Anthocyanin-Rich Jamun (Syzygium cumini L.) Pulp Transported on Protein-Coated Ionic Gelation Microparticles of Calcium Alginate: Production and Morphological Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Zeta Potential of Biopolymer Solutions
2.2.2. Experimental Design
2.2.3. Ionic Gelation Microparticles (IG) Production
2.2.4. Protein-Coating (PC) by Electrostatic Interaction
2.3. Microparticles Characterization
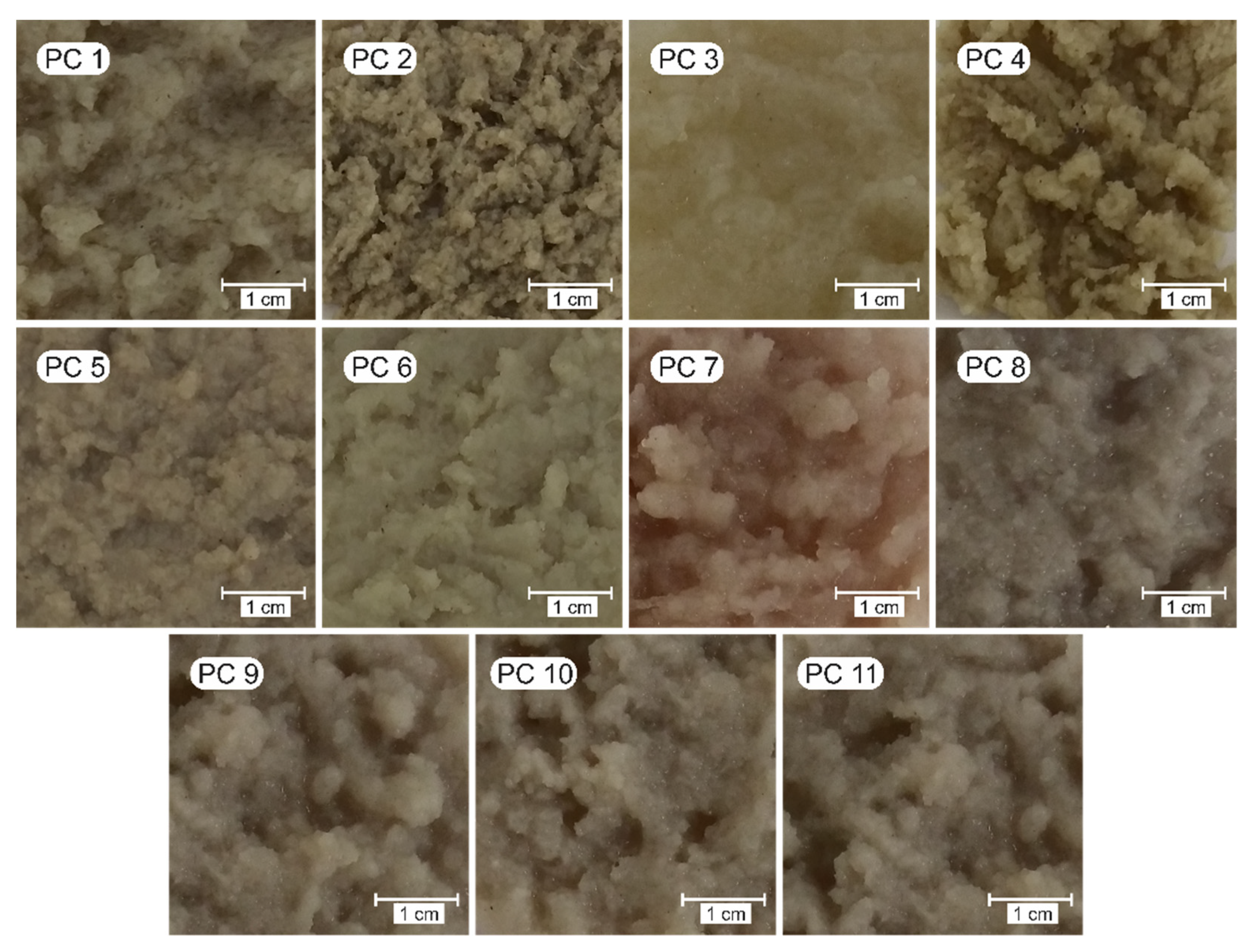
2.3.1. Visual Aspect and Morphology
2.3.2. Moisture and Protein Contents
2.3.3. Total Anthocyanins Content
2.4. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Zeta Potential of Biopolymer Solutions
3.2. Microparticles Characterization
3.2.1. Visual Aspect
3.2.2. Morphology
3.2.3. Moisture Content
3.2.4. Protein Content
3.2.5. Anthocyanins Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Zengin, G.; Mocan, A.; Simirgiotis, M.J.; Ceylan, R.; Uysal, S.; Aktumsek, A. Evaluation of Antioxidant Potential, Enzyme Inhibition Activity and Phenolic Profile of Lathyrus Cicera and Lathyrus Digitatus: Potential Sources of Bioactive Compounds for the Food Industry. Food Chem. Toxicol. 2017, 107, 609–619. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef] [PubMed]
- Dimou, C.; Karantonis, H.C.; Skalkos, D.; Koutelidakis, A.E. Valorization of Fruits By-Products to Unconventional Sources of Additives, Oil, Biomolecules and Innovative Functional Foods. Curr. Pharm. Biotechnol. 2019, 20, 776–786. [Google Scholar] [CrossRef]
- Chaudhary, A.; Bag, S.; Banerjee, P.; Chatterjee, J. Wound Healing Efficacy of Jamun Honey in Diabetic Mice Model through Reepithelialization, Collagen Deposition and Angiogenesis. J. Tradit. Complement. Med. 2020, 10, 529–543. [Google Scholar] [CrossRef]
- Kumar, M.; Hasan, M.; Lorenzo, J.M.; Dhumal, S.; Nishad, J.; Rais, N.; Verma, A.; Changan, S.; Barbhai, M.D.; Radha; et al. Jamun (Syzygium cumini (L.) Skeels) Seed Bioactives and Its Biological Activities: A Review. Food Biosci. 2022, 50, 102109. [Google Scholar] [CrossRef]
- Migliato, K.F.; Moreira, R.R.D.; Mello, J.C.P.; Sacramento, L.V.S.; Corrêa, M.A.; Salgado, H.R.N. Controle Da Qualidade Do Fruto de Syzygium cumini (L.) Skeels. Braz. J. Pharmacogn. 2007, 17, 94–101. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds and Pharmacological and Food Applications of: Syzygium cumini—A Review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Pradhan, R.C.; Mishra, S.; Patel, A.S.; Kar, A. Physicochemical and Nutritional Characterization of Jamun (Syzygium cuminii). Curr. Res. Nutr. Food Sci. 2017, 5, 25–35. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; de Oliveira, R.A. Bioactive Films of Arrowroot Starch and Blackberry Pulp: Physical, Mechanical and Barrier Properties and Stability to PH and Sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.F.; Soares, I.H.B.T.; Soares, C.T.; Fakhouri, F.M.; de Oliveira, R.A. Development and Characterization of Arrowroot Starch Films Incorporated with Grape Pomace Extract. Polysaccharides 2022, 3, 250–263. [Google Scholar] [CrossRef]
