Abstract
Objective: The aim of this systematic review is to compare the survival rates of zirconia and titanium dental implants, by evaluating the most recent scientific evidence, in order to comprehend the behaviour of zirconia implants as an alternative to titanium, due to the latter’s biological properties. Methods: An electronic search was performed on the Pubmed/MEDLINE and Scopus databases in November 2023 to identify clinical trials that investigated zirconia and titanium implants’ behaviour with a follow-up of at least 5 years. The primary outcome was the implant survival rate—defined as the maintenance of the implant in situ during the period of study. The secondary outcome was the implant success rate, which is associated with the values of the peri-implant variables—the probing depth, marginal bone loss, gingival recession, bleeding on probing, plaque index, and aesthetics scores. Results: A total of 17 articles were selected from the search, resulting in a sample of 364 studies. A total of 15 articles fulfilled the selection criteria. Zirconia implants showed satisfactory results. Due to the lack of data available with follow-up times of more than five years, it is not possible to conclusively describe the benefits of zirconia in comparison with titanium implants. Conclusions: While zirconia implants show promise as a future alternative to metal implants, more research is needed to understand their long-term benefits and peri-implant behaviour.
1. Introduction
Peri-implantitis is a chronic inflammatory disease caused by bacterial activity that leads to the destruction of supporting implant tissue—compromising the peri-implant assessment parameters. Although bacterial activity is the primary cause, it is important to note that the condition is often aggravated by excessive immune responses against bacterial invasion [1].
1.1. Titanium Implants
Titanium is currently the most commonly used material in the implant industry due to its favourable characteristics—biocompatibility and mechanical and corrosion resistance [1,2]. However, Nakagawa M et al. [3] found that this corrosion resistance decreases in low oxygen conditions, such as in an intra-gingival environment. Titanium implants’ osseointegration is also slow and could be accompanied by aseptic loosening or insufficient osseointegration [4]. Several studies have shown that titanium particles can be released during osseointegration, which may cause allergic reactions and damage to the tissues around the implant. This happens because the particles trigger inflammation, increase cytokine levels, and affect the bone around the implant, making osseointegration more difficult. Contrary to this, Wachi T et al. [2] found a reduced level of ion release from titanium materials owing to the formation of a non-stoichiometric titanium dioxide (TiO2) film; on the other hand, these materials are not safe from failure because of a fibrous layer that forms at the bone–tissue interface. Additionally, nano-engineered titanium implants, such as those enhanced by electrochemical anodization (EA), promote the formation of a thickened TiO2 film with distinctive nano-microgeometries, which could potentially benefit soft-tissue integration at the gingival–abutment interface, improving the overall performance and reducing the likelihood of failure [5]. Despite the current knowledge, no hard evidence exists of a causal relation between the release of titanium particles and implant failure [6,7,8,9,10,11,12,13,14].
1.2. Zirconia Implants
Zirconia implant research has been undertaken for more than fifty years because of their aesthetic outcomes and since titanium implants are not always the preferred choice to rehabilitate anterior regions [7,8,15]. These aesthetic concerns, combined with the possible biological complications induced by titanium particles, have led to zirconia becoming an increasingly studied material in recent years as an opportunity to implement metal-free implant procedures [16]. Despite the fact that zirconia implants have shown optimal responses during the early healing phase and osseointegration, because of the low rate of bone loss and peri-implant infections, these responses were not always statistically significant [6,7,16].
Compared to titanium, Y-TZP has a lower elasticity modulus (around 100 GPa vs. 200 GPa) combined with a high level of fracture resistance due to its distinctive characteristic, stress-induced transformation [7,15,17,18]. Y-TZP synthesis includes the addition of yttria to stabilise the tetragonal phase of pure zirconia, and this addition makes it more stable to the influence of thermal fluctuations [19].
Kohal RJ et al. [20] reported that a one-piece zirconia implant was twice as resistant to fracture than a two-piece. In spite of the evolution of zirconia resistance to fracture through the years, Gahlert M et al. [21] showed that the foremost cause of implant failure was a reduced implant diameter of 3.25 mm.
Osman RB et al. [22] emphasised the difficulty of placing zirconia implants in dense bone tissue and the necessity of improving surgical protocols to reduce the failure rate, such as to minimise the risk of implant fracture.
1.3. Objective
The aim of this systematic review is to compare the survival rate of zirconia and titanium dental implants by evaluating the most recent scientific evidence in order to comprehend the behaviour of zirconia implants, as an alternative to titanium, due to the latter’s biological properties.
