Abstract
Fluorescent proteins find application as biocompatible, genetically encoded labels for visualization of living organisms tissues. Green fluorescent proteins (GFPs) are the most diverse, but proteins with red fluorescence have advantages, such as lower phototoxicity and better penetration into biological tissues. A promising approach is to obtain red fluorescent proteins (RFPs) from GFPs by introducing mutations that stabilize the oxidized chromophore state with an extended conjugated π-system. However, to date this remains a non-trivial task and experimental developments are carried out mainly by random mutagenesis. Development of descriptors obtained in molecular modeling can rationalize this field. Herein, we rely on experimental data on the AzamiGreen fluorescent protein and its variants that are oxidized to the red form. We perform classical molecular dynamics (MD) and combined quantum mechanics/molecular mechanics (QM/MM) simulations to determine structural and dynamic features that govern oxidation. We demonstrate that the red state is predominantly stabilized by interactions of polar lysine residues with chromophore oxygen atoms. Dynamic network analysis demonstrates that in red fluorescent proteins the chromophore motions are correlated with the movement of surrounding protein side chains to a higher extent than in green variants. The presence of different resonance forms of the chromophore determines the fluorescence band maximum value: a decrease in the phenolate form population leads to the red shift.
1. Introduction
Fluorescent proteins (FPs) were first obtained from the jellyfish Aequorea victoria [1] and since have found wide application in various fields of chemistry. FPs have a β-barrel structure containing chromophores formed by autocatalysis from three consecutive amino acid residues in the polypeptide chain [2]. FPs are widely used in biochemistry as fluorescent labels for visualization of biological objects [3,4,5]. Their versatility and diverse photophysical properties provide other areas of application, such as the creation of ion- and potential-dependent sensors and protein–protein interaction probes [6,7,8]. There is a wide variety of fluorescent proteins with different spectral properties of absorption, emission and Stokes shift, while the chromophore has a similar chemical structure [9,10]. Natural FPs are able to demonstrate properties such as photoactivation, reversible photoswitching and irreversible phototransformation [11,12,13]. One of the main advantages of FPs as fluorescent labels is their biocompatibility and possibility of expression directly in living cells. At the same time, green fluorescent proteins (GFPs) have the best photophysical properties, which are most widely used as tools for biovisualization, ultra-high-resolution spectroscopy, and biosensors [2].
Although natural fluorescent proteins cover the entire optical range of the electromagnetic spectrum, great research efforts are focused on modifying existing FPs. Red fluorescent proteins (RFPs), which have absorption and emission bands shifted to the red region, have advantages such as reduced phototoxicity and better penetration into tissues of biological samples [14,15,16] compared to GFPs. However, natural RFPs have lower brightness and a lower maturation rate under physiological conditions. In addition, natural FPs consist of several subunits and for practical applications require monomerization, which also impairs the photophysical properties, which is especially critical for RFPs. In this regard, it is of interest to create red fluorescent proteins with specified properties by bioengineering methods.
Green fluorescent proteins have the greatest structural diversity. Therefore, a promising direction is the creation of RFPs from GFPs by site-directed mutagenesis. In past decades, various mutant proteins with fluorescence in the spectrum range from blue to yellow were obtained from GFPs [17,18,19]. However, the creation of a protein with red fluorescence based on GFPs still faces difficulties. One of the possibilities for creating new RFPs is the search for and improvement of photoconvertible proteins that form a red chromophore under the influence of ultraviolet light due to the peptide bond cleavage with the protein backbone and the formation of additional double bonds. Examples include the EosFP [20] and SAASoti [21] proteins. Another direction is the modification of natural proteins such as Discosoma sp. RFP (DsRed) and related proteins [22,23,24,25] in order to improve the photophysical properties and stability in the tissues of living organisms.
The DsRed-type chromophore is formed from the GFP-type chromophore by oxidation by atmospheric oxygen with formation of additional double bonds and expansion of the conjugate π-system. At the same time, the peptide bond with a protein backbone is maintained. Therefore, it is expected that introduction of certain mutations near the chromophore will promote stabilization of the red form. Mutations of residues can change the local chromophore environment and lead to changes in spectral characteristics, photostability, chemical stability, and so on [26]. The formation and stability of specific forms are determined by the influence of the protein environment on both the thermodynamic characteristics and the accessibility of the β-barrel central part for the oxygen molecule. However, it has not yet been possible to obtain a fully fledged RFP by point mutations of GFP. The first successful attempt at mutagenesis of the green protein avGFP to form a DsRed-type chromophore was the work of Mishin et al. [27], but the resulting mutant protein had an extremely low intensity of red fluorescence. In addition, the mechanism of red chromophore formation has not been elucidated. Among the latest advances in this field, it is possible to obtain RFP by introducing a non-standard amino acid 3-aminotyrosine with additional mutations around the chromophore and selected expression conditions [28].
In a recent study [29], variants of a green fluorescent protein AzamiGreen that are able to convert to the oxidized form with red fluorescence were obtained. The amino acid residues, mutations in which are important in stabilizing a specific form of the chromophore, were identified and interpreted based on crystal structures. Also, the protein variant with both green and red forms was obtained.
Development of computational chemistry methods allows one to deepen the understanding of the origin of changes related to amino acid substitutions with atomic resolution [30,31,32,33,34,35,36]. Herein, we perform simulations of AzamiGreen and its variants that demonstrate red fluorescence using molecular dynamic simulations with both classical and combined quantum mechanics/molecular mechanics (QM/MM) potentials. We determine key structural and dynamic differences that are responsible for stabilization of the oxidized or reduced form.
