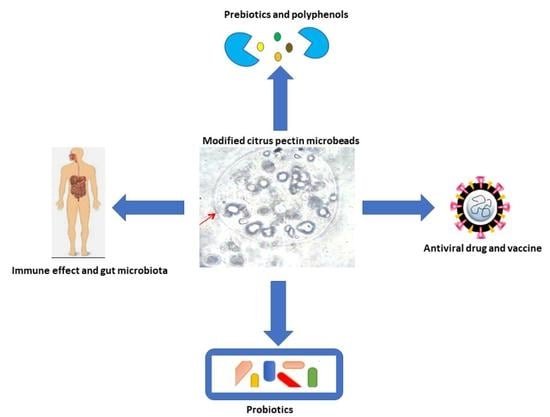
Potential Biomedical Applications of Modified Pectin as a Delivery System for Bioactive Substances
Abstract
1. Introduction
2. Structural Properties of Pectin
2.1. Gelling Components
2.2. Non-Gelling Components
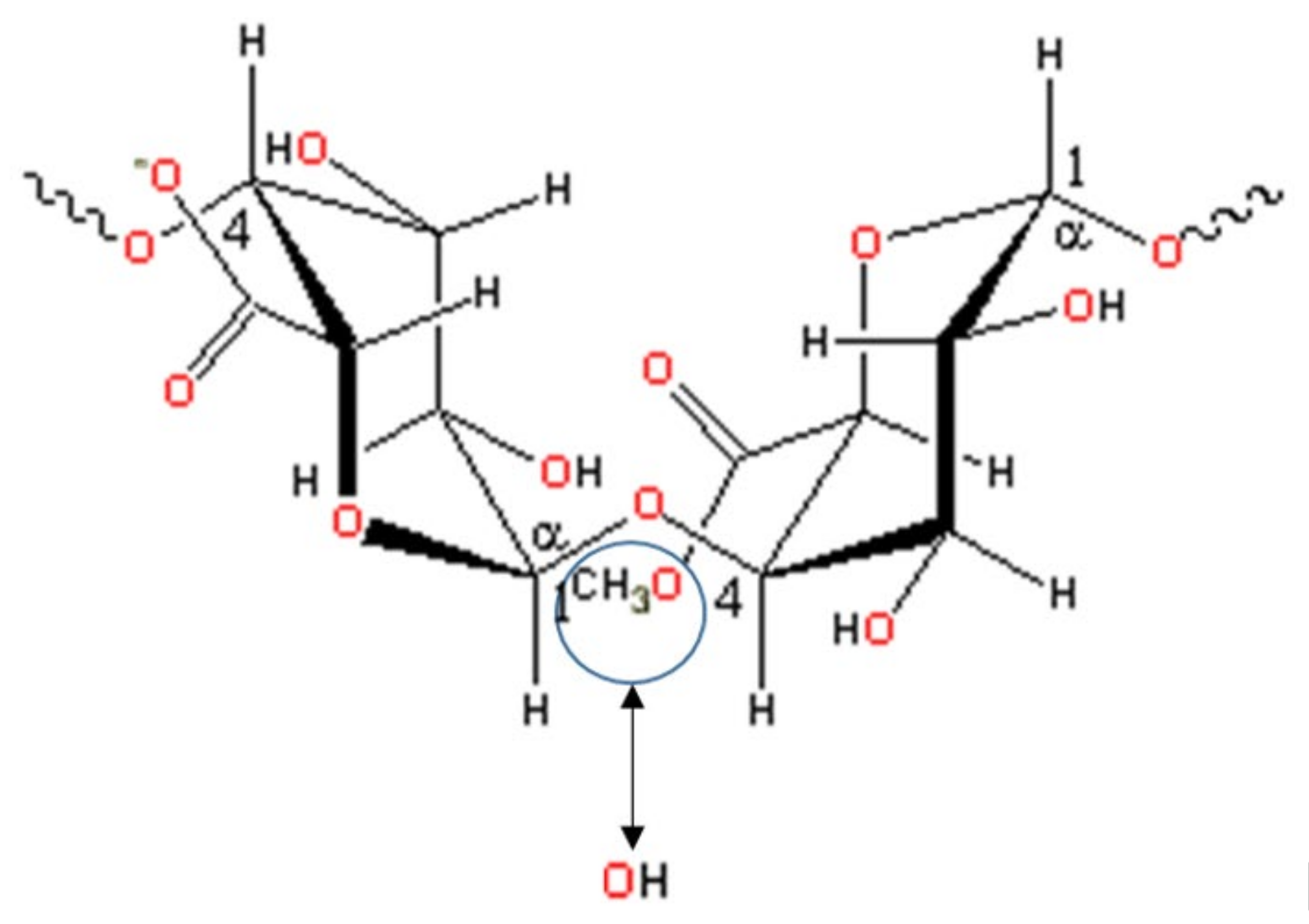
3. Degree of Esterification in Modified Pectin
4. Recent Extraction Techniques of Modified Pectin
4.1. Ultrasound
4.2. Ohmic Heating
4.3. Microwave
4.4. Other Techniques
5. Modification Techniques of Pectin
5.1. Chemical Modification
5.2. Enzymatic Modification
5.3. Ultrasound Irradiation
6. Applications of Modified Pectin in Biomedicals
6.1. MP Immune Interaction Effect
6.2. MP Micro- and/or Nano-Encapsulation and Delivery System
6.2.1. Probiotics
6.2.2. Vaccine Products
6.2.3. Polyphenols
6.3. Prebiotic Effect
6.4. Gut Microbiota Effect
6.5. Synergistic Effect
6.6. Anti-Viral Effect
6.7. Other Applications
7. Conclusions and Future Directions
8. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Market and Market Inc. Bioplastics & Biopolymers Market by Type (Non-Biodegradable/Bio-Based, Biodegradable), End-Use Industry (Packaging, Consumer Goods, Automotive & Transportation, Textiles, Agriculture & Horticulture), Region—Global Forecast to 2026. 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/biopolymers-bioplastics-market-88795240.html?gclid (accessed on 6 September 2022).