2. Materials and Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [23].
2.1. Research Conduct
The search process was carried out during November 2023 and included two databases: PubMed/MEDLINE and Scopus, with a time restriction for paper publish after 2009. The search strategy combined the following keywords: “Implants”, “Zirconia”, “Titanium”, “Ceramic”, “Survival”, and “Survival rate”. In PubMed, filters were applied to include only clinical trials and randomised controlled trials (N = 263), whereas in Scopus, the search was limited to research articles, case reports, and studies within the fields of medicine and dentistry (N = 77). A manual search was also performed.
2.2. Study Selection
The sample selection strategy followed the Population, Intervention, Comparison, Outcome and Study Design (PICOS) method:
P—patients undergoing implant surgery;
I—zirconia dental implants;
C—titanium dental implants;
O—survival rate (SR), success rate (ScR), periodontal variables (peri-implant probing depth (PPD), marginal bone loss (MBL), gingival recession (GR), bleeding on probing (BoP), plaque index (PI), and aesthetics scores (PINK));
S—clinical trials and randomised controlled trials.
2.3. Inclusion Criteria
The following inclusion criteria were applied:
- (a)
- Studies involving adult human participants;
- (b)
- Reporting the survival rate of zirconia or titanium implants;
- (c)
- Clinical trials and randomised controlled trials published from 2010 onward;
- (d)
- A minimum follow-up period of five years.
2.4. Exclusion Criteria
Studies in which implants and bone grafts were placed simultaneously were excluded to minimise the risk of regeneration-related failure. Additionally, studies evaluating implants composed of both titanium and zirconia were not considered. Studies comparing only abutment materials were also excluded. The detailed exclusion criteria are provided in Appendix A.
2.5. Screening Method
The screening was performed by two reviewers (F.A. and T.C.) in accordance with the inclusion and exclusion criteria explained above. In cases of uncertainty, a third reviewer (R.F.-A.) assessed the study.
The screening began with the removal of duplicated articles. Titles and abstracts were then reviewed for relevance, followed by a full-text assessment to determine whether the studies addressed the research question. The PRISMA 2020 flow diagram is presented in Figure 1.
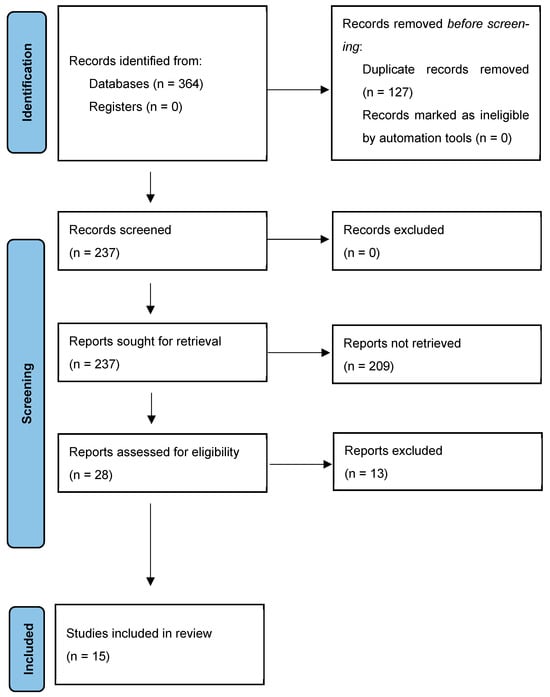
Figure 1.
PRISMA 2020 flow diagram.
2.6. Data Extraction and Outcomes
The primary outcome is implant SR, defined as the maintenance of the implant in situ during the study period.
The secondary outcome is the implant ScR, which is associated with peri-implant variables values—PPD, MBL, GR, BoP, and PINK scores.
Data extraction was performed by one reviewer (F.A.) using data extraction tables; only intelligible information was selected. The data extracted were organised in four sections:
- (1)
- Study characteristics—author, year, type of design (randomised clinical trial [RCT]/clinical trial [CT], follow-up (years), intervention (titanium/zirconia/comparing both), patient sample (sample size and mean age [years]), implant sample (sample size, restoration type [partial/full edentulous; fixed dental prosthesis (FDP)/single crown (SC)], and location (maxilla/mandible/both).
- (2)
- Implant design—material, characteristics (type of implant [one/two-piece], diameter [mm], and length [mm]), brand.
- (3)
- Evaluation criteria—SR, ScR, BoP, PPD, GR, PI, MBL, PI, and PINK Score.
- (4)
- Outcomes divided into three subsections—(i) comparative studies, (ii) titanium studies, (iii) zirconia studies—periodontal parameters (BoP, PPD, GR, PI, and MBL), PINK Score, SR, and ScR.
For periodontal and aesthetic parameters, only results expressed as mean/median and standard deviation were considered.
For SR and ScR, results were accepted if expressed as a percentage (%).