2. Computational Details
2.1. Computational Procedure
Classical molecular dynamics (MD) simulations were performed using the NAMD3 [37] program. For standard amino acid residues, the CHARMM36 [38] force field parameters were used, and the chromophore parameters were obtained from the CGenFF [39] force field parameters, which have previously been successfully used to model fluorescent proteins [34,40,41], as well as the standard CHARMM parameters for the Gln62 and Met62 residues. MD simulations were performed in a rectangular periodic box with TIP3P [42] water molecules and neutralized by adding chloride anions if necessary. In constructing the unit cell, the minimum distance between the boundary and a protein molecule was 10 Å. Before starting the MD simulation, a minimization of 5000 steps was performed for each system, and then the water shell was relaxed for 5 ps at 300 K. The productive MD runs were carried out for 500 ns with velocity reinitialization every 100 ns at P = 1 atm and T = 300 K in an isobaric–isothermal ensemble using a Nosé–Hoover barostat and a Langevin thermostat. The integration time step was 1 fs, the cutoff distance of electrostatic and van der Waals interactions was 12 Å, and the switching distance was 10 Å.
Molecular dynamics simulations with quantum mechanics/molecular mechanics potentials (QM/MM) were performed using NAMD [37] and TeraChem [43] programs. MM parameters for MD simulations were similar to those described above. The QM subsystem was composed of the chromophore and nearby residues, Asn65/Ser65 residue and the side chains of the nearest polar residues (Arg66/Lys66, Arg91, Lys159, His193, Glu211) and 2–3 water molecules that directly interact with the chromophore or nearest polar residues. The QM part was described at the PBE0-D3/6-31G** [44,45] level. The analysis of trajectories and structures was carried out using the Chimera [46] and VMD [47] molecular editors. Covariance and correlation matrices for dynamical network analysis were calculated with the Carma [48] program as well as the NetworkView [49] service for data analysis. Clustering of MD trajectories was carried out using the TTClust [50] program with the choice of the hierarchical grouping algorithm [51].
2.2. Preparation of Model Systems
Crystal structures of AzamiGreen (AG, PDB ID 8I4J) and its variant AzamiRed1.0 (AR1.0, PDB ID 8I4K) [29] were used as sources of initial coordinates for classical MD simulations. The remaining models, including AR0.1, AR0.6 and AR1.6, were obtained from AR1.0 by introducing in silico amino acid substitutions. For all considered systems the protonation states of residues containing ionizable groups were the following: side chains of glutamic and aspartic acids were negatively charged; side chains of arginine and lysine residues were positively charged. For histidine residues, protonation state was selected from analysis of the local environment. Thus, histidine residues 21, 88, and 168 were protonated at the ε-nitrogen; 189 and 200 were protonated at the δ-nitrogen; and histidine 193, which forms π–π-stacking interactions with the chromophore, was protonated at both ε- and δ-nitrogen atoms. The chromophore in AG protein is formed from Gln62, Tyr63 and Gly64 residues and has charge −1. Thus, the total charge of the protein is 0 and the system does not require neutralization.
The AR1.0 variant differs from AG by 29 amino acids. Protonation states of ionizable groups were selected. The crystal structure contains an intramolecular bond between the carboxyl group of Glu211 and the N2 atom of the chromophore imidazoline ring, which is not typical for DsRed RFPs [52]. So the Glu211 residue was considered protonated in the modeling. The AR1.0 chromophore differs from AG due to the presence of Met62 residue instead of Gln62, the presence of a double bond between the Cα-atom of Met62 and N-atom of Phe61, and the cis-conformation of the peptide bond between Phe61 and the chromophore. The protein total charge in the simulation was +1, and a chloride anion was added for neutralization.
For modeling, we also considered proteins with absorption and fluorescence characteristics that occupy an intermediate position between the RFP AR1.0 and the GFP AG. In [29], they are designated as AR0.1, AR0.6, and AR1.6. AR0.1 was experimentally obtained from AG and differed from it by 35 mutations. Its amino acid sequence is closer to AR1.0 and differs from it by 13 mutations. The most significant mutation that changes the chromophore environment is the substitution of Lys159 for Met159, which brings AR0.1 closer to the green protein AG. However, unlike AG, instead of Arg66, the structure contains Lys66, as in AR1.0. Worth noting is the substitution of Tyr71 for His71, which was assumed protonated at the ε-nitrogen. The AR0.6 protein differs from AR1.0 by only 3 mutations and was considered in this work because it exhibits more a intense absorption band in the red region than AR0.1, but also a more intense band in the green region than AR1.0. The AR1.6 protein is obtained by introducing reverse mutations and differs from the original AR1.0 by 15 residues and from the green AG by 14 residues, while maintaining the red band in the absorption and fluorescence spectrum. All these proteins had +1 in the simulations and were neutralized by the addition of a chloride anion.
3. Results and Discussion
3.1. Classical Molecular Dynamics Simulations
Classical MD simulation of the proteins was performed for 500 ns at 300 K and the chromophore interactions with the protein environment were analyzed. Based on the structure analysis, the most conservative intramolecular interactions were hydrogen bonds between the O3 and O25 atoms of the chromophore and the polar residues of protein (Figure 1), which are retained along the entire trajectory. If a disruption of these bonds was noticed in the beginning of a trajectory, preliminary 50 ns MD relaxation with constraints on these hydrogen bonds was performed.
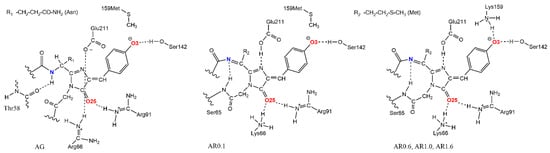
Figure 1.
Protein environment of chromophore for five considered proteins.