- Abu-Elsaad, N.M.; Elkashef, W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharmacol. 2016, 94, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chen, X.; Tang, T. Recent progress in controlled carbonization of (waste) polymers. Prog. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Sen, A.; Manuel, S.; Kale, R. Fruit waste pectin in enhancing the establishment of probiotic bacteria. J. Nutr. Health Food Eng. 2014, 1, 124–126. [Google Scholar]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Yapo, B.M. Pineapple and banana pectins comprise fewer homogalacturonan building blocks with a smaller degree of polymerization as compared with yellow passion fruit and lemon pectins: Implication for gelling properties. Biomacromolecules 2009, 10, 717–721. [Google Scholar] [CrossRef]
- Freitas, C.; Costa, A.; Rodrigues, F.; Júnior, M.; Dias, M.; Sousa, R. Optimization of pectin extraction from passion fruit (Passiflora edulis flavicarpa) using the response surface method. Braz. J. Dev. 2020, 6, 25609–25625. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.; Wang, Z.; Lu, M.; Yue, K. Modified citrus pectins by UV/H2O2 oxidation at acidic and basic conditions: Structures and in vitro anti-inflammatory, anti-proliferative activities. Carbohydr. Polym. 2020, 247, 116742. [Google Scholar] [CrossRef]
- Qi, X.; Al-Ghazzewi, F.H.; Tester, R.F. Dietary fiber, gastric emptying, and carbohydrate digestion: A mini-review. Starch-Stärke 2018, 70, 1700346. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Kennedy, R.A. Swelling and diffusion studies of calcium polysaccharide gels intended for film coating. Int. J. Pharm. 2008, 358, 205–213. [Google Scholar] [CrossRef]
- Majee, S.B.; Avlani, D.; Ghosh, P.; Biswas, G.R. Therapeutic and pharmaceutical benefits of native and modified plant pectin. J. Med. Plant Res. 2018, 12, 1–6. [Google Scholar]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The emulsifying and emulsion-stabilizing properties of pectin: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic effects of modified citrus pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef]
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329. [Google Scholar] [CrossRef]
- Hossein, G.; Keshavarz, M.; Ahmadi, S.; Naderi, N. Synergistic effects of PectaSol-C modified citrus pectin an inhibitor of Galectin-3 and paclitaxel on apoptosis of human SKOV-3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 7561–7568. [Google Scholar] [CrossRef]
- Keizman, D.; Frenkel, M.A.; Peer, A.; Rosenbaum, E.; Margel, D.; Sarid, D.; Neiman, V.; Leibovitch, I.; Sternberg, I.A.; Boursi, B. Effect of pectasol-c modified citrus pectin (P-MCP) treatment (tx) on PSA dynamics in non-metastatic biochemically relapsed prostate cancer (BRPC) patients (pts): Primary outcome analysis of a prospective phase II study. J. Clin. Oncol. 2019, 37, e16609. [Google Scholar] [CrossRef]
- Tehranian, N.; Sepehri, H.; Mehdipour, P.; Biramijamal, F.; Hossein-Nezhad, A.; Sarrafnejad, A.; Hajizadeh, E. Combination effect of PectaSol and Doxorubicin on viability, cell cycle arrest and apoptosis in DU-145 and LNCaP prostate cancer cell lines. Cell Biol. Int. 2012, 36, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Katz, A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and-independent prostate cancer cells. Integr. Cancer Ther. 2010, 9, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Voragen, A.G.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Diaz, J.V.; Anthon, G.E.; Barrett, D.M. Nonenzymatic degradation of citrus pectin and pectate during prolonged heating: Effects of pH, temperature, and degree of methyl esterification. J. Agric. Food Chem. 2007, 55, 5131–5136. [Google Scholar] [CrossRef]
- Morris, V.J. Pectin galactans, galectins and health Bioactive roles for pectin. Agro Food Ind. Hi-Tech 2009, 20, 37–40. [Google Scholar]
- Gunning, A.P.; Bongaerts, R.J.; Morris, V.J. Recognition of galactan components of pectin by galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and characterization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Yapo, B.M.; Koffi, K.L. Yellow passion fruit rind a potential source of low-methoxyl pectin. J. Agric. Food Chem. 2006, 54, 2738–2744. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Munyensanga, C.; Celus, M.; Dewettinck, K.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Molar mass influence on pectin-Ca2+ adsorption capacity, interaction energy and associated functionality: Gel microstructure and stiffness. Food Hydrocoll. 2018, 85, 331–342. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Schols, H.; Voragen, A. Complex pectins: Structure elucidation using enzymes. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 14, pp. 3–19. [Google Scholar]
- Vincken, J.-P.; Schols, H.A.; Oomen, R.J.; McCann, M.C.; Ulvskov, P.; Voragen, A.G.; Visser, R.G. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol. 2003, 132, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin–an emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Moore, J.P.; Farrant, J.M.; Driouich, A. A role for pectin-associated arabinans in maintaining the flexibility of the plant cell wall during water deficit stress. Plant Signal. Behav. 2008, 3, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.H.; Mottern, H.; Nutting, G.; Speiser, R. Enzyme-demethylated pectinates and their gelation. Food Technol. 1949, 3, 90–94. [Google Scholar]
- Rinaudo, M. Physicochemical properties of pectins in solution and gel states. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 14, pp. 21–33. [Google Scholar]
- Eliaz, I. The potential role of modified citrus pectin in the prevention of cancer metastasis. Clin. Pract. Altern. Med. 2001, 2, 177–180. [Google Scholar]
- Lee, T.; Chang, Y.H. Structural, physicochemical, and in-vitro release properties of hydrogel beads produced by oligochitosan and de-esterified pectin from yuzu (Citrus junos) peel as a quercetin delivery system for colon target. Food Hydrocoll. 2020, 108, 106086. [Google Scholar] [CrossRef]
- Jung, J.; Arnold, R.D.; Wicker, L. Pectin and charge modified pectin hydrogel beads as a colon-targeted drug delivery carrier. Colloids Surf. B. 2013, 104, 116–121. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Jabarah, Z. Preparation and characterization of maleate, tartarate, and phthalate modified pectin. J. Food Ind. Nutr. Sci. 2012, 2, 57–64. [Google Scholar]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Li, D.-Q.; Du, G.-M.; Jing, W.-W.; Li, J.-F.; Yan, J.-Y.; Liu, Z.-Y. Combined effects of independent variables on yield and protein content of pectin extracted from sugar beet pulp by citric acid. Carbohydr. Polym. 2015, 129, 108–114. [Google Scholar] [CrossRef]