- Carmona, L.; Sulli, M.; Diretto, G.; Alquézar, B.; Alves, M.; Peña, L. Improvement of Antioxidant Properties in Fruit from Two Blood and Blond Orange Cultivars by Postharvest Storage at Low Temperature. Antioxidants 2022, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, D.; Gürel, D.B.; Çağındı, Ö.; Kayaardı, S. Heat Treatment and Microwave Applications on Homemade Sour Cherry Juice: The Effect on Anthocyanin Content and Some Physicochemical Properties. Curr. Plant Biol. 2022, 29, 100242. [Google Scholar] [CrossRef]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and Characteristics of Alginate and Anthocyanin Complexes. Int. J. Biol. Macromol. 2020, 164, 726–734. [Google Scholar] [CrossRef]
- Codevilla, C.F.; Bazana, M.T.; Da Silva, C.; Barin, J.; Ragagnin, C. Nanostructures Containing Bioactive Compounds Extracted from Plants. Ciência Nat. 2015, 37, 142–151. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of Antioxidant Phenolic Compounds Extracted from Spent Coffee Grounds by Freeze-Drying and Spray-Drying Using Different Coating Materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Wang, R.; Tian, Z.; Chen, L. A Novel Process for Microencapsulation of Fish Oil with Barley Protein. Food Res. Int. 2011, 44, 2735–2741. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Ma, S.; Yang, M.; Zhang, H.; Zhang, T.; Yu, Y.; Du, Z. Co-Assembly of Egg White-Derived Peptides and Protein-Polysaccharide Complexes for Curcumin Encapsulation: The Enhancement of Stability, Redispersibility, and Bioactivity. Food Chem. 2022, 394, 133496. [Google Scholar] [CrossRef]
- Leong, J.-Y.; Lam, W.-H.; Ho, K.-W.; Voo, W.-P.; Lee, M.F.-X.; Lim, H.-P.; Lim, S.-L.; Tey, B.-T.; Poncelet, D.; Chan, E.-S. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolye Complex Formation between Alginate and Chitosan as a Function of PH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Tankhiwale, R. Investigation of Water Uptake Behavior and Stability of Calcium Alginate/Chitosan Bi-Polymeric Beads: Part-1. React. Funct. Polym. 2006, 66, 645–658. [Google Scholar] [CrossRef]
- Ćujić, N.; Trifković, K.; Bugarski, B.; Ibrić, S.; Pljevljakušić, D.; Šavikin, K. Chokeberry (Aronia melanocarpa L.) Extract Loaded in Alginate and Alginate/Inulin System. Ind. Crops Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Albarri, R.; Torun, M.; Şahin, S. Encapsulation of Moringa Oleifera Leaf Extract in Chitosan-Coated Alginate Microbeads Produced by Ionic Gelation. Food Biosci. 2022, 50, 102158. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K.; Badwaik, L.S. Physicochemical and Release Behaviour of Phytochemical Compounds Based on Black Jamun Pulp Extracts-Filled Alginate Hydrogel Beads through Vibration Dripping Extrusion. Int. J. Biol. Macromol. 2022, 194, 715–725. [Google Scholar] [CrossRef]
