Evidence of the Differences Between Human and Bovine Serum Albumin Through the Interaction with Coumarin-343: Experimental (ICD) and Theoretical Studies (DFT and Molecular Docking)
Abstract
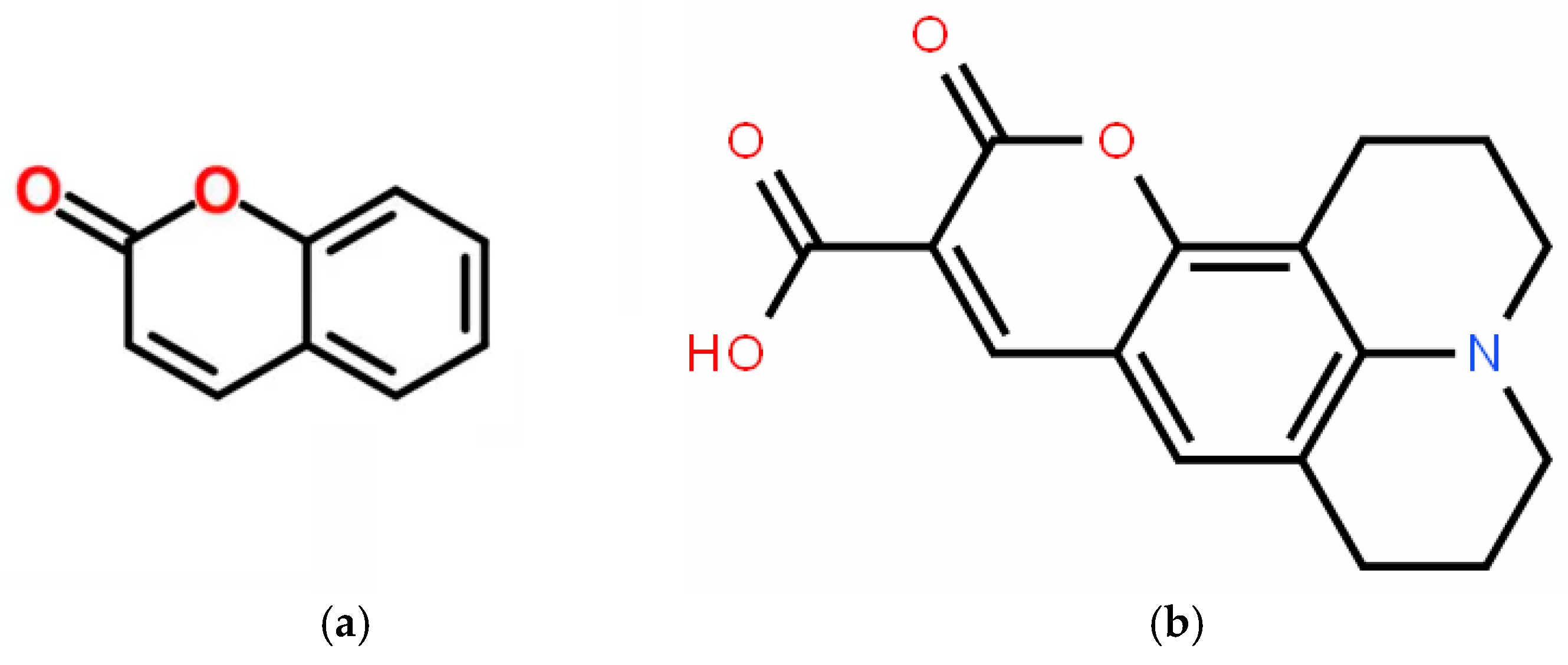
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
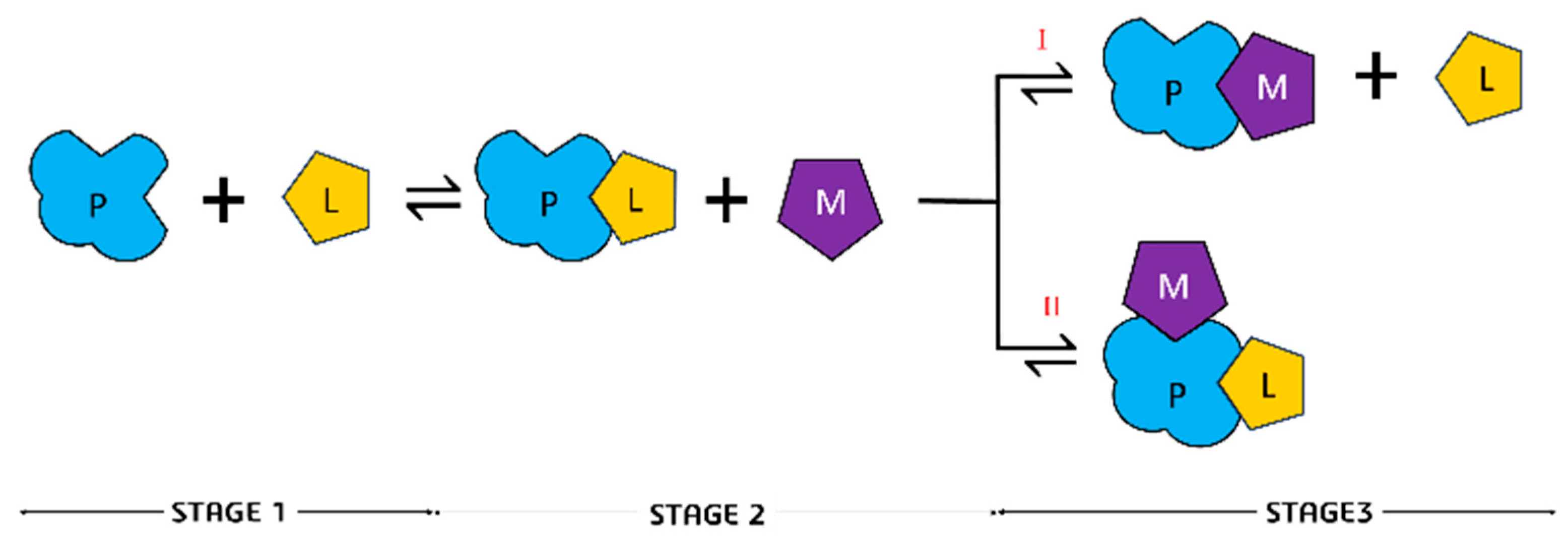
2.2.1. Binding of C343 and Proteins
2.2.2. C343 and Albumin Protein Stoichiometry
2.2.3. Electronic Circular Dichroism Spectroscopy
2.2.4. Molecular Docking Studies
3. Results
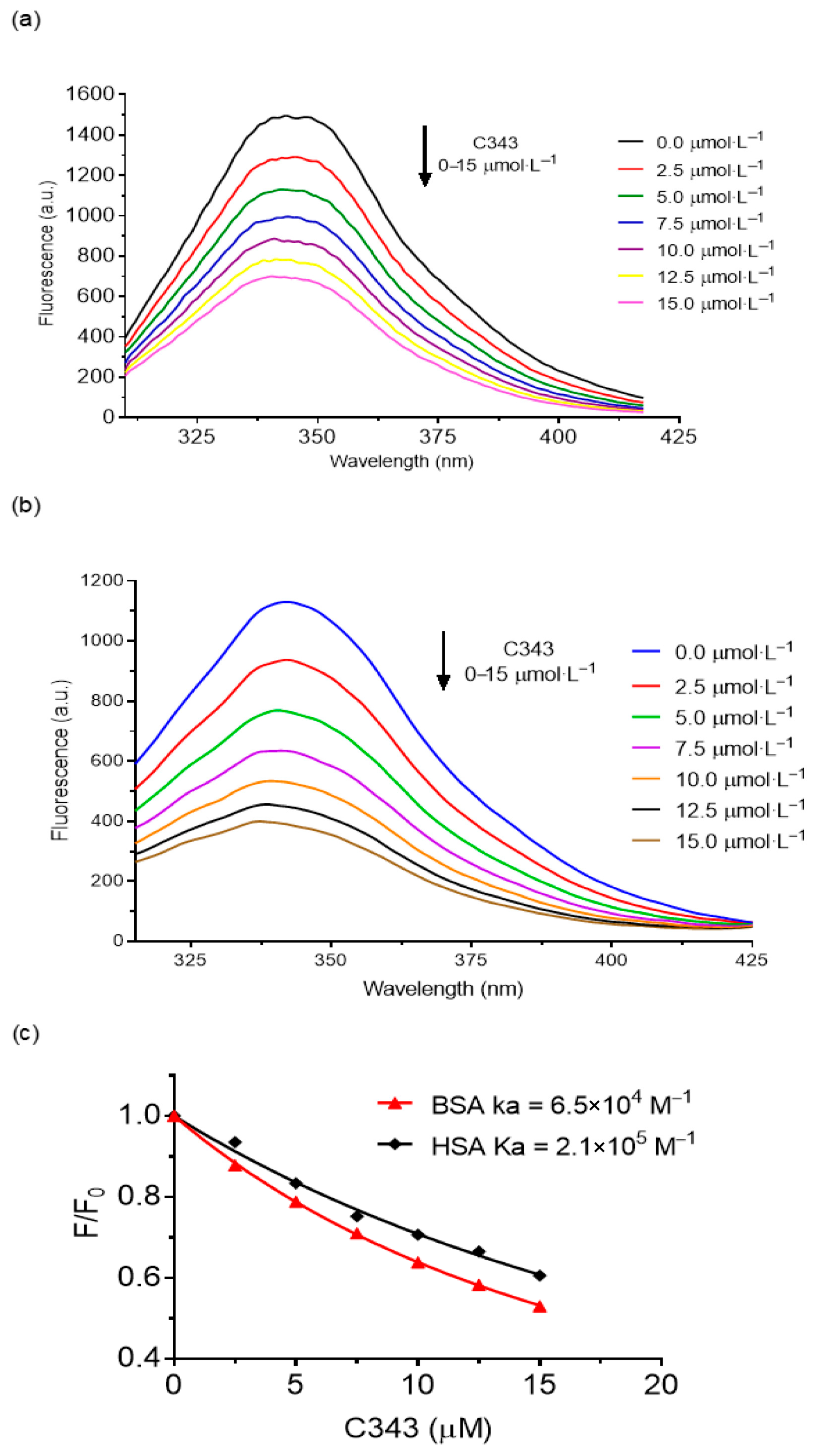
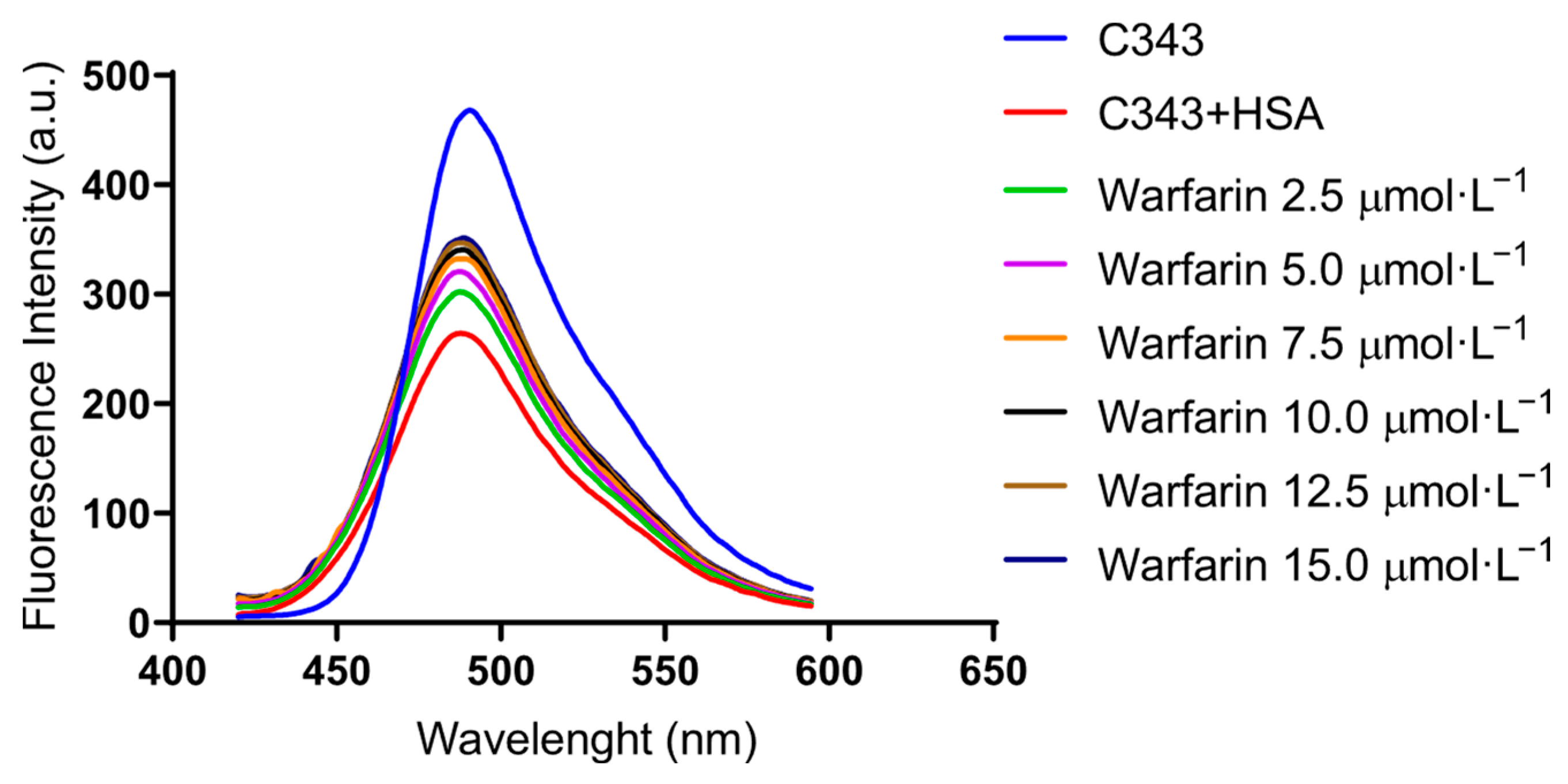
3.1. Binding of C343 in HSA and BSA
3.2. Stern–Volmer
3.3. Determination of Stoichiometry
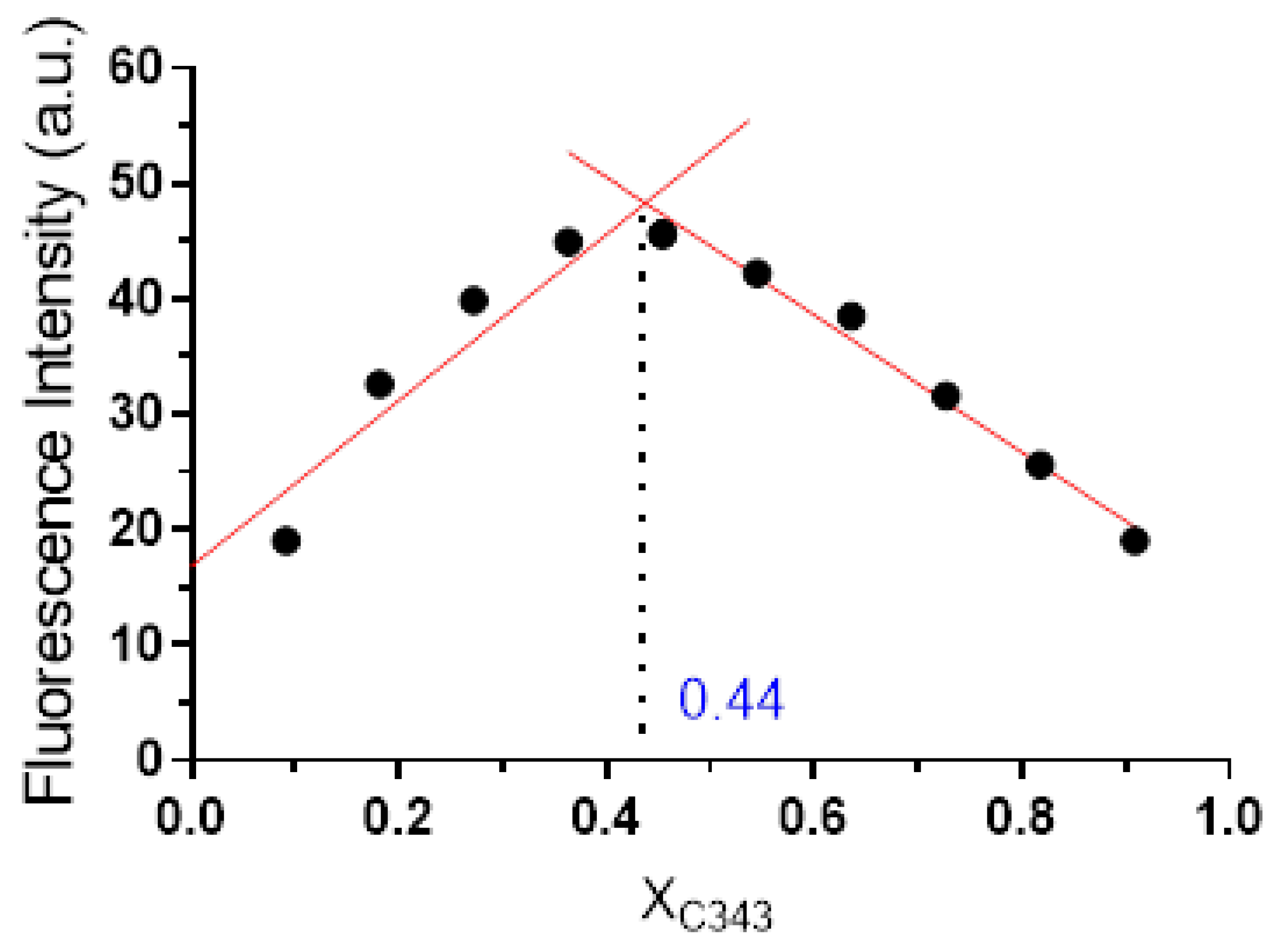
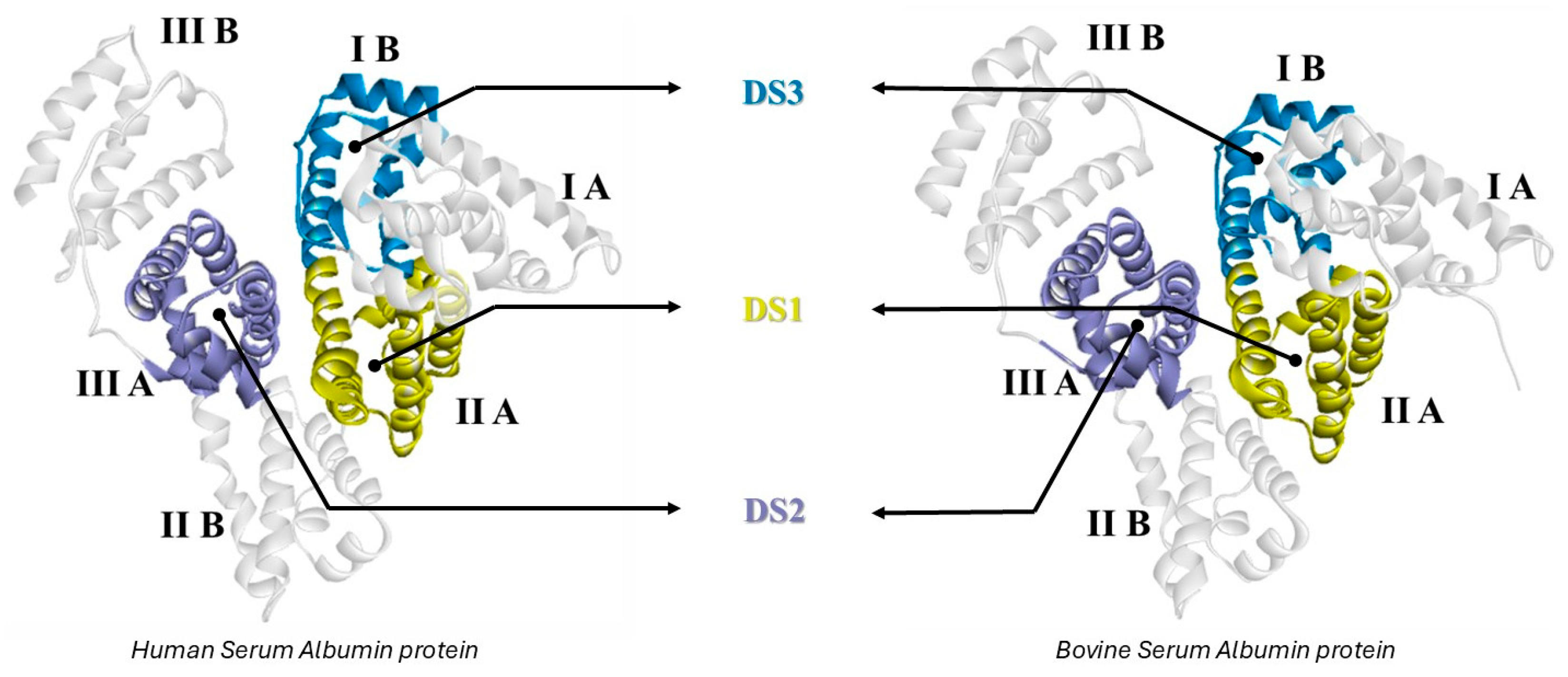
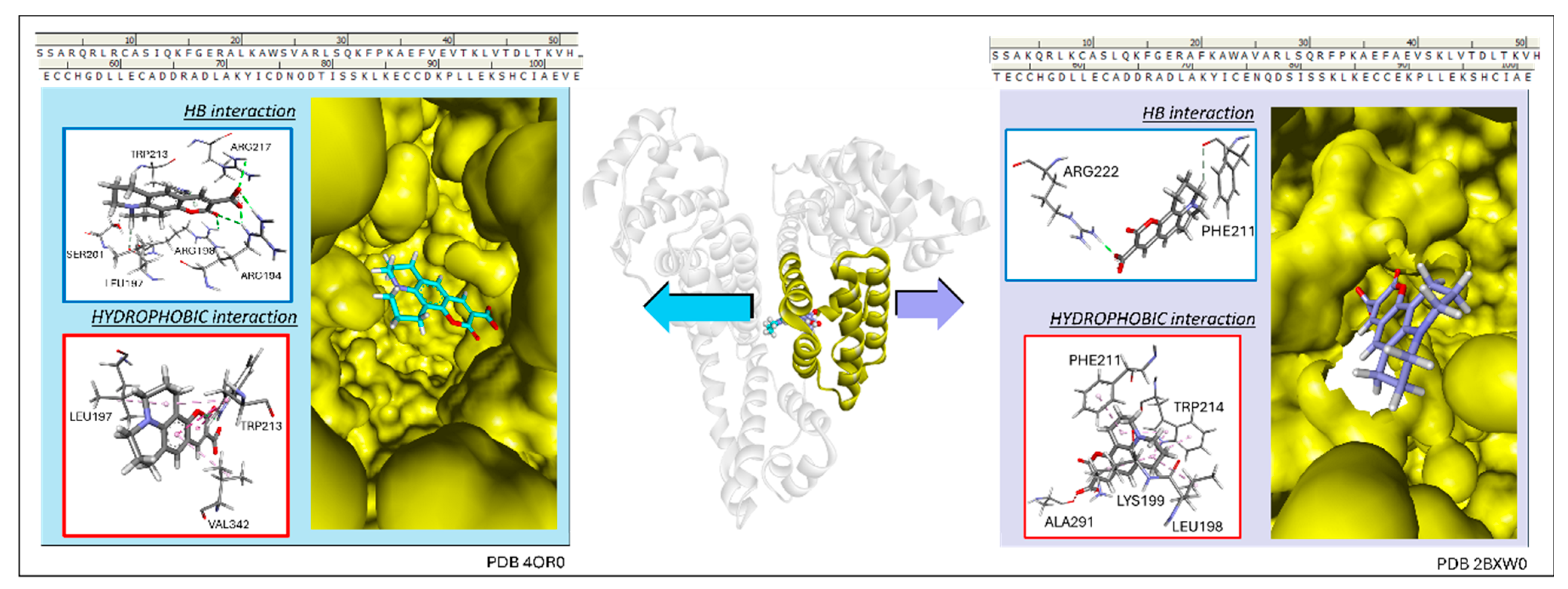
3.4. Determination of Preferential Binding Sites
3.5. Molecular Docking Results
3.6. Density Functional Theory Calculations
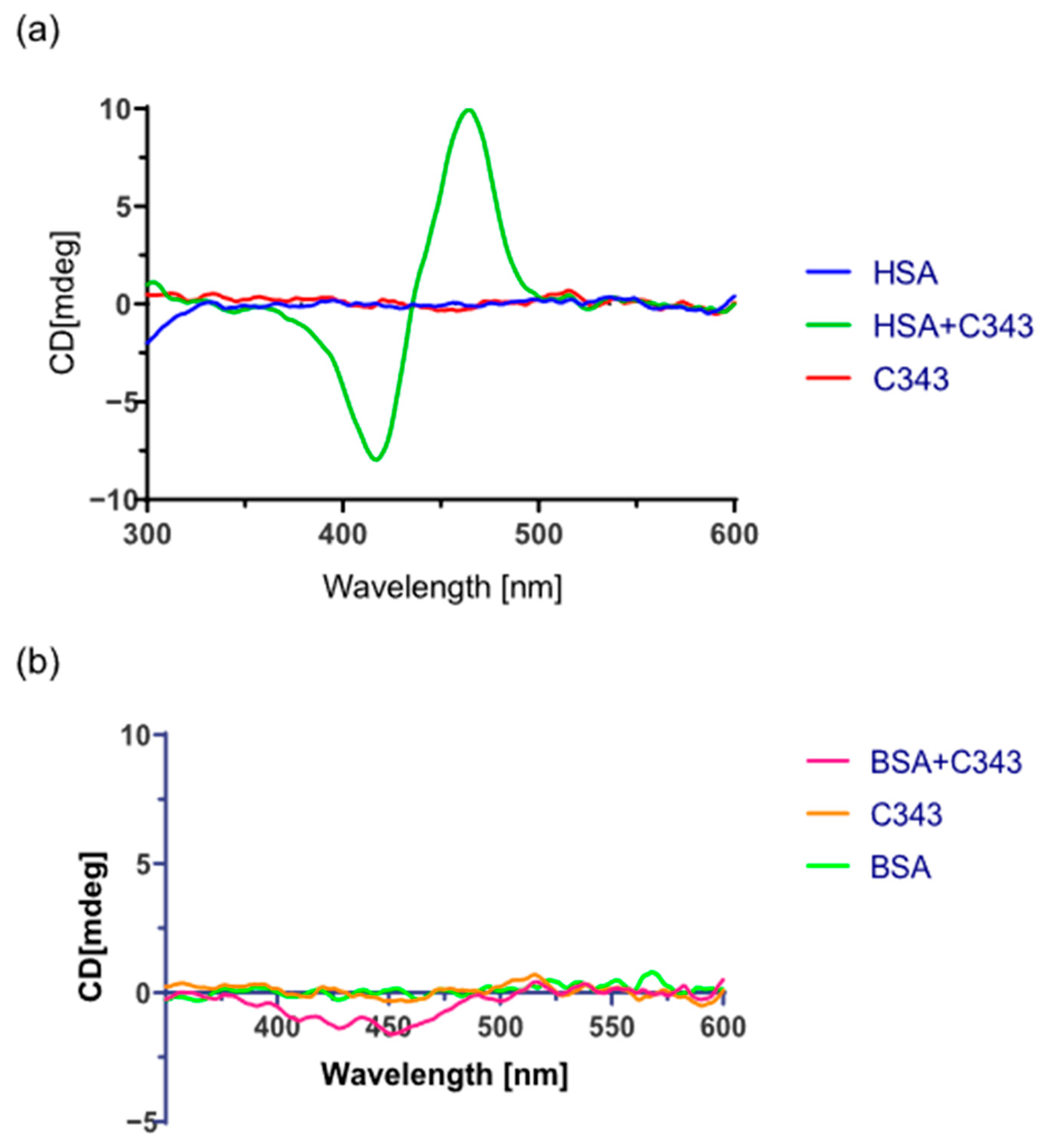
3.7. Induced Circular Dichroism Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guido, R.V.C.; Andricopulo, A.D.; Oliva, G. Planejamento de fármacos, biotecnologia e química medicinal: Aplicações em doenças infecciosas. Estud. Av. 2010, 24, 81–98. [Google Scholar] [CrossRef]
- Yoguim, M.I. Estudos da Interação da Rosa de Bengala com a Proteína Albumina do Soro Humano (HSA) sob Aspectos Experimentais e Teóricos na Caracterização dos Sítios de Ligação; Universidade Estadual Paulista “Júlio de Mesquita Filho”: Bauru, Brazil, 2021. [Google Scholar]