- Tan, J.; Hua, X.; Liu, J.; Wang, M.; Liu, Y.; Yang, R.; Cao, Y. Extraction of sunflower head pectin with superfine grinding pretreatment. Food Chem. 2020, 320, 126631. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnick, P. Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocoll. 2019, 87, 237–244. [Google Scholar] [CrossRef]
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Mesbahi, G.; Jamalian, J.; Farahnaky, A. A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocoll. 2005, 19, 731–738. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, J.-F.; Li, D.-Q. Comparative studies of combined influence of variables on the esterification degree of pectin extracted by sulphuric acid and citric acid. Adv. Polym. Technol. 2019, 2019, 6313241. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Zhang, F.; Yang, X.; Ni, L.; Zhang, W.; Liu, Z.; Zhang, Y. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocoll. 2020, 98, 105246. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Pulse Electric Field-Assisted Extraction; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci. 2018, 21, 20–26. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Broxterman, S.E.; Picouet, P.; Schols, H.A. Acetylated pectins in raw and heat processed carrots. Carbohydr. Polym. 2017, 177, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Polanco-Lugo, E.; Martínez-Castillo, J.I.; Cuevas-Bernardino, J.C.; González-Flores, T.; Valdez-Ojeda, R.; Pacheco, N.; Ayora-Talavera, T. Citrus pectin obtained by ultrasound-assisted extraction: Physicochemical, structural, rheological and functional properties. CyTA-J. Food 2019, 17, 463–471. [Google Scholar] [CrossRef]
- Yu, M.; Xia, Y.; Zhou, M.; Guo, Y.; Zheng, J.; Zhang, Y. Effects of different extraction methods on structural and physicochemical properties of pectins from finger citron pomace. Carbohydr. Polym. 2021, 258, 117662. [Google Scholar] [CrossRef]
- Umaña, M.M.; Dalmau, M.E.; Eim, V.S.; Femenia, A.; Rosselló, C. Effects of acoustic power and pH on pectin-enriched extracts obtained from citrus by products. Modelling of the extraction process. J. Sci. Food Agric. 2019, 99, 6893–6902. [Google Scholar] [CrossRef]
- Patience, N.; Schieppati, D.; Boffito, D. Continuous and pulsed ultrasound pectin extraction from navel orange peels. Ultrason. Sonochem. 2021, 73, 105480. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S.; Kasapis, S.; Boye, J.I. Novel Food Processing: Effects on Rheological and Functional Properties; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Varghese, K.S.; Pandey, M.; Radhakrishna, K.; Bawa, A. Technology, applications and modelling of ohmic heating: A review. J. Food Sci. Technol. 2014, 51, 2304–2317. [Google Scholar] [CrossRef]
- Saberian, H.; Hamidi-Esfahani, Z.; Gavlighi, H.A.; Barzegar, M. Optimization of pectin extraction from orange juice waste assisted by ohmic heating. Chem. Eng. Process. 2017, 117, 154–161. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef]
- Arrutia, F.; Adam, M.; Calvo-Carrascal, M.Á.; Mao, Y.; Binner, E. Development of a continuous-flow system for microwave-assisted extraction of pectin-derived oligosaccharides from food waste. J. Chem. Eng. 2020, 395, 125056. [Google Scholar] [CrossRef]
- Li, D.-Q.; Jia, X.; Wei, Z.; Liu, Z.-Y. Box–Behnken experimental design for investigation of microwave-assisted extracted sugar beet pulp pectin. Carbohydr. Polym. 2012, 88, 342–346. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Su, D.-L.; Li, P.-J.; Quek, S.Y.; Huang, Z.-Q.; Yuan, Y.-J.; Li, G.-Y.; Shan, Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem. 2019, 286, 1–7. [Google Scholar] [CrossRef]
- Liew, S.Q.; Teoh, W.H.; Tan, C.K.; Yusoff, R.; Ngoh, G.C. Subcritical water extraction of low methoxyl pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Int. J. Biol. Macromol. 2018, 116, 128–135. [Google Scholar] [CrossRef]
- Lal, A.N.; Prince, M.V.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74, 102844. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Liu, J.-P.; Huang, X.; Du, L.-P.; Shi, F.-L.; Dong, R.; Huang, X.-T.; Zheng, K.; Liu, Y.; Cheong, K.-L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Fracasso, A.F.; Perussello, C.A.; Carpiné, D.; de Oliveira Petkowicz, C.L.; Haminiuk, C.W.I. Chemical modification of citrus pectin: Structural, physical and rheologial implications. Int. J. Biol. Macromol. 2018, 109, 784–792. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, W.; Gao, W.; Tian, G.; Zhao, C.; Bao, Y.; Cui, J.; Lian, Y.; Zheng, J. Effect of mesoscopic structure of citrus pectin on its emulsifying properties: Compactness is more important than size. J. Colloid Interface Sci. 2020, 570, 80–88. [Google Scholar] [CrossRef]
- Kurita, O.; Miyake, Y.; Yamazaki, E. Chemical modification of citrus pectin to improve its dissolution into water. Carbohydr. Polym. 2012, 87, 1720–1727. [Google Scholar] [CrossRef]
- Sun, Q.; Wicker, L. Hydrogel Encapsulation of Lactobacillus casei by Block Charge Modified Pectin and Improved Gastric and Storage Stability. Foods 2021, 10, 1337. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Verkempinck, S.H.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Targeted modifications of citrus pectin to improve interfacial properties and the impact on emulsion stability. Food Hydrocoll. 2022, 132, 107841. [Google Scholar] [CrossRef]
- Georgiev, Y.; Ognyanov, M.; Yanakieva, I.; Kussovski, V.; Kratchanova, M. Isolation, characterization and modification of citrus pectins. J. Biosci. Biotechnol. 2012, 1, 223–233. [Google Scholar]
- Celus, M.; Salvia-Trujillo, L.; Kyomugasho, C.; Maes, I.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Structurally modified pectin for targeted lipid antioxidant capacity in linseed/sunflower oil-in-water emulsions. Food Chem. 2018, 241, 86–96. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Chen, T.-T.; Zhang, Z.-H.; Wang, Z.-W.; Chen, Z.-L.; Ma, H.; Yan, J.-K. Effects of ultrasound modification at different frequency modes on physicochemical, structural, functional, and biological properties of citrus pectin. Food Hydrocoll. 2021, 113, 106484. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Montilla, A.; Moreno, F.J.; Villamiel, M. Modification of citrus and apple pectin by power ultrasound: Effects of acid and enzymatic treatment. Ultrason. Sonochem. 2017, 38, 807–819. [Google Scholar] [CrossRef]