For all 10 obtained trajectories, RMSD was calculated for the protein backbone (except for five terminal residues) and separately for the chromophore with the nearest residues shown in Figure 1 (heavy atoms only). Since all structures are rigid β-barrels, no significant differences were found; RMSD does not exceed 3 Å. The structure relaxation takes 20–50 ns. At the same time, for AG and AR1.6 in green form, a gradual increase in RMSD is observed, while for the other structures the graph reaches a plateau (see Figure S1).
RMSF values for Cα-atoms of all protein residues were also calculated. A comparison of the results for AG (green form) and other proteins in red form is shown in Figure 2. The main difference is that Asp74 residue for AR1.0 and AR1.6 has a lower RMSF than for other structures. We propose that this occurs due to stochastic interactions of the loop including residues 71–77 with the solvent and the C-terminus of the protein. But the chromophore and its environment do not demonstrate significant difference.
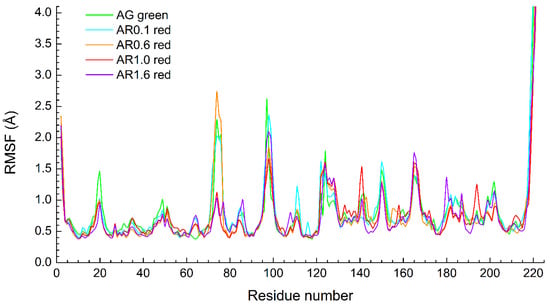
Figure 2.
The comparison of Cα-atom RMSF for classical MD trajectory (500 ns) of five proteins.
Close contacts of protein side chain polar groups with chromophore atoms were analyzed. These are primarily positively charged residues Arg66/Lys66, Arg91, and Lys159 and polar residues Ser142 and Glu211 (see Figure 1). Table S1 of the Supplementary Data presents the populations of N–H∙∙∙O hydrogen bonds between the protonated guanidine and amino groups of the residues and the oxygen atom O25 or O3 of the chromophore along the MD trajectory. The criterion for a strong N–H∙∙∙O H-bond was the condition d(H∙∙∙O) ≤ 2.1 Å, and that for a weak H-bond was the condition 2.1 Å < d(H∙∙∙O) ≤ 3.1 Å. Figure 3 shows d(H∙∙∙O) distance distributions along the trajectory for the Lys66 and Lys159 residues.
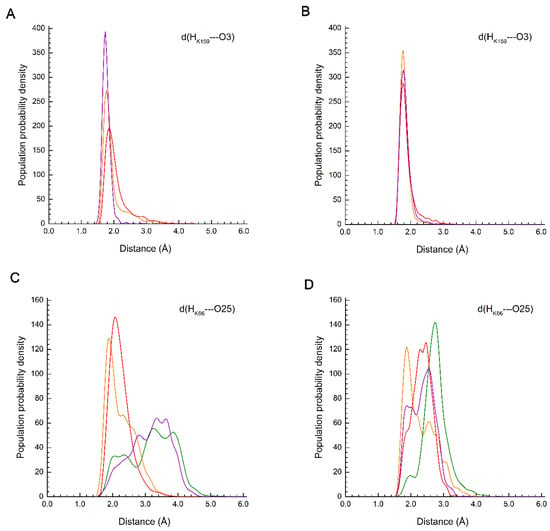
Figure 3.
Distribution of distances d(H∙∙∙O) between polar groups of protein side chains and chromophore oxygen atoms for proteins AR0.1 (green line), AR0.6 (orange line), AR1.0 (red line) and AR1.6 (violet line). (A,B) Distance of Lys159-NH3+∙∙∙O3 for green and red form, respectively; (C,D) distance of Lys66-NH3+∙∙∙O25 for green and red form, respectively.
The results of trajectory analysis show that the most stable interaction is between O25 atom and Arg91 residue, which is present in all considered structures: a strong H-bond is present in more than 88% of the trajectory, and the total occupancy is more than 98%. The Lys159 residue is present in AR0.6, AR1.0, and AR1.6 proteins, while in the AG and AR0.1 it is replaced by Met159, which does not participate in electrostatic interactions with chromophores. The presence of the Lys159 residue determines the maturation rate of the red chromophore, so the K→M reverse mutation leads to the appearance of a fluorescence maximum in the green region [29].
In proteins containing Lys159, this residue stably interacts with chromophore O3 atoms in both red and green forms. For red forms, the strong H-bond is more pronounced than for the green form (Figure 3A,B). The Lys66 residue is more labile, since, except for the chromophore O25 atom, it can also interact with the Glu211 carboxyl group, carbonyl oxygen of Ser65 main chains and water molecules located above the plane of the chromophore imidazoline ring. In AG structure, Lys66 residue is replaced by Arg66, which is not coordinated to O25 atoms, but interacts only with negatively charged Glu211 throughout the entire trajectory for AG in both green and red forms. In AR0.1 structure, a weak H-bond populates 83% of the trajectory in red form, a strong H-bond populates 6%, and in green form the H-bond occupancy is 34% for strong and 13% for weak H-bonds. For AR0.6 structure, both red and green forms, the occupancies of strong and weak hydrogen bonds are close and make up about 50% of the trajectory. In AR1.0 protein, a similar situation is observed for green form, while for the red form the weak H-bond dominates with occupancy of 71%, and the occupancy of strong H-bonds is 28%. For AR1.6 protein, both in green and red forms, the weak hydrogen bond dominates, and for the red form its contribution is 65% against 40% for the green form.