2.7. Quality Assessment and Risk of Bias
Bias assessment was conducted by two researchers (F.A. and T.C.) by submitting the sample to the criteria of RoB 2 (randomised clinical trials) [24] and ROBINS-I (non-randomised clinical trials) [25] tools, which follow the Cochrane Handbook for Systematic Reviews recommendations. Non-randomised clinical trials, labelled as non-randomised studies of interventions (NRSI) by Cochrane, include cohort studies, case–control studies, controlled before-and-after studies, and interrupted-time-series studies.
3. Results
The search resulted in 364 articles—340 articles from the primary database search, and 24 from the secondary manual research. After the removal of duplicate articles, 237 studies were screened, and only 15 articles fulfilled the selection criteria, which were included in the qualitative synthesis. The agreement level between the reviewers was strong (k = 0.85).
3.1. Studies Characteristics
Of the fifteen articles included, four were randomised clinical trials (RCT), and 11 were non-randomised clinical trials (nRCT), whose characteristics included in Table 1. The studies were published between 2011 and 2023; only one article directly compared titanium and zirconia implants (published in 2020) [Koller 2020] [6], six articles studied titanium implants’ behaviour (published between 2011 and 2018) [S. Ma DClinDent 2018 [26]; Cochran, D.L. 2011 [27]; Ravald, N 2013 [28]; Müller, F 2015 [29]; van Velzen, FJ 2015 [30]; Buser, D 2012 [9]]; and eight studies analysed zirconia implants (published between 2015 and 2023) [Balmer, M 2020 [31]; Brunello, G 2022 [32]; Grassi, FR 2015 [33]; Cionca, N 2021 [34]; Spies, BC 2015 [35]; Lorenz, J 2019 [36]; Kiechle S 2023 [37]; Kohal RJ 2023 [38]]. The follow-up ranged between 5 and 15 years.

Table 1.
Study characteristics.
The sample size was heterogeneous among studies, ranging from 16 to 303 patients; the minimum mean age was 46, and the maximum was 76 years old.
This systematic review included the evaluation of 2496 implants—1986 titanium implants and 464 zirconia implants. These implants were part of partial and full edentulous restorations, located on the maxilla and/or mandibula; partial edentulous restorations include single crowns (SCs) and fixed dental prostheses (FDPs), and full edentulous restorations were restored with FDPs.
3.2. Implant Characteristics
This review included seven titanium articles [6,9,26,27,28,29,30] and nine zirconia articles [6,31,32,33,34,35,36,37,38]; the zirconia ones were composed of Y-TZP or ZrO2. The titanium implants were all two-pieces, with diameters ranging from 3.3 to 4.8 mm and lengths between 8.0 and 14.0 mm, from the brands Ziteron®, Southern Implants®, Astra Tech®, Brånemark®, and Straumann®. The zirconia ones were made of Y-TZP or ZrO2. Regarding zirconia implants, only one was a one-piece ZrO2 implant with a 4.1 mm diameter and available in 8.0, 10.0, and 12.0 mm lengths, manufactured by Straumann®. The remaining zirconia implants were composed of Y-TZP and included both one- and two-piece designs, with diameters ranging from 3.25 to 5.5 mm and lengths between 8.0 and 16.0 mm, from Nobel Biocare®, Straumann®, Oensingen®, Bredent Medical®, Zircon Medical®, and VITA Zahnfabrik®.
3.3. Output Measurement Methods and Results (Table 2 and Table 3)

Table 2.
Titanium studies’ output.

Table 3.
Zirconia studies’ output.
3.3.1. Survival Rate Criteria (SR)
The survival rate was calculated in 13 studies [Koller et al. [6], Cochran D.L. 2011 [27], Ravald, N et al. [28], F. Müller 2015 [29], van Velzen, FJ 2015 [30], Buser, D et al. [9], Balmer, M et al. [31], Brunello, G et al. [32], Grassi, FR et al. [33], N. Cionca 2021 [34], Lorenz, J et al. [36], Kiechle S 2023 [37], Kohal RJ 2023 [38]] and was defined as the implant remaining in situ.
The implant survival rate ranged from 71.9% to 99.7% for titanium implants and 55% to 100% for zirconia implants.
The lowest survival for both materials was reported by Koller et al. [6] for maxillary implants, although the patient sample size was small (N = 15).
Cochran D.L et al. [27] described one of the lowest SRs, 87%, after 5.0 years, for 626 titanium implants. In contrast, Buser, D et al. [9] reported a survival rate of 98.8% in 511 titanium implants after 10.0 years.
Koller et al. [6] compared titanium and zirconia implants, and the SR difference was not statistically significant between the groups (p > 0.05).