- Wang, W.; Feng, Y.; Chen, W.; Adie, K.; Liu, D.; Yin, Y. Citrus pectin modified by microfluidization and ultrasonication: Improved emulsifying and encapsulation properties. Ultrason. Sonochem. 2021, 70, 105322. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kirkland, R.; Grunewald, Z.I.; Sun, Q.; Wicker, L.; de La Serre, C.B. Beneficial effects of non-encapsulated or encapsulated probiotic supplementation on microbiota composition, intestinal barrier functions, inflammatory profiles, and glucose tolerance in high fat fed rats. Nutrients 2019, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.; Jammal, J.; Bianco-Peled, H. Insights into the nanostructure of low-methoxyl pectin–calcium gels. Carbohydr. Polym. 2013, 97, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus pectin research advances: Derived as a biomaterial in the construction and applications of micro/nano-delivery systems. Food Hydrocoll. 2022, 133, 107910. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Wang, W.; Zou, M.; Ding, T.; Ye, X.; Liu, D. Synergistic effect and mechanisms of combining ultrasound and pectinase on pectin hydrolysis. Food Bioproc. Technol. 2016, 9, 1249–1257. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Kyomugasho, C.; Gutiérrez-Ortiz, A.A.; De Bie, M.; Panozzo, A.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Production and molecular characterization of tailored citrus pectin-derived compounds. Food Chem. 2022, 367, 130635. [Google Scholar] [CrossRef]
- Larsen, L.R.; Buerschaper, J.; Schieber, A.; Weber, F. Interactions of anthocyanins with pectin and pectin fragments in model solutions. J. Agric. Food Chem. 2019, 67, 9344–9353. [Google Scholar] [CrossRef]
- Do Prado, S.B.R.; Shiga, T.M.; Harazono, Y.; Hogan, V.A.; Raz, A.; Carpita, N.C.; Fabi, J.P. Migration and proliferation of cancer cells in culture are differentially affected by molecular size of modified citrus pectin. Carbohydr. Polym. 2019, 211, 141–151. [Google Scholar] [CrossRef]
- Odun-Ayo, F.; Reddy, L. Potential Roles of Modified Pectin Targeting Galectin-3 against Severe Acute Respiratory Syndrome Coronavirus-2. J 2021, 4, 824–837. [Google Scholar] [CrossRef]
- Merheb, R.; Abdel-Massih, R.M.; Karam, M.C. Immunomodulatory effect of natural and modified citrus pectin on cytokine levels in the spleen of BALB/c mice. Int. J. Biol. Macromol. 2019, 121, 1–5. [Google Scholar] [CrossRef]
- Garcia-Crespo, K.E.; Chan, C.C.; Gabryszewski, S.J.; Percopo, C.M.; Rigaux, P.; Dyer, K.D.; Domachowske, J.B.; Rosenberg, H.F. Lactobacillus priming of the respiratory tract: Heterologous immunity and protection against lethal pneumovirus infection. Antivir. Res. 2013, 97, 270–279. [Google Scholar] [CrossRef]
- Barbosa, J.R.; de Carvalho Junior, R.N. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef]
- Sekhon, B.S. Food nanotechnology–an overview. Nanotechnol. Sci. Appl. 2010, 3, 1. [Google Scholar]
- Liu, H.; Xie, M.; Nie, S. Recent trends and applications of polysaccharides for microencapsulation of probiotics. Food Front. 2020, 1, 45–59. [Google Scholar] [CrossRef]
- Wicker, L.; Kim, Y.; Kim, M.-J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a bioactive polysaccharide–Extracting tailored function from less. Food Hydrocoll. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Bai, F.; Diao, J.; Wang, Y.; Sun, S.; Zhang, H.; Liu, Y.; Wang, Y.; Cao, J. A new water-soluble nanomicelle formed through self-assembly of pectin–curcumin conjugates: Preparation, characterization, and anticancer activity evaluation. J. Agric. Food Chem. 2017, 65, 6840–6847. [Google Scholar] [CrossRef]
- Hwang, S.W.; Shin, J.S. Pectin-coated curcumin-chitosan microparticles crosslinked with Mg2+ for delayed drug release in the digestive system. Int. J. Polym. Sci. 2018, 2018, 2071071. [Google Scholar] [CrossRef]
- Tian, L.; Singh, A.; Singh, A.V. Synthesis and characterization of pectin-chitosan conjugate for biomedical application. Int. J. Biol. Macromol. 2020, 153, 533–538. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S.; Nagy, K.S.; Eweis, M. Surface modification of polypropylene films by chitosan and chitosan/pectin multilayer. Carbohydr. Polym. 2008, 71, 187–195. [Google Scholar] [CrossRef]
- Hiorth, M.; Versland, T.; Heikkilä, J.; Tho, I.; Sande, S.A. Immersion coating of pellets with calcium pectinate and chitosan. Int. J. Pharm. 2006, 308, 25–32. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Y.; Ferguson, L.R.; Shu, Q.; Garg, S. Evaluation of mucoadhesive coatings of chitosan and thiolated chitosan for the colonic delivery of microencapsulated probiotic bacteria. J. Microencapsul. 2013, 30, 103–115. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A. Binding behavior of calcium to polyuronates: Comparison of pectin with alginate. Carbohydr. Polym. 2008, 72, 334–341. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, L. Multiple steps and critical behaviors of the binding of calcium to alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef]
- Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food; World Health Organization: London, UK; Food and Agriculture Organization: Quebec City, QC, Canada, 2002. [Google Scholar]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Short, M.D.; Basit, A.W. An in vivo comparison of intestinal pH and bacteria as physiological trigger mechanisms for colonic targeting in man. J. Control. Release 2008, 130, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Gebara, C.; Chaves, K.S.; Ribeiro, M.C.E.; Souza, F.N.; Grosso, C.R.; Gigante, M.L. Viability of Lactobacillus acidophilus La5 in pectin–whey protein microparticles during exposure to simulated gastrointestinal conditions. Int. Food Res. J. 2013, 51, 872–878. [Google Scholar] [CrossRef]
- Gerez, C.L.; Font de Valdez, G.; Gigante, M.L.; Grosso, C. Whey protein coating bead improves the survival of the probiotic Lactobacillus rhamnosus CRL 1505 to low pH. Lett. Appl. Microbiol. 2012, 54, 552–556. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; García-Galindo, H.; Alvarez-Ramírez, J.; Vernon-Carter, E.J. Textural properties of alginate–pectin beads and survivability of entrapped Lb. casei in simulated gastrointestinal conditions and in yoghurt. Food Res. J. 2010, 43, 111–117. [Google Scholar] [CrossRef]
- Brinques, G.B.; Ayub, M.A.Z. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J. Food Eng. 2011, 103, 123–128. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Odun-Ayo, F.; Mellem, J.; Reddy, L. Improving the survival of probiotic in simulated conditions and azoxymethane-induced colon tumour bearing mice using modified citrus pectin-alginate microencapsulation. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 101–109. [Google Scholar] [CrossRef]
- Odun-Ayo, F.; Mellem, J.; Reddy, L. The effect of modified citrus pectin-probiotic on faecal lactobacilli in Balb/c mice. Food Sci. Technol. 2017, 37, 478–482. [Google Scholar] [CrossRef]