- Lehn, J.-M.; Sanders, J.K.M. Supramolecular chemistry: Concepts and perspectives. Angew. Chem. Int. Ed. Engl. 1995, 34, 2563. [Google Scholar] [CrossRef]
- Pacheco, I.B. Estudo Molecular e Espectroscópico da Interação de Derivados Dihidropiridínicos com o DNA.; Universidade Federal de Santa Catarina: Florianópolis, Brazil, 2023. [Google Scholar]
- Lindup, W.E.; Orme, M.C. Clinical pharmacology: Plasma protein binding of drugs. Br. Med. J. 1981, 282, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, X.; Yang, H.; Martinez, A.; Feng, M.; Dong, Z.; Gao, G. Acridine-based macrocyclic fluorescent sensors: Self-assembly behavior characterized by crystal structures and a tunable bathochromic-shift in emission induced by via adjusting the ring size and rigidity. Org. Biomol. Chem. 2013, 11, 3375–3381. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, S.; Lachowicz, J.I.; Nowakowski, M.E.; Jaremko, M.; Jaremko, Ł. Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin. J. Inorg. Biochem. 2019, 198, 110716. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Singh, S.; Kumar, P.; Kumar, R. Review of post-translational modification of human serum albumin: A potential marker with altered structure and function. Int. J. Biol. Macromol. 2020, 164, 482–492. [Google Scholar]
- Nelson, D.L.; Cox, M.M.; Lehninger, A.L. Princípios de Bioquímica de Lehninger, 5th ed.; Artmed: Porto Alegre, Brazil, 2011; p. 1273. ISBN 9788536324180. [Google Scholar]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Ghuman, J.; Zunszain, P.A.; Chung, C.