- da Silva Carvalho, A.G.; da Costa Machado, M.T.; de Freitas Queiroz Barros, H.D.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Hubinger, M.D. Anthocyanins from Jussara (Euterpe edulis Martius) Extract Carried by Calcium Alginate Beads Pre-Prepared Using Ionic Gelation. Powder Technol. 2019, 345, 283–291. [Google Scholar] [CrossRef]
- Dallabona, I.D.; de Lima, G.G.; Cestaro, B.I.; Tasso, I.D.S.; Paiva, T.S.; Laureanti, E.J.G.; Jorge, L.M.d.M.; da Silva, B.J.G.; Helm, C.V.; Mathias, A.L.; et al. Development of Alginate Beads with Encapsulated Jabuticaba Peel and Propolis Extracts to Achieve a New Natural Colorant Antioxidant Additive. Int. J. Biol. Macromol. 2020, 163, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- de Moura, S.C.S.R.; Berling, C.L.; Garcia, A.O.; Queiroz, M.B.; Alvim, I.D.; Hubinger, M.D. Release of Anthocyanins from the Hibiscus Extract Encapsulated by Ionic Gelation and Application of Microparticles in Jelly Candy. Food Res. Int. 2019, 121, 542–552. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-Loaded Polysaccharides-Based Complex Particles Obtained by Polyelectrolyte Complexation and Ionic Gelation. I-Particles Obtaining and Characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Dong, Y.; Fan, Q.; Bao, Y.; Yan, H. Waterborne Polyurethane/Silica Nanocomposites Based on Electrostatic Interaction: Interfacial Interactions and Properties. Prog. Org. Coat. 2022, 171, 107052. [Google Scholar] [CrossRef]
- Wang, W.; Narain, R.; Zeng, H. Hydrogels. Polym. Sci. Nanotechnol. Fundam. Appl. 2020, 10, 203–244. [Google Scholar] [CrossRef]
- Herrman, D.A.; Brantsen, J.F.; Awika, J.M. Interactions of 3-Deoxyanthocyanins with Gum Arabic and Sodium Alginate Contributing to Improved Pigment Aqueous Stability. Food Chem. 2022, 372, 131233. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Prata, A.S.; Grosso, C.R.F. Alginate and Whey Protein Based-Multilayered Particles: Production, Characterisation and Resistance to PH, Ionic Strength and Artificial Gastric/Intestinal Fluid. J. Microencapsul. 2017, 34, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, J.C.; Nogueira, G.F.; Prata, A.S.; Grosso, C.R.F. Effect of Molar Weight of Gelatin in the Coating of Alginate Microparticles. Polimeros 2021, 31, 1–9. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Souza, F.N.; Gebara, C.; Ribeiro, M.C.E.; Chaves, K.S.; Gigante, M.L.; Grosso, C.R.F. Production and Characterization of Microparticles Containing Pectin and Whey Proteins. Food Res. Int. 2012, 49, 560–566. [Google Scholar] [CrossRef]
- Tello, F.; Falfan-Cortés, R.N.; Martinez-Bustos, F.; Martins da Silva, V.; Hubinger, M.D.; Grosso, C. Alginate and Pectin-Based Particles Coated with Globular Proteins: Production, Characterization and Anti-Oxidative Properties. Food Hydrocoll. 2015, 43, 670–678. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Arlington, TX, USA, 1998. [Google Scholar]