- Sandolo, C.; Péchiné, S.; Le Monnier, A.; Hoys, S.; Janoir, C.; Coviello, T.; Alhaique, F.; Collignon, A.; Fattal, E.; Tsapis, N. Encapsulation of Cwp84 into pectin beads for oral vaccination against Clostridium difficile. Eur. J. Pharm. Biopharm. 2011, 79, 566–573. [Google Scholar] [CrossRef]
- Bruxelle, J.; Tsapis, N.; Hoys, S.; Collignon, A.; Janoir, C.; Fattal, E.; Péchiné, S. Protection against Clostridium difficile infection in a hamster model by oral vaccination using flagellin FliC-loaded pectin beads. Vaccine 2018, 36, 6017–6021. [Google Scholar] [CrossRef]
- Vidhyalakshmi, R.; Bhakyaraj, R.; Subhasree, R. Encapsulation “the future of probiotics”—A review. Adv. Biol. Res. 2009, 3, 96–103. [Google Scholar]
- Anosova, N.G.; Brown, A.M.; Li, L.; Liu, N.; Cole, L.E.; Zhang, J.; Mehta, H.; Kleanthous, H. Systemic antibody responses induced by a two-component Clostridium difficile toxoid vaccine protect against C. difficile-associated disease in hamsters. J. Med. Microbiol. 2013, 62, 1394–1404. [Google Scholar] [CrossRef]
- Kociolek, L.K.; Gerding, D.N. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 150–160. [Google Scholar] [CrossRef]
- Ghose, C.; Eugenis, I.; Sun, X.; Edwards, A.N.; McBride, S.M.; Pride, D.T.; Kelly, C.P.; Ho, D.D. Immunogenicity and protective efficacy of recombinant Clostridium difficile flagellar protein FliC. Emerg. Microbes Infect. 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Reddy, L. Gastrointestinal microbiota dysbiosis associated with SARS-CoV-2 infection in colorectal cancer: The implication of probiotics. Gastroenterol. Insights 2022, 13, 35–59. [Google Scholar] [CrossRef]
- Odun-Ayo, F.; Mellem, J.; Naicker, T.; Reddy, L. Chemoprevention of azoxymethane-induced colonic carcinogenesis in Balb/c mice using a modified pectin alginate probiotic. Anticancer Res. 2015, 35, 4765–4775. [Google Scholar] [PubMed]
- Vos, A.P.; Haarman, M.; VanGinkel, J.W.H.; Knol, J.; Garssen, J.; Stahl, B.; Boehm, G.; M’Rabet, L. Dietary supplementation of neutral and acidic oligosaccharides enhances Th1-dependent vaccination responses in mice. Pediatr. Allergy Immunol. 2007, 18, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020, 132, 109098. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef]
- Huang, W.; Wang, L.; Wei, Y.; Cao, M.; Xie, H.; Wu, D. Fabrication of lysozyme/κ-carrageenan complex nanoparticles as a novel carrier to enhance the stability and in vitro release of curcumin. Int. J. Biol. Macromol. 2020, 146, 444–452. [Google Scholar] [CrossRef]
- Moideen, M.M.; Karuppaiyan, K.; Kandhasamy, R.; Seetharaman, S. Skimmed milk powder and pectin decorated solid lipid nanoparticle containing soluble curcumin used for the treatment of colorectal cancer. J. Food Process Eng. 2020, 43, e13246. [Google Scholar] [CrossRef]
- Sabra, R.; Billa, N.; Roberts, C.J. An augmented delivery of the anticancer agent, curcumin, to the colon. React. Funct. Polym. 2018, 123, 54–60. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wu, J.-Y. Biocompatible polyelectrolyte complex nanoparticles from lactoferrin and pectin as potential vehicles for antioxidative curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef]
- Cheewatanakornkool, K.; Niratisai, S.; Manchun, S.; Dass, C.R.; Sriamornsak, P. Characterization and in vitro release studies of oral microbeads containing thiolated pectin–doxorubicin conjugates for colorectal cancer treatment. Asian J. Pharm. Sci. 2017, 12, 509–520. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Khaniki, M.; Azizian, S.; Mohaghgheghi, M.A.; Sadeghizadeh, M.; Najafi, F. Chemoprevention of azoxymethane-initiated colon cancer in rat by using a novel polymeric nanocarrier–curcumin. Eur. J. Pharmacol. 2012, 689, 226–232. [Google Scholar] [CrossRef]
- Cai, R.; Pan, S.; Li, R.; Xu, X.; Pan, S.; Liu, F. Curcumin loading and colon release of pectin gel beads: Effect of different de-esterification method. Food Chem. 2022, 389, 133130. [Google Scholar] [CrossRef]
- Pourasgari, F.; Ahmadian, S.; Salmanian, A.H.; Sarbolouki, M.N.; Massumi, M. Low cytotoxicity effect of dendrosome as an efficient carrier for rotavirus VP2 gene transferring into a human lung cell line. Mol. Biol. Rep. 2009, 36, 105–109. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef]
- Sharma, A.; Agarwal, V.; Kumar, R.; Chaurasia, H.; Chaurasia, D.; Bhardwaj, P. Prebiotics: A review of therapeutic potential. J. Pharm. Innov. 2011, 1, 28–40. [Google Scholar]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Dantas, A.; Verruck, S.; de Liz, G.R.; Hernandez, E.; Prudencio, E.S. Lactose-free skim milk and prebiotics as carrier agents of Bifidobacterium BB-12 microencapsulation: Physicochemical properties, survival during storage and in vitro gastrointestinal condition behaviour. Int. J. Food Sci. 2021, 56, 2132–2145. [Google Scholar] [CrossRef]
- Dos Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Pinto, S.S.; Muñoz, I.B.; Amboni, R.D. Effect of microencapsulation on survival of Bifidobacterium BB-12 exposed to simulated gastrointestinal conditions and heat treatments. LWT-Food Sci. Technol. 2013, 50, 39–44. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Walter, J.; Hutkins, R.W. Synbiotics for improved human health: Recent developments, challenges, and opportunities. Annu. Rev. Food. Sci. Technol. 2018, 9, 451–479. [Google Scholar] [CrossRef]
- Ouwehand, A.; Tiihonen, K.; Mäkivuokko, H.; Rautonen, N. Synbiotics: Combining the benefits of pre-and probiotics. J. Funct. Foods. 2007, 2, 195–213. [Google Scholar]
- Watson, R.; Preedy, V.R. Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Azagra-Boronat, I.; Rodríguez-Lagunas, M.J.; Castell, M.; Pérez-Cano, F.J. Prebiotics for gastrointestinal infections and acute diarrhea. In Dietary Interventions in Gastrointestinal Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–191. [Google Scholar]
- Licht, T.R.; Ebersbach, T.; Frøkiær, H. Prebiotics for prevention of gut infections. Trends Food Sci. Technol. 2012, 23, 70–82. [Google Scholar] [CrossRef]
- Chackoshian, K.A.; Shojaosadati, S.A. Improvement of probiotic survival in fruit juice and under gastrointestinal conditions using pectin-nanochitin-nanolignocellulose as a novel prebiotic gastrointestinal-resistant matrix. J. Carbaridian Biotechnol. 2017, 4, 179–191. [Google Scholar]
- Hotchkiss, A.T.; Liu, L.; Call, J.; Cooke, P.; Luchansky, J.B.; Rastall, R. (Eds.) Synbiotic matrices derived from plant oligosaccharides and polysaccharides. In New Delivery Systems for Controlled Drug Release from Naturally Occurring Materials; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008. [Google Scholar]
