An Engineered Cargo-Transport Molecular Motor Composed of a Kinesin Monomer and a Diffusing Microtubule-Associated Protein
Abstract
1. Introduction
2. The Engineered Motor
2.1. Description
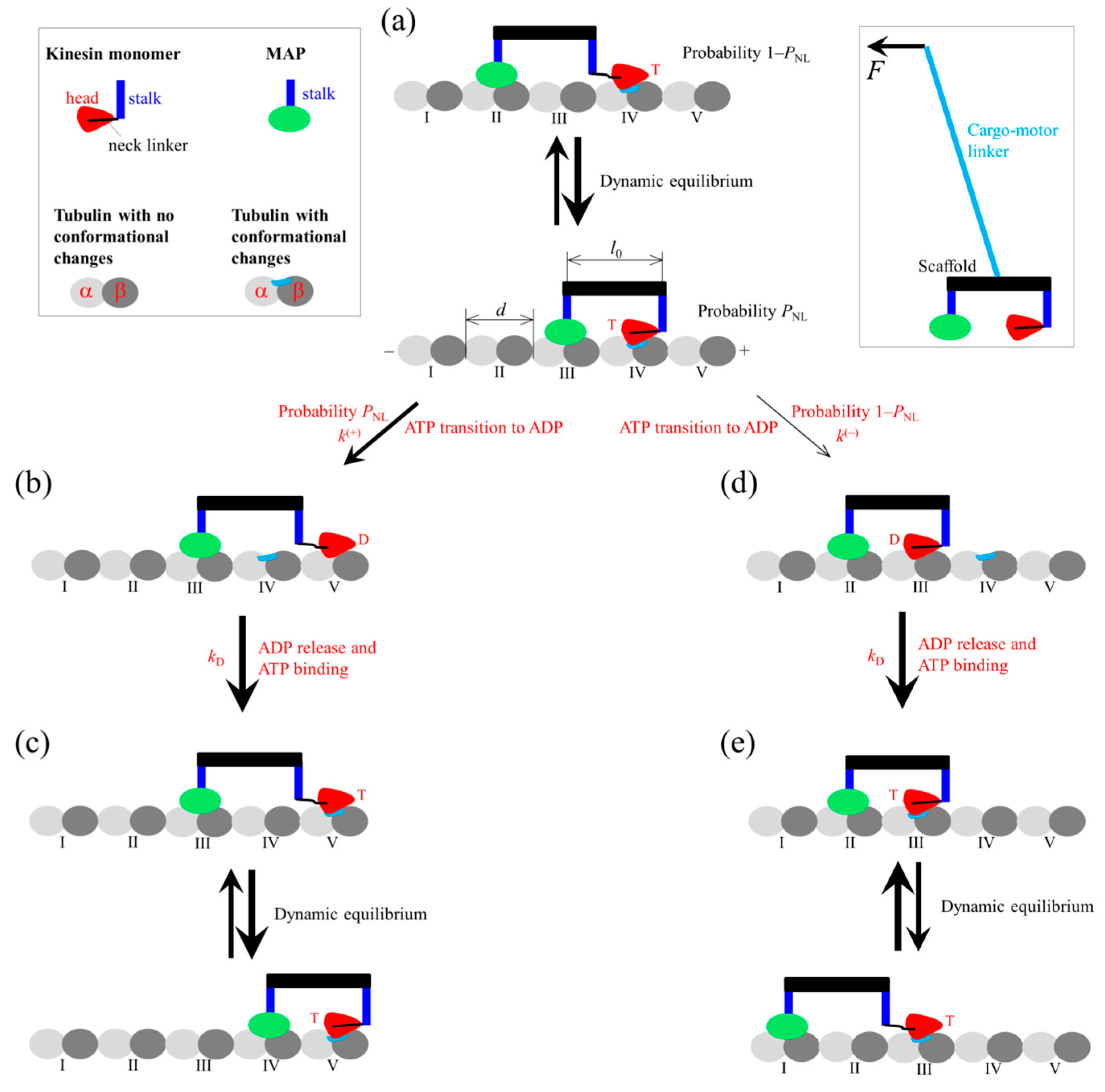
2.2. Chemomechanical Pathway
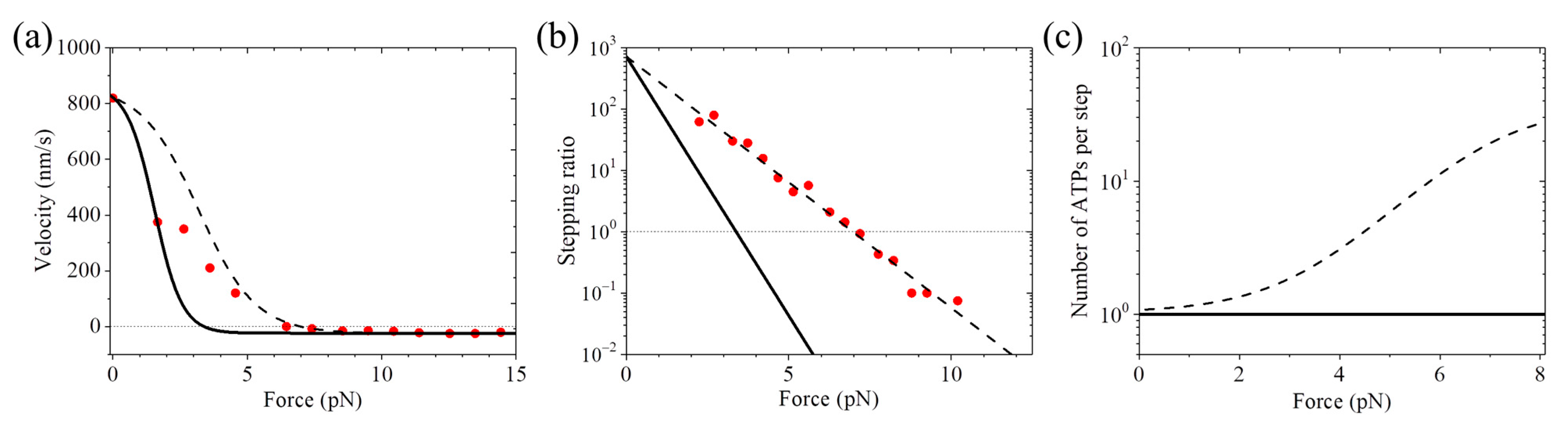
3. Results and Discussion
4. Concluding Remarks
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Vale, R.D.; Milligan, R.M. The way things move: Looking under the hood of molecular motor proteins. Science 2000, 288, 88–95. [Google Scholar] [CrossRef]
- Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 1998, 279, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Vale, R. The molecular motor toolbox for intracellular transport. Cell 2003, 112, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Insight into the chemomechanical coupling mechanism of kinesin molecular motors. Commun. Theor. Phys. 2021, 73, 057601. [Google Scholar] [CrossRef]
- Howard, J. The movement of kinesin along microtubules. Annu. Rev. Physiol. 1996, 58, 703–729. [Google Scholar] [CrossRef]
- Iino, R.; Kinbara, K.; Bryant, Z. Introduction: Molecular motors. Chem. Rev. 2020, 120, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Modeling of chemomechanical coupling of cytoplasmic dynein motors. J. Phys. Chem. B 2024, 128, 10063–10074. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Pierce, N.A. A synthetic DNA walker for molecular transport. J. Am. Chem. Soc. 2004, 126, 10834–10835. [Google Scholar] [CrossRef]
- Green, S.J.; Bath, J.; Turberfield, A.J. Coordinated chemomechanical cycles: A mechanism for autonomous molecular motion. Phys. Rev. Lett. 2008, 101, 238101. [Google Scholar] [CrossRef]
- Valero, J.; Skugor, M. Mechanisms, methods of tracking and applications of DNA walkers: A review. Chem. Phys. Chem. 2020, 21, 1971–1988. [Google Scholar] [CrossRef]
- Vale, R.D.; Reese, T.S.; Sheetz, M.P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 1985, 42, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, F.; Sack, S.; Marx, A.; Thormählen, M.; Schönbrunn, E.; Biou, V.; Thompson, A.; Mandelkow, E.M.; Mandelkow, E. The crystal structure of dimeric kinesin and implications for microtubule-dependent motility. Cell 1997, 91, 985–994. [Google Scholar] [CrossRef]
- Meyhöfer, E.; Howard, J. The force generated by a single kinesin molecule against an elastic load. Proc. Natl. Acad. Sci. USA 1005, 92, 574–578. [Google Scholar] [CrossRef]