- Philipp, B.; Dautzenberg, H.; Linow, K.-J.; Kötz, J.; Dawydoff, W. Polyelectrolyte Complexes—Recent Developments and Open Problems. Prog. Polym. Sci. 1989, 14, 91–172. [Google Scholar] [CrossRef]
- Jun-xia, X.; Hai-yan, Y.; Jian, Y. Microencapsulation of Sweet Orange Oil by Complex Coacervation with Soybean Protein Isolate/Gum Arabic. Food Chem. 2011, 125, 1267–1272. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Comparison of Protein–Polysaccharide Nanoparticle Fabrication Methods: Impact of Biopolymer Complexation before or after Particle Formation. J. Colloid Interface Sci. 2010, 344, 21–29. [Google Scholar] [CrossRef]
- You, G.; Niu, G.; Long, H.; Zhang, C.; Liu, X. Elucidation of Interactions between Gelatin Aggregates and Hsian-Tsao Gum in Aqueous Solutions. Food Chem. 2020, 319, 126532. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Influence of Concentration, Ionic Strength and PH on Zeta Potential and Mean Hydrodynamic Diameter of Edible Polysaccharide Solutions Envisaged for Multinanolayered Films Production. Carbohydr. Polym. 2011, 85, 522–528. [Google Scholar] [CrossRef]
- Canto, A.R.; Kumon, T. Efeito Do PH e Da Homogeneização a Ultra Alta Pressão Sobre a Estabilidade Física Do Suco de Caju (Anacardium occidentale). Acta Tecnológica 2013, 8, 44–49. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Alginate-Based Encapsulation of Polyphenols from Clitoria ternatea Petal Flower Extract Enhances Stability and Biological Activity under Simulated Gastrointestinal Conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Benítez, E.I.; Genovese, D.B.; Lozano, J.E. Effect of PH and Ionic Strength on Apple Juice Turbidity: Application of the Extended DLVO Theory. Food Hydrocoll. 2007, 21, 100–109. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Peng, D.; Jin, W.; Sagis, L.M.C.; Li, B. Adsorption of Microgel Aggregates Formed by Assembly of Gliadin Nanoparticles and a β-Lactoglobulin Fibril-Peptide Mixture at the Air/Water Interface: Surface Morphology and Foaming Behavior. Food Hydrocoll. 2022, 122, 107039. [Google Scholar] [CrossRef]
- Xi, C.; Sun, Z.; Chen, X.; Ding, X.; Zhang, T. Characterization of Coacervation Behavior between Whey Protein Isolate and Propylene Glycol Alginate: A Morphology, Spectroscopy, and Thermodynamics Study. Food Chem. X 2022, 15, 100402. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; Gao, Y. Interaction and Formation Mechanism of Binary Complex between Zein and Propylene Glycol Alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef]
- Lago, E.S.; Gomes, E.; Da Silva, R. Produção de Geléia de Jambolão (Syzygium cumini Lamarck): Processamento, Parâmetros Físico—Químicos e Avaliação Sensorial. Cienc. Tecnol. Aliment. 2006, 26, 847–852. [Google Scholar] [CrossRef]
