional designs, which do not allow for causal inferences, or lacked pre-diagnostic adiposity data. In addition, measures of adiposity varied widely—ranging from adipose tissue biopsies to anthropometrics, LAP, VAI, or imaging—hindering comparability. The scarcity of longitudinal assessments and inconsistent characterization of inflammatory markers further complicate interpretation. These methodological constraints underline the need for more robust, standardized, and temporally sensitive study designs.
To sum up, the present synthesis also points up the role of sociodemographic factors in shaping the adiposity–endometriosis relationship. Differences related to age, race or ethnicity, educational attainment, and socioeconomic status do exist, but remain highly fragmented across the literature. Studies vary widely in the variables included, the analytical depth applied, and the theoretical frameworks used. As a result, sociodemographic determinants are rarely integrated into biological models despite their potential relevance for variability in symptom burden, inflammatory profiles, and disease severity [1,34,35,36]. In addition, inconsistent measurement and limited research of sociodemographic variables constrain the ability to distinguish biological heterogeneity from variability driven by differential exposure to structural conditions, because many studies control for—rather than examine—factors such as income, education, race or ethnicity, or reproductive history. Addressing these limitations requires more systematic and theoretically informed incorporation of sociodemographic indicators, including intersectional frameworks that recognise how multiple social positions jointly shape biological vulnerability, symptom trajectories, and access to care.
In sum, the evidence reviewed indicates that the relationship between adiposity and endometriosis reflects a dynamic interplay of biological, metabolic, inflammatory, and sociodemographic factors. Depot-specific immune activation, alterations in adipocyte function, and visceral adiposity-related metabolic dysregulation converge with differential risk patterns linked to age, fat distribution, and lifestyle determinants. At the same time, sociodemographic disparities—fragmented across existing studies and rarely incorporated into disease models—suggest that biological vulnerability cannot be fully understood without considering structural conditions, access to care, and lived experiences that influence diagnosis, symptom trajectories, and disease severity. Integrating these dimensions within a unified biopsychosocial and intersectional framework may therefore offer a more comprehensive understanding of endometriosis pathophysiology and clinical presentation, improve the identification of metabolic and inflammatory phenotypes, and guide more equitable, personalized, and contextually informed prevention and treatment strategies.
3.2. Clinical Relevance and Therapeutic Implications
This narrative review has provided an opportunity to examine several factors that directly influence clinical practice due to their relevance for the diagnosis and treatment of endometriosis, as well as their potential to improve the quality of life of affected women.
A central consideration is that VAT plays a key role in the progression of endometriosis, owing to its pronounced inflammatory and metabolic activity. This observation suggests that therapeutic strategies aimed at reducing inflammation and improving VAT metabolic function might help mitigate symptoms and complement standard treatments without compromising reproductive objectives [30].
In addition, the potential use of metabolic indices as clinical tools merits attention. Indicators such as the Lipid Accumulation Product (LAP) and the Visceral Adiposity Index (VAI) have been proposed as valuable metrics for identifying inflammatory risk profiles associated with visceral adiposity. These indices may facilitate the early identification of women at increased risk of metabolic dysfunction and comorbidities linked to endometriosis. Adapting therapeutic strategies to each patient’s metabolic phenotype (e.g., elevated VAT activity or high LAP levels) and social context may further enhance the precision and clinical relevance of treatment decisions, thereby supporting more inclusive and individualized care [17,33].
Given the considerable heterogeneity in disease presentation, it is also essential to advance diagnostic and therapeutic approaches that are as personalized as possible and responsive to the specific clinical circumstances and needs of each woman.
The expansion of programs and interventions that promote lifestyle modification is equally important. Although still an emerging field of study, dietary changes, regular physical activity, and stress-reduction practices—such as mindfulness—may contribute to symptom management and improve general well-being. These psychological interventions can also help address modifiable risk factors and promote both metabolic and reproductive health [1,17,52].
Furthermore, additional longitudinal and multidisciplinary research is required to more fully elucidate the interactions between metabolic dysfunction, hormonal regulation, and psychosocial factors in endometriosis. Such work may contribute to the development of more effective prevention and treatment strategies. The specific contexts and needs of different countries should also be considered, as these may influence access to diagnostic services and treatment options. In fact, in many regions, access to early diagnosis and effective care remains limited, and it is therefore crucial to examine these realities as well [1].