-W.; Curry, S. Structural basis of binding of fluorescent, site-specific dansylated amino acids to human serum albumin. J. Struct. Biol. 2011, 174, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Trynda-Lemiesz, L.; Wiglusz, K. Interactions of human serum albumin with meloxicam: Characterization of binding site. J. Pharm. Biomed. Anal. 2010, 52, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Leboffe, L.; di Masi, A.; Trezza, V.; Fanali, G.; Gioia, M.; Coletta, M.; Fasano, M.; Permyakov, E.A. Ligand binding to the FA3-FA4 cleft inhibits the esterase-like activity of human serum albumin. PLoS ONE 2015, 10, e0120603. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.A.; Curry, S.; Franks, N.P. Binding of the general anesthetics propofol and halothane to human serum albumin: High resolution crystal structures. J. Biol. Chem. 2000, 275, 38731–38738. [Google Scholar] [CrossRef] [PubMed]
- Bolattin, M.B.; Nandibewoor, S.T.; Joshi, S.D.; Dixit, S.R.; Chimatadar, S.A. Interaction between carisoprodol and bovine serum albumin and effect of β-cyclodextrin on binding: Insights from molecular docking and spectroscopic techniques. RSC Adv. 2016, 6, 63463–63471. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, Y.; Hu, D.; Gu, L.; Fei, X.; Zhang, J. Effect of Rapamycin Microspheres in Sjögren Syndrome Dry Eye: Preparation and Outcomes. Ocul. Immunol. Inflamm. 