- Cuadros, T.R.; Skurtys, O.; Aguilera, J.M. Mechanical Properties of Calcium Alginate Fibers Produced with a Microfluidic Device. Carbohydr. Polym. 2012, 89, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid Gel Particles: Formation, Characterization, and Application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; De Haan, B.J.; Kamps, J.A.A.M.; Faas, M.M.; Kitano, T. Zeta-Potentials of Alginate-PLL Capsules: A Predictive Measure for Biocompatibility? J. Biomed. Mater. Res.—Part A 2007, 80, 813–819. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-Based Polyelectrolyte Multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Guo, J.; Giusti, M.M.; Kaletunç, G. Encapsulation of Purple Corn and Blueberry Extracts in Alginate-Pectin Hydrogel Particles: Impact of Processing and Storage Parameters on Encapsulation Efficiency. Food Res. Int. 2018, 107, 414–422. [Google Scholar] [CrossRef]
- Haynes, C.A.; Norde, W. Globular Proteins at Solid/Liquid Interfaces. Colloids Surfaces B Biointerfaces 1994, 2, 517–566. [Google Scholar] [CrossRef]
- Silverio, G.B.; Sakanaka, L.S.; Alvim, I.D.; Shirai, M.A.; Grosso, C.R.F. Production and Characterization of Alginate Microparticles Obtained by Ionic Gelation and Electrostatic Adsorption of Concentrated Soy Protein. Cienc. Rural 2018, 48. [Google Scholar] [CrossRef]
- Molina Ortiz, S.E.; Puppo, M.C.; Wagner, J.R. Relationship between Structural Changes and Functional Properties of Soy Protein Isolates-Carrageenan Systems. Food Hydrocoll. 2004, 18, 1045–1053. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Ribeiro, C.P.; Aguirre, J.M. de Secagem Por Atomização de Polpa de Amora-Preta Usando Maltodextrina Como Agente Carreador. Braz. J. Food Technol. 2012, 15, 157–165. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Drying Air Temperature and Carrier Agent Concentration on the Physicochemical Properties of Açai Juice Powder. Cienc. Tecnol. Aliment. 2009, 29, 444–450. [Google Scholar] [CrossRef]





| Test | Experimental Design | IG Solution | PC Solution | ||
|---|---|---|---|---|---|
| Alginate:Jamun Ratio 1 (w/w) | Coating 1 (%) | Alginate (g Solids 100 g−1) | Jamun 2 (g Solids 100 g−1) | Gelatin (g Solids 100 g−1) | |
| 1 | 1:1.26 (−1) | 1.46 (−1) | 2.00 | 2.52 | 1.46 |
| 2 | 1:1.26 (−1) | 8.54 (1) | 2.00 | 2.52 | 8.54 |
| 3 | 1:0.45 (1) | 1.46 (−1) | 2.00 | 0.91 | 1.46 |
| 4 | 1:0.45 (1) | 8.54 (1) | 2.00 | 0.91 | 8.54 |
| 5 | 1:2.00 (−1.41) | 5.00 (0) | 2.00 | 4.00 | 5.00 |
| 6 | 1:0.40 (1.41) | 5.00 (0) | 2.00 | 0.80 | 5.00 |
| 7 | 1:0.67 (0) | 0.00 (−1.41) | 2.00 | 1.33 | 0.00 |
| 8 | 1:0.67 (0) | 10.00 (1.41) | 2.00 | 1.33 | 10.00 |
| 9 | 1:0.67 (0) | 5.00 (0) | 2.00 | 1.33 | 5.00 |
| 10 | 1:0.67 (0) | 5.00 (0) | 2.00 | 1.33 | 5.00 |
| 11 | 1:0.67 (0) | 5.00 (0) | 2.00 | 1.33 | 5.00 |
| Solutions (0.2% w/w) | pH | ZP (mV) |
|---|---|---|
| Alginate | 6.0 | −74.40 ± 7.35 |
| Gelatin | 4.0 | 8.96 ± 1.15 |
| Jamun pulp | 4.0 | −17.20 ± 1.84 |