There is also a critical need to integrate biopsychosocial and intersectional perspectives into research, prevention, diagnosis, and clinical management of endometriosis. Sociodemographic factors—such as age, race/ethnicity, educational attainment, and socioeconomic status—should be systematically considered during clinical assessment, as they may influence risk profiles and contribute support more equitable and individualized care [53,54]. It is also essential to examine the health disparities experienced by women with endometriosis, including those arising from gender-based inequities and overlapping forms of discrimination. For example, the needs of a woman with endometriosis who has not experienced any form of victimization differ greatly from those of a woman who also faces discrimination on the basis of race, gender, and/or age. Such analyses promote more person-centered care and a more nuanced understanding of each clinical scenario. This approach is consistent with key Sustainable Development Goals (SDGs), particularly SDG 3: ensure healthy lives and promote well-being for all at all ages; SDG 5: achieve gender equality and empower all women and girls); and SDG 10: reduce inequality within and among countries.
In this context, sexual education plays a crucial role in enabling women to better understand their bodies and the pain associated with endometriosis, challenging the common misconception that menstrual or pelvic pain is “normal.” Sexual education also improves access to healthcare and may facilitate earlier diagnosis, which is crucial for improving prognosis. As previously noted, educational attainment is an important factor in endometriosis [17,34,55]. Women with higher levels of education tend to have better employment opportunities, higher income, and greater knowledge of healthcare systems, which may enable earlier access to care and timelier diagnosis and treatment [55]. Although no definitive preventive strategies exist for endometriosis, improving awareness of the condition—along with timely diagnosis and early treatment—may help slow or interrupt its natural progression, reduce long-term symptoms, and, in some cases, reduce the likelihood of central nervous system sensitization to [1].
All these recommendations for clinical practice reinforce the need for a multidisciplinary approach that allows patients to benefit from the expertise of diverse professionals. Furthermore, multidisciplinary teams with specialists across health and social care disciplines are better equipped to implement comprehensive biopsychosocial models and intersectional frameworks in the assessment and management of women with endometriosis. Ongoing training and professional development regarding chronic pain, particularly endometriosis, and its biopsychosocial and intersectional management are essential for healthcare and social care providers (see Table 2 for further details).

Table 2.
Future research axes and practical recommendations based on the evidence clinical and research gaps highlighted in the current review.
In sum, endometriosis should be approached through a comprehensive, multidisciplinary framework that brings together gynecology, endocrinology, psychology, nutrition, and other related professions to address the condition’s biopsychosocial, inflammatory, and metabolic dimensions. Given the heterogeneity of clinical presentations and the interplay between symptom burden, hormonal–metabolic factors, lifestyle determinants, and psychosocial factors, coordinated care is essential to optimize outcomes and improve quality of life.
Future research should prioritize longitudinal studies to clarify causal pathways and to identify clinically meaningful phenotypes that can guide risk stratification and targeted interventions. In parallel, healthcare systems should advance the implementation of more inclusive and personalized models of care—grounded in patient-centered outcomes and equitable access—to move beyond one-size-fits-all management and support tailored prevention and treatment strategies.
3.3. Main Contributions and Novelty of the Present Review
This review adds to prior work by explicitly integrating adiposity-related biology, metabolic dysfunction, and inflammation within a biopsychosocial and intersectional framework, offering a more comprehensive understanding of endometriosis as a systemic condition. Unlike previous studies that primarily focused on isolated biological mechanisms, this synthesis highlights the interplay between biological, metabolic, and structural determinants.
Moreover, the review’s findings on depot-specific inflammatory responses in VAT versus SAT provide novel insights into the localized and systemic inflammatory mechanisms underlying endometriosis. The identification of adipokines such as leptin and adiponectin as key modulators of inflammation and metabolic dysfunction further underscores the complexity of the disease and opens new avenues for targeted therapeutic interventions.
Along the same line, the review’s emphasis on the role of VAT in endometriosis progression highlights the potential for developing therapeutic strategies that target VAT inflammation and metabolic dysfunction. Additionally, the proposed use of metabolic indices such as LAP and VAI for risk stratification represents a novel approach to identifying women at higher risk for endometriosis-related comorbidities.