In addition to the considered positively charged residues, the crystal structure of AG and AR1.0 contains close contacts of the Ser142 OH group with chromophore O3 atoms. Analysis of MD trajectories for all simulated proteins showed that this interaction is not strong, but Ser142 residue is located near the chromophore and can be coordinated to O3 by both hydroxyl groups and NH groups of the main chain. It should also be noted that there are always one to three water molecules near the O3 atom, which demonstrates the chromophore accessibility for small molecules. The Glu211 residue, remaining near the chromophore above the imidazoline ring plane, can interact with N15 nitrogen atom, as in the crystal structure of AR1.0, and with polar residues His193, Arg66/Lys66 and water molecules, as in the AG structure (Figure 4A). Since in all structures except AG the Glu211 residue is considered protonated, the COOH∙∙∙N15 bond was present in these structures, whereas for AG the Glu211 residue was located above the chromophore parallel to the imidazoline ring plane throughout the MD trajectory.
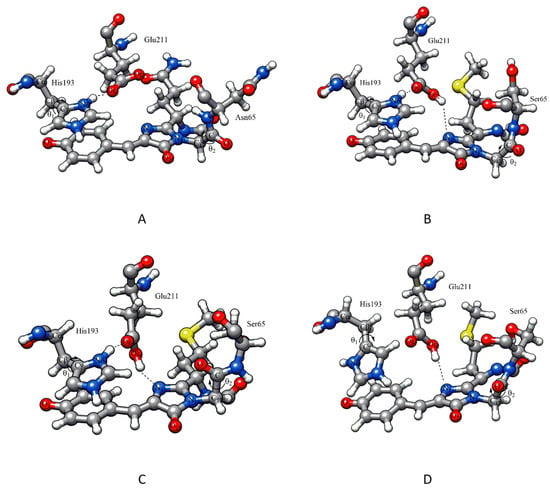
Figure 4.
The arrangement of residues above the chromophore plane: (A,B) for crystal structures of AG and AR1.0, respectively, after hydrogen addition and minimization; (C,D) for AR0.1 structures from MD trajectories with green and red forms of chromophore, respectively. Color code for molecular models: carbon – grey, oxygen – red, nitrogen – blue, sulfur – yellow and hydrogen – white.
The conformations of protonated histidine His193 (angle θ1) and backbone atoms linking chromophore to residue 65 (angle θ2) shown in Figure 4 were also analyzed. In the crystal structures of AG and AR1.0, the angle θ1 (Cα-Cβ-Cγ-Nδ) is approximately −100°, which corresponds to the gauche- conformation of His193. Molecular dynamics showed that in all proteins, the histidine side chain rotates around the Cβ-Cγ bond with formation of gauche+ conformation (θ1 +30°), when the protonated Nδ atom is located near the chromophore phenolate oxygen O3 (Figure 4D) and surrounding water molecules. For AG protein, the initial gauche- conformation is dominated (the average value θ1 is 89 ± 37° for green form and −98 ± 21° for red form); for AR0.1 protein, the green form also realizes the gauche- conformation over a significant part of the trajectory, while in red form the gauche+ conformation is predominated. AR0.6, AR1.0, and AR1.6 proteins demonstrate different behavior: in green form, the gauche+ conformation is more populated than in red form. Although rotation of histidine side chains is slow and we have not obtained a representative distribution of θ1 values, we expect that the gauche+ conformation contributes to the stabilization of the phenolate form of the chromophore. This is supported by further QM/MM modeling.
Another structural parameter that changes during MD simulations is the dihedral angle θ2 (N15-C17-C-O) in Gly64 residue of the chromophore (Figure 4). In the crystal structures of AG and AR1.0, it is close to 110° and 180°, respectively (trans conformation). In dynamics of AG, the angle θ2 retains its original orientation (the average value is 114 ± 14° for green form and 100 ± 16° for red form), whereas for other proteins, carbonyl oxygen of the main chain tends to turn towards the chromophore (Figure 4C) taking values from 0° to 60°.
The considered structural parameters determine the influence of the nearest environment on the chromophore. To evaluate the effect of mutations in residues that do not directly interact with chromophores, but determine the spectral characteristics of mutant proteins, a dynamic network analysis (DNA) of all 500 ns MD trajectories was performed. DNA allows us to assess the correlated motion of different residues, which is expected to help us better understand the differences in the behavior of proteins with a similar chemical structure.
3.2. Dynamic Network Analysis
The DNA results show clustering of the modeled system (protein) into residue groups with coordinated motion. For all proteins, both in red and green form, clusters containing the chromophore were analyzed. A comparison of the results is shown in Figure 5.
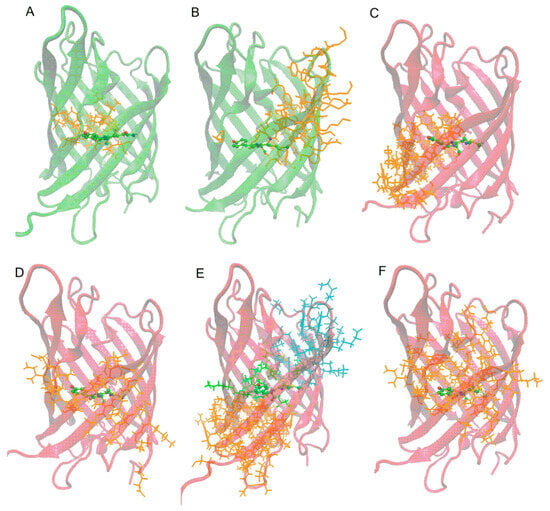
Figure 5.
Clusters of correlated motions including chromophore for the considered proteins: (A) AG in green form; (B) AR0.1 in green form; (C) AR0.1 in red form; (D–F) AR0.6, AR1.0, and AR1.6 in red form, respectively. Colors of the cartoon representation of the protein β-barrel correspond to the chromophore form.