- Pimentel, T.C.; de Oliveira, L.I.G.; Macedo, E.d.L.C.; Costa, G.N.; Dias, D.R.; Schwan, R.F.; Magnani, M. Understanding the potential of fruits, flowers, and ethnic beverages as valuable sources of techno-functional and probiotics strains: Current scenario and main challenges. Trends Food Sci. Technol. 2021, 114, 25–59. [Google Scholar] [CrossRef]
- Sánchez, B.; Ruiz, L.; Gueimonde, M.; Ruas-Madiedo, P.; Margolles, A. Toward improving technological and functional properties of probiotics in foods. Trends Food Sci. Technol. 2012, 26, 56–63. [Google Scholar] [CrossRef]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Gibson, G.R.; Rastall, R. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef]
- Serban, D.E. Gastrointestinal cancers: Influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 2014, 345, 258–270. [Google Scholar] [CrossRef]
- Orii, S.; Yamaguchi, T.; Anzai, H.; Saito, S.; Chiba, T.; Suzuki, K. Chemoprevention for colorectal tumorigenesis associated with chronic colitis in mice via apoptosis. J. Exp. Clin. Cancer Res. 2003, 22, 41–46. [Google Scholar]
- Zampa, A.; Silvi, S.; Fabiani, R.; Morozzi, G.; Orpianesi, C.; Cresci, A. Effects of different digestible carbohydrates on bile acid metabolism and SCFA production by human gut micro-flora grown in an in vitro semi-continuous culture. Anaerobe 2004, 10, 19–26. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Nicolaus, B.; Poli, A.; Orlando, P. The prebiotic source influences the growth, biochemical features and survival under simulated gastrointestinal conditions of the probiotic Lactobacillus acidophilus. Anaerobe 2012, 18, 280–285. [Google Scholar] [CrossRef]
- Succi, M.; Tremonte, P.; Pannella, G.; Tipaldi, L.; Cozzolino, A.; Romaniello, R.; Sorrentino, E.; Coppola, R. Pre-cultivation with selected prebiotics enhances the survival and the stress response of Lactobacillus rhamnosus strains in simulated gastrointestinal transit. Front. Microbiol. 2017, 8, 1067. [Google Scholar] [CrossRef]
- Shinohara, K.; Ohashi, Y.; Kawasumi, K.; Terada, A.; Fujisawa, T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 2010, 16, 510–515. [Google Scholar] [CrossRef]
- Larsen, N.; Cahú, T.B.; Saad, S.M.I.; Blennow, A.; Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Larsen, N.; Bussolo de Souza, C.; Krych, L.; Barbosa Cahú, T.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef]
- Sulek, K.; Vigsnaes, L.K.; Schmidt, L.R.; Holck, J.; Frandsen, H.L.; Smedsgaard, J.; Skov, T.H.; Meyer, A.S.; Licht, T.R. A combined metabolomic and phylogenetic study reveals putatively prebiotic effects of high molecular weight arabino-oligosaccharides when assessed by in vitro fermentation in bacterial communities derived from humans. Anaerobe 2014, 28, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Jonkers, D.M.; Troost, F.J.; Roeselers, G.; Venema, K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS ONE 2014, 9, e113864. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.; Aguirre, M.; Venema, K.; Bosch, G.; Gruppen, H.; Schols, H.A. In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula. J. Agric. Food Chem. 2014, 62, 1079–1087. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Tuncil, Y.E.; Nakatsu, C.H.; Kazem, A.E.; Arioglu-Tuncil, S.; Reuhs, B.; Martens, E.C.; Hamaker, B.R. Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J. Funct. Foods 2017, 32, 347–357. [Google Scholar] [CrossRef]
- Li, P.-J.; Xia, J.-L.; Nie, Z.-Y.; Shan, Y. Pectic oligosaccharides hydrolyzed from orange peel by fungal multi-enzyme complexes and their prebiotic and antibacterial potentials. LWT-Food Sci. Technol. 2016, 69, 203–210. [Google Scholar] [CrossRef]
- Tian, L.; Bruggeman, G.; van den Berg, M.; Borewicz, K.; Scheurink, A.J.; Bruininx, E.; de Vos, P.; Smidt, H.; Schols, H.A.; Gruppen, H. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol. Nutr. Food Res. 2017, 61, 1600186. [Google Scholar] [CrossRef] [PubMed]
- Khailova, L.; Baird, C.H.; Rush, A.A.; Barnes, C.; Wischmeyer, P.E. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates inflammatory response and homeostasis of spleen and colon in experimental model of Pseudomonas aeruginosa pneumonia. Clin. Nutr. 2017, 36, 1549–1557. [Google Scholar] [CrossRef]
- Mountzouris, K.C. Nutritional strategies targeting the beneficial modulation of the intestinal microflora with relevance to food safety: The role of probiotics and prebiotics. In Food Safety; Springer: Boston, MA, USA, 2007; pp. 133–152. [Google Scholar]
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L.; Chaturvedi, R.; Peek, R.M., Jr.; Wilson, K.T.; Polk, D.B. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Investig. 2011, 121, 2242. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017, 152, 111–123.e118. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Huxley, V.H.; Glinsky, G.V.; Pienta, K.J.; Raz, A.; Glinsky, V.V. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia 2005, 7, 522–527. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Huang, Z.-L.; Yang, G.-H.; Lu, W.-Q.; Yu, N.-R. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 2008, 14, 7386. [Google Scholar] [CrossRef]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Conti, S.; Vexler, A.; Hagoel, L.; Kalich-Philosoph, L.; Corn, B.W.; Honig, N.; Shtraus, N.; Meir, Y.; Ron, I.; Eliaz, I. Modified citrus pectin as a potential sensitizer for radiotherapy in prostate cancer. Integr. Cancer Ther. 2018, 17, 1225–1234. [Google Scholar] [CrossRef]
- Jiang, J.; Eliaz, I.; Sliva, D. Synergistic and additive effects of modified citrus pectin with two polybotanical compounds, in the suppression of invasive behavior of human breast and prostate cancer cells. Integr. Cancer Ther. 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in cardiac fibrosis and inflammation: Mechanistic insights and clinical implications. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Calvier, L.; Fernández-Celis, A.; Rousseau, E.; Jurado-López, R.; Rossoni, L.V.; Jaisser, F.; Zannad, F.; Rossignol, P.; Cachofeiro, V. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension 2015, 66, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Dahdouh, E.; El-Khatib, S.; Baydoun, E.; Abdel-Massih, R.M. Additive Effect of MCP in Combination with Cefotaxime against Staphylococcus aureus. Med. Chem. 2017, 13, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Wilk, B.; Melnick, S.J.; Eliaz, I. Synergistic antioxidant and anti-inflammatory effects between modified citrus pectin and honokiol. Evid. Based Complement. Alternat. Med. 2017, 2017, 8379843. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.; Hao, X.; Zhang, Y.; Deng, W. Perindopril and a galectin-3 inhibitor improve ischemic heart failure in rabbits by reducing Gal-3 expression and myocardial fibrosis. Front. Physiol. 2019, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and cancer stemness. Glycobiology 2018, 28, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and fibrosis in severe COVID-19 patients: Galectin-3, a target molecule to consider. Front. Immunol. 2020, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Zhang, T.; Xue, H.; Wang, X.; Foday, A.D.; Tai, G.; Zhou, Y. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj. J. 2012, 29, 159–165. [Google Scholar] [CrossRef]