This review also identifies critical evidence gaps, including the need for prospective studies to establish causality and the relevance of integrating sociodemographic factors into biological models. By addressing these gaps, future research can build on the novel insights presented here to develop more effective and equitable diagnostic and therapeutic strategies.
By integrating adiposity-related biology, metabolic dysfunction, and sociodemographic determinants, this review provides a novel framework for understanding endometriosis as a systemic and multifactorial condition. This approach has the potential to guide future research, improve diagnostic precision, and support the development of more personalized and equitable care models for women with endometriosis.
4. Materials and Methods
This study follows a comprehensive narrative review methodology to explore the current evidence on the relationship between adiposity, adipose tissue function, and metabolic markers in women with endometriosis from a biopsychosocial and intersectional perspective. The search was conducted across PubMed, Scopus, and Web of Science databases, focusing on peer-reviewed articles published in English over the past ten years. The search strategy was based on Medical Subject Headings (MeSH) terms to enhance precision and consistency in identifying relevant literature. The followed key terms were included: Endometriosis, Inflammation, Adiposity, and Metabolic. Moreover, Boolean operators were used to combine terms and refine the search results.
Inclusion criteria included empirical studies focusing on (I) adiposity or body composition indicators (e.g., BMI, fat distribution, etc.); and (II) metabolic or inflammatory biomarkers (e.g., cytokines, TNF-α, IL-6, etc.). In addition, given the main objective of this research, sociodemographic factors such as age, race or ethnicity, socioeconomic status, and educational attainment were also included when such variables were available.
Exclusion criteria comprise (I) case reports, conference abstracts, non-human studies; (II) Publications lacking primary data; and (III) duplicate records.
Due to it is a narrative review, a formal risk-of-bias assessment was not performed. Instead, the selected studies were critically evaluated to analyse their methodological quality and main limitations. In particular, the following issues were appraised: the clarity of study design, adequacy of sample size, validity of measurement instruments, and consistency of reported outcomes. In addition, the methodological limitations and potential sources of bias acknowledged by the original authors were also analysed during the process.
As part of the data analysis, the main findings extracted from the final selection of the studies were organized into thematic categories. Descriptive and comparative methods were used to identify the principal patterns and trends in the reviewed literature.
5. Conclusions
In this narrative review, the relationship between adiposity, metabolic dysfunction, and inflammation in the pathophysiology of endometriosis is examined from a biopsychosocial and intersectional perspective. The findings indicate that endometriosis cannot be conceptualized solely as a purely gynecological disease but rather should be considered a systemic disorder characterized by complex metabolic and inflammatory interactions. The differential inflammatory activity observed in visceral and subcutaneous adipose tissue underline that adipose depots are not passive energy stores but might act as active endocrine and immune organ capable of modulating local and systemic homeostasis. Moreover, in women with endometriosis, alterations in adipocyte morphology and function, together with a pro-inflammatory adipokine and cytokine profile, appear to sustain a chronic inflammatory milieu that favors lesion implantation, survival, and angiogenesis. There is a bidirectional interaction between adipose tissue and endometriotic lesions, in which metabolic and inflammatory signals mutually amplify each other and are associated with the chronic progression of the disease.
In addition to these biological and metabolic pathways, the evidence reviewed indicates that sociodemographic factors—such as age, race or ethnicity, educational attainment, and socioeconomic status—also influence the adiposity–endometriosis relationship. However, these influences remain fragmented across studies and are rarely integrated into biological models, despite their relevance for understanding variability in symptom burden, inflammatory profiles, and diagnostic trajectories. This gap underscores the relevance of incorporating biopsychosocial and intersectional perspectives to better capture how structural conditions, lived experiences, and access to healthcare interact with adiposity-related mechanisms in endometriosis.
In sum, recognizing adiposity-related mechanisms alongside psychosocial determinants may improve diagnostic approaches and inform treatment development. Future research and clinical strategies should jointly address metabolic–inflammatory pathways and the broader structural factors that shape disease expression, to advance more equitable and person-centered endometriosis care. This also underscores the value of inclusive, personalized pathways delivered through multidisciplinary teams integrating gynecologic, endocrine, psychological, and nutritional expertise along with other related professionals.