| Run | Moisture Content (%) | Protein Content 1 (%) | Anthocyanins Content (mg 100 g−1) | ||
|---|---|---|---|---|---|
| IG | PC | PC | IG | PC | |
| 1 | 97.29 ± 0.64 | 95.53 ± 0.12 | 21.82 ± 0.72 | 6.61 ± 0.87 | 1.96 ± 0.47 |
| 2 | 97.29 ± 0.64 | 88.24 ± 0.29 | 37.89 ± 5.88 | 6.61 ± 0.87 | 1.24 ± 0.20 |
| 3 | 97.22 ± 0.13 | 96.64 ± 0.07 | 29.33 ± 1.74 | 4.51 ± 0.64 | 3.64 ± 0.73 |
| 4 | 97.22 ± 0.13 | 87.04 ± 0.22 | 36.62 ± 4.33 | 4.51 ± 0.64 | 1.15 ± 0.09 |
| 5 | 96.40 ± 0.05 | 91.41 ± 0.08 | 23.75 ± 0.23 | 6.59 ± 1.53 | 1.23 ± 0.04 |
| 6 | 97.02 ± 0.05 | 91.36 ± 0.06 | 36.87 ± 0.81 | 2.26 ± 0.16 | 1.89 ± 0.45 |
| 7 | 97.06 ± 0.12 | 97.06 ± 0.12 | 0.00 ± 0.00 | 5.84 ± 0.62 | 5.84 ± 0.62 |
| 8 | 97.06 ± 0.12 | 93.50 ± 0.08 | 35.99 ± 3.06 | 5.84 ± 0.62 | 0.78 ± 0.14 |
| 9 | 97.06 ± 0.12 | 94.36 ± 0.05 | 55.87 ± 4.23 | 5.82 ± 0.30 | 1.88 ± 0.28 |
| 10 | 97.06 ± 0.12 | 94.38 ± 0.04 | 45.01 ± 1.03 | 5.82 ± 0.30 | 1.86 ± 0.08 |
| 11 | 97.06 ± 0.12 | 94.37 ± 0.05 | 49.00 ± 1.40 | 5.82 ± 0.30 | 1.84 ± 0.19 |
| Run | Protein Content (%) | Relative Deviation (%) | |
|---|---|---|---|
| Experimental Value | Predicted Value | ||
| 1 | 21.82 | 21.28 | 2.48 |
| 2 | 37.89 | 39.86 | 5.20 |
| 3 | 29.33 | 21.28 | 27.45 |
| 4 | 36.62 | 39.86 | 8.85 |
| 5 | 23.75 | 42.44 | 78.68 |
| 6 | 36.87 | 42.44 | 15.10 |
| 7 | 0.00 | 5.74 | - |
| 8 | 35.99 | 31.95 | 11.24 |
| 9 | 55.87 | 42.44 | 24.05 |
| 10 | 45.01 | 42.44 | 5.72 |
| 11 | 49.00 | 42.44 | 13.40 |
| Mean relative deviation (%) | 19.22 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.G.d.; Nogueira, G.F.; Soares, C.T.; Oliveira, R.A.d. Anthocyanin-Rich Jamun (Syzygium cumini L.) Pulp Transported on Protein-Coated Ionic Gelation Microparticles of Calcium Alginate: Production and Morphological Characteristics. Polysaccharides 2023, 4, 33-50. https://doi.org/10.3390/polysaccharides4010002
Silva VGd, Nogueira GF, Soares CT, Oliveira RAd. Anthocyanin-Rich Jamun (Syzygium cumini L.) Pulp Transported on Protein-Coated Ionic Gelation Microparticles of Calcium Alginate: Production and Morphological Characteristics. Polysaccharides. 2023; 4(1):33-50. https://doi.org/10.3390/polysaccharides4010002
Chicago/Turabian StyleSilva, Vitor Gonçalves da, Gislaine Ferreira Nogueira, Cyntia Trevisan Soares, and Rafael Augustus de Oliveira. 2023. "Anthocyanin-Rich Jamun (Syzygium cumini L.) Pulp Transported on Protein-Coated Ionic Gelation Microparticles of Calcium Alginate: Production and Morphological Characteristics" Polysaccharides 4, no. 1: 33-50. https://doi.org/10.3390/polysaccharides4010002
APA StyleSilva, V. G. d., Nogueira, G. F., Soares, C. T., & Oliveira, R. A. d. (2023). Anthocyanin-Rich Jamun (Syzygium cumini L.) Pulp Transported on Protein-Coated Ionic Gelation Microparticles of Calcium Alginate: Production and Morphological Characteristics. Polysaccharides, 4(1), 33-50. https://doi.org/10.3390/polysaccharides4010002