For AG protein in green form, a chromophore is included in a small cluster of 23 residues located mainly on one side of the imidazoline ring, while the phenolate fragment is located at the border of this cluster. This means that phenolic oxygen practically does not interact with the protein environment and the phenolic fragment movements are determined by protein main chain mobility and the chromophore backbone. Such dynamic behavior stabilizes the green form of the chromophore. A similar pattern of movements is observed for AR0.1 protein. Despite a large number of mutations compared to AG, the absence of the Lys159 residue near the phenolic oxygen determines the phenolic fragment location at the border of the cluster. The cluster is larger than for AG and contains 42 residues.
For AR0.6, AR1.0 and AR1.6 proteins in red form, a significant difference is observed: the chromophore is located in the center of cluster; i.e., the chromophore motions are correlated with the movement of all surrounding protein side chains. Moreover, this tendency is most clearly expressed for AR1.0, where the chromophore falls into three clusters at once: from the C-terminus, N-terminus and phenolate fragment. Thus, the formation of hydrogen bonds between the chromophore and nearest polar residues Lys66, Lys159 and Glu211 contributes to a change in the protein dynamic behavior and leads to stabilization of the red form.
3.3. QM/MM Molecular Dynamics
To analyze the electron density distribution in chromophore molecules, QM/MM dynamics of five proteins in red and green forms was studied. The starting structures were selected from clustering of classical MD trajectory using RMSD of residues (heavy atoms only) that play an important role in green to red form conversion. According to [29], these are residues 60–66, 69, 105, 107, 158, and 159. The location of His193 and Glu211 residues, which are in close proximity to the chromophore, was also taken into account. For all proteins, starting structures were selected from fairly populated clusters in which the residue’s orientation was close to the crystal structure of AG and AR1.0, and hydrogen bonds with the chromophore atoms were retained. However, the conformations of His193 and the main chain of residues 64 and 65 could differ from the crystalline structure. The structural parameters of the QM part for the starting geometries are presented in Table 1. Note that the hydrogen bonds and close contacts obtained from classical MD were retained throughout the entire trajectory of QM/MM dynamics.

Table 1.
Structural parameters of the chromophore environment for starting geometries used in QM/MM dynamics. “-” correspond to the absence of a hydrogen bond.
Based on the QM/MM dynamics results, the Mulliken charges of O3 and O25 atoms, as well as the bond lengths characterizing the predominance of resonance forms associated with the negative charge localization, were analyzed (Figure 6). Since the electron density distribution in chromophores is determined mainly by interactions with the nearest polar residues, the differences in the mutant protein behavior are associated primarily with Lys66 and Lys159 residues, as well as with His193 conformation.
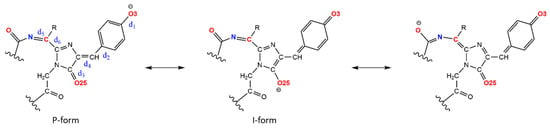
Figure 6.
The chromophore resonance forms in red form with indicated characteristic bonds. For green form, only P- and I-forms are possible.
To estimate the contribution of P-(phenolate) and I-(imidazolinolate) resonance forms to the chromophore electronic structure, a structural criterion based on the d1–d4 bond length comparison was used. If d1 > d3 and d2 > d4, the structure was considered P-form. If d1 < d3 and d2 < d4, the structure was considered I-form. In other cases, it was assumed that intermediate states of electron density distribution are realized. The results of the resonance P- and I-form population analysis for AG-AR1.6 proteins with the chromophore in the unoxidized (green form) and oxidized (red form) states are shown in Figure 7. The average values of d1–d6 bond lengths and Mulliken charges on the O3 and O25 atoms are given in the Supporting Information (Figure S2 and Tables S2 and S3).
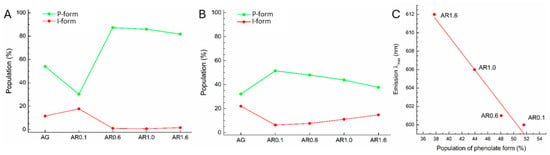
Figure 7.
The populations of chromophore resonance P- and I-forms (see Figure 6) along QM/MM MD trajectory of 5 ps for proteins in green form (A) and red form (B). (C) Correlation between phenolate resonance form of chromophore in oxidized (red) state and emission band maximum wavelength.
The P-form in which negative charge is localized on the O3 atom predominates for AG protein and is observed in 54% of the trajectory, while the I-form is found only in 12% of the trajectory (Figure 7A). For AR0.1 protein, which demonstrates both green and red fluorescence, the occupancy of phenolate P-form is lower at 30% vs. 18% for I-form. This result is observed due to the introduction of R66K mutation when the positively charged NH3+ group of lysine stabilizes the negative charge on the O25 atom. Stabilization of resonance I-form shifts the fluorescence maximum to the red region, which is in agreement with experimental measurements: the maximum emission of AR0.1 in green form is at 512 nm, while for AG it corresponds to 505 nm [29]. For AR0.6, AR1.0 and AR1.6 proteins, due to the introduction of M159K mutation, the phenolate form predominates in more than 80% of the trajectory, while the expressed I-form is virtually absent. The influence of Lys159 is also evident from the analysis of the Mulliken charges of the chromophore O3 atom: for AR0.6, AR1.0, and AR1.6 proteins in the green state, the charge is 0.02–0.11 a.u. lower than for AG and AR0.1 (see Tables S2 and S3). However, this effect is absent in the red form due to greater charge delocalization. Since these proteins do not exhibit fluorescence in the green region of the spectrum, but have absorption maxima around 500 nm, it can be assumed that the unoxidized green form of the chromophore is present as a neutral protonated structure, while the negatively charged phenolate is converted to oxidized red form.