2019, 27, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.S.; Dinda, A.K.; Pandey, N.K.; Dasgupta, S. Effects of Urea, Metal Ions and Surfactants on the Binding of Baicalein with Bovine Serum Albumin. J. Pharm. Anal. 2016, 6, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ksenofontov, A.A.; Bocharov, P.S.; Ksenofontova, K.V.; Antina, E.V. Water-soluble BODIPY-based fluorescent probe for BSA and HSA detection. J. Mol. Liq. 2021, 345, 117031. [Google Scholar] [CrossRef]
- Sulkowska, A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002, 616, 227–232. [Google Scholar] [CrossRef]
- Kandagal, P.B.; Seetharamappa, J.; Ashoka, S.; Shaikh, S.; Manjunatha, D. Study of the interaction between doxepin hydrochloride and bovine serum albumin by spectroscopic techniques. Int. J. Biol. Macromol. 2006, 39, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Halder, M. Detailed scrutiny of the anion receptor pocket in subdomain IIA of serum proteins toward individual response to specific ligands: HSA-pocket resembles flexible biological slide-wrench unlike BSA. J. Phys. Chem. B 2014, 118, 6071–6085. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.P.; Pereira, T.; Vitorio, F.; Nadur, N.; Lacerda, R.; Kümmerle, A. The importance of cumarins for medicinal chemistry and the development of bioactive compounds in the last years. Quím. Nova 2021, 44, 180–197. [Google Scholar] [CrossRef]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending topics on coumarin and its derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.R.; Boza, I.; Ximenes, V.; Yoguin, M.; Dávila-Rodriguez, M.-J.; Morgon, N.; Caracelli, I. Elucidação da quiralidade induzida na molécula dansilglicina na complexação com a proteína albumina do soro humano (HSA). Quím. Nova 2019, 42, 135–142. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Fang, Y.; Ramani, K. Using least median of squares for structural superposition of flexible proteins. BMC Bioinform. 2009, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Falcone, R.D.; Silber, J.J.; Correa, N.M. Role of the medium on the C343 inter/intramolecular hydrogen bond interactions: An absorption, emission, and 1H NMR investigation of C343 in benzene/n-heptane mixtures. J. Phys. Chem. A 2010, 114, 7326–7330. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, A.; Zielinski, K.; Sekula, B. Structural studies of bovine, equine, and leporine serum albumin complexes with naproxen. Proteins 2014, 82, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Kozuch, S.; Martin, J.M.L. Halogen bonds: Benchmarks and theoretical analysis. J. Chem. Theory Comput. 2013, 9, 1918–1931. [Google Scholar] [CrossRef] [PubMed]
- Peters, T., Jr. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Jiskoot, W.; Visser, A.J.W.G.; Herron, J.N.; Sutter, M. Fluorescence Spectroscopy. In Methods for Structural Analysis of Protein Pharmaceuticals; AAPS Press: Arlington, TX, USA, 2005; p. 27. [Google Scholar]
- Guizado, T.R.C. Estudos Computacionais da Interação de Porfirinas e Seus Complexos de Ferro com Albumina Sérica Humana. Master’s Thesis, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Neto, G.L.B.; Baptista, E.; Becca, G.; Nakatani, H.; Souza, V. Interações competitivas de complexos de rutênio contendo dimetilsulfóxido e ligantes N-heterocíclicos com albumina de soro humano. Quím. Nova 2020, 43, 261–270. [Google Scholar] [CrossRef]