- Liu, C.; Tang, J.; Ma, Y.; Liang, X.; Yang, Y.; Peng, G.; Qi, Q.; Jiang, S.; Li, J.; Du, L. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015, 89, 6121–6125. [Google Scholar] [CrossRef]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant–pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef]
- De Godoi, A.M.; Faccin-Galhardi, L.C.; Rechenchoski, D.Z.; Arruda, T.B.M.G.; Cunha, A.P.; de Almeida, R.R.; Rodrigues, F.E.A.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Structural characterization and antiviral activity of pectin isolated from Inga spp. Int. J. Biol. Macromol. 2019, 139, 925–931. [Google Scholar] [CrossRef]
- Dong, C.-X.; Hayashi, K.; Mizukoshi, Y.; Lee, J.-B.; Hayashi, T. Structures and anti-HSV-2 activities of neutral polysaccharides from an edible plant, Basella rubra L. Int. J. Biol. Macromol. 2012, 50, 245–249. [Google Scholar] [CrossRef]
- Yapo, B.M.; Koffi, K.L. The polysaccharide composition of yellow passion fruit rind cell wall: Chemical and macromolecular features of extracted pectins and hemicellulosic polysaccharides. J. Sci. Food Agric. 2008, 88, 2125–2133. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.; Rather, S.A.; Wani, S.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- Yapo, B.M.; Wathelet, B.; Paquot, M. Comparison of alcohol precipitation and membrane filtration effects on sugar beet pulp pectin chemical features and surface properties. Food Hydrocoll. 2007, 21, 245–255. [Google Scholar] [CrossRef]
- Marenda, F.R.B.; Mattioda, F.; Demiate, I.M.; de Francisco, A.; de Oliveira Petkowicz, C.L.; Canteri, M.H.G.; de Mello Castanho Amboni, R.D. Advances in studies using vegetable wastes to obtain pectic substances: A review. J. Polym. Environ. 2019, 27, 549–560. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: A review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Edashige, Y.; Murakami, N.; Tsujita, T. Inhibitory effect of pectin from the segment membrane of citrus fruits on lipase activity. J. Nutr. Sci. Vitaminol. 2008, 54, 409–415. [Google Scholar] [CrossRef]

| Source of Pectin | Mode of Extraction(s) | Outcome | DE (%) | Refs |
|---|---|---|---|---|
| Grapefruit | Ultrasound-microwave assisted | GalA and DE increase with an increase in microwave power and heating time | N/A | [55,56] |
| Citrus (Tangerine) | Ultrasound | Decreased content level of GalA to 71% | N/A | [57] |
| Finger citron pomace | Ultrasound | High GalA content (75–90%) when exposed to NaOH and HCl | 47–51 | [58] |
| Citrus | Ultrasound | The high yield of pectin extraction by ultrasound is dependent on the pH condition | N/A | [59] |
| Citrus (Orange) | Ultrasound | Increased power density leads to maximum yield of pectin extraction | LMP, HMP | [60] |
| Citrus (Orange) | Ohmic heating | The highest yield of pectin was ob-tained at the optimum conditions of 67.18 ± 3.77%. | ≥50 | [63] |
| Pomelo peels | Ultrasound-microwave assisted | Higher GalA content was obtained from combined technique compared to single technique. | 56.88 | [64] |
| Potato pulp | Microwave | 59.75% higher yield of pectin with high GalA content compared to the heating method. | N/A | [65] |
| Beet pulp | Microwave | Reduced duration of extraction has effect on the mass and amount of pectin obtained. | N/A | [66] |
| Citrus (Lime) | Microwave and conventional heating | Energy-saving that speeds up the extraction process, a lower pectin yield and could be improved by longer irradiation time. | N/A | [67] |
| Grapefruit | Microwave and ultrasound | High holding capacity for water, oil, cholesterol adsorption, glucose and nitrite ions. | N/A | [68] |
| Citrus (Orange) | Surfactant and microwave-assisted | A higher yield of pectin (32.8%) and GalA content (78.1%). | 69.8 | [69] |
| Citrus (Pomelo) | Subcritical water | Extraction (pectin) yield and the rate was influenced by the temperature. | LMP | [70] |
| Citrus (Pomelo) | Deep Eutectic Solvents/Citric Acid | 39.72% pectin yield was obtained and influenced by pH resulting in HMP | 57.56 | [70] |
| Jackfruit | Pulsed electric and microwave | Higher pectin yield was obtained compared to conventional extraction. | N/A | [71] |
| Jackfruit | Ultrasound-microwave- ohmic heating assisted | Combined techniques demonstrated significant antioxidant activity of pectin, however in some experiments, ultrasound microwave performed better than the conventional. | 62–65 | [72] |
| Source of Pectin | Modification | Outcome | Ref |
|---|---|---|---|
| Citrus | Chemical (Alkali and acidic hydrolytic) | Good room-temperature stability, improved water solubility, and pseudoplastic behaviour with lower viscosity | [74] |
| Commercial citrus | Chemical (TFA and H2O2) | HG: RGI ratio determines the anti-inflammatory activity and emulsion stability. H2O2 modified pectin promotes the selective growth of specific probiotics | [75] |
| Citrus | Chemical (NaOH and HCl) | The total charge density of pectin was raised and improved the interaction with the pea protein. | [75] |
| Citrus | Chemical (NaCl) | Reduced Mw and viscosity and increased MP density favour interfacial properties | [76] |
| Citrus | Chemical (glycine, glycine methyl ester, or glycylglycine) | The glycine methyl ester bound to the carboxyl groups of pectin molecules which led to the improved dissolution of pectin. | [77] |
| Citrus pulp | Enzymatic (Pectinmethylesterase) | The integrity of charged modify pectin hydrogel was maintained under simulated GI conditions showing good vehicles for colon-targeted delivery for probiotics with longer stability | [78] |
| Citrus | Enzymatic and chemical demethylesterification | The low methyl esterified low Mw pectin materials showed improved interfacial characteristics. | [79] |
| Citrus (Orange and lemon) | Enzymatic and endopolygalacturonase | Pectin showed complement activation in the classical pathway at 1.25 and 2.5 mg/mL stimulating the immune system. | [80] |
| Citrus | Enzymatic, and chemical demethylesterification | Due to its greater ability to chelate pro-oxidative metal ions (Fe2+), low demethylesterified pectin displayed a higher antioxidant capacity than high demethylesterified pectin, methyl esters distribution pattern along the pectin chain only slightly affected the antioxidant capacity. | [81] |