S. Ma DClinDent et al. [26] and Lorenz, J et al. [36] did not report survival rate data.
3.3.2. Success Rate Criteria (ScR)
Success rate data were reported in eight articles [S. Ma DClinDent 2018 [26], Cochran D.L. 2011 [27], F. Müller 2015 [29], Buser D 2012 [9], Grassi FR 2015 [33], N. Cionca 2021 [34], BC Spies 2015 [35], Kiechle S 2023 [37]]. Among them, only Kiechle S. et al. [37] did not specify the criteria used. The criteria for defining success varied among authors. S. Ma DClinDent et al. [26] classified cases as successful despite marginal bone loss exceeding 1.8 mm over a 5-year follow-up. Cochran, DL et al. [27] and BC Spies et al. [35] used Kaplan–Meier survival analysis [39], while the remaining studies adopted the Albrektsson et al. [40] criteria, which define success as follows: the absence of implant mobility, suitability for prosthetic restoration, no evidence of persistent complaints, no continuous radiolucency, and vertical bone loss of less than 0.2 mm per year [Müller, F 2015 [29], Buser, D 2012 [9], Grassi, FR 2015 [33], N. Cionca 2021 [34]].
3.3.3. Bleeding on Probing (BoP)
The BoP parameter was assessed in studies by Koller et al. [6], Ravald, N et al. [28], Buser, D et al. [9], Balmer, M et al. [31], Brunello, G et al. [32], Grassi, FR et al. [33], and Lorenz, J et al. [36]. With the exception of Ravald, N et al. [28], Balmer, M et al. [31], and Brunello, G et al. [32], who recorded bleeding scores after probing at six sites around the implant, and Lorenz, J et al. [36], who used sulcus bleeding index (SBI), which recorded four sites per implant, Koller et al. [6], Buser D et al. [9], and Grassi, FR et al. [33] classified BoP according to the modified Sulcus Bleeding Index (mSBI) and measured bleeding at six sites around the implant—0, no bleeding when a periodontal probe is passed along the gingival margin; 1, isolated bleeding spots visible; 2, blood forms a confluent red line on margin; 3, heavy or profuse bleeding.
Due to the discrepancies in the measurement methods, we found it difficult to compare the BoP through the studies. Nonetheless, the zirconia group exhibited the lowest values, with 12.9 ± 15.8% reported by Brunello G. et al. [32] and 0.47 ± 0.51 using the mSBI by Lorenz J. et al. [36]. However, Koller et al. [6] found lower BoP values in titanium implants compared to zirconia, although the difference was not statistically significant (p = 0.130).
3.3.4. Peri-Implant Probing Depth (PPD)
Seven articles reported data on PPD using a periodontal probe [van Velzen, FJ 2015 [30], Buser D 2012 [9], Balmer, M 2020 [31], Brunello, G 2022 [32], Grassi, FR 2015 [33], Cionca, N 2021 [34], Lorenz, J 2019 [36]]. With the exception of Lorenz J. et al. [36], who measured PPD at four sites per implant, all other studies assessed recorded PPD values at six sites around the implant.
The lowest PPD value was observed in the zirconia group—2.2 ± 0.53 mm, with 96.9% ScR [Grassi FR, 2022 [33]]. The highest PPD for zirconia was 3.5 ± 1.0 mm, as reported by Cionca N. et al. [34] ], with a success rate of only 63%. In contrast, in the titanium group, although PPD values were approximately 4.0 mm, the success rate was higher than that of zirconia—97% [Buser D, 2012 [9]], in a study with a larger sample size.
3.3.5. Gingival Recession (GR)
Among the seven articles that evaluated the GR parameter [Koller 2020 [6], S. Ma DClinDent 2018 [26], Buser, D 2012 [9], Balmer, M 2020 [31], Brunello, G 2022 [32], Grassi, FR 2015 [33], Lorenz, J 2019 [36]], GR was generally measured as the distance from the implant shoulder to the mucosal margin (DIM). S. Ma DClinDent [26] used the cervical margins of adjacent natural teeth as a reference, while Grassi, FR et al. [33] also considered the epimucosal position of the crown margin. Given the absence of a cementoenamel junction in implants, Grassi F.R. et al. [33] assessed GR by measuring the distance from the incisal edge to the most apical point of the vestibular margin. Koller et al. [6] did not provide a clear explanation.
GR values were comparable between groups; however, the titanium group exhibited the lowest GR value (0.08 ± 0.20 mm) [S. Ma DClinDent 2018 [26]], whereas the zirconia group presented the highest value (1.27 ± 0.81 mm) [Koller 2020 [6]].
3.3.6. Plaque Index (PI)
The plaque index was assessed using different methodologies across the seven studies that reported results. The measurement method was not specified by Koller et al. [