- Nakata, A.; Imamura, Y.; Otsuka, T.; Nakai, H. Time-dependent density functional theory calculations for core-excited states: Assessment of standard exchange-correlation functionals and development of a novel hybrid functional. J. Chem. Phys. 2006, 124, 094105. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.H.; Benesi, H.A. Interaction of iodine with aromatic hydrocarbons. Nature 1949, 164, 963. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Fluorescence quenching methods to study protein-nucleic acid interactions. Methods Enzymol. 2004, 379, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Hazai, E.; Sawyer, L. Binding of the pepper alkaloid piperine to bovine β-lactoglobulin: Circular dichroism spectroscopy and molecular modeling study. J. Agric. Food Chem. 2005, 53, 10179–10185. [Google Scholar] [CrossRef] [PubMed]
- Góes Filho, L.S. Estudo do Efeito de Solventes nas Propriedades Espectroscópicas do Antibiótico Norfloxacina: Absorção, Fluorescência Estacionária e Resolvida No Tempo. Ph.D. Thesis, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Dasgupta, A. Immunoassays and Issues with Interference in Therapeutic Drug Monitoring. In Clinical Challenges in Therapeutic Drug Monitoring; Dasgupta, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–44. [Google Scholar] [CrossRef]
- Huang, C.Y. Determination of binding stoichiometry by the continuous variation method: The Job plot. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1982; Volume 87, pp. 509–525. [Google Scholar] [CrossRef]