Dynamics results for AG-AR1.6 proteins in red form (Figure 7B) show that for AG, P-form is present in 32% of the trajectory and I-form in 22%. This is the lowest population value of phenolate form in a number of considered proteins. Since AG is a natural GFP and does not exhibit emission in the red region, we consider only AR0.1-AR0.6 series in red form. In AR0.1 protein, P-form is stabilized by His193 residue, which protonated δ-nitrogen is oriented to the O3 atom of a chromophore. This conformation dominates in classical MD trajectory, because the AR0.1 structure lacks the polar residue Lys159, as in other mutant proteins, and Glu211 residue, as expected, interacts with the nitrogen atom of the chromophore imidazoline ring, as in the observed AR1.0 structure. In this regard, on the AR0.1 QM/MM trajectory, the occupancy of resonance form I is 52%, and that of I-form is 6%. For the AR0.6-AR1.6 proteins, His193 protonated δ-nitrogen interacts with Glu211 residue, as in AG structure, while the negatively charged chromophore O3 atom interacts with protonated Lys159 residue. In the series AR0.6–AR1.0–AR1.6, the occupancy of resonance P-form decreases at 48, 44 and 38%, respectively, while the occupancy of I-form increases from 8% for AR0.6 to 11% for AR1.0 and 15% for AR1.6. The obtained results are correlated with the experimental fluorescence spectrum (Figure 7C). The emission maximum in the series AR0.1–AR0.6–AR1.0–AR1.6 shifts to the red region and corresponds to wavelengths of 600, 601, 606, 612 nm, respectively [29].
Analysis of QM/MM MD trajectories confirmed the influence of chromophore environment on the spectral characteristics of fluorescence proteins. At the same time, differences associated with mutations in distant residues that do not directly interact with the chromophore also have a significant effect on the electronic structure of the protein photoactive part.
4. Conclusions
Amino acid substitutions in AzamiGreen are able to bring a new photophysical property, red fluorescence. We performed extensive molecular modeling and reveal that these changes affect the dynamic behavior of both the chromophore and the entire protein. R91K and M159K mutations close to the chromophore lead to the formation of stable hydrogen bonds with chromophore oxygen atoms and stabilization of the form with red fluorescence. However, the introduction of individual mutations around the chromophore is not enough to obtain RFPs, although a single reverse mutation K159M in AR1.0 protein leads to the appearance of a green fluorescent form along with the red one. Therefore, appearance of a positive charge in the 159th position is not obligatory for red form formation.
Dynamic network analysis revealed groups of residues with correlated motions involving chromophores. For green-emitting AG and AR0.1 proteins, the chromophore motion is correlated with the motion of side chains located on the imidazoline ring side and not interacting with movement of the phenolic fragment. For red-emitting AR0.6, AR1.0, and AR1.6 proteins, the chromophore is contained in a group of side chain motions that form hydrogen bonds with both imidazoline and phenolate oxygens. This result emphasizes the importance of the chromophore’s immediate environment in stabilizing the green or red form.
QM/MM dynamics showed the effect of changes in protein amino acid sequence on the electronic structure of chromophore. Despite the similar β-barrel structure and conformation of the residues surrounding the chromophore, in the series of proteins AR0.1-AR0.6-AR1.0-AR1.6 there is a decrease in the contribution of the phenolate form and an increase in the proportion of the form where negative charge is localized on imidazoline oxygen. This effect leads to a red shift of the emission band maxima, which is consistent with experimental studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biophysica5040053/s1, Figure S1: The comparison of backbone and chromophore environment (resid) RMSD along the classical MD trajectory 500 ns. A,B—AG in green and red form, respectively; C,D—AR0.1 in green and red form, respectively; E,F—AR0.6 in green and red form, respectively; G,H—AR1.0 in green and red form, respectively; I,J—AR1.6 in green and red form, respectively; Table S1: Populations (%) of hydrogen bonds between the side chains polar groups and the chromophore oxygen atoms based on the classical MD trajectory 500 ns; Figure S2: The bond lengths dependences determining the predominance of phenolate or imidazolinolate form of chromophore proteins along the QM/MM MD trajectory. A,B—AG in green and red form, respectively; C,D—AR0.1 in green and red form, respectively; E,F—AR0.6 in green and red form, respectively; G,H—AR1.0 in green and red form, respectively; I,J—AR1.6 in green and red form, respectively; Table S2: Average parameters obtained from QM/MM MD trajectories analysis of proteins with chromophore in green form; Table S3: Average parameters obtained from QM/MM MD trajectories analysis of proteins with chromophore in red form.
Author Contributions
Conceptualization, V.B.K. and M.G.K.; methodology, V.B.K. and M.G.K.; validation, V.B.K. and R.A.S.; formal analysis, V.B.K., M.G.K. and R.A.S.; investigation, V.B.K. and R.A.S.; data curation, V.B.K. and R.A.S.; writing—original draft preparation, V.B.K. and M.G.K.; writing—review and editing, V.B.K. and M.G.K.; visualization, V.B.K. and R.A.S.; supervision, M.G.K.; project administration, M.G.K.; funding acquisition, M.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (# 19-73-20032).
Data Availability Statement
The structures and topologies of the simulated systems are available in the general-purpose, open-access repository ZENODO (https://doi.org/10.5281/zenodo.17176618).