| Apple | Ultrasonic irradiation | The primary structure could not be altered; however, the viscosity was high. | [82] |
| Citrus | Mono and dual frequency ultrasound irradiations | GalA content increased, but its intrinsic vis molecular weight and DE decreased. | [83] |
| Citrus and apple | Enzymatic and ultrasonic irradiation | Higher depolymerisation in pectin treated by ultrasound in the presence of nitric and citric acids than in water; high-methoxylated pectin has a degree of esterification > 50%, hence suitable as a gelling agent. | [84] |
| Citrus | Ultrasonication and Microfluidization | MP showed enhanced encapsulation capacity to shield cholecalciferol (vitamin D3) from UV deterioration | [85] |
| Citrus | Charge modification | Pectin could cover the entire surface and encase the probiotic cell in a hydrogel matrix, reducing its accessibility. | [86] |
| Commercial pectin | Cross-linking | LMP was found to be ~700 nm in size compared to high methoxylated pectin (~850 nm) | [28] |
| Citrus and Apple | Cross-linking | LMP–calcium gels showed rod-like junctions and point-like cross-links zones formed between surrounding chains and monocomplexes. | [87] |
| Biopolymer | Bioactive Substance | Model | Type of Encapsulation | References |
|---|---|---|---|---|
| Citrus pectin (modified) | L. paracasei LPC-37, B. bifidum ATCC 29521 | Broth medium | Emulsification/freeze drying | [75] |
| Pectin methylesterase modified pectin; | Lactobacillus casei W8 | SGI | calcium ionotropic gelation | [78] |
| Charge-modified citrus pectin | L. paracasei subsp. paracasei L. casei W8 | Wistar rat | Iontropic gelation by extrusion | [86] |
| Alginate; modified pectin; Chitosan | L. acidophilus | SGI | Emulsification | [119,120] |
| Pectin/gelatine | Cysteine protease (Clostridium difficile) | Hamster and SGI | Cross-linking | [121] |
| Pectin/gelatine | Flagellin (Clostridium difficile) | Hamster and SGI | Cross-linking | [122,123,124,125,126] |
| Alginate-modified pectin- Chitosan | L. acidophilus | Balb/c Mice | Emulsification | [127,128] |
| Pectin-derived oligosaccharides | Galacto-and fructo-oligosaccharides | Influenza vaccinated mouse model | N/A | [129] |
| Pectin-like polysaccharides | Blueberry anthocyanin extract | SGI | Emulsification | [130] |
| Pectin Strawberry fibre | Phenolic compounds | SGI | N/A | [131] |
| Lysozyme and κ-carrageenan | Curcumin | SGI | Emulsification | [132] |
| Pectin and biopolymeric skimmed milk powder | Curcumin | Caco-2 cells | Dispersion and homogenization | [133] |
| Modified citrus pectin and chitosan | Curcumin | SGI | Extrusion | [134] |
| Pectin and lactoferrin | Curcumin | In vitro | N/A | [135] |
| Pectin and doxorubicin | Doxorubicin | SGI | Ionotropic gelation and extrusion | [136] |
| Polymeric nanocarrier–curcumin | Curcumin | Azoxymethane-induced rat model | Emulsification | [137] |
| Pectin/calcium | Curcumin | SGI | calcium ionotropic gelation | [138] |
| Pectin/calcium | Epigallocatechin gallate and curcumin | Bacterial cell/human cell | N/A | [139,140] |
| MP | Disease Type | Model Used | Studied Type | Outcome | References |
|---|---|---|---|---|---|
| MCP and paclitaxel | Ovarian cancer | In vitro | Human SKOV-3 cells | Synergistic cytotoxic effects with an increase in caspase-3 activity, and reduced cell viability | [19] |
| MCP (PectaSol) and Dox | Prostate | In vitro | DU-145 and LNCaP cells | Dox and PectaSol’s cumulative cytotoxicity impact quickly causes cell death in DU-145 cells through apoptosis and in LNCaP cells through cell cycle arrest. | [21] |
| MCP | Prostate | In vitro | LNCaP and PC3 cells | MCPs prevent MAP kinase from becoming activated, boost the expression of its pro-apoptotic protein downstream target Bim, and cause Caspase-3 to be cleaved in PC3 and CASP1.r | [22] |
| MCP + Lactobacillus paracasei LPC-37 and Bifidobacterium bifidum ATCC 29521. | Prebiotic activity | In vitro | Broth cells | Prebiotic activity scores increases with selective growth of probiotic bacterial. | [75] |
| Charged MCP and L. paracasei subsp. paracasei L. casei W8®; L. casei W8 | Obesity and gut disorder | In vivo | Wistar rats | Pectin-encapsulated probiotic supplementation positively modulated gut microbiota composition in HF-fed male rats | [86] |
| MCP + L. acidophillus ATCC 4356 + alginate | Azoxymethane-induced colon tumour | In vivo, In vitro | SGI, and Balb/c Mice | MCP and alginate significantly enhanced the viability of L. acidophilus ATCC 4356 compared to the control (p < 0.05) both in vitro and in vivo and increased faecal lactobacilli. | [119,120] |
| MCP + L. acidophillus ATCC 4356 + alginate | Colon cancer | In vivo | Balb/c Mice | Probiotics improve the bioactivity of MCP by chemopreventive effects against pre-cancerous colonic lesions and adenocarcinoma. | [128] |
| MCP and IR | Prostate | In vitro | PCa cells | MCP sensitizes prostate cancer cells towards radiotherapy enhancing cytotoxicity. | [185] |
| MCP + BreastDefend and ProstaCaid | Breast and prostate cancers | In vitro | Breast (MDA-MB-231) and prostate (PC-3) cancer cells | MCP reduces the metastatic characteristics of human breast and prostate cancer cells synergistically when combined with BD and PC, respectively. | [186] |
| MCP and cefotaxime | Antimicrobial resistance | In vitro | Assay | Some isolates of S. aureus are inhibited | [189] |
| MCP and Honokiol | Cancer and cardiovasular | In vitro | Assay/cell lines | Improved antioxidant and anti-inflammatory properties | [190] |
| MCP and perindopril | Myocardial fibrosis | In vivo | Rabbits | Perindopril and MCP significantly reduce myocardial fibrosis and ameliorate ischemic heart failure. | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odun-Ayo, F.; Reddy, L. Potential Biomedical Applications of Modified Pectin as a Delivery System for Bioactive Substances. Polysaccharides 2023, 4, 1-32. https://doi.org/10.3390/polysaccharides4010001
Odun-Ayo F, Reddy L. Potential Biomedical Applications of Modified Pectin as a Delivery System for Bioactive Substances. Polysaccharides. 2023; 4(1):1-32. https://doi.org/10.3390/polysaccharides4010001
Chicago/Turabian StyleOdun-Ayo, Frederick, and Lalini Reddy. 2023. "Potential Biomedical Applications of Modified Pectin as a Delivery System for Bioactive Substances" Polysaccharides 4, no. 1: 1-32. https://doi.org/10.3390/polysaccharides4010001
APA StyleOdun-Ayo, F., & Reddy, L. (2023). Potential Biomedical Applications of Modified Pectin as a Delivery System for Bioactive Substances. Polysaccharides, 4(1), 1-32. https://doi.org/10.3390/polysaccharides4010001