- Visscher, K.; Schnitzer, M.J.; Block, S.M. Single kinesin molecules studied with a molecular force clamp. Nature 1999, 400, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Baig, F.; Bakdaleyeh, M.; Bazzi, H.M.; Cao, L.; Tripathy, S.K. Dissecting the pH sensitivity of kinesin-driven transport. J. Phys. Chem. B 2024, 128, 11855–11864. [Google Scholar] [CrossRef]
- Carter, N.J.; Cross, R.A. Mechanics of the kinesin step. Nature 2005, 435, 308–312. [Google Scholar] [CrossRef]
- Berliner, E.; Young, E.C.; Anderson, K.; Mahtani, H.K.; Gelles, J. Failure of a single-headed kinesin to track parallel to microtubule protofilaments. Nature 1995, 373, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Young, E.C.; Mahtani, H.K.; Gelles, J. One-headed kinesin derivatives move by a nonprocessive, low-duty ratio mechanism unlike that of two-headed kinesin. Biochemistry 1998, 37, 3467–3479. [Google Scholar] [CrossRef]
- Kamei, T.; Kakuta, S.; Higuchi, H. Biased binding of single molecules and continuous movement of multiple molecules of truncated single-headed kinesin. Biophys. J. 2005, 88, 2068–2077. [Google Scholar] [CrossRef]
- Diehl, M.R.; Zhang, K.; Lee, H.J.; Tirrell, D.A. Engineering cooperativity in biomotor-protein assemblies. Science 2006, 311, 1468–1471. [Google Scholar] [CrossRef]
- Schimert, K.I.; Budaitis, B.G.; Reinemann, D.N.; Lang, M.J.; Verhey, K.J. Intracellular cargo transport by single-headed kinesin motors. Proc. Natl. Acad. Sci. USA 2019, 116, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Okada, Y.; Schadschneider, A.; Chowdhury, D. Intracellular transport of single-headed molecular motors KIF1A. Phys. Rev. Lett. 2005, 95, 118101. [Google Scholar] [CrossRef]
- Oriola, D.; Casademunt, J. Cooperative force generation of KIF1A Brownian motors. Phys. Rev. Lett. 2013, 111, 048103. [Google Scholar] [CrossRef] [PubMed]
- Oriola, D.; Casademunt, J. Cooperative action of KIF1A Brownian motors with finite dwell time. Phys. Rev. E 2014, 89, 032722. [Google Scholar] [CrossRef]
- Xie, P. Mechanochemical coupling of two coupled kinesin monomers: Comparison with that of the single dimer. RSC Mechanochem. 2025, 2, 127–141. [Google Scholar] [CrossRef]
- Bath, J.; Green, S.J.; Allen, K.E.; Turberfield, A.J. Mechanism for a directional, processive, and reversible DNA motor. Small 2009, 5, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Young, E.C.; Fleming, M.L.; Gelles, J. Coupling of kinesin steps to ATP hydrolysis. Nature 1997, 388, 390–393. [Google Scholar] [CrossRef]
- Coy, D.L.; Wagenbach, M.; Howard, J. Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J. Biol. Chem. 1999, 274, 3667–3671. [Google Scholar] [CrossRef]