Acknowledgments
The research was carried out using the equipment of the shared research facilities of HPC computing resources at Lomonosov Moscow State University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Johnson, F.H.; Shimomura, O.; Saiga, Y.; Gershman, L.C.; Reynolds, G.T.; Waters, J.R. Quantum efficiency of Cypridina luminescence, with a note on that of Aequorea. J. Cell. Comp. Physiol. 1962, 60, 85–103. [Google Scholar] [CrossRef]
- Khrenova, M.G.; Savitsky, A.P. Fluorescent proteins. In Theoretical and Computational Photochemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 337–349. [Google Scholar]
- Shaner, N.C.; Patterson, G.H.; Davidson, M.W. Advances in fluorescent protein technology. J. Cell Sci. 2007, 120, 4247–4260. [Google Scholar] [CrossRef]
- Day, R.N.; Davidson, M.W. The fluorescent protein palette: Tools for cellular imaging. Chem. Soc. Rev. 2009, 38, 2887–2921. [Google Scholar] [CrossRef] [PubMed]
- Frommer, W.B.; Davidson, M.W.; Campbell, R.E. Genetically encoded biosensors based on engineered fluorescent proteins. Chem. Soc. Rev. 2009, 38, 2833. [Google Scholar] [CrossRef]
- Zhou, X.X.; Lin, M.Z. Photoswitchable fluorescent proteins: Ten years of colorful chemistry and exciting applications. Curr. Opin. Chem. Biol. 2013, 17, 682–690. [Google Scholar] [CrossRef]
- Germond, A.; Fujita, H.; Ichimura, T.; Watanabe, T.M. Design and development of genetically encoded fluorescent sensors to monitor intracellular chemical and physical parameters. Biophys. Rev. 2016, 8, 121–138. [Google Scholar] [CrossRef]
- Romei, M.G.; Boxer, S.G. Split Green Fluorescent Proteins: Scope, Limitations, and Outlook. Annu. Rev. Biophys. 2019, 48, 19–44. [Google Scholar] [CrossRef]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Olenych, S.G.; Claxton, N.S.; Ottenberg, G.K.; Davidson, M.W. The Fluorescent Protein Color Palette. Curr. Protoc. Cell Biol. 2006, 33, 21.5. [Google Scholar] [CrossRef]
- Dickson, R.M.; Cubitt, A.B.; Tsien, R.Y.; Moerner, W.E. On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature 1997, 388, 355–358. [Google Scholar] [CrossRef]
- Lukyanov, K.A.; Fradkov, A.F.; Gurskaya, N.G.; Matz, M.V.; Labas, Y.A.; Savitsky, A.P.; Markelov, M.L.; Zaraisky, A.G.; Zhao, X.; Fang, Y.; et al. Natural animal coloration can be determined by a nonfluorescent green fluorescent protein homolog. J. Biol. Chem. 2000, 275, 25879–25882. [Google Scholar] [CrossRef]
- Hoi, H.; Shaner, N.C.; Davidson, M.W.; Cairo, C.W.; Wang, J.; Campbell, R.E. A Monomeric Photoconvertible Fluorescent Protein for Imaging of Dynamic Protein Localization. J. Mol. Biol. 2010, 401, 776–791. [Google Scholar] [CrossRef]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Akerboom, J.; Carreras Calderón, N.; Tian, L.; Wabnig, S.; Prigge, M.; Tolö, J.; Gordus, A.; Orger, M.B.; Severi, K.E.; Macklin, J.J.; et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Kumar, M.; Zhang, J.; Zhao, S.; Drobizhev, M.; McCollum, M.; Anderson, C.T.; Wang, Y.; Pokorny, A.; Tian, X.; et al. A genetically encoded far-red fluorescent indicator for imaging synaptically released Zn2+. Sci. Adv. 2023, 9, eadd2058. [Google Scholar] [CrossRef]
- Heim, R.; Prasher, D.C.; Tsien, R.Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA 1994, 91, 12501–12504. [Google Scholar] [CrossRef]
- Ormö, M.; Cubitt, A.B.; Kallio, K.; Gross, L.A.; Tsien, R.Y.; Remington, S.J. Crystal Structure of the Aequorea victoria Green Fluorescent Protein. Science 1996, 273, 392–395. [Google Scholar] [CrossRef]
- Tomosugi, W.; Matsuda, T.; Tani, T.; Nemoto, T.; Kotera, I.; Saito, K.; Horikawa, K.; Nagai, T. An ultramarine fluorescent protein with increased photostability and pH insensitivity. Nat. Methods 2009, 6, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Paez-Segala, M.G.; Sun, M.G.; Shtengel, G.; Viswanathan, S.; Baird, M.A.; Macklin, J.J.; Patel, R.; Allen, J.R.; Howe, E.S.; Piszczek, G.; et al. Fixation-resistant photoactivatable fluorescent proteins for CLEM. Nat. Methods 2015, 12, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, I.; Gavshina, A.; Savitsky, A. Reversible photobleaching of photoconvertible SAASoti-FP. J. Biomed. Photon. Eng. 2017, 3, 040303. [Google Scholar] [CrossRef]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.G.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef]
- Shcherbo, D.; Merzlyak, E.M.; Chepurnykh, T.V.; Fradkov, A.F.; Ermakova, G.V.; Solovieva, E.A.; Lukyanov, K.A.; Bogdanova, E.A.; Zaraisky, A.G.; Lukyanov, S.; et al. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods 2007, 4, 741–746. [Google Scholar] [CrossRef]
- Kredel, S.; Oswald, F.; Nienhaus, K.; Deuschle, K.; Röcker, C.; Wolff, M.; Heilker, R.; Nienhaus, G.U.; Wiedenmann, J. mRuby, a Bright Monomeric Red Fluorescent Protein for Labeling of Subcellular Structures. PLoS ONE 2009, 4, e4391. [Google Scholar] [CrossRef]