Author Contributions
C.M.G.-S.: Conceptualization; Formal analysis; Methodology; Resources; Project administration; Supervision; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing; Funding acquisition. J.A.C.-R.: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing. A.M.C.-M.: Investigation; Methodology; Resources; Validation; Visualization; Roles/Writing—original draft. R.M.L.-G.: Conceptualization; Formal analysis; Resources; Project administration; Supervision; Validation; Visualization; Writing—review & editing: Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project 12590: Evaluation, Counseling, and Psychological Intervention in the Context of Health. IDCQ HOSPITALES Y SANIDAD, S.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization (WHO). Endometriosis. Available online: https://www.who.int/es/news-room/fact-sheets/detail/endometriosis (accessed on 17 November 2025).
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Johnson, N.P.; Hummelshoj, L.; Adamson, G.D.; Keckstein, J.; Taylor, H.S.; Abrao, M.S.; Bush, D.; Kiesel, L.; Tamimi, R.; Sharpe-Timms, K.L.; et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 2017, 32, 315–324. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- De Corte, P.; Klinghardt, M.; von Stockum, S.; Heinemann, K. Time to Diagnose Endometriosis: Current Status, Challenges and Regional Characteristics—A Systematic Literature Review. BJOG 2025, 132, 118–130. [Google Scholar] [CrossRef]
- Li, W.; Feng, H.; Ye, Q. Factors contributing to the delayed diagnosis of endometriosis—A systematic review and meta-analysis. Front. Med. 2025, 12, 1576490. [Google Scholar] [CrossRef]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [PubMed]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The social and psychological impact of endometriosis on women’s lives: A critical narrative review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Sims, O.T.; Gupta, J.; Missmer, S.A.; Aninye, I.O. Stigma and Endometriosis: A Brief Overview and Recommendations to Improve Psychosocial Well-Being and Diagnostic Delay. Int. J. Environ. Res. Public Health 2021, 18, 8210. [Google Scholar] [CrossRef]
- Koller, D.; Pathak, G.A.; Wendt, F.R.; Tylee, D.S.; Levey, D.F.; Overstreet, C.; Gelernter, J.; Taylor, H.S.; Polimanti, R. Epidemiologic and Genetic Associations of Endometriosis With Depression, Anxiety, and Eating Disorders. JAMA Netw. Open 2023, 6, e2251214. [Google Scholar] [CrossRef]
- Van Barneveld, E.; Manders, J.; van Osch, F.H.M.; van Poll, M.; Visser, L.; van Hanegem, N.; Lim, A.C.; Bongers, M.Y.; Leue, C. Depression, Anxiety, and Correlating Factors in Endometriosis: A Systematic Review and Meta-Analysis. J. Womens Health 2022, 31, 219–230. [Google Scholar] [CrossRef]
- Ma, N.; Hu, Y.; Xu, Y. Association between lipid accumulation product and visceral adiposity index with endometriosis: Evidence from NHANES 1999–2006. BMC Womens Health 2025, 25, 307. [Google Scholar] [CrossRef]
- Gonnella, F.; Konstantinidou, F.; Donato, M.; Gatta, D.M.P.; Peserico, A.; Barboni, B.; Stuppia, L.; Nothnick, W.B.; Gatta, V. The Molecular Link between Obesity and the Endometrial Environment: A Starting Point for Female Infertility. Int. J. Mol. Sci. 2024, 25, 6855. [Google Scholar] [CrossRef]
- Hong, J.; Yi, K.W. What is the link between endometriosis and adiposity? Obstet. Gynecol. Sci. 2022, 65, 227–233. [Google Scholar] [CrossRef]