- Xie, P. Non-tight and tight chemomechanical couplings of biomolecular motors under hindering loads. J. Theor. Biol. 2020, 490, 110173. [Google Scholar] [CrossRef]
- Umbreit, N.T.; Gestaut, D.R.; Tien, J.F.; Vollmar, B.S.; Gonen, T.; Asbury, C.L.; Davis, T.N. The Ndc80 kinetochore complex directly modulates microtubule dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 16113–16118. [Google Scholar] [CrossRef]
- Chakraborty, M.; Tarasovetc, E.V.; Zaytsev, A.V.; Godzi, M.; Figueiredo, A.C.; Ataullakhanov, F.I.; Grishchuk, E.L. Microtubule end conversion mediated by motors and diffusing proteins with no intrinsic microtubule end-binding activity. Nat. Commun. 2019, 10, 1673. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Modeling study of the dynamics of kinesin-14 molecular motors. J. Phys. Chem. B 2022, 126, 8720–8734. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Modeling studies of the mechanism of context-dependent bidirectional movements of kinesin-14 motors. Molecules 2024, 29, 1792. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Lansky, Z.; Szuba, A.; Schwarz, F.W.; Mitra, A.; Gao, M.; Lüdecke, A.; Wolde, P.R.T.; Diez, S. Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding. Nat. Chem. Biol. 2017, 13, 1245–1252. [Google Scholar] [CrossRef]
- Tseng, K.-F.; Mickolajczyk, K.J.; Feng, G.; Feng, Q.; Kwok, E.S.; Howe, J.; Barbar, E.J.; Dawson, S.C.; Hancock, W.O.; Qiu, W. The tail of kinesin-14a in giardia is a dual regulator of motility. Curr. Biol. 2020, 30, 3664–3671. [Google Scholar] [CrossRef]
- Janson, M.E.; Loughlin, R.; Loiodice, I.; Fu, C.; Brunner, D.; Nedelec, F.J.; Tran, P.T. Crosslinkers and motors organize dynamic microtubules to form stable bipolar arrays in fission yeast. Cell 2007, 128, 357–368. [Google Scholar] [CrossRef]
- Gaska, I.; Armstrong, M.E.; Alfieri, A.; Forth, S. The mitotic crosslinking protein PRC1 acts like a mechanical dashpot to resist microtubule sliding. Dev. Cell 2020, 54, 367–378. [Google Scholar] [CrossRef]
- Clancy, B.E.; Behnke-Parks, W.M.; Andreasson, J.O.L.; Rosenfeld, S.S.; Block, S.M. A universal pathway for kinesin stepping. Nature Struct. Mol. Biol. 2011, 18, 1020–1027. [Google Scholar]
- Crevel, I.M.T.C.; Lockhart, A.; Cross, R.A. Weak and strong states of kinesin and Ncd. J. Mol. Biol. 1996, 257, 66–76. [Google Scholar] [CrossRef]
- Sosa, H.; Peterman, E.J.G.; Moerner, W.E.; Goldstein, L.S.B. ADP-induced rocking of the kinesin motor domain revealed by single-molecule fluorescence polarization microscopy. Nat. Struc. Biol. 2001, 8, 540–544. [Google Scholar] [CrossRef]
