Abstract
Ceria-based nanostructures, employed as catalytic supports for noble and non-noble metals, are well-known for their remarkable activity in steam-reforming reactions, exceptional resistance to degradation, and thermal stability. However, the catalytic activity and selectivity of such systems are strongly dependent on the size and shape of ceria, making it possible to tune the oxide properties, affecting catalyst design and performance. The rational manipulation of ceria nanostructures offers various features that directly impact steam-reforming transformations, including the possibility of tuning oxygen vacancies, redox properties, and oxygen storage capacity. Thus, the importance of shape control in ceria nanomaterials is highlighted herein, emphasizing how the surface atomic configurations (exposure of different facets) significantly impact their efficiency. Although the main focus of this review is to discuss how the catalyst design may affect the performance of hydrogen production, some other elemental studies are shown, when necessary, to exemplify the level of deepness (or not) that literature has reached. Thus, an overview of ceria properties and how the physicochemical control of nanostructures contributes to their tuning will be presented, as well as a discussion regarding elemental materials design and the most prominent synthetic procedures; then, we select some metals (Ni, Co, and Pt) to discuss the understanding of such aspects for the field. Finally, challenges and perspectives for nanoengineering catalysts based on shape-controlled ceria nanostructures will be described to possibly improve the performance of designed catalysts for steam-reforming reactions. Although there are other literature reviews on ceria-based catalysts for these reactions, they do not specifically focus on the influence of the size and shape of the oxide.
1. Introduction
Energy is vital in driving economic growth as all production operations and activities rely on it as a fundamental input. Hence, with the expansion of economies, energy demand has a corresponding rise, with renewable energy resources emerging as promising options for sustainable energy production [1]. In this scenario, energy sources such as solar, wind, hydro, geothermal, and biomass play a significant role as they are naturally replenished within a human timescale [2]. However, it is crucial to recognize that these energy sources are not directly manageable as their primary energy cannot be stored.
Although some strategies are being studied to overcome potential offer shortages, hydrogen (H2) has emerged as part of the solution due to its chemical energy carrier properties [3], contributing significantly to decarbonizing dominant sectors and facilitating the achievement of net-zero CO2 emissions by 2050 [4]. However, H2 is predominantly found in compounds rather than in its free form, requiring the utilization of technologies that require substantial energy consumption for its obtaining [5]. In addition to the technical challenges associated with H2 production, other undeniable concerns must be addressed, which include storage, transportation, safety, the integration of H2 into the energy matrix, and the impact on greenhouse gas emissions [6]. Therefore, high costs, high energy consumption, and technical barriers present obstacles to the widespread adoption of H2 as an energy reservoir.
Considering the challenges mentioned and the present circumstances, H2 has not been widely regarded as a viable solution on a large scale due to persistent economic concerns and environmental impacts. However, its consumption is essential for fertilizer production in the ammonia industry, oil refining, methanol production, and iron and steel manufacturing [7,8,9]. Thus, attaining technological advancements and achieving economies of scale are crucial to making H2 economically feasible. Technologies such as photochemical water splitting, bio-photolysis, photo- and dark-fermentation, and thermochemical and biomass-to-energy processes have attracted attention toward producing green H2 that can help mitigate global temperature increases within the 2 °C limit [10,11,12]. Nevertheless, these technologies are still maturing for commercial viability.
Currently, the most prominent methods for H2 production are electrochemical water splitting (electrolysis) and steam-reforming reactions, with steam–methane reforming being the dominant process [13]. The primary factor behind the limited utilization of electrolysis is the high cost associated with electricity, a crucial requirement for its operation; reforming reactions do not require such input. The non-utilization of electricity is especially relevant for sectors such as the production of chemicals and certain segments of the transportation industry where alternative decarbonization methods may not be feasible. In the long run, attention will also be directed toward addressing portions of the heat market. However, a recent study stated that direct H2 technologies always increase the energy system’s cost and cannot be considered large-scale solutions for heating and transport, at least for now, and such sectors are critical for replacing the global carbon-based energy matrix [14]. Thus, it is crucial to continue reducing the cost of H2 production, and many efforts are being directed toward steam-reforming reactions due to its stage of development.
The application of nanostructures in steam reforming for H2 obtaining has witnessed significant recent advances, opening up new possibilities for improving the efficiency and effectiveness of this process, making it less costly [15,16]. Fundamentally, hydrogen gas generation through steam reforming entails the reaction of different feedstocks, including natural gas, higher hydrocarbons, methanol, ethanol, acetone, biomass, and biogas, with steam [16,17]. Shape-controlled nanostructures, which possess well-defined shapes and sizes at the nanoscale, have emerged as a promising area of research, offering distinct advantages due to their unique geometric properties. These nanostructures allow for precise control over catalytic activity, selectivity, and thermal stability [18].
One notable breakthrough involves the synthesis of metal oxides with specific shapes, such as cubes, rods, plates, spheres, and polyhedral nanostructures. Innovative techniques have been developed to engineer these nanomaterials precisely. This allows the tailoring of their properties to optimize steam-reforming reactions once they can be used as a support for noble and non-noble metals. These catalysts then exhibit superior catalytic performance compared to conventional structures [19]. Interestingly, more complex morphologies than those cited before can be obtained. For example, Li et al. introduced a novel crystallographic-oriented epitaxial growth technique for synthesizing a cerium oxide homojunction with specific crystal facets. The homojunction was composed of a hexahedron prism with exposed (100) facets and an anchored octahedron with (111) facets on the surface (by using different H2PO4− concentrations), as depicted in Figure 1 [20]. Thus, by maneuvering shapes, researchers can optimize the dispersion and accessibility of active sites, thereby enhancing catalytic performance [19,21,22,23]. Additionally, integrating shape-controlled nanostructures as support materials can improve thermal stability, minimizing catalyst deactivation and extending the operational lifespan of steam-reforming reactors [24].

Figure 1.
SEM images of CeO2: (a1,a2) regular octahedron morphology; (b1,b2) octahedron surface textured with nanorod-like arms with the addition of 0.5% H2PO4−; octahedron surface textured with thicker nanorod-like arms with the concentration increase of H2PO4− to (c1,c2) 1% and (d1,d2) 2%; (a3, b3,c3,d3) schematic representation of the prepared nanostructures. Reproduced with permission from [20].
Cerium dioxide (CeO2, ceria) has gained significant attention as a catalyst for steam-reforming reactions as it exhibits unique properties; among many, it possesses a high oxygen storage capacity, enabling it to participate in redox reactions, and excellent thermal stability and resistance to sintering, which are crucial factors for maintaining catalytic activity over prolonged operations without undergoing significant structural changes or degradation [25]. The term “cerium oxides” is of significant importance as it encompasses dicerium trioxide (Ce2O3), cerium dioxide (CeO2), and their non-stoichiometric hybrids. For the general concept, we will use the term “cerium oxides.” However, in the context of specific publications, we will adopt the designation chosen by the respective authors cited herein.
Their significance lies in the versatility of synthesizing cerium oxide catalysts in various morphologies. Based on different synthetic strategies—which allow for the selection of stabilizers, growth-directing reagents, solvents, and metal precursors—it is possible to precisely modulate the properties of ceria-based nanocatalysts [26]. In addition, other strategies can increase the surface area. An alternative way of improving the surface area is to use a template of another oxide, as Das et al. have shown (Figure 2) [27]. Using an intelligent approach, they prepared SiO2 nanospheres using the Stöber method before nickel phyllosilicate was formed on the synthesized spheres using the ammonia evaporation method. Then, cerium nitrate was precipitated on the previous material before calcination and reduction at 800 °C. Thus, a novel core-shell structured catalyst, Ni-SiO2@CeO2, was developed for the dry reforming of biogas (CH4/CO2 = 1.5) at low temperatures (600 °C). The catalyst exhibited a high surface area and provided a dual confinement effect, enhancing coke resistance and avoiding sintering.
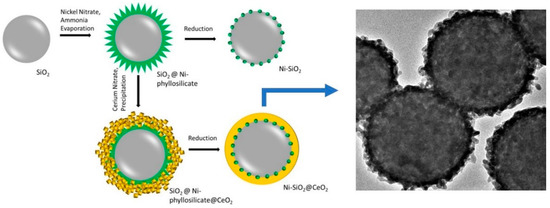
Figure 2.
The preparation of the Ni-SiO2@CeO2 catalyst is depicted in the scheme, with a TEM image of the final material shown on the right side of the figure. Modified with permission from [27].
Additionally, cerium oxides are usually employed as a support for nanoparticles (noble or non-noble metals) and can be combined with oxides or promoters to enhance their catalytic activity and selectivity in steam-reforming reactions. While existing reviews discuss cerium-oxide-based heterogeneous catalysts for steam reforming, we have observed a distinct lack of reviews dedicated to synthesizing shape-controlled cerium oxide catalysts and analyzing the implications of such synthesis methods. Thus, this review begins with a discussion of the suitability of ceria for steam-reforming reactions and the fundamentals of preparing cerium-oxide-based nanomaterials, emphasizing how size and shape influence their performance. Subsequently, attention is shifted to the most promising cerium oxide shapes in interaction with Ni, Co, and Pt, highlighting the potential applications of controlled nanomaterials in this field and addressing current challenges, concentrated in the last 5 to 8 years. The significance of developing highly efficient and stable catalysts is also underscored. Then, based on the articles that compose this review, concluding remarks and perspectives will be presented, showing the limitations in the field.
The Motivation Driving This Review
Steam-reforming reactions have been extensively investigated using metal-based catalysts—including noble and non-noble ones. Among them, Ni is often considered the preferred choice due to cost and activity [28,29,30,31]; however, other metals also present significant performances. In particular, Ni nanoparticles supported on different inorganic oxides like Al2O3, CeO2, ZnO, La2O3, SiO2, MgO, ZrO2, and TiO2, have been the subject of research for H2 production through steam-reforming reactions of various renewable feedstocks [32,33,34,35,36,37,38].
Remarkably, Ni catalysts supported on Al2O3 and CeO2 have shown the most promising results, and alumina demonstrates exceptional performances, presenting an attractive metal–support interface that acts as a highly active site for the cleavage of C-C and C-H bonds in these reactions; ceria offers important redox properties (among other benefits, which will be discussed) [39]. Taking into account these findings and other related outcomes, previous reviews have focused on CeO2 and Al2O3 as supports for steam-reforming reactions [40,41]. Nevertheless, given our focus on topics related to shape control, cerium oxides were chosen as the primary subject due to the well-established knowledge regarding their morphology (but, when possible, examples of catalysts composed of Al2O3 and CeO2 will be discussed). Also, cerium-oxide-based catalysts are suggested to play an essential role in preventing coke formation [42], and such materials possess crucial characteristics that can be modulated to improve H2 production from steam-reforming reactions, which will be discussed in this section. However, it is vital to highlight that, in the following sections, we will extend our considerations beyond Ni-based catalysts as we will also dive into a discussion of catalysts comprising other metals, both noble and non-noble.
Cerium-oxide-based catalysts in steam-reforming reactions highlight several key characteristics inherent to oxide support itself that play a crucial role in the synergistic interplay between cerium oxides’ support and the metals that are immobilized on them. For instance, CeO2 demonstrates the ability to accommodate substantial oxygen deficiencies within its structure [25], leading to high oxygen ion conductivities that can be further enhanced through doping processes [43]. In addition, oxygen vacancies are the prevailing defects significantly influencing ceria’s electronic and chemical properties and are also related to the size of the nanostructure [44]. Such vacancies in the CeO2 crystal lattice enhance catalytic activity by promoting oxygen diffusion and facilitating redox reactions [45,46]. Notably, a material with a higher Ce3+/Ce4+ ratio tends to offer a more significant number of oxygen vacancies. To determine this ratio, X-ray photoelectron spectroscopy (XPS) is commonly employed [47,48]. The analysis is performed by area integration of the peaks after applying fitting parameters, which usually provide a complicated XPS Ce 3d spectrum, with three 3d3/2-3d5/2 spin-orbit-split doublets associated with Ce4+ and two double peaks related to Ce3+ [49,50]. By associating specific peaks to each Ce species, it is possible to obtain the required Ce3+/Ce4+ ratio. It is worth noting that X-ray absorption near-edge spectroscopy (XANES) can also be used once some authors consider that XPS overestimates the Ce3+ concentration [47,51].
Hao et al. conducted a study on the distribution of Ce4+/Ce3+ across CeO2 nanocubes perpendicular to the (100) facet using STEM (scanning transmission electron microscopy) and EELS (electron energy loss spectroscopy) [52]. They discovered that reducing the diameter of the CeO2 nanostructures led to lattice expansion, enabling the creation of oxygen vacancies as the energy needed decreased with smaller sizes. Specifically, in Figure 3a, a noticeable shift toward lower energy loss positions was observed only for the topmost two layers of the larger nanocubes. Conversely, in Figure 3b, for smaller CeO2 nanocubes, the spectral shift occurred in all atomic layers, including the center layers. Consequently, a major Ce3+ content of 53.7% (with an oxygen vacancy concentration of 17.5%) was measured in the smaller CeO2 nanostructures (5.4 nm) compared to the larger ones.

Figure 3.
(a,b) Layer-by-layer EELS detection on CeO2 nanocubes consisting of 35 and 20 layers. The dotted lines in the Ce M edge spectra represent the position of the center layer (20) in the larger nanocube. The colors represent variations in the M5 and M4 peaks position and height layer by layer, relating the left images with the center ones. The accompanying graphs on the right depict each layer’s distribution maps of the M5/M4 ratio (blue dots). Reproduced with permission from [52].
Interestingly, these atomic-scale and quantitative characterizations of the Ce4+/Ce3+ distribution and local environment changes provide fundamental insights into the intrinsic properties of CeO2 nanostructures; however, such studies are still lacking in the steam-reforming reactions field. One can notice that the properties mentioned above are closely related to improved steam-reforming activity and remarkable stability in terms of carbon formation due to the oxygen storage capacity (OSC) of Ce and its role as a site for water dissociation [53,54,55,56]. This property is crucial in steam-reforming reactions because it allows ceria to act as an oxygen buffer.
The OSC is closely associated with the ease at which cerium can transition between different oxidation states. This phenomenon can be attributed, in part, to the comparable energy levels of the 4f and 5d electronic states and the low potential energy barrier for electron density distribution between them [57]. The ability to switch between different oxidation states makes ceria an excellent catalyst in steam-reforming reactions. An important property raised from the above is the surface oxygen mobility that CeO2 possesses, which enables efficient oxygen transfer during the reaction and contributes to the catalytic activity.
Considering the shape control of cerium oxide nanostructures, one can bear in mind that the physical and chemical performance of cerium oxides in heterogeneous catalysis applications relies on their surface atomic configurations, which necessitates a process of surface faceting. Then, visualizing the Ce and O atoms on the oxides’ surface is crucial for understanding catalytic efficiency. Through HTEM (high-resolution transmission electron microscopy) imaging, Lin et al. determined the atomic structures of the (100), (110), and (111) surfaces of CeO2 nanocubes [58]. The (100) surface of CeO2, which is predominantly exposed, exhibits a complex structure with a mixture of Ce, O, and reduced CeO terminations. The (110) surface revealed a combination of flat CeO2-x layers and (111) nanofacets, with numerous surface O vacancies due to low oxygen vacancy formation energy. The (111) facet appeared to be O-terminated based on a comparison between simulated and experimental images (Figure 4). These findings suggest that the surface structure of CeO2 is more complex than previously assumed, necessitating further research to elucidate surface properties, especially in the context of surface-selective catalysis. However, the literature reflects a scarcity of knowledge regarding these aspects too. Furthermore, the understanding of CeO2-based materials serving as supports for metal nanoparticles is even more limited.
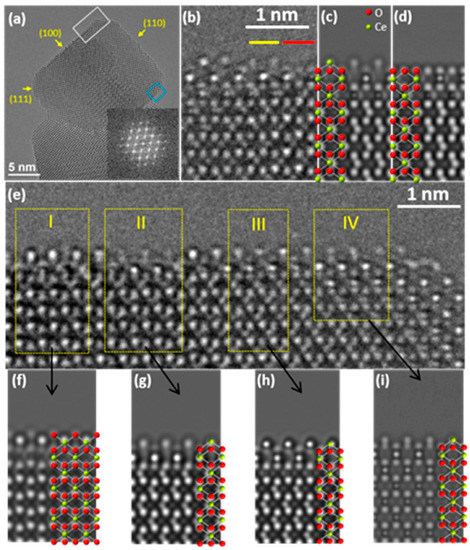
Figure 4.
(a) An HREM image of a typical CeO2 nanocube. (b) A magnified HREM image of the (100) surface of the nanocube, indicated by the blue box in (a). (c,d) Simulated HREM images of the (100) surface in (b), representing Ce and O terminations, superimposed on the atom positions of the HREM images. (e) A further magnified HREM image of the (100) surface of the nanocube, highlighted by the white box in (a). (f) A simulated image of the region I in (e), using a (√2 × √2)R45° reconstructed CeO model overlaid. (g–i) Simulated HREM images of regions II, III, and IV in (e) using a Ce-terminated model. Reproduced with permission from [58].
Another notable characteristic is the exceptional thermal stability of cerium oxides once they exhibit high resistance to degradation and sintering, making them well-suited for steam-reforming reactions that require elevated temperatures without experiencing significant structural deterioration. Thus, manipulating the oxide structure presents an interesting opportunity to enhance cerium oxide properties in all the aspects mentioned above; insights regarding synthesis procedures are essential and must be discussed more in the literature for the hydrogen production field. Consequently, a comprehensive review was conducted herein to identify and select the most pertinent publications for our proposal, aiming to elucidate the crucial role of morphology in enhancing the application of steam-reforming processes.
2. Designing Nanocatalysts for H2 Production
One fundamental aspect of nanocatalyst design is the optimization of particle size once reducing the size of nanostructures has led to an increased surface area, which enhances catalytic activity improvements [59]. By precisely controlling the size of nanostructures, one can maximize the number of active sites available for H2 generation due to quantum confinement and the proportions of atoms at corners, edges, and faces, resulting in improved efficiency [12,60]. Thus, the design of nanocatalysts has emerged as a crucial concept in H2 production as the fine control of various parameters (apart from the size) such as composition (mono-, bi-, or multimetallic materials) and structure (solid, hollow, or porous) is essential to reach unique physical and chemical properties [12]. However, besides such variables, the ability to precisely manipulate the shape of nanostructures provides a versatile approach for fine-tuning particle performance based on the faceting or arrangement of atoms on the surface [61]. Such phenomena can be explained based on the distinct surface energy exhibited by each crystal facet, affecting the interaction between the target molecules and the nanostructure’s surface, resulting in increased catalytic activity. It is important to highlight that surface energy (γ) can be defined as the energy needed to create a unit area of a “new” surface or as the surplus-free energy per unit area for a specific crystallographic face, as described by Equation (1) [62]:
γ = (1/2)Nbερ
Nb represents the number of bonds that must be broken to generate the surface, ε denotes the bond strength, and ρ represents the density of surface atoms. As an illustration, Araiza et al. conducted a study investigating the influence of precipitation or hydrothermal-prepared CeO2 nanostructures (particles, rods, and cubes) on carbon deposition during steam reforming of ethanol when used to create 10 wt. % Ni/CeO2 catalysts. In addition to the size and dispersion of the metal particles, the interaction between the metal and ceria with different shapes played a crucial role. The researchers found that Ni supported on CeO2 rods exhibited improved activity and higher H2 yields at 550 °C for 24 h under a stream, along with reduced carbon deposits. These favorable outcomes were attributed to the catalysts’ enhanced oxygen storage capacity and improved Ni dispersion (Figure 5) [63].

Figure 5.
TEM images of CeO2 support with different shapes: (a) particles, (b) rods, and (c) cubes; TEM images of the supports with impregnated nickel oxide NPs: (d) Ni/CeO2—particles, (e) Ni/CeO2—rods, (f) and Ni/CeO2—cubes, showing EDX (energy-dispersive X-ray spectroscopy) spectra in the insets. Reproduced with permission from [63].
This understanding allows us to gain valuable insights into the relationships between structure and performance in H2 production. Consequently, these insights enable performance enhancements and facilitate the deliberate creation of nanocatalysts with desired activities and selectivity for H2 production. Thus, researchers carefully engineer nanomaterials to achieve desired characteristics, such as high stability and selectivity, as controlled nanostructures can enhance mass transport and promote efficient reactant interactions, leading to enhanced H2 production rates. It is worth mentioning that such morphologies arise from planar features showing crystal-structure-controlled growth orientation, although this is not always discussed. Padi et al. prepared a nano-sized NiO-CeO2 solid solution in which Ni nanoparticles’ growth occurred via an exsolution mechanism. Interestingly, STEM-EDX elemental mapping revealed the presence of grain boundaries and stacking faults in the Ni/CeO2 catalyst after 90 h of the methane-dry-reforming reaction (Figure 6). Remarkably, the catalyst exhibited both high activity and stability during the reaction at 800 °C for 50 h, confirming the strong metal–support interaction and the absence of coking [64].
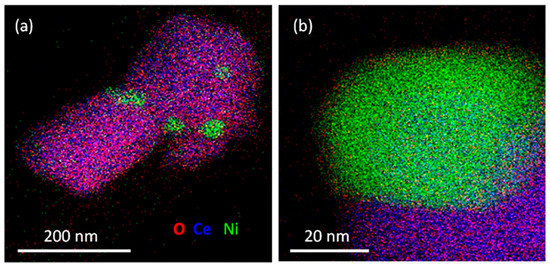
Figure 6.
(a) Low and (b) high magnification STEM-EDX elemental mapping images reveal the presence of exsolved Ni/CeO2 from Ce0.97Ni0.33O2-δ after 90 h of dry reforming of methane at 800 °C. Reproduced with permission from [64].
3. Main Synthetic Strategies for Shape-Controlled Cerium Oxide Nanostructures and Metal Immobilization
By carefully selecting the synthesis method and meticulously optimizing experimental conditions, nanomaterials can be created, with precise control over their morphology; this includes the synthesis of nanocubes, nanorods, nanowires, nanooctahedra, and a variety of other shapes, as cited in the introduction [65,66,67,68,69,70]. Thus, synthesizing cerium oxide nanostructures has been successfully achieved through various methods, such as sol–gel, precipitation, electrochemical deposition, and hydrothermal processes [65]. Among them, the hydrothermal process seems to be the most versatile as experimental conditions such as temperature, pressure, precursor solution (and concentration), precipitating agent, and pH are easily manageable [70]. After obtaining the desired morphology, one can incorporate noble or non-noble metals on the surface of the cerium oxides obtained, improving the catalytic properties of cerium oxide for various applications. Hereafter, we have focused on the most notable and straightforward synthesis methods for achieving the shape control of cerium oxide nanostructures. It is important to note that the literature contains numerous other examples, and even slight modifications in the synthetic procedures can lead to significant morphological variations.
3.1. Hydrothermal Method
In the hydrothermal method, the reactants are combined in a sealed vessel and heated to above room temperature, with high hydrostatic pressure promoting the formation of pure, controlled-size nanomaterials. Such a process is recognized as a versatile and effective technique for synthesizing cerium oxide nanomaterials with different morphologies. The hydrothermal method regulates composition and morphology by adjusting factors such as temperature, pressure, reaction time, and the composition of the reaction solution [71,72,73]. However, adding components such as organic molecules, surfactants, polymers, and inorganic compounds is critical as they can act as growth-directing reagents. Also known as shape-controlling or structure-directing agents, they guide the growth and formation of specific nanomaterial shapes by modifying the growth kinetics of precursor molecules. Thus, it is possible to achieve nanomaterials with tailored shapes by carefully selecting the appropriate growth-directing reagent and controlling the reaction conditions. It is worth mentioning that alternative solvents can be used, integrating the so-called solvothermal method; however, the hydrothermal method is more widely applicable for oxide syntheses.
The formation of spherical CeO2 particles using a hydrothermal approach is challenging as the process typically leads to the formation of irregular-shaped nanostructures or agglomerates rather than well-defined spherical particles. However, it is still possible to obtain spherical CeO2 particles by modifying the synthesis conditions or incorporating additional steps. One approach to obtaining CeO2 spheres is through a template-assisted hydrothermal method. In this method, a sacrificial template material, such as a surfactant like cetyltrimethylammonium bromide (CTAB), is used as a template for the deposition of CeO2 [74]. Microspheres were synthesized hydrothermally by diluting them in equimolar amounts Ce(NO3)3.6H2O, urea, and polyvinylpyrrolidone (PVP) in 160 mL of deionized water before transferring them to an autoclave and heating them at 110 °C for 12 h. Then, the solid was centrifuged and washed with water followed by ethanol, dried at 100 °C overnight, and calcined at 500 °C for 5 h. The authors added nickel from wet impregnation using Ni(NO)3)2·6H2O as a Ni precursor for the steam reforming of glycerol [75].
While achieving perfect spherical cerium oxide particles through hydrothermal synthesis alone may be challenging, it is important to note that specific conditions and techniques may alter the size, morphology, and properties of the cerium oxide nanostructures. Some examples will be shown here, displaying the flexibility of the method. Wang et al. synthesized CeO2 nanorods using the hydrothermal method, using Ce(NO3)3.6H2O as the metal precursor [76]. Aiming to obtain a specific morphology (nanorods), the authors used NaOH as a precipitating agent. Although they did not discuss the base function further, it can be anticipated that NaOH can act to maintain a specific pH range for the formation and growth of nanostructures, help in the dissolution and reactivity of the precursor, and tailor the anisotropic growth along specific crystallographic directions, favoring the formation of elongated nanorods. For the hydrothermal process itself, the solution was transferred to an autoclave (100 mL) made of Teflon-coated stainless steel and gradually heated to 100 °C and kept at that temperature for 14 h. The solid obtained was washed with hot water and dried at 100 °C overnight. Finally, it was calcined at 300 °C for 6 h. After washing with hot water and drying, they used such nanorods as supports for Ir nanoparticles, immobilized by a deposition method using an aqueous solution of H2IrCl6·6H2O as an Ir source and Na2CO3 as a precipitating agent, and the resulting material was used for the ethanol steam-reforming reaction.
Papavasiliou et al. used the hydrothermal method to obtain nanorods using the same metal precursor and NaOH as a precipitating agent, with a different concentration from the previously shown method; however, they employed different experimental conditions: the autoclave was heated at 110 °C for 24 h; after, the material was washed with water several times and dried under a vacuum. The material was calcined at 400 °C for 4 h. The resulting material was characterized by TEM, STEM, and SEM (Figure 7). Interestingly, the authors obtained nanorods with a higher surface area than those obtained by the previously discussed method. Platinum was incorporated into the nanorods using the wet impregnation method, using an aqueous solution of H2PtCl6∙6H2O [77]. One can notice that the specific concentration of NaOH, reaction temperature, reaction time, and other synthesis parameters should be carefully optimized to achieve the desired morphology, affecting the materials’ properties, even reaching the same morphology.
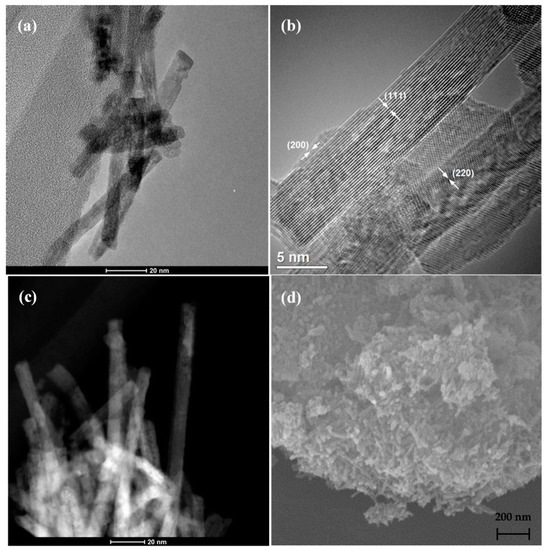
Figure 7.
(a,b) TEM, (c) STEM, and (d) SEM images of CeO2 nanorods after calcination at 400 °C. Reproduced with permission from [77].
Vecchietti et al. prepared nanocubes and nanooctahedra CeO2 nanostructures [24]. Both materials were synthesized hydrothermally; the nanocubes were prepared from a mixture of Ce(NO3)3.6H2O and NaOH solutions and kept under magnetic stirring for 30 min in a Teflon vessel before being transferred to a stainless-steel autoclave and kept under heating at 180 °C for 24 h. After curing at room temperature, the solid was separated by centrifugation and washed several times with water and ethanol. Then, it was oven-dried at 60 °C for 24 h and calcined at 450 °C for 5 h in a tube furnace. Nanooctahedra materials were synthesized by diluting Ce(NO3)3.6H2O in deionized water and adding a solution of NaOH drop by drop under magnetic stirring for 15 min before the same hydrothermal protocol employed for the nanocubes was used. Then, the material was dried at 80 °C for 12 h and calcined at 500 °C for 2 h. The materials are shown in Figure 8.
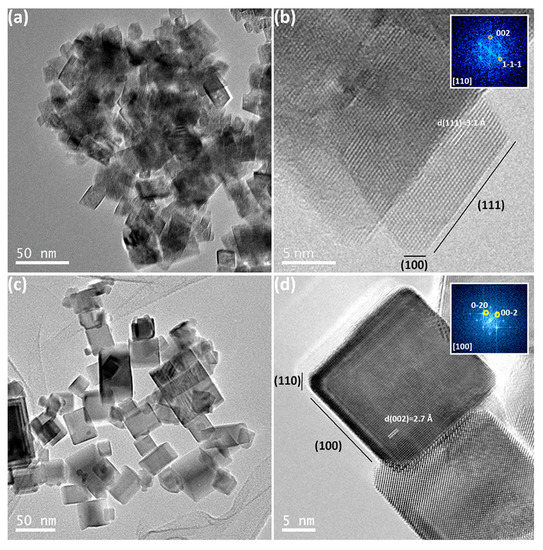
Figure 8.
TEM and HREM images for CeO2 (a,b) nanooctraedra and (c,d) nanocubes. The inset in (b) and (d) are the fast Fourier transform (FFT) of that image. Reproduced with permission from [24].
CeO2 nanowires are commonly prepared in the literature. As an example, Rodrigues et al. prepared nanowires composed of Ce and Sm by diluting NaOH in deionized water before gradually adding Ce(NO3) 3·6H2O and Sm(NO3)3·6H2O solutions. The resulting mixture was transferred to an autoclave and heated at 110 °C for 24 h; after cooling to room temperature, the solid was collected and washed three times with deionized water by centrifugation and dried at 110 °C for 2 h. After finely dispersing Ni particles, they obtained high yields for H2 production from ethanol steam reforming (~60% of selectivity) and stability with no activity loss after 192 h of reaction at 550 °C [78]. Although most of the examples here have included NaOH as a precipitating agent, and it seems to be the most common choice in the studies performed for steam-reforming processes, others such as NH4OH, ammonia, and tri-sodium phosphate, to cite just a few, can be used [79,80,81].
3.2. Sol–gel Method
The sol–gel method has found extensive use in the synthesis of ceramic materials, including metal oxides (including ZrO2, Al2O3, TiO2, ZnO, WO3, Nb2O5, and rare earth oxides), nitrides, and carbides [82]. This technique offers advantages compared to the hydrothermal method, such as low reaction temperatures, precise control over the composition of the resulting material, ease of scaling up for large-scale production, and a high purity level [83]. The key mechanism in sol–gel synthesis involves a precursor’s hydrolysis and condensation reactions, which can be inorganic or metal-organic. These reactions give rise to a sol, a colloidal suspension of nanoscale particles. The sol gradually transforms into a gel, a solid-like network, through subsequent chemical reactions and mild thermal treatments. The final material is obtained by calcining the gel, resulting in the desired ceramic product [84].
One of the main benefits of the sol–gel method is its adaptability and control over the properties of the obtained nanomaterials. In the context of ceria nanostructure preparation, limited research has focused on using this method to tailor the morphologies of ceria nanostructures for steam-reforming processes in recent years. However, a recent study conducted by Yang et al. prepared a sponge-like structure of ceria with the sol–gel method using Ce(NO3) 3·6H2O as the metal precursor and citric acid as a stabilizer and ethylene glycol that could help in controlling hydrolysis and polymerization. After calcination at 450 °C for 5 h, the material was obtained and used to support CuO immobilization. Although the material showed interesting catalytic performance for hydrogen production by methanol steam reforming, the best catalytic activity was obtained by a CuO/CeO2 nanorod catalyst prepared by a hydrothermal approach [85]. More examples of this technique are available in the literature, but they are concentrated on obtaining sphere/quasi-sphere nanostructures and not always for steam-reforming technologies, which is interesting as the hydrothermal method is often used for other morphologies [86,87,88].
3.3. Co-Precipitation Method
The co-precipitation method is a widely used technique for obtaining nanomaterials, probably due to its easy and efficient chemical pathway. The process is conducted with two or more precursor salts containing the desired metal ions diluted, generally, in water, forming a solution. Sometimes this process is also known by the designation of “precipitation” due to the possibility of using just one metal precursor. After the dilution of the precursors, a precipitating agent, usually a strong base like ammonium hydroxide or sodium hydroxide, is added gradually to the solution, promoting the formation of insoluble metal hydroxide or carbonate precipitates. However, the precipitation process can be triggered by altering various conditions, including temperature, pH value, evaporation rate, and salt concentration [89]. The method’s simplicity and ease of use have advantages, such as cheap and accessible chemical reagents. Furthermore, the method allows for control over the nanomaterials’ size, composition, and shape. Nanostructures can be obtained by adjusting the addition of the precipitating agent, temperature, and concentration of reagents [26].
Hernández et al. synthesized nanowires of Ag-CeO2 by preparing Ag nanowires through the polyol method, followed by Ce(NO3)3·6H2O precipitation, in which NH4OH was added dropwise to complete the process. After 24 h of aging, the mixture was heated at 50 °C for 24 h and 100 °C for a further 24 h before calcination at 500 °C at a rate of 5 °C/min for 5 h under air. The controlled material was successfully applied for hydrogen production from methanol steam reforming [90].
Despite the effectiveness of the co-precipitation method in producing efficient catalysts, it often does not yield CeO2 with a well-defined, regular form. This limitation highlights the need for further exploration and utilization of the previously discussed synthesis methods, such as sol–gel and hydrothermal methods, which offer more control over the morphology of CeO2.
4. Shape-Controlled Cerium-Oxide-Based Nanocatalysts with Ni and Co
Low-cost and available non-noble catalysts, particularly Ni-based catalysts, are extensively used in steam-reforming reactions. However, there is a limited understanding in the literature regarding the mechanisms of their active sites and the significance of metal–support interactions in Ni and cerium oxide systems. Furthermore, when such discussions are present, they often refer to specific reaction conditions and substrates, making it challenging to generalize the findings. However, as a general assumption, the combination of highly dispersed metal nanoparticles on oxide surfaces exhibits high reactivity [91]. In this context, ceria is crucial in preventing metal sintering by establishing strong metal–support interactions, demonstrating high reducibility. As a result, ceria serves as a vital promoter for maintaining the stability and activity of metal catalysts [92]. In low-loaded Ni catalysts supported on CeO2, the presence of the ceria facilitates strong metal–support interactions, leading to the partial oxidation of Ni atoms in direct contact with the support [93,94,95], bringing significant changes in the chemical and catalytic properties of these systems, particularly in the cleavage of C–H and O–H bonds [96,97,98,99,100]. Moreover, the high reducibility of the ceria support allows it to act as an oxygen reservoir, enabling unique reaction pathways such as the reverse spillover of oxygen from ceria to metal sites, as discussed before. As a result, the remarkable decoking activity observed in ceria-supported Ni catalysts for methane steam reforming can be attributed to a mechanism involving the supply of oxygen from the support, facilitating the removal of carbon as CO, and water plays a crucial role as one of the reactants, reloading the oxygen vacancies generated during the reverse spillover step [42].
However, the scenario can be more complicated depending on the temperature at which the reaction is conducted and the substrate as Ni active species tend to sinter and suffer from carbon deposition, resulting in their deactivation [101]. In this context, shape-controlled CeO2 is one of many options and is highly used to avoid such drawbacks [102]. Luo and co-workers proposed a strategic approach to finely adjust the metal–support interaction by employing different shapes of CeO2—spheres, rods, and cubes—as supports for Ni nanoparticles (Figure 9a–i) [103]. This approach also influences the specific surface area exposed by the Ni nanoparticles, metal–support interactions, concentration of oxygen vacancies, and catalytic performance in glycerol steam reforming. The molar ratio of nC3H8O to nH2O was 1:9, with a gas hourly space velocity (GHSV) of 23,700 mL h−1 gcat−1, and the reaction was carried out at a pressure of 1 atm and a maximum temperature of 600 °C.

Figure 9.
HRTEM images depict the reduced (a,b) sphere-shaped Ni/CeO2, (d,e) rod-shaped Ni/CeO2, and (g,h) cube-shaped Ni/CeO2 catalysts. In addition, scanning STEM images along with mappings illustrate the distribution of Ni, Ce, and O in the reduced (c) sphere-shaped Ni/CeO2, (f) rod-shaped Ni/CeO2, and (i) cube-shaped Ni/CeO2 samples. TEM images of spent catalysts: (j) sphere-shaped Ni/CeO2, (k) rod-shaped Ni/CeO2, and (l) cube-shaped Ni/CeO2 catalysts. Modified with permission from [103].
Interestingly, they observed that the sphere-shaped Ni/CeO2 catalyst did not change its morphology after the reaction (and promoted higher production of H2), unlike the rod- and cube-shaped Ni/CeO2 catalysts. Furthermore, the researchers noted distinct carbon deposition characteristics among the catalysts. The Ni/CeO2 catalyst supported on CeO2 spheres exhibited abundant filamentous carbon surrounding the catalyst, while encapsulated carbon was scarce, leaving the remaining facets still available for reactant adsorption (Figure 9j). On the other hand, the catalysts based on rod- and cube-shaped Ni/CeO2 structures displayed amorphous carbon on their surfaces (Figure 9k,l). This carbon layer covered the metal particles, leading to catalyst deactivation. These experimental findings corroborate the significance of the CeO2 shape in determining overall catalytic efficiency.
Although specific reaction conditions support morphology, and substrate utilization may highly account for the results, it should be noted that ceria enhances the catalytic activity of Ni through metal–support interactions and plays an essential role in the dissociation of water and prevention of coke formation, as discussed before for methane and glycerol steam reforming, also for ethanol steam reforming [42]. Thus, this seems to be the case for steam-reforming reactions.
The presence of oxygen vacancies on the catalyst surface is an important feature that can be tailored to enhance redox properties and oxygen mobility. Bearing that in mind, Ni et al. conducted a study to examine the correlation between Ni and CeO2 in Ni/CeO2 catalysts and its impact on the CO2 reforming of CH4. They employed various synthesis methods, including hydrothermal and precipitation techniques, and compared the results with a commercial counterpart. The CeO2 nanostructures obtained through these methods exhibited different shapes, although the control over the shape was not precise. Interestingly, the researchers observed variations in the oxygen vacancies between the different synthesis approaches after immobilizing Ni0 on the supports. They found that the material prepared through hydrothermal synthesis (the material with a higher shape control) exhibited higher efficiency, which they attributed to improved Ni0 dispersion and the presence of a more significant number of oxygen vacancies [104].
According to density functional theory (DFT) and experimental studies, it has been observed that the chemical activity of various planes in CeO2 follows the sequence (110) < (100) < (111), suggesting that the (110) planes of CeO2 are more prone to the formation of oxygen vacancies compared to (100) and (111) planes [105,106,107]. Thus, Wang et al. specifically controlled the shape of CeO2-based Ni catalysts and obtained nanorods, nanocubes, nanooctahedra, and nanoparticles of ceria, exposing mainly (110)/(100), (100), (111), and (111)/(100) facets, and used such materials for methane dry reforming. They found that CeO2-based nanorods used for Ni immobilization presented higher concentrations of oxygen vacancies than the other three ceria materials [108]. Once again, it is crucial to emphasize the need for further research on the data related to hydrogen production. In-depth studies in this area can enhance performance and enable the development of more tailored catalysts to meet specific requirements.
The Ce3+/Ce4+ redox cycle enables CeO2 to undergo a reversible transformation into a nonstoichiometric oxide, leading to its high OSC and enhanced oxygen mobility [109]. Tu et al. prepared paper-structured catalysts with dispersed CeO2–Ni flowers for dry reforming of methane and observed that the high oxygen storage capacity and oxygen mobility of their material contributed to the partial removal of coke formation on the catalyst particles during the reforming process, allowing for an improved catalytic activity compared to other Ni-based catalyst systems with higher Ni contents without the CeO2 flowers [110]. Based on their findings, it can be inferred that the shape of CeO2 has a significant impact on its overall OSC. It is commonly understood that rod- or cube-shaped CeO2 structures possess higher OSC than spherical particles in other CeO2-based catalytic systems than those discussed here [107,111,112], suggesting the shape effect. The same trend appears to hold for steam-reforming reactions as well. Further examples will support this observation.
Qian and co-workers studied attractive core-shell catalysts (Ni in the core and alumina and ceria in the inner and outer shells, respectively) that provided a high hydrogen yield in the steam reforming of acetic acid. They prepared, with different Ni/Ce ratios, alumina over ceria catalysts (designated as Ni@AlxCe, x = 05, 10, 30), and the same was performed with ceria added to the alumina shell but with just one Ni/Ce ratio, designated as Ni@Al10Ce-a (Figure 10) [39]. They started the preparation by obtaining Ni nanoparticles using PVP as a stabilizer, NiCl2·6H2O as a metal precursor, and NaBH4 as a reductant that was added after the mixture was ultrasonicated for 15 min; then, Ce(NO3)3·6H2O (or Al2O3) was added to the suspension and underwent 1 h of ultrasonication and the Ni nanoparticles were coated with an alumina (or ceria) shell. The authors attributed the enhanced catalytic activity of the Ni@Al10Ce catalyst to the mobility of oxygen within the ceria lattice, allowing it to migrate to the surface of the Ni catalyst and react with the dissociated acetic acid, associated with optimized reaction conditions. Additionally, they attributed the minimal formation of coke to the oxygen storage property of ceria. The mobile oxygen released from ceria could migrate to the surface of Ni nanoparticles, where it reacted with the coke precursor or the already formed coke. The diffusion of acetic acid and water from the bulk solution to the surface of nickel nanoparticles, where dissociation took place, was attributed to the pathway provided by the alumina coating, having a better function at a specific ratio.
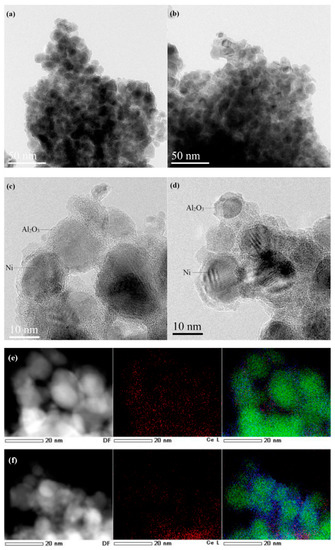
Figure 10.
HRTEM images of (a) Ni@Al10Ce and (b) Ni@Al10Ce-a with low magnification. (c) Ni@Al10Ce and (d) Ni@Al10Ce-a with high magnification. STEM images with cerium EDS mappings and overlaid mappings of the (e) Ni@Al10Ce and (f) Ni@Al10Ce-a catalysts reduced at 600 °C. The green and blue spots represent Ni and Al elements, respectively. Modified with permission from [39].
Also, using the synergy between CeO2 and Al2O3, Lai et al. prepared Ni−CeO2−Al2O3 hybrid nanoparticle clusters as catalysts for hydrogen production via low-temperature steam-methane reforming [113]. The authors obtained a significant metal–support interface generation in the hybrid nanostructure using aerosol-based evaporation-induced self-assembly, in which the addition of Al2O3 was responsible for the surficial area increase, suppression of Ni sintering, and lowering of coke formation. Tu et al. developed a novel two-step hydrothermal process to produce flower-like nanostructures comprised of ceria–zirconia with an OSC of 536 µmol O2 g−1, which was twice the OSC of pure flower-like CeO2 (284 µmol O2 g−1) [114]. By forming a solid solution with zirconia, the function of ceria as a support material for Ni catalysts in the dry reforming of methane was enhanced at 750 °C, with an initial methane conversion of 88.4%. The authors concluded that the flower-like catalyst’s petal-shaped structure improved the Ni metal’s sintering resistance and enhanced coking resistance. It is important to highlight that the manipulation of oxygen vacancies (and OSC) has been achieved through doping methods [115,116]. However, to the best of our knowledge, no studies have been conducted on the control or rational effect of the different morphology or shape of CeO2-based catalysts in this regard. Moreover, we feel that such studies can provide interesting insights if they include experimental data and DFT calculations to support their findings.
Extensive research has been conducted on using Co as a catalyst (or co-catalyst) for steam-reforming reactions [117,118,119,120], Its combination with CeO2 structures has shown a synergistic effect, enhancing the active surface area, inhibiting sintering, and facilitating oxygen storage. The catalytic properties have long been studied as CeO2-morphology-dependent for Co-based catalysts [121]. However, our search indicated a recent trend of focusing on ethanol steam reforming and more profound knowledge regarding this substrate; thus, we will focus on the recent aspects of the interaction of Co and ceria, beyond simply speaking of a specific morphology and including the effect of ceria facets. Interestingly, the catalysts’ activity and selectivity are influenced by the acid-base properties of the oxide support, as discussed in Noronha’s excellent publication [122]. Thus, choosing the support correctly is of high priority, and ceria properties are remarkable. For example, Ferencz et al. reported a notable influence of the support on product selectivity during ethanol reforming for Co-based catalysts. Due to ceria’s basic nature, hydrogen production was accompanied by the generation of aldehyde and crotonaldehyde as byproducts. Therefore, the acidity/basicity properties of the support play a crucial role in determining the products’ formation [123]. In the same way, the oxidation state of the active species also can play a pivotal role in the activity and selectivity of the process [124].
The literature has already shown that the Co content influences the interaction between metal and cerium, favoring the catalytic properties in ethanol steam reforming, and the highest Co content proved to be when the catalyst was prepared by a co-precipitation method under optimized conditions (500 °C, H2O/EtOH = 12/1) [125]. However, metal dispersion plays a crucial role in the catalytic process, as expected in heterogeneous catalysis, and it is generally recognized as an essential factor. For that matter, the preparation method choice and adding additives can be beneficial. Greluk et al. prepared Co/CeO2 and Ni/CeO2 catalysts by employing various techniques—the impregnation method, different active phase precursors, impregnation solvents, and the presence of organic additives—in an attempt to enhance the dispersion of the metals (Co or Ni) and promote strong metal–support interactions [126]. Adding citric acid to the precursor metal salt solution has proven effective in achieving improved dispersion and smaller metal particle sizes for both catalysts.
Conversely, catalysts prepared using an ammonia solution resulted in larger metal particle sizes. Interestingly, although the authors did not precisely control the shape of the CeO2 (they obtained rectangular-like shape ceria; Figure 11), the crucial step in the steam reforming of ethanol at 420 °C was using both catalysts is the dehydrogenation of ethanol, which predominantly takes place on the terrace sites of cobalt/nickel. However, the cleavage of the C–C bond is more favorable at the edge/step sites, leading to higher efficiency in the overall process. In the case of Co-based catalysts, apart from size effects, they found that their performance was based on the oxidation state of the Co nanoparticles.

Figure 11.
HRTEM images and corresponding FFT patterns of (a) Co/CeO2 (obtained in the presence of citric acid) and (b) Co/CeO2 (obtained from ammonia solution). Modified with permission from [126].
Following a similar trajectory of exploration, Li et al. recently studied Co/CeO2 catalysts with an exposure preference of (111) and (100) facets of CeO2, aiming to understand how to regulate the Co chemical state [127]. They discovered that the Co supported on ceria with (111) facets presented a lower oxidation state and displayed an enhanced capability for cleaving C–C bonds, resulting in superior catalytic performance for the ethanol steam-reforming reaction compared to the Co supported on ceria with (100) facets. Using in situ synchrotron radiation photoionization mass spectrometry and DFT calculations, it has been revealed that Co sites in a low oxidation state exhibit a strong preference for carbon chain shortening through C–C cleavage rather than carbon chain lengthening via condensation reactions. This unique property enhances the efficiency of hydrogen production in the ethanol steam-reforming reaction. Additional information regarding DFT calculations provided the knowledge that the O-terminated (111) facet allowed for the stable support of metallic Co, while the O-terminated (100) facet led to the formation of cobalt oxide, controlling the chemical states of the Co sites, indicating that the oxidation state of Co could be regulated by the CeO2 support with a specific facet exposure (Figure 12).
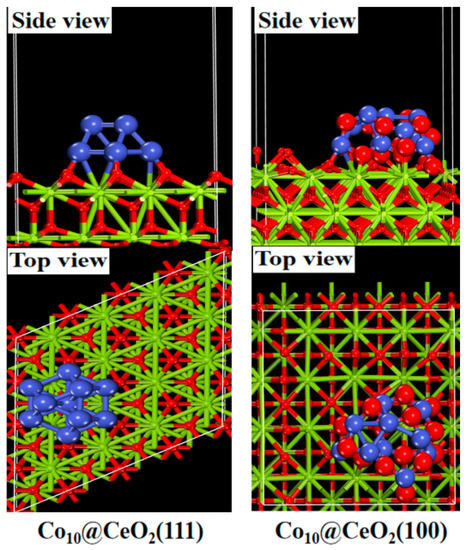
Figure 12.
Side and top views of optimized geometries of Co10 clusters over CeO2 with the exposure of (111) and (100) facets. Reproduced with permission from [127].
One may notice that the Co species may undergo differences during the reaction. Interestingly, Huck-Iriart et al. studied the chemical state of Co in Co-based catalysts during the ethanol steam-reforming reaction by near-ambient pressure XPS (NAP-XPS), extended X-ray absorption fine structure (EXAFS), and X-ray absorption near-edge structure (XANES) [128]. They realized that differences in synthesis methods and precursors affected the Co species during the reaction, which is fascinating. Although their studies did not focus on CeO2-based catalysts, they provided valuable insights that could be applied to investigate supported cobalt on CeO2, particularly with different morphologies, opening possibilities for future research in this area. Although with another aim, Sohn et al. employed AP-XPS and XANES techniques to observe the effect of Co on the reduction characteristics of ceria under ethanol steam-reforming conditions. The authors found that the reducibility of ceria particles is influenced by their particle size, with smaller particles exhibiting a greater degree of surface reduction. Moreover, the presence of fully oxidized Co nanoparticles on the ceria support hinders the surface reducibility of ceria as the reduction of cobalt oxide phases (Co3O4 and CoO) takes priority over the reduction of ceria [129].
Researchers used La2O3 as a promoter for Co/CeO2 and Ni/CeO2 catalysts for the conversion of hydrogen at a temperature of 500 °C, obtaining a complete conversion of ethanol in 21 h [130]. However, the Co/CeO2 catalyst prepared with a La/Co molar ratio equal to 0.1 by co-impregnation proved selective during ethanol steam reforming, showing hydrogen and carbon dioxide selectivity of 94% and 88%, respectively. Although this showed good conversion, methane and carbon monoxide were produced in low amounts, and trace level acetaldehyde was detected among the reaction products. Incorporating La in the structure resulted in forming smaller particle sizes of Co and good dispersion, improving the catalytic activity; however, there was no control of ceria. Thus, it is crucial to understand the effect of promoters, like La and other lanthanides as Zr [131], but there is a lack of studies associating the doping process with shape controlling. The doping of Co into the lattice of CeO2 was studied for the auto-thermal reforming of acetic acid. Although it was unrelated to ethanol reforming, the authors’ findings may be interesting for other substrates and open up further possibilities.
As a final example considering Co-based catalysts and showing the importance of the shape control of ceria, Hu et al. prepared Co-Ce-O by different hydrothermal methods; however, the material that offered the best response was based on ceria nanorods [132], which exhibited a stable hydrogen yield of 2.69 mol-H2/mol acetic acid and a H2 production rate of 332.3 mmol-H2/(gcat·h), even at 600 °C. The acetic acid conversion was initially close to 98.6% and remained stable. In addition, the authors obtained CO2 and CO selectivity of 69.2% and 29.9%, respectively, with no records of by-products. Its greater reactivity concerning the other structures was related to controlling the material’s morphology, exhibiting a greater surface area than the others. It is important to emphasize that a comprehensive understanding of the catalysts’ surface processes requires further research. Therefore, a combination of theoretical and experimental studies becomes essential. For example, Varga et al. observed a strong dissolution of Co into ceria and a certain extent of Co encapsulation by ceria through XRD, XPS, and LEIS (low energy ion scattering) [133]. These observations can significantly influence the material’s performance, indicating the need for further investigations in this area.
5. Shape-Controlled Cerium-Oxide-Based Nanocatalysts with Pt
Despite their high value, noble metals are commonly used as catalysts for steam-reforming reactions due to their high catalytic activity, resistance to catalyst poisoning, thermal stability, and ability to control selectivity [134]. Research is ongoing to explore alternative non-noble metal catalysts, as discussed before; however, the fine-tuning of ceria as a support for noble metals can be used to improve metal–support interactions, avoiding high concentrations of the metal on the support, improving efficiency and resistance to deactivation. Thus, the influence of the noble metal used is important, and deeper knowledge regarding its effect is still the focus of much research [134]. In this context, Vayssilov et al. observed that the electron transfer is favorable on ceria supports regardless of their morphology, while oxygen transfer requires nanostructured ceria in close proximity to Pt. These findings provide valuable insights into the formation mechanism of Pt–O species on ceria and the structure-activity dependence of ceria-based catalysts [135].
Thus, through the reduction of nanoparticle size, catalytic activities can be enhanced while reducing costs. However, smaller noble nanostructures tend to aggregate during catalytic processes, and significant efforts have been made to develop sinter-resistant, single-site catalysts. Jones et al. conducted research using various CeO2 nanostructures to identify the most effective facet for trapping Pt, forming highly thermally stable, single-atom Pt on CeO2 nanostructures [136]. Atomically dispersed Pt was observed on CeO2 nanocubes and polyhedra, and, after various catalytic cycles at temperatures of up to 300 °C, the Pt on CeO2 nanorods remained atomically dispersed, showing CeO2’s role in suppressing catalyst sintering and offering insights for the design of single-atom nanocatalysts. A recent study by Wang et al. showed that steam treatment had a significant impact on catalytic properties by accelerating the formation of active surface lattice oxygen near Pt single atoms [137]. This treatment led to a substantial enhancement in catalytic performance. Unfortunately, we could not find similar studies for steam-reforming reactions, but they prove to be promising to the field.
However, other studies show that the shape-control of ceria is important in Pt-based catalysts. For example, CeO2 nanorods have been studied as a support for Pt nanoparticles for the steam reforming of various substrates. Claudio-Piedras et al. conducted a study on Pt/CeO2 nanorod (hydrothermal method) catalysts for methanol steam reforming [138]. They discovered that the choice of Pt precursor (Pt(NH3)4(NO3)2), (CH3-COCHCO-CH3)2Pt, and H2PtCl6.6H2O) immobilized onto the ceria by the incipient impregnation method and that synthesis solvent influenced the catalyst’s performance, indicating that further research is required to establish a common trend. Additionally, the impact of ceria shape on catalytic properties suggests that there is still much to explore in this area.
Studying the catalytic properties of Pt/CeO2 nanorods (hydrothermally prepared) for the steam reforming of methanol, Papavasiliou et al. used the H2PtCl6.6H2O as the metal precursor for the immobilization of Pt by wet impregnation and deposition–precipitation methods. The latter technique provided highly dispersed Pt (mean particle size of less than 1 nm) over the support, which was correlated by the authors due to oxygen vacancies (although no characterizations were provided to prove such a relationship). Interestingly, the catalyst with just 0.3 wt.% Pt strongly interacted with the support surface, in agreement with the STEM, HR-TEM, XRD, and Raman analyses, resulting in an efficient material for the steam reforming of methanol reactions [77].
Verykios and co-workers gave us an overview of the catalyst performance of differently shaped CeO2 supports as carriers of Pt nanoparticles for low-temperature steam reforming of methanol [139]. Comparing CeO2 bulk (PPT), nanocubes (NC), nanorods (NR), and flower-like (FL) structures (Figure 13), the Pt/CeO2–PPT catalyst demonstrated the highest activity for the dehydrogenation of ethanol to acetaldehyde. On the other hand, the Pt/CeO2–FL and Pt/CeO2–NC catalysts exhibited a higher rate of decomposition of acetaldehyde into CH4 and CO. The ethanol dehydrogenation activity followed the order PPT > NR > NC > FL, while Pt/CeO2–NR presented the best stability and Pt/CeO2–PPT deactivated quickly. XPS analysis showed the presence of Ce3+ and Pt0 on the Pt/CeO2–NR catalyst after a reaction at 300 °C. However, the Pt/CeO2–PPT catalyst exhibited a lower degree of reduction related to the accumulation of acetate species, which can be attributed to the imbalance between the decomposition rate of acetate species to CHx species on Ptδ+ and their desorption as CH4. The authors believe that this was caused by the lower metal–support interface of the Pt/CeO2–PPT catalyst, which had a lower Pt dispersion. The blockage of the Pt–support interface by these CHx species leads to catalyst deactivation. Curiously, nanorods are the focus of great interest, probably due to their best performances.
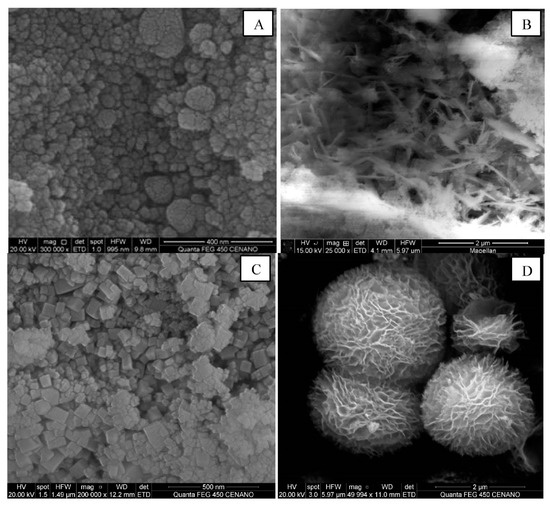
Figure 13.
SEM images for the (A) CeO2–PPT, (B) CeO2–NR, (C) CeO2–NC, and (D) CeO2–FL catalysts. Reproduced with permission from [139].
Raúl Pérez-Hernández researched Pt/Ni catalysts supported on CeO2 nanorods for methanol steam reforming. The study revealed that the enhanced catalytic activity was not solely attributed to the synergy between the metals; CO-DRIFTS (CO diffuse reflectance infrared Fourier transform spectroscopy) experiments indicated that the bimetallic PtNi nanoparticles were predominantly Pt-terminated, which contributed to the improved performance. TPD-DRIFTS (temperature-programmed desorption associated with diffuse reflectance infrared Fourier transform spectroscopy) experiments conducted in the presence of methanol demonstrated that methoxy species and C-H groups strongly interacted with the support’s surface oxygen near Pt. This interaction, facilitated by Pt, resulted in the formation of formate species and suggested Pt’s important role as an intermediary in the reaction [140].
Based on a sophisticated synthesis approach, Dai et al. created a CeO2@Pt-Beta yolk-shell (“Beta”—the name of the material refers to the zeolite Beta) catalyst for low-temperature ethanol steam reforming (Figure 14) [141].
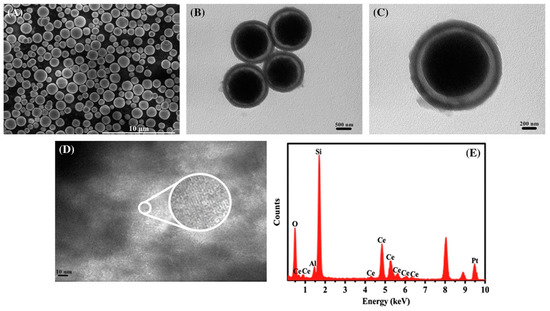
Figure 14.
SEM image of (A) CeO2; TEM images of (B–D) CeO2@Pt-Beta catalyst view at different magnifications. (E) The EDX spectrums of the catalyst. Reproduced with permission from [141].
To guarantee the superior performance of the prepared catalyst, the authors compared it with the Pt-Beta and CeO2-Pt-Beta counterparts. They attributed the better performance of the synthesized material to the synergistic effect between the movable CeO2 core and the Pt nanoparticles and the high dispersion of the metal, which achieved 100% conversion with 67% hydrogen selectivity. They also believe that the interstitial hollow space within the catalyst allows for the high loading of reactant molecules, particularly promoting the reaction of larger molecules such as ethanol and acetaldehyde with the CeO2 core and metal Pt active sites. Throughout the 20 h test at 350 °C, the catalyst remained stable, without any significant decrease in activity. Once more, it is crucial to emphasize that the synthesis of shape-controlled nanostructures is an actively developing field. Ongoing research and advancements are necessary to gain a comprehensive understanding of the intrinsic processes occurring during reactions, including the significance of doping techniques. This is also pertinent when considering Pt-based catalysts [116].
Although extensive research has focused on Pt as a catalyst for steam reforming, leading to a comprehensive understanding of its performance and behavior in this application, the established catalytic response of Pt makes it the preferred choice for researchers and industry professionals. However, other noble metals also are promising candidates for steam-reforming reactions, although there is a relatively limited volume of studies exploring their utilization with shape-controlled CeO2 nanostructures. For instance, Kolb et al. conducted a study on the impact of ceria oxide supports’ morphology and crystalline facets on the performance of Rh/CeO2 and Rh/CeO2/Al2O3 catalysts in the oxidative steam reforming of propylene glycol (630 °C, with a steam-to-carbon ratio of 3.5 and an oxygen-to-carbon ratio of 0.15) [142]. A hydrothermal method was employed to synthesize well-defined ceria nanorods and nanocubes, which were then deposited onto α-Al2O3. TEM images revealed that the ceria nanorods exhibited exposed (110) and (100) planes, while the nanocubes possessed only (100) planes; such structures were used as supports for Rh nanoparticles, providing a large surface area and efficient heat and mass transfer. The superior performance of the Rh catalyst supported on ceria nanorods could be attributed to the improved dispersion of Rh species and the presence of higher surface oxygen vacancies associated with the exposed (100) and (110) crystal planes. Also, the catalyst design allowed Rh nanoparticles to stabilize, prevented sintering, and minimized carbon deposition. Unfortunately, little is known about the shape-controlling of ceria for other noble metals, which is the reason for the emphasis on Pt nanoparticles herein presented.
6. Concluding Remarks and Perspectives
In this discussion, we have emphasized the significance of controlling the characteristics of ceria-based catalysts to optimize their catalytic performance for steam-reforming reactions. Specifically, we have explored control over size and shape. The ability to engineer precise shapes at the nanoscale provides unprecedented control over catalytic properties, enabling researchers to enhance reaction efficiency, selectivity, and stability. As this field progresses, it holds tremendous promise for advancing clean energy technologies and driving the transition toward a sustainable hydrogen economy.
Initially, we examined the importance of ceria as a support material for metal nanoparticles due to its properties (oxygen vacancies, redox properties, and oxygen storage capacities) and how they can be manipulated regarding the control of the shape of the ceria nanostructures. Subsequently, we delved into experimental strategies, showing how different facets are essential to provide strong metal–support interactions, affording catalyst stability with lower coke deposition. Then, we chose some metals (Ni, Co, and Pt) for ceria-based catalysts to show how such physicochemical controlling can be essential to achieve better results.
We observed that significant progress has been made in understanding catalysts through synthetic and characterization technical advancements. This knowledge has paved the way for the rational design and synthesis of catalysts with enhanced performance, making them suitable for industrial-scale applications, pilot studies, and eventual commercialization. To achieve cost reduction in steam-reforming reactions, there is a need for more efficient materials and breakthroughs in technological development, keeping in mind that alternative and environmentally benign processes for H2 production still need substantial industrial maturing; thus, achieving advancements in the reforming field is crucial from both economic and ecological perspectives as reforming technologies will present progress at a faster rate compared to the development of sustainable technologies.
However, much work must be conducted in the field. By comprehensively searching for the most recent studies regarding shape-controlled ceria, we observed that the literature lacks a fundamental understanding of steam-reforming reactions based on morphological issues. By saying that, we mean that almost no studies regarding mechanisms and DFT calculations were available, making it difficult to better understand the reason for differences in catalysts’ performances due to the ceria’s morphology. Of course, the literature brings such insights for other reactions; nevertheless, we cannot assume that the findings apply to steam-reforming reactions. Also, the shape-controlled ceria literature seems to apply mostly to reactions with alcohol. Although such substrates are attractive, methane counts for about 95% of the market; therefore, more studies must be conducted, especially due to the increasing levels of biogas production. Apart from the abovementioned, studies performed with different ceria morphologies are concentrated on non-noble metals (or present more studies). This is a valid approach due to a cost reduction and availability perspective; however, noble metals must be explored more due to their potential. In addition, by employing nanoscience tools, noble metal utilization can be more efficient, reducing costs through the use of lower loadings or by mixing them with non-noble metals. The literature brings examples of this; however, not for shape-controlled studies.
These proposals aim to elevate processes to a higher level, enabling a design-driven approach where catalytic systems can be strategically planned at earlier stages, paving the way for energy generation and advancements.
Author Contributions
Conceptualization, writing-original draft preparation, M.A.S.G.; writing-review and editing, S.d.S.E., J.P.M.; P.N.R., J.M.A.R.d.A., and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Maranhão and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001 (CAPES Portaria No 206, de 4 de setembro de 2018).
Data Availability Statement
All data used in this review article are from publicly available sources, published literature, and scientific databases, which are appropriately cited. No new data were generated for this study.
Acknowledgments
The authors acknowledge FAPEMA and Porto do Itaqui for J.P.M. and S.S.E scholarships.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Shetwi, A.Q. Sustainable development of renewable energy integrated power sector: Trends, environmental impacts, and recent challenges. Sci. Total Environ. 2022, 822, 153645. [Google Scholar] [CrossRef] [PubMed]
- Dincer, I.; Bicer, Y. Integration of renewable energy systems for multigeneration. In Integrated Energy Systems for Multigeneration; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–402. [Google Scholar] [CrossRef]
- Egeland-Eriksen, T.; Hajizadeh, A.; Sartori, S. Hydrogen-based systems for integration of renewable energy in power systems: Achievements and perspectives. Int. J. Hydrog. Energy 2021, 46, 31963–31983. [Google Scholar] [CrossRef]
- Deutch, J. Is Net Zero Carbon 2050 Possible? Joule 2020, 4, 2237–2240. [Google Scholar] [CrossRef] [PubMed]
- McCay, M.H.; Shafiee, S. Hydrogen. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 475–493. [Google Scholar] [CrossRef]
- Sgarbossa, F.; Arena, S.; Tang, O.; Peron, M. Renewable hydrogen supply chains: A planning matrix and an agenda for future research. Int. J. Prod. Econ. 2023, 255, 108674. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- AbouSeada, N.; Hatem, T.M. Climate action: Prospects of green hydrogen in Africa. Energy Rep. 2022, 8, 3873–3890. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 economy realizable in the foreseeable future? Part II: H2 storage, transportation, and distribution. Int. J. Hydrog. Energy 2020, 45, 20693–20708. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrog. Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Gothe, M.L.; Kohlrausch, E.C.; Zardo, M.L.; Tanaka, A.A.; de Lima, R.B.; Silva, A.A.M.; Garcia, M.A.S.; Vidinha, P.; Machado, G. Nanoengineering of Catalysts for Enhanced Hydrogen Production. Hydrogen 2022, 3, 218–254. [Google Scholar] [CrossRef]
- Reeve, J.; Grasham, O.; Mahmud, T.; Dupont, V. Advanced Steam Reforming of Bio-Oil with Carbon Capture: A Techno-Economic and CO2 Emissions Analysis. Clean Technol. 2022, 4, 309–328. [Google Scholar] [CrossRef]
- Korberg, A.D.; Thellufsen, J.Z.; Skov, I.R.; Chang, M.; Paardekooper, S.; Lund, H.; Mathiesen, B.V. On the feasibility of direct hydrogen utilisation in a fossil-free Europe. Int. J. Hydrog. Energy 2023, 48, 2877–2891. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B 2021, 296, 120210. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrog. Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2016, 8, 1237–1259. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Shen, W. Shape Engineering of Oxide Nanoparticles for Heterogeneous Catalysis. Chem. Asian J. 2016, 11, 1470–1488. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, Y.; Zhao, Z.; Xu, Q.; Wang, X.; Xiao, M.; Zou, Z. Hexahedron Prism-Anchored Octahedronal CeO2: Crystal Facet-Based Homojunction Promoting Efficient Solar Fuel Synthesis. J. Am. Chem. Soc. 2015, 137, 9547–9550. [Google Scholar] [CrossRef]
- Chupradit, S.; Kavitha, M.; Suksatan, W.; Ansari, M.J.; Al Mashhadani, Z.I.; Kadhim, M.M.; Mustafa, Y.F.; Shafik, S.S.; Kianfar, E. Morphological Control: Properties and Applications of Metal Nanostructures. Adv. Mater. Sci. Eng. 2022, 2022, 1971891. [Google Scholar] [CrossRef]
- Chen, C.; Wylie, R.A.L.; Klinger, D.; Connal, L.A. Shape Control of Soft Nanoparticles and Their Assemblies. Chem. Mater. 2017, 29, 1918–1945. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Nguyen, Q.N.; Xia, Y. Noble-Metal Nanocrystals with Controlled Shapes for Catalytic and Electrocatalytic Applications. Chem. Rev. 2021, 121, 649–735. [Google Scholar] [CrossRef]
- Vecchietti, J.; Pérez-Bailac, P.; Lustemberg, P.G.; Fornero, E.L.; Pascual, L.; Bosco, M.V. Shape-Controlled Pathways in the Hydrogen Production from Ethanol Steam Reforming over Ceria Nanoparticles. ACS Catal. 2022, 12, 10482–10498. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Ghaffari Saeidabad, N.; Noh, Y.S.; Alizadeh Eslami, A.; Song, H.T.; Kim, H.D.; Fazeli, A.; Moon, D.J. A Review on Catalysts Development for Steam Reforming of Biodiesel Derived Glycerol; Promoters and Supports. Catalysts 2020, 10, 910. [Google Scholar] [CrossRef]
- García, L. Hydrogen production by steam reforming of natural gas and other nonrenewable feedstocks. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 83–107. [Google Scholar] [CrossRef]
- Idriss, H.; Scott, M.; Subramani, V. Introduction to hydrogen and its properties. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–19. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Menegazzo, F.; Ghedini, E.; Innocenti, G.; Di Michele, A.; Cruciani, G.; Cavani, F.; Signoretto, M. Increase of Ceria Redox Ability by Lanthanum Addition on Ni Based Catalysts for Hydrogen Production. ACS Sustain. Chem. Eng. 2018, 6, 13867–13876. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Matras, J.; Niewiadomski, M.; Ruppert, A.; Grams, J. Activity of Ni catalysts for hydrogen production via biomass pyrolysis. Kinet. Catal. 2012, 53, 565–569. [Google Scholar] [CrossRef]
- Pant, K.K.; Mohanty, P.; Agarwal, S.; Dalai, A.K. Steam reforming of acetic acid for hydrogen production over bifunctional Ni–Co catalysts. Catal. Today 2013, 207, 36–43. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukewicz, W.; Czylkowska, A.; Maniecki, T. Steam reforming of ethanol for hydrogen production: Influence of catalyst composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and process conditions. React. Kinet. Mech. Catal. 2021, 132, 907–919. [Google Scholar] [CrossRef]
- Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G. CeO2-supported Pt/Ni catalyst for the renewable and clean H2 production via ethanol steam reforming. Appl. Catal. B 2014, 145, 73–84. [Google Scholar] [CrossRef]
- Pu, J.; Luo, Y.; Wang, N.; Bao, H.; Wang, X.; Qian, E.W. Ceria-promoted Ni@Al2O3 core-shell catalyst for steam reforming of acetic acid with enhanced activity and coke resistance. Int. J. Hydrog. Energy 2018, 43, 3142–3153. [Google Scholar] [CrossRef]
- Manan, W.N.; Wan Isahak, W.N.R.; Yaakob, Z. CeO2-Based Heterogeneous Catalysts in Dry Reforming Methane and Steam Reforming Methane: A Short Review. Catalysts 2022, 12, 452. [Google Scholar] [CrossRef]
- Anil, S.; Indraja, S.; Singh, R.; Appari, S.; Roy, B. A review on ethanol steam reforming for hydrogen production over Ni/Al2O3 and Ni/CeO2 based catalyst powders. Int. J. Hydrog. Energy 2022, 47, 8177–8213. [Google Scholar] [CrossRef]
- Liu, Z.; Senanayake, S.D.; Rodriguez, J.A. Elucidating the interaction between Ni and CeOx in ethanol steam reforming catalysts: A perspective of recent studies over model and powder systems. Appl. Catal. B 2016, 197, 184–197. [Google Scholar] [CrossRef]
- Guo, X.; Waser, R. Electrical properties of the grain boundaries of oxygen ion conductors: Acceptor-doped zirconia and ceria. Prog. Mater. Sci. 2006, 51, 151–210. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Hickey, N.; Fornasiero, P.; Kašpar, J.; Gatica, J.M.; Bernal, S. Effects of the Nature of the Reducing Agent on the Transient Redox Behavior of NM/Ce0.68Zr0.32O2 (NM = Pt, Pd, and Rh). J. Catal. 2001, 200, 181–193. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, P.; Koberstein, J.; Khalid, S.; Chan, S.-W. Cerium oxidation state in ceria nanoparticles studied with X-ray photoelectron spectroscopy and absorption near edge spectroscopy. Surf. Sci. 2004, 563, 74–82. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Borges, L.R.; Silva, A.G.M.; Braga, A.H.; Rossi, L.M.; Suller Garcia, M.A.; Vidinha, P. Towards the Effect of Pt0/Ptδ+ and Ce3+ Species at the Surface of CeO2 Crystals: Understanding the Nature of the Interactions under CO Oxidation Conditions. ChemCatChem 2021, 13, 1340–1354. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, H.; Zhu, G.; Wu, H.; He, L. Ceria supported Ru0-Ru+ clusters as efficient catalyst for arenes hydrogenation. Chin. Chem. Lett. 2021, 32, 770–774. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.-H.; Raitano, J.M.; Hanson, J.C.; Caliebe, W.A.; Khalid, S.; Chan, S.-W. Phase stability in ceria-zirconia binary oxide nanoparticles: The effect of the Ce3+ concentration and the redox environment. J. Appl. Phys. 2006, 99, 084313. [Google Scholar] [CrossRef]
- Hao, X.; Yoko, A.; Chen, C.; Inoue, K.; Saito, M.; Seong, G.; Adschiri, T.; Ikuhara, Y. Atomic-Scale Valence State Distribution inside Ultrafine CeO2 Nanocubes and Its Size Dependence. Small 2018, 14, 1802915. [Google Scholar] [CrossRef]
- Waldvogel, A.; Fasolini, A.; Basile, F.; Thomas, S.; Roger, A.-C. Effect of the Support Synthetic Method on the Activity of Ni/CeZrPr Mixed Oxide in the Co-Methanation of CO2/CO Mixtures for Application in Power-to-Gas with Co-Electrolysis. Energy Fuels 2021, 35, 13304–13314. [Google Scholar] [CrossRef]
- Fasolini, A.; Ruggieri, S.; Femoni, C.; Basile, F. Highly Active Catalysts Based on the Rh4(CO)12 Cluster Supported on Ce0.5Zr0.5 and Zr Oxides for Low-Temperature Methane Steam Reforming. Catalysts 2019, 9, 800. [Google Scholar] [CrossRef]
- Auxéméry, A.; Frias, B.B.; Smal, E.; Dziadek, K.; Philippot, G.; Legutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous supercritical solvothermal preparation of nanostructured ceria-zirconia as supports for dry methane reforming catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- Basile, F.; Mafessanti, R.; Fasolini, A.; Fornasari, G.; Lombardi, E.; Vaccari, A. Effect of synthetic method on CeZr support and catalytic activity of related Rh catalyst in the oxidative reforming reaction. J. Eur. CerAm Soc. 2019, 39, 41–52. [Google Scholar] [CrossRef]
- Holgado, J.P.; Alvarez, R.; Munuera, G. Study of CeO2 XPS spectra by factor analysis: Reduction of CeO2. Appl. Surf. Sci. 2000, 161, 301–315. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Z.; Wen, J.; Poeppelmeier, K.R.; Marks, L.D. Imaging the Atomic Surface Structures of CeO2 Nanoparticles. Nano Lett. 2014, 14, 191–196. [Google Scholar] [CrossRef]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef]
- Aguilera González, E.N.; Estrada Flores, S.; Martínez Luévanos, A. Nanomaterials: Recent Advances for Hydrogen Production. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–27. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lu, X. (Eds.) Metallic Nanostructures; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Araiza, D.G.; Gómez-Cortés, A.; Díaz, G. Effect of ceria morphology on the carbon deposition during steam reforming of ethanol over Ni/CeO2 catalysts. Catal. Today 2020, 349, 235–243. [Google Scholar] [CrossRef]
- Padi, S.P.; Shelly, L.; Komarala, E.P.; Schweke, D.; Hayun, S.; Rosen, B.A. Coke-free methane dry reforming over nano-sized NiO-CeO2 solid solution after exsolution. Catal. Commun. 2020, 138, 105951. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, S.; Yan, S.; Hu, J.; Dan, Y. Fabrication and Application of CeO2 Nanostructure with Different Morphologies: A Review. J. Renew Mater. 2020, 8, 1443–1472. [Google Scholar] [CrossRef]
- Maya-Johnson, S.; Gracia, L.; Longo, E.; Andres, J.; Leite, E.R. Synthesis of Cuboctahedral CeO2 Nanoclusters and Their Assembly into Cuboid Nanoparticles by Oriented Attachment. ChemNanoMat 2017, 3, 228–232. [Google Scholar] [CrossRef]
- Gou, W.; Xia, Z.; Tan, X.; Xue, Q.; Ye, F.; Dai, S.; Zhang, M.; Si, R.; Zou, Y.; Ma, Y.; et al. Highly active and stable amorphous IrOx/CeO2 nanowires for acidic oxygen evolution. Nano Energy 2022, 104, 107960. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Damma, D.; Jampaiah, D.; Mukherjee, D.; Vithal, M.; Reddy, B.M. Cr-Doped CeO2 Nanorods for CO Oxidation: Insights into Promotional Effect of Cr on Structure and Catalytic Performance. Catal. Lett. 2020, 150, 948–962. [Google Scholar] [CrossRef]
- Farhang-Sahlevani, S.; Pandiyarajan, T.; Sanhueza, F.; Akbari-Fakhrabadi, A.; Mansilla, H.D.; Contreras, D.; Mangalaraja, R.V.; Gracia-Pinilla, M.A. A facile hydrothermal synthesis of CeO2 nanocubes decorated ZnO nanostructures: Optical and enhanced photocatalytic properties. J. Mater. Sci. Mater. Electron. 2019, 30, 11643–11651. [Google Scholar] [CrossRef]
- Lin, M.; Fu, Z.Y.; Tan, H.R.; Tan, J.P.Y.; Ng, S.C.; Teo, E. Hydrothermal Synthesis of CeO2 Nanocrystals: Ostwald Ripening or Oriented Attachment? Cryst. Growth Des. 2012, 12, 3296–3303. [Google Scholar] [CrossRef]
- Soler, L.; Casanovas, A.; Ryan, J.; Angurell, I.; Escudero, C.; Pérez-Dieste, V.; Llorca, J. Dynamic Reorganization of Bimetallic Nanoparticles under Reaction Depending on the Support Nanoshape: The Case of RhPd over Ceria Nanocubes and Nanorods under Ethanol Steam Reforming. ACS Catal. 2019, 9, 3641–3647. [Google Scholar] [CrossRef]
- Modragón-Galicia, G.; Toledo Toledo, M.; Morales-Anzures, F.; Salinas-Hernández, P.; Gutiérrez-Martínez, A.; García, M.E.F.; Barrera, F.T.A.; Reyna-Alvarado, J.; López-Galán, O.A.; Ramos, M.; et al. Catalytic Aspects of Pt/Pd Supported on ZnO Rods for Hydrogen Production in Methanol Steam Reforming. Top Catal. 2022, 65, 1556–1569. [Google Scholar] [CrossRef]
- Abbas, T.; Tahir, M.; Saidina Amin, N.A. Enhanced Metal–Support Interaction in Ni/Co3O4/TiO2 Nanorods toward Stable and Dynamic Hydrogen Production from Phenol Steam Reforming. Ind. Eng. Chem. Res 2019, 58, 517–530. [Google Scholar] [CrossRef]
- Yuejuan, W.; Jingmeng, M.; Mengfei, L.; Ping, F.; Mai, H. Preparation of High-Surface Area Nano-CeO2 by Template-Assisted Precipitation Method. J. Rare Earths 2007, 25, 58–62. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; Lu, J.; He, S.; Lu, H.; Song, D.; Chen, D.; Luo, Y. Novel nanowire self-assembled hierarchical CeO2 microspheres loaded with nickel-based catalysts for hydrogen production from steam reforming of glycerol. Fuel Process. Technol. 2023, 243, 107677. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Zhu, J.; Han, B.; Zhao, L.; Yu, H.; Deng, Z.; Shi, W. Study on different CeO2 structure stability during ethanol steam reforming reaction over Ir/CeO2 nanocatalysts. Appl. Catal. A Gen. 2018, 564, 226–233. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Paxinou, A.; Słowik, G.; Neophytides, S.; Avgouropoulos, G. Steam Reforming of Methanol over Nanostructured Pt/TiO2 and Pt/CeO2 Catalysts for Fuel Cell Applications. Catalysts 2018, 8, 544. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Moura, A.B.L.; e Silva, F.A.; Candido, E.G.; da Silva, A.G.M.; de Oliveira, D.C.; Quiroz, J.; Camargo, P.H.C.; Bergamaschi, V.S.; Ferreira, J.C.; et al. Ni supported Ce0.9Sm0.1O2-δ nanowires: An efficient catalyst for ethanol steam reforming for hydrogen production. Fuel 2019, 237, 1244–1253. [Google Scholar] [CrossRef]
- Khadar, Y.A.S.; Balamurugan, A.; Devarajan, V.P.; Subramanian, R. Hydrothermal Synthesis of Gadolinium (Gd) Doped Cerium Oxide (CeO2) Nanoparticles: Characterization and Antibacterial Activity. Orient. J. Chem. 2017, 33, 2405–2411. [Google Scholar] [CrossRef]
- Ortega, P.P.; Hangai, B.; Moreno, H.; Rocha, L.S.R.; Ramírez, M.A.; Ponce, M.A.; Longo, E.; Simões, A.Z. Tuning structural, optical, and gas sensing properties of ceria-based materials by rare-earth doping. J. Alloys Compd. 2021, 888, 161517. [Google Scholar] [CrossRef]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B 2015, 174–175, 403–412. [Google Scholar] [CrossRef]
- Sakka, S. Sol–Gel Process and Applications. In Handbook of Advanced Ceramics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 883–910. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Scotti, R.; Di Credico, B.; Redaelli, M. Synthesis and Characterization of Morphology-Controlled TiO2 Nanocrystals. Stud. Surf. Sci. Catal. 2017, 177, 477–540. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming. Int. J. Hydrog. Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- Pundir, S.; Priya, R.; Singh, K.; Kaur, H.; Choudhary, P. A systematic study on synthesis of CeO2 nanoparticles by various routes. IOP Conf. Ser. Earth Environ. Sci. 2023, 1110, 012030. [Google Scholar] [CrossRef]
- Ioannou, M.E.; Pouroutzidou, G.K.; Chatzimentor, I.; Tsamesidis, I.; Florini, N.; Tsiaoussis, I.; Lymperaki, E.; Komninou, P.; Kontonasaki, E. Synthesis and Characterization of Cerium Oxide Nanoparticles: Effect of Cerium Precursor to Gelatin Ratio. Appl. Sci. 2023, 13, 2676. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Maleki, M.; Rahemi, N. Sequential impregnation vs. sol-gel synthesized Ni/Al2O3-CeO2 nanocatalyst for dry reforming of methane: Effect of synthesis method and support promotion. Mol. Catal. 2017, 431, 39–48. [Google Scholar] [CrossRef]
- Bajaj, N.S.; Joshi, R.A. Energy materials: Synthesis and characterization techniques. In Energy Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 61–82. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Gutiérrez-Wing, C.; Mondragón-Galicia, G.; Gutiérrez-Martínez, A.; Deepak, F.L.; Mendoza-Anaya, D. Ag nanowires as precursors to synthesize novel Ag-CeO2 nanotubes for H2 production by methanol reforming. Catal. Today 2013, 212, 225–231. [Google Scholar] [CrossRef]
- Freund, H.-J.; Pacchioni, G. Oxide ultra-thin films on metals: New materials for the design of supported metal catalysts. Chem. Soc. Rev. 2008, 37, 2224. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.A.; Campbell, C.T. Ceria Maintains Smaller Metal Catalyst Particles by Strong Metal-Support Bonding. Science 2010, 329, 933–936. [Google Scholar] [CrossRef]
- Mao, Z.; Lustemberg, P.G.; Rumptz, J.R.; Ganduglia-Pirovano, M.V.; Campbell, C.T. Ni Nanoparticles on CeO2 (111): Energetics, Electron Transfer, and Structure by Ni Adsorption Calorimetry, Spectroscopies, and Density Functional Theory. ACS Catal. 2020, 10, 5101–5114. [Google Scholar] [CrossRef]
- Carrasco, J.; Barrio, L.; Liu, P.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. Theoretical Studies of the Adsorption of CO and C on Ni(111) and Ni/CeO2 (111): Evidence of a Strong Metal–Support Interaction. J. Phys. Chem. C 2013, 117, 8241–8250. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Ramírez, P.J.; Liu, Z.; Gutiérrez, R.A.; Grinter, D.G.; Carrasco, J.; Senanayake, D.S.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. Room-Temperature Activation of Methane and Dry Re-forming with CO2 on Ni-CeO2 (111) Surfaces: Effect of Ce3+ Sites and Metal–Support Interactions on C–H Bond Cleavage. ACS Catal. 2016, 6, 8184–8191. [Google Scholar] [CrossRef]
- Carrasco, J.; López-Durán, D.; Liu, Z.; Duchoň, T.; Evans, J.; Senanayake, S.D.; Crumlin, E.J.; Matolín, V.; Rodríguez, J.A.; Ganduglia-Pirovano, M.V. In Situ and Theoretical Studies for the Dissociation of Water on an Active Ni/CeO2 Catalyst: Importance of Strong Metal-Support Interactions for the Cleavage of O-H Bonds. Angew. Chem. Int. Ed. 2015, 54, 3917–3921. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V. The non-innocent role of cerium oxide in heterogeneous catalysis: A theoretical perspective. Catal. Today 2015, 253, 20–32. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Zhang, F.; Gutiérrez, R.A.; Ramírez, P.J.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, V. Breaking Simple Scaling Relations through Metal–Oxide Interactions: Understanding Room-Temperature Activation of Methane on M/CeO2 (M = Pt, Ni, or Co) Interfaces. J. Phys. Chem. Lett. 2020, 11, 9131–9137. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of Metal-Support Interactions on C−H Bond Breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Sanchez, M.C.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Wang, Y.; Younesi, A.; Arandiyan, H. The evaluation of autothermal methane reforming for hydrogen production over Ni/CeO2 catalysts. Int. J. Hydrog. Energy 2018, 43, 22340–22346. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Zhu, S.; Lu, J.; He, S.; Lu, H.; Song, D.; Luo, Y. Insight into the effect of CeO2 morphology on catalytic performance for steam reforming of glycerol. Fuel 2023, 334, 126587. [Google Scholar] [CrossRef]
- Ni, Z.; Djitcheu, X.; Gao, X.; Wang, J.; Liu, H.; Zhang, Q. Effect of preparation methods of CeO2 on the properties and performance of Ni/CeO2 in CO2 reforming of CH4. Sci. Rep. 2022, 12, 5344. [Google Scholar] [CrossRef]
- Sayle, D.C.; Maicaneanu, S.A.; Watson, G.W. Atomistic Models for CeO2 (111), (110), and (100) Nanoparticles, Supported on Yttrium-Stabilized Zirconia. J. Am. Chem. Soc. 2002, 124, 11429–11439. [Google Scholar] [CrossRef]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron Localization Determines Defect Formation on Ceria Substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Wang, N.; Qian, W.; Chu, W.; Wei, F. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming. Catal. Sci. Technol. 2016, 6, 3594–3605. [Google Scholar] [CrossRef]
- Migani, A.; Vayssilov, G.N.; Bromley, S.T.; Illas, F.; Neyman, K.M. Greatly facilitated oxygen vacancy formation in ceria nanocrystallites. Chem. Commun. 2010, 46, 5936. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.H.; Le, D.N.; Dao, T.D.; Tran, Q.-T.; Doan, T.C.D.; Shiratori, Y.; Dang, C.M. Paper-structured catalyst containing CeO2–Ni flowers for dry reforming of methane. Int. J. Hydrog. Energy 2020, 45, 18363–18375. [Google Scholar] [CrossRef]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Sreeremya, T.S.; Krishnan, A.; Remani, K.C.; Patil, K.R.; Brougham, D.F.; Ghosh, S. Shape-Selective Oriented Cerium Oxide Nanocrystals Permit Assessment of the Effect of the Exposed Facets on Catalytic Activity and Oxygen Storage Capacity. ACS Appl. Mater. Interfaces 2015, 7, 8545–8555. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.-H.; Lak, J.H.; Tsai, D.-H. Hydrogen Production via Low-Temperature Steam–Methane Reforming Using Ni–CeO2–Al2O3 Hybrid Nanoparticle Clusters as Catalysts. ACS Appl. Energy Mater. 2019, 2, 7963–7971. [Google Scholar] [CrossRef]
- Tu, P.H.; Sakamoto, M.; Sasaki, K.; Shiratori, Y. Synthesis of flowerlike ceria–zirconia solid solution for promoting dry reforming of methane. Int. J. Hydrog. Energy 2022, 47, 42171–42184. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Hou, F.; Wu, C.; Pan, L.; Zou, J.; Wang, L.; Zhang, X.; Liu, G.; Li, G. Engineering oxygen vacancies and nickel dispersion on CeO2 by Pr doping for highly stable ethanol steam reforming. Appl. Catal. B 2019, 258, 117940. [Google Scholar] [CrossRef]
- Vecchietti, J.; Lustemberg, P.; Fornero, E.L.; Calatayud, M.; Collins, S.E.; Mohr, S. Controlled selectivity for ethanol steam reforming reaction over doped CeO2 surfaces: The role of gallium. Appl. Catal. B 2020, 277, 119103. [Google Scholar] [CrossRef]
- Sohn, H.; Ozkan, U.S. Cobalt-Based Catalysts for Ethanol Steam Reforming: An Overview. Energy Fuels 2016, 30, 5309–5322. [Google Scholar] [CrossRef]
- Wolf, M. Thermodynamic assessment of the stability of bulk and nanoparticulate cobalt and nickel during dry and steam reforming of methane. RSC Adv. 2021, 11, 18187–18197. [Google Scholar] [CrossRef]
- Wong, Y.; Halim, H.H.; Khairudin, N.F.; Pham, T.N.; Putra, S.E.M.; Hamamoto, Y.; Inagaki, K.; Hamada, I.; Mohamed, A.R.; Morikawa, Y. Dry Reforming of Methane on Cobalt Catalysts: DFT-Based Insights into Carbon Deposition Versus Removal. J. Phys. Chem. C 2021, 125, 21902–21913. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Mustapa, S.I.; Bin Mohd Yassin, M.Y.; Abdullah, S. Experimental and optimization studies of hydrogen production by steam methane reforming over lanthanum strontium cobalt ferrite supported Ni catalyst. Int. J. Energy Res. 2019, 43, 8118–8135. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Li, M.; Liu, Y.; Bai, X. Co/CeO2 for ethanol steam reforming: Effect of ceria morphology. J. Rare Earths 2013, 31, 565–571. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of Hydrogen from Ethanol: Review of Reaction Mechanism and Catalyst Deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef]
- Ferencz, Z.s.; Erdőhelyi, A.; Baán, K.; Oszkó, A.; Óvári, L.; Kónya, Z.; Papp, C.; Steinrück, H.-P.; Kiss, J. Effects of Support and Rh Additive on Co-Based Catalysts in the Ethanol Steam Reforming Reaction. ACS Catal. 2014, 4, 1205–1218. [Google Scholar] [CrossRef]
- Varga, E.; Ferencz, Z.; Oszkó, A.; Erdőhelyi, A.; Kiss, J. Oxidation states of active catalytic centers in ethanol steam reforming reaction on ceria based Rh promoted Co catalysts: An XPS study. J. Mol. Catal. A Chem. 2015, 397, 127–133. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S. Hydrogen production by steam reforming of ethanol over Co/CeO2 catalysts: Effect of cobalt content. J. Energy Inst. 2019, 92, 222–238. [Google Scholar] [CrossRef]
- Greluk, M.; Gac, W.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S. Co/CeO2 and Ni/CeO2 catalysts for ethanol steam reforming: Effect of the cobalt/nickel dispersion on catalysts properties. J. Catal. 2021, 393, 159–178. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.; Li, L.; Xu, J.; Ma, J.; Ni, J.; Yan, J.; Han, J.; Pan, Y.; Lui, Y.; et al. Regulating cobalt chemical state by CeO2 facets preferred exposure for improved ethanol steam reforming. Fuel 2023, 336, 126758. [Google Scholar] [CrossRef]
- Huck-Iriart, C.; Soler, L.; Casanovas, A.; Marini, C.; Prat, J.; Llorca, J.; Escudero, C. Unraveling the Chemical State of Cobalt in Co-Based Catalysts during Ethanol Steam Reforming: An in Situ Study by Near Ambient Pressure XPS and XANES. ACS Catal. 2018, 8, 9625–9636. [Google Scholar] [CrossRef]
- Sohn, H.; Soykal, I.I.; Zhang, S.; Shan, J.; Tao, F.; Miller, J.T.; Ozkan, U.S. Effect of Cobalt on Reduction Characteristics of Ceria under Ethanol Steam Reforming Conditions: AP-XPS and XANES Studies. J. Phys. Chem. C 2016, 120, 14631–14642. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Turczyniak-Surdacka, S. Enhanced catalytic performance of La2O3 promoted Co/CeO2 and Ni/CeO2 catalysts for effective hydrogen production by ethanol steam reforming. Renew Energy 2020, 155, 378–395. [Google Scholar] [CrossRef]
- Ishihara, A.; Tsujino, H.; Hashimoto, T. Effects of the addition of CeO2 on the steam reforming of ethanol using novel carbon-Al2O3 and carbon-ZrO2 composite-supported Co catalysts. RSC Adv. 2021, 11, 8530–8539. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ding, C.; Wang, Q.; Chen, H.; Jia, X.; Huang, L. Preparation of Co-Ce-O catalysts and its application in auto-thermal reforming of acetic acid. Inorg. Chem. Commun. 2022, 141, 109537. [Google Scholar] [CrossRef]
- Varga, E.; Pusztai, P.; Óvári, L.; Oszkó, A.; Erdőhelyi, A.; Papp, C.; Steinrück, H.-P.; Kónyabc, Z.; Kiss, J. Probing the interaction of Rh, Co and bimetallic Rh–Co nanoparticles with the CeO2 support: Catalytic materials for alternative energy generation. Phys. Chem. Chem. Phys. 2015, 17, 27154–27166. [Google Scholar] [CrossRef] [PubMed]
- Charisiou, N.D.; Siakavelas, G.I.; Papageridis, K.N.; Motta, D.; Dimitratos, N.; Sebastian, V. The Effect of Noble Metal (M: Ir, Pt, Pd) on M/Ce2O3-γ-Al2O3 Catalysts for Hydrogen Production via the Steam Reforming of Glycerol. Catalysts 2020, 10, 790. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebanga, M.H.; Wang, Y.L.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef]
- Claudio-Piedras, A.; Ramírez-Zamora, R.M.; Alcántar-Vázquez, B.C.; Gutiérrez-Martínez, A.; Mondragón-Galicia, G.; Morales-Anzures, F.; Pérez-Hérnandez, R. One dimensional Pt/CeO2-NR catalysts for hydrogen production by steam reforming of methanol: Effect of Pt precursor. Catal. Today 2021, 360, 55–62. [Google Scholar] [CrossRef]
- Kourtelesis, M.; Moraes, T.S.; Mattos, L.V.; Niakolas, D.K.; Noronha, F.B.; Verykios, X. The effects of support morphology on the performance of Pt/CeO2 catalysts for the low temperature steam reforming of ethanol. Appl. Catal. B 2021, 284, 119757. [Google Scholar] [CrossRef]
- Pérez-Hernández, R. Reactivity of Pt/Ni supported on CeO2-nanorods on methanol steam reforming for H2 production: Steady state and DRIFTS studies. Int. J. Hydrog. Energy 2021, 46, 25954–25964. [Google Scholar] [CrossRef]
- Dai, R.; Zheng, Z.; Lian, C.; Li, X.; Wu, X.; An, X.; Xie, X. A high-performance CeO2@Pt-Beta yolk-shell catalyst used in low-temperature ethanol steam reforming for high-purity hydrogen production. Int. J. Energy Res. 2019, 43, 2075–2085. [Google Scholar] [CrossRef]
- Shanmugam, V.; Zapf, R.; Hessel, V.; Pennemann, H.; Kolb, G. Nano-architectured CeO2 supported Rh with remarkably enhanced catalytic activity for propylene glycol reforming reaction in microreactors. Appl. Catal. B 2018, 226, 403–411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).