- Pantelis, A.; Machairiotis, N.; Lapatsanis, D.P. The Formidable yet Unresolved Interplay between Endometriosis and Obesity. Sci. World J. 2021, 2021, 6653677. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W. Association between body mass index and endometriosis risk: A meta-analysis. Oncotarget 2017, 8, 46928–46936. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, M.; Lin, L.; Gao, Y.; Chen, G.Q.; Chen, S.; Chen, Q. Is body mass index associated with the incidence of endometriosis and the severity of dysmenorrhoea: A case-control study in China? BMJ Open 2020, 10, e037095. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M. Social determinants of health inequalities. Lancet 2005, 365, 1099–1104. [Google Scholar] [CrossRef]
- Hankivsky, O. Women’s health, men’s health, and gender and health: Implications of intersectionality. Soc. Sci. Med. 2012, 74, 1712–1720. [Google Scholar] [CrossRef]
- Bougie, O.; Yap, M.I.; Sikora, L.; Flaxman, T.; Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: A systematic review and meta-analysis. BJOG 2019, 126, 1104–1115. [Google Scholar] [CrossRef]
- Røssell, E.-L.; Melgaard, A.; Saraswat, L.; Horne, A.W.; Josiasen, M.; Rytter, D. Sociodemographic characteristics of women with endometriosis: A Danish register-based case-control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 310, 113968. [Google Scholar] [CrossRef]
- Raffone, A.; Doglioli, M.; Aguzzi, A.; Girardi, L.; De Meis, L.; Neola, D.; Travaglino, A.; Giorgi, M.; Vastarella, M.G.; Cobellis, L.; et al. Race and ethnicity reporting in endometriosis literature: A systematic review. Facts Views Vis. Obgyn 2025, 17, 263–270. [Google Scholar] [CrossRef]
- Anekwe, C.V.; Jarrell, A.R.; Townsend, M.J.; Gaudier, G.I.; Hiserodt, J.M.; Stanford, F.C. Socioeconomics of Obesity. Curr. Obes. Rep. 2020, 9, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Baez, A.S.; Ortiz-Whittingham, L.R.; Tarfa, H.; Osei Baah, F.; Thompson, K.; Baumer, Y.; Powell-Wiley, T.M. Social determinants of health, health disparities, and adiposity. Prog. Cardiovasc. Dis. 2023, 78, 17–26. [Google Scholar] [CrossRef]
- Abobeleira, J.P.; Neto, A.C.; Mauersberger, J.; Salazar, M.; Botelho, M.; Fernandes, A.S.; Martinho, M.; Serrão, M.P.; Rodrigues, A.R.; Almeida, H.; et al. Evidence of Browning and Inflammation Features in Visceral Adipose Tissue of Women with Endometriosis. Arch. Med. Res. 2024, 55, 103064. [Google Scholar] [CrossRef]
- Kubo, K.; Kamada, Y.; Hasegawa, T.; Sakamoto, A.; Nakatsuka, M.; Matsumoto, T.; Masuyama, H. Inflammation of the adipose tissue in the retroperitoneal cavity adjacent to pelvic endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 3598–3606. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Chu, T.; Chen, X.; Zhou, H.; Xu, D.; Dong, C.; Wu, Y. Association between visceral adiposity index and endometriosis: A population-based study. Front. Nutr. 2025, 12, 1602288. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Peterson, C.M.; Backonja, U.; Taylor, R.N.; Stanford, J.B.; Allen-Brady, K.L.; Smith, K.R.; Louis, G.M.B.; Schliep, K.C. Adiposity and Endometriosis Severity and Typology. J. Minim. Invasive Gynecol. 2020, 27, 1516–1523. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Ren, Y.F.; Li, B.B.; Wei, C.; Yu, B. The mysterious association between adiponectin and endometriosis. Front. Pharmacol. 2024, 15, 1396616. [Google Scholar] [CrossRef]
- Venkatesh, S.S.; Ferreira, T.; Benonisdottir, S.; Rahmioglu, N.; Becker, C.M.; Granne, I.; Wittemans, L.B. Obesity and risk of female reproductive conditions: A Mendelian randomisation study. PLoS Med. 2022, 19, e1003679. [Google Scholar] [CrossRef]
- Jiang, J.F.; Jiang, Z.Y.; Xue, M. Serum and peritoneal fluid levels of inter- leukin-6 and interleukin-37 as biomarkers for endometriosis. Gynecol. Endocrinol. 2019, 35, 571–575. [Google Scholar] [CrossRef]