- Guo, W.; Gao, Y.; Du, D.; Sanchez, J.E.; Li, Y.; Qiu, W.; Li, L. Elucidating the interactions between Kinesin-5/BimC and the microtubule: Insights from TIRF microscopy and molecular dynamics simulations. Brief. Bioinform. 2025, 26, bbaf144. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Yajima, H.; Nitta, R.; Inoue, S.; Ogura, T.; Sato, C.; Hirokawa, N. X-ray and Cryo-EM structures reveal mutual conformational changes of kinesin and GTP-state microtubules upon binding. EMBO J. 2015, 34, 1270–1286. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-X.; Wang, P.-Y.; Chen, H.; Xie, P. Studies of conformational changes of tubulin induced by interaction with kinesin using atomistic molecular dynamics simulations. Int. J. Mol. Sci. 2021, 22, 6709. [Google Scholar] [CrossRef]
- Shang, Z.; Zhou, K.; Xu, C.; Csencsits, R.; Cochran, J.C.; Sindelar, C.V. High-resolution structures of kinesin on microtubules provide a basis for nucleotide-gated force-generation. eLife 2014, 3, e04686. [Google Scholar] [CrossRef][Green Version]
- Rice, S.; Lin, A.W.; Safer, D.; Hart, C.L.; Naber, N.; Carragher, B.O.; Cain, S.M.; Pechatnikova, E.; Wilson-Kubalek, E.M.; Whittaker, M.; et al. A structural change in the kinesin motor protein that drives motility. Nature 1999, 402, 778–784. [Google Scholar] [CrossRef]
- Asenjo, A.B.; Weinberg, Y.; Sosa, H. Nucleotide binding and hydrolysis induces a disorder-order transition in the kinesin neck-linker region. Nat. Struc. Mol. Biol. 2006, 13, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-X.; Guo, S.-K.; Wang, P.-Y.; Chen, H.; Xie, P. All-atom molecular dynamics simulations reveal how kinesin transits from one-head-bound to two-heads-bound state. Proteins 2020, 88, 545–557. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W.; Jiang, Q.; Wang, C.; Knossow, M.; Gigant, B. The structure of apo-kinesin bound to tubulin links the nucleotide cycle to movement. Nat. Comm. 2014, 5, 5364. [Google Scholar] [CrossRef]
- Nitta, R.; Okada, Y.; Hirokawa, N. Structural model for strain-dependent microtubule activation of Mg-ADP release from kinesin. Nat. Struct. Mol. Biol. 2008, 15, 1067–1075. [Google Scholar] [CrossRef]
- Yildiz, A.; Tomishige, M.; Gennerich, A.; Vale, R.D. Intramolecular strain coordinates kinesin stepping behavior along microtubules. Cell 2008, 134, 1030–1041. [Google Scholar] [CrossRef]
- Moyer, M.L.; Gilbert, S.P.; Johnson, K.A. Pathway of ATP hydrolysis by monomeric and dimeric kinesin. Biochemistry 1998, 37, 800–813. [Google Scholar] [CrossRef]
- Rosenfeld, S.S.; Fordyce, P.M.; Jefferson, G.M.; King, P.H.; Block, S.M. Stepping and stretching: How kinesin uses internal strain to walk processively. J. Biol. Chem. 2003, 278, 18550–18556. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Hirokawa, N. A processive single-headed motor: Kinesin superfamily protein KIF1A. Science 1999, 283, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.F.; Choi, M.H. Rectified Brownian motion and kinesin motion along microtubules. Phys. Rev. E 2001, 63, 051901. [Google Scholar] [CrossRef]
- Muir, K.W.; Batters, C.; Dendooven, T.; Yang, J.; Zhang, Z.; Burt, A.; Barford, D. Structural mechanism of outer kinetochore Dam1-Ndc80 complex assembly on microtubules. Science 2023, 382, 1184–1190. [Google Scholar] [CrossRef]
- Rice, S.; Cui, Y.; Sindelar, C.; Naber, N.; Matuska, M.; Vale, R.; Cooke, R. Thermodynamic properties of the kinesin neck region docking to the catalytic core. Biophys. J. 2003, 84, 1844–1854. [Google Scholar] [CrossRef]
- Asbury, C.L.; Fehr, A.N.; Block, S.M. Kinesin moves by an asymmetric hand-over-hand mechanism. Science 2003, 302, 2130–2134. [Google Scholar] [CrossRef]
- Yildiz, A.; Tomishige, M.; Vale, R.D.; Selvin, P.R. Kinesin walks hand-over-hand. Science 2004, 303, 676–678. [Google Scholar] [CrossRef]
- Wolff, J.O.; Scheiderer, L.; Engelhardt, T.; Engelhardt, J.; Matthias, J.; Hell, S.W. MINFLUX dissects the unimpeded walking of kinesin-1. Science 2023, 379, 1004–1010. [Google Scholar] [CrossRef]
- Kita, T.; Sasaki, K.; Niwa, S. Biased movement of monomeric kinesin-3 KLP-6 explained by a symmetric Brownian ratchet model. Biophys. J. 2025, 124, 205–214. [Google Scholar] [CrossRef]
- Vale, R.D.; Funatsu, T.; Pierce, D.W.; Romberg, L.; Harada, Y.; Yanagida, T. Direct observation of single kinesin molecules moving along microtubules. Nature 1996, 380, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-X.; Fu, Y.-B.; Guo, S.-K.; Wang, P.-Y.; Chen, H.; Xie, P. Investigating role of conformational changes of microtubule in regulating its binding affinity to kinesin by all-atom molecular dynamics simulation. Proteins 2018, 86, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-K.; Wang, P.-Y.; Xie, P. A model of processive movement of dimeric kinesin. J. Theor. Biol. 2017, 414, 62–75. [Google Scholar] [CrossRef]
- Guo, S.-K.; Shi, X.-X.; Wang, P.-Y.; Xie, P. Processivity of dimeric kinesin-1 molecular motors. FEBS Open Bio 2018, 8, 1332–1351. [Google Scholar] [CrossRef]
- Verhey, K.J.; Ohi, R. Causes, costs and consequences of kinesin motors communicating through the microtubule lattice. J. Cell Sci. 2023, 136, jcs260735. [Google Scholar] [CrossRef]
- Wijeratne, S.S.; Fiorenza, S.A.; Neary, A.E.; Subramanian, R.; Betterton, M.D. Motor guidance by long-range communication on the microtubule highway. Proc. Natl. Acad. Sci. USA 2022, 119, e2120193119. [Google Scholar] [CrossRef]
- Xie, P. A model for cooperativity of kinesin-4 motors by communicating through the microtubule track. Chem. Phys. 2024, 581, 112274. [Google Scholar] [CrossRef]
- Sindelar, C.V.; Downing, K.H. An atomic-level mechanism for activation of the kinesin molecular motors. Proc. Natl. Acad. Sci. USA 2010, 107, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.P.M.H.; Asenjo, A.B.; Paydar, M.; Dhakal, S.; Kwok, B.H.; Sosa, H. Structural basis of mechano-chemical coupling by the mitotic kinesin KIF14. Nat. Commun. 2021, 12, 3637. [Google Scholar] [CrossRef]
- Xie, P.; Guo, S.-K.; Chen, H. A generalized kinetic model for coupling between stepping and ATP hydrolysis of kinesin molecular motors. Int. J. Mol. Sci. 2019, 20, 4911. [Google Scholar] [CrossRef]
- Xie, P. Effect of the neck linker on processive stepping of kinesin motor. Biophysica 2023, 3, 46–68. [Google Scholar] [CrossRef]
- Xie, P. Dynamics of kinesin motor proteins under longitudinal and sideways loads. J. Theor. Biol. 2021, 530, 110879. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Description | Value |
|---|---|---|
| k(+) | Rate of ATP transition to ADP in monomer with forward NL | 108 s–1 |
| k(–) | Rate of ATP transition to ADP in monomer without forward NL | 3 s–1 |
| E0 | Energy change of NL docking and large conformational change of ATP monomer | 3 kBT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P. An Engineered Cargo-Transport Molecular Motor Composed of a Kinesin Monomer and a Diffusing Microtubule-Associated Protein. Biophysica 2025, 5, 26. https://doi.org/10.3390/biophysica5030026
Xie P. An Engineered Cargo-Transport Molecular Motor Composed of a Kinesin Monomer and a Diffusing Microtubule-Associated Protein. Biophysica. 2025; 5(3):26. https://doi.org/10.3390/biophysica5030026
Chicago/Turabian StyleXie, Ping. 2025. "An Engineered Cargo-Transport Molecular Motor Composed of a Kinesin Monomer and a Diffusing Microtubule-Associated Protein" Biophysica 5, no. 3: 26. https://doi.org/10.3390/biophysica5030026
APA StyleXie, P. (2025). An Engineered Cargo-Transport Molecular Motor Composed of a Kinesin Monomer and a Diffusing Microtubule-Associated Protein. Biophysica, 5(3), 26. https://doi.org/10.3390/biophysica5030026