- Olson, E.J.; Bühlmann, P. Getting more out of a Job plot: Determination of reactant to product stoichiometry in cases of displacement reactions and n:n complex formation. J. Org. Chem. 2011, 76, 8406–8412. [Google Scholar] [CrossRef] [PubMed]
- Pitman, S.J.; Evans, A.K.; Ireland, R.T.; Lempriere, F.; McKemmish, L.K. Benchmarking basis sets for density functional theory thermochemistry calculations: Why unpolarised basis sets and the polarised 6-311G family should be avoided. J. Phys. Chem. A 2023, 127, 10834–10848. [Google Scholar] [CrossRef] [PubMed]
- Mahamiya, V.; Bhattacharyya, P.; Shukla, A. Benchmarking Gaussian basis sets in quantum-chemical calculations of photo-absorption spectra of light atomic clusters. ACS Omega 2022, 7, 48261–48271. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J.; Ziegler, T.; van Gisbergen, S.J.A.; Baerends, E.J. Chiroptical properties from time-dependent density functional theory. I. Circ. Dichroism Spectra Org. molecules. J. Chem. Phys. 2002, 116, 6930–6940. [Google Scholar] [CrossRef]
- Graciani, F.S.; Ximenes, V.F. Investigation of human albumin-induced circular dichroism in dansylglycine. PLoS ONE 2013, 8, e76849. [Google Scholar] [CrossRef] [PubMed]
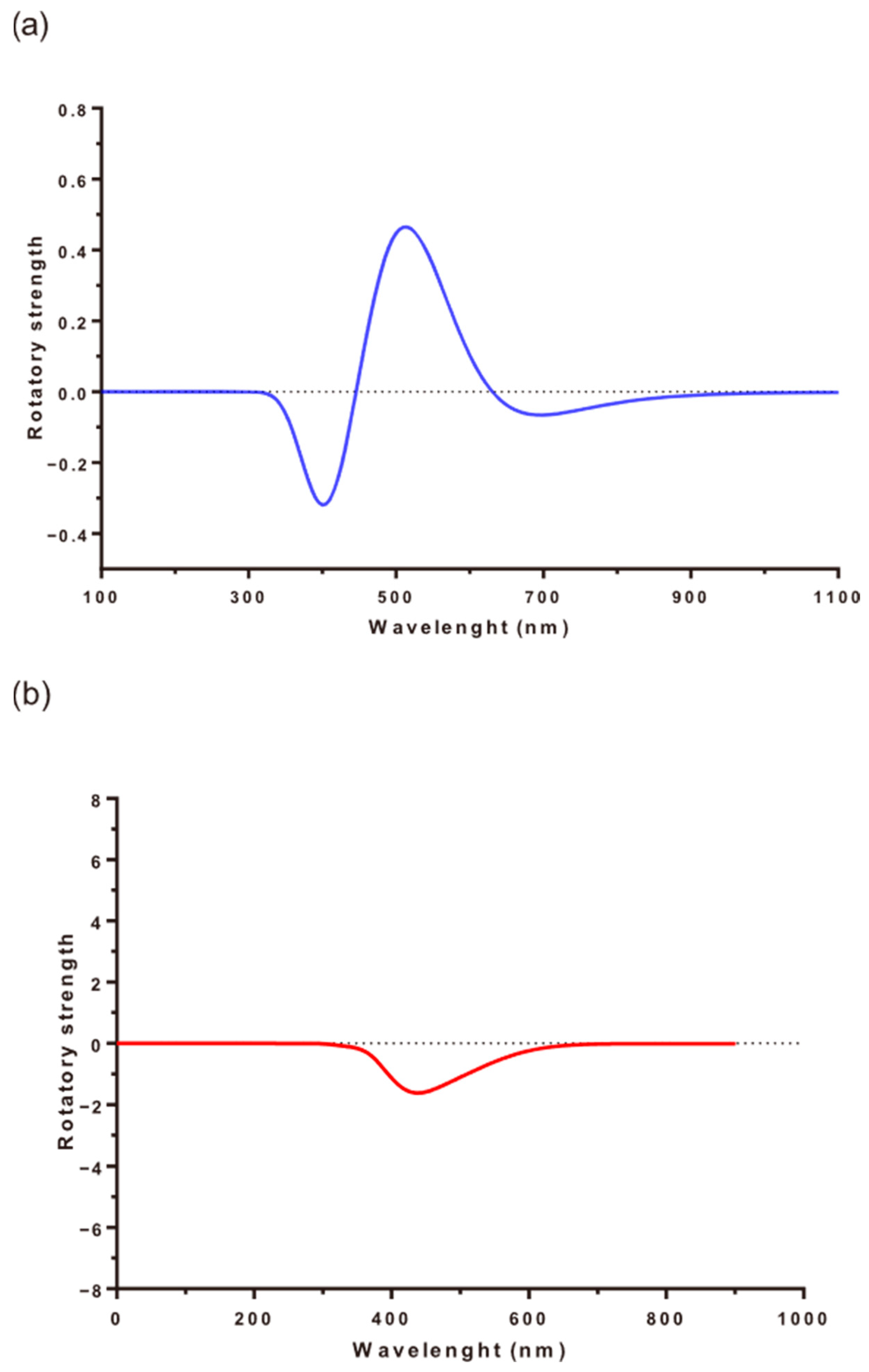
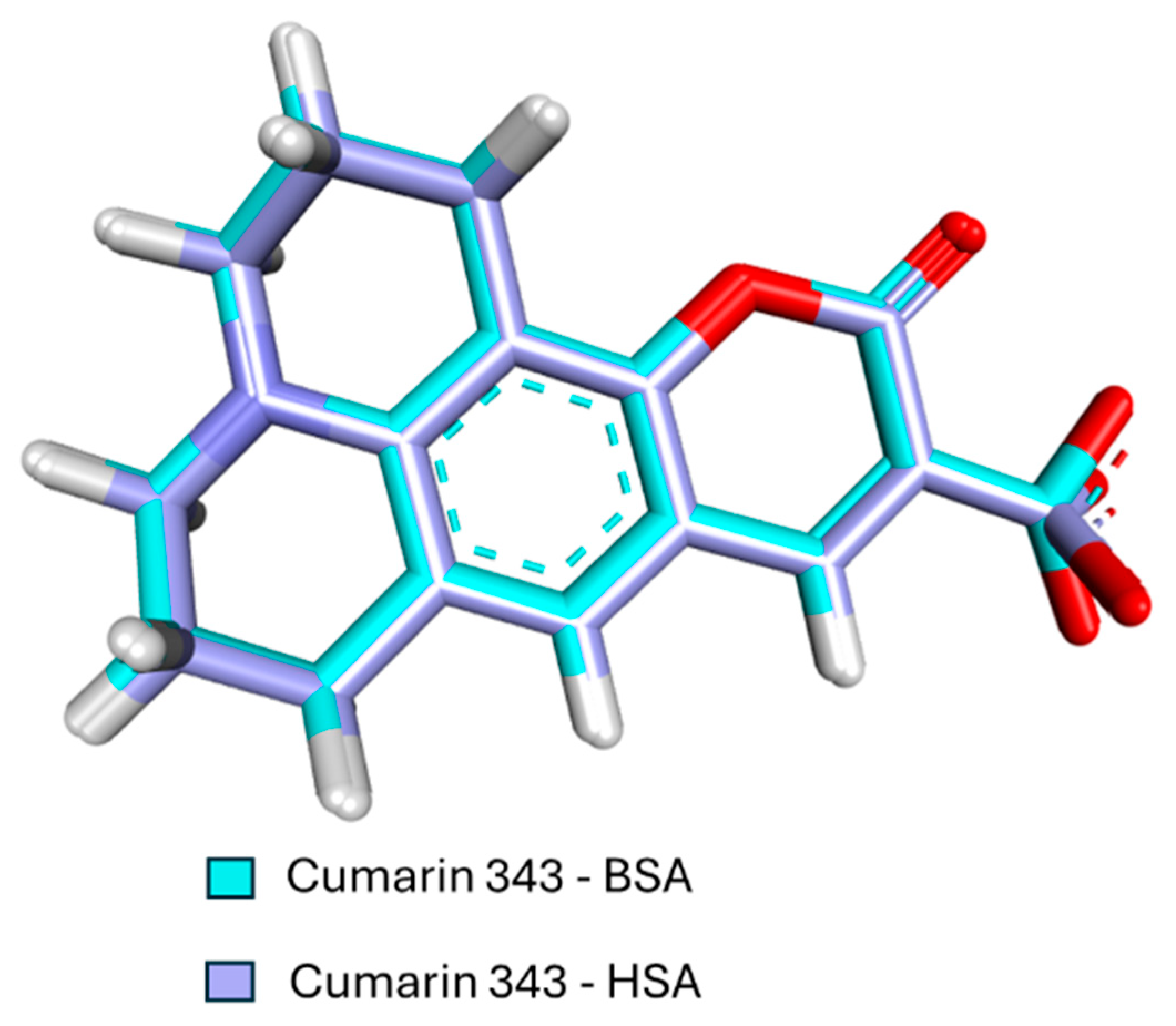
| Temperature (K) | KSV (mol−1·L) | Kq (mol−1·L·s−1) | R2 |
|---|---|---|---|
| 293 | 3.09 × 105 | 3.09 × 1013 | 0.9903 |
| 3.01 × 105 | 3.01 × 1013 | 0.9925 | |
| 3.05 × 105 | 3.05 × 1013 | 0.9926 | |
| 313 | 1.57 × 105 | 1.57 × 1013 | 0.9979 |
| 1.52 × 105 | 1.52 × 1013 | 0.9986 | |
| 1.37 × 105 | 1.37 × 1013 | 0.9994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, C.R.; Yoguim, M.I.; Pavan, N.M.; Morgon, N.H.; Ximenes, V.F.; de Souza, A.R. Evidence of the Differences Between Human and Bovine Serum Albumin Through the Interaction with Coumarin-343: Experimental (ICD) and Theoretical Studies (DFT and Molecular Docking). Biophysica 2025, 5, 27. https://doi.org/10.3390/biophysica5030027
de Souza CR, Yoguim MI, Pavan NM, Morgon NH, Ximenes VF, de Souza AR. Evidence of the Differences Between Human and Bovine Serum Albumin Through the Interaction with Coumarin-343: Experimental (ICD) and Theoretical Studies (DFT and Molecular Docking). Biophysica. 2025; 5(3):27. https://doi.org/10.3390/biophysica5030027
Chicago/Turabian Stylede Souza, Carmen Regina, Maurício Ikeda Yoguim, Nathalia Mariana Pavan, Nelson Henrique Morgon, Valdecir Farias Ximenes, and Aguinaldo Robinson de Souza. 2025. "Evidence of the Differences Between Human and Bovine Serum Albumin Through the Interaction with Coumarin-343: Experimental (ICD) and Theoretical Studies (DFT and Molecular Docking)" Biophysica 5, no. 3: 27. https://doi.org/10.3390/biophysica5030027
APA Stylede Souza, C. R., Yoguim, M. I., Pavan, N. M., Morgon, N. H., Ximenes, V. F., & de Souza, A. R. (2025). Evidence of the Differences Between Human and Bovine Serum Albumin Through the Interaction with Coumarin-343: Experimental (ICD) and Theoretical Studies (DFT and Molecular Docking). Biophysica, 5(3), 27. https://doi.org/10.3390/biophysica5030027