- Neves, D.; Neto, A.C.; Salazar, M.; Fernandes, A.S.; Martinho, M.; Charrua, A.; Rodrigues, A.R.; Gouveia, A.M.; Almeida, H. A narrative review about the intricate crosstalk among endometrium, adipose tissue, and neurons in endometriosis. The multifaceted role of leptin. Obes. Rev. 2025, 26, e13879. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Cipriani, S.; Ricci, E.; Roncella, E.; Mauri, P.A.; Parazzini, F.; Vercellini, P. Endometriosis and inflammatory bowel disease: A systematic review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 246–251. [Google Scholar] [CrossRef]
- Karaskova, E.; Velganova-Veghova, M.; Geryk, M.; Foltenova, H.; Kucerova, V.; Karasek, D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 4226. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef]
- Petraglia, F.; Vannuccini, S.; Donati, C.; Jeljeli, M.; Bourdon, M.; Chapron, C. Endometriosis and comorbidities: Molecular mechanisms and clinical implications. Trends Mol. Med. 2025, in press. [Google Scholar] [CrossRef]
- Aziz, M.; Beaton, M.A.; Aziz, M.A.; Opoku-Anane, J.; Elhadad, N. Endometriosis and autoimmunity: A large-scale case-control study of endometriosis and 10 distinct autoimmune diseases. NPJ Womens Health 2025, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Dereziński, T.; Zozulińska-Ziółkiewicz, D.; Uruska, A.; Dąbrowski, M. Visceral adiposity index as a useful tool for the assessment of cardiometabolic disease risk in women aged 65 to 74. Diabetes Metab. Res. Rev. 2018, 34, e3052. [Google Scholar] [CrossRef]
- Brown, K.A.; Scherer, P.E. Update on adipose tissue and cancer. Endocr. Rev. 2023, 44, 961–974. [Google Scholar] [CrossRef]
- Van den Bosch, A.A.S.; Pijnenborg, J.M.A.; Romano, A.; Winkens, B.; van der Putten, L.J.M.; Kruitwagen, R.F.P.M.; Werner, H.M.J. The impact of adipose tissue distribution on endometrial cancer: A systematic review. Front. Oncol. 2023, 13, 1182479. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.J.; Ahn, H.S. Does endometriosis increase the risks of endometrial hyperplasia and endometrial cancer? Gynecol. Oncol. 2023, 169, 147–153. [Google Scholar] [CrossRef]
- Barnard, M.E.; Farland, L.V.; Yan, B.; Wang, J.; Trabert, B.; Doherty, J.A.; Meeks, H.D.; Madsen, M.; Guinto, E.; Collin, L.J.; et al. Endometriosis Typology and Ovarian Cancer Risk. JAMA 2024, 332, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.; Ramos, P.; Solano, J.A. Transition between endometriosis and ovarian endometrioid carcinoma [Transición entre endometriosis y carcinoma endometrioide de ovario]. Clínica Investig. Ginecol. Obstet. 2007, 34, 24–26. [Google Scholar] [CrossRef]
- Ennab, F.; Atiomo, W. Obesity and female infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 89, 102336. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, L.; Guo, Z.; Yao, N.; Zhang, S.; Pu, P. Obesity and its impact on female reproductive health. Front. Endocrinol. 2024, 14, 1326546. [Google Scholar] [CrossRef]
- Martire, F.G.; Costantini, E.; Ianes, I.; d’Abate, C.; De Bonis, M.; Capria, G.; Piccione, E.; Andreoli, A. Nutrition and Uterine Fibroids: Clinical Impact and Emerging Therapeutic Perspectives. J. Clin. Med. 2025, 14, 7140. [Google Scholar] [CrossRef]
- Abdi, Y.H.; Abdi, M.S.; Bashir, S.G.; Ahmed, N.I.; Abdullahi, Y.B. Understanding Global Health Inequality and Inequity: Causes, Consequences, and the Path Toward Justice in Healthcare. Public Health Chall. 2025, 4, e70156. [Google Scholar] [CrossRef]
- OECD. Education at a Glance 2025: OECD Indicators; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- London, S.; Temporelli, K.L.; Monterubbianesi, P.D. Vinculación entre salud, ingreso y educación: Un análisis comparativo para América Latina. Econ. Soc. 2009, 14, 125–146. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.