- Mo, G.C.H.; Posner, C.; Rodriguez, E.A.; Sun, T.; Zhang, J. A rationally enhanced red fluorescent protein expands the utility of FRET biosensors. Nat. Commun. 2020, 11, 1848. [Google Scholar] [CrossRef] [PubMed]
- Cranfill, P.J.; Sell, B.R.; Baird, M.A.; Allen, J.R.; Lavagnino, Z.; de Gruiter, H.M.; Kremers, G.-J.; Davidson, M.W.; Ustione, A.; Piston, D.W. Quantitative assessment of fluorescent proteins. Nat. Methods 2016, 13, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Mishin, A.S.; Subach, F.V.; Yampolsky, I.V.; King, W.; Lukyanov, K.A.; Verkhusha, V.V. The First Mutant of the Aequorea victoria Green Fluorescent Protein That Forms a Red Chromophore. Biochemistry 2008, 47, 4666–4673. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, X.; Zhang, J.; Ai, H. Engineering and Characterization of 3-Aminotyrosine-Derived Red Fluorescent Variants of Circularly Permutated Green Fluorescent Protein. Biosensors 2024, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Otsubo, S.; Nishida, M.; Takekawa, N.; Imada, K. Red fluorescent proteins engineered from green fluorescent proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2307687120. [Google Scholar] [CrossRef]
- Nemukhin, A.V.; Grigorenko, B.L.; Savitsky, A.P. Computer Modeling of the Structure and Spectra of Fluorescent Proteins. Acta Nat. 2009, 1, 33–43. [Google Scholar] [CrossRef]
- Bravaya, K.B.; Grigorenko, B.L.; Nemukhin, A.V.; Krylov, A.I. Quantum Chemistry Behind Bioimaging: Insights from Ab Initio Studies of Fluorescent Proteins and Their Chromophores. Acc. Chem. Res. 2012, 45, 265–275. [Google Scholar] [CrossRef]
- Grigorenko, B.L.; Krylov, A.I.; Nemukhin, A.V. Molecular Modeling Clarifies the Mechanism of Chromophore Maturation in the Green Fluorescent Protein. J. Am. Chem. Soc. 2017, 139, 10239–10249. [Google Scholar] [CrossRef]
- Khrenova, M.G.; Nemukhin, A.V.; Tsirelson, V.G. Origin of the π-stacking induced shifts in absorption spectral bands of the green fluorescent protein chromophore. Chem. Phys. 2019, 522, 32–38. [Google Scholar] [CrossRef]
- Marynich, N.K.; Khrenova, M.G.; Gavshina, A.V.; Solovyev, I.D.; Savitsky, A.P. First biphotochromic fluorescent protein moxSAASoti stabilized for oxidizing environment. Sci. Rep. 2022, 12, 7862. [Google Scholar] [CrossRef] [PubMed]
- Stepanyuk, R.A.; Polyakov, I.V.; Kulakova, A.M.; Marchenko, E.I.; Khrenova, M.G. Towards machine learning prediction of the fluorescent protein absorption spectra. Mendeleev Commun. 2024, 34, 788–791. [Google Scholar] [CrossRef]
- Grigorenko, B.L.; Khrenova, M.G.; Jones, D.D.; Nemukhin, A.V. Histidine-assisted reduction of arylnitrenes upon photo-activation of phenyl azide chromophores in GFP-like fluorescent proteins. Org. Biomol. Chem. 2024, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ and Side-Chain χ1 and χ2 Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field (CGenFF): A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Gavshina, A.V.; Marynich, N.K.; Khrenova, M.G.; Solovyev, I.D.; Savitsky, A.P. The role of cysteine residues in the allosteric modulation of the chromophore phototransformations of biphotochromic fluorescent protein SAASoti. Sci. Rep. 2021, 11, 24314. [Google Scholar] [CrossRef]
- Gavshina, A.V.; Solovyev, I.D.; Khrenova, M.G.; Boyko, K.M.; Varfolomeeva, L.A.; Minyaev, M.E.; Popov, V.O.; Savitsky, A.P. The role of the correlated motion(s) of the chromophore in photoswitching of green and red forms of the photoconvertible fluorescent protein mSAASoti. Sci. Rep. 2024, 14, 8754. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Seritan, S.; Bannwarth, C.; Fales, B.S.; Hohenstein, E.G.; Isborn, C.M.; Kokkila-Schumacher, S.I.L.; Li, X.; Liu, F.; Luehr, N.; Snyder, J.W.; et al. TeraChem: A graphical processing unit-accelerated electronic structure package for large-scale ab initio molecular dynamics. WIREs Comput. Mol. Sci. 2021, 11, e1494. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable adiabatic connection models free from adjustable parameters. Chem. Phys. Lett. 1997, 274, 242–250. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Glykos, N.M. Software news and updates carma: A molecular dynamics analysis program. J. Comput. Chem. 2006, 27, 1765–1768. [Google Scholar] [CrossRef]
- Eargle, J.; Luthey-Schulten, Z. NetworkView: 3D display and analysis of protein·RNA interaction networks. Bioinformatics 2012, 28, 3000–3001. [Google Scholar] [CrossRef]
- Tubiana, T.; Carvaillo, J.-C.; Boulard, Y.; Bressanelli, S. TTClust: A Versatile Molecular Simulation Trajectory Clustering Program with Graphical Summaries. J. Chem. Inf. Model. 2018, 58, 2178–2182. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236. [Google Scholar] [CrossRef]
- Yarbrough, D.; Wachter, R.M.; Kallio, K.; Matz, M.V.; Remington, S.J. Refined crystal structure of DsRed, a red fluorescent protein from coral, at 2.0-Å resolution. Proc. Natl. Acad. Sci. USA 2001, 98, 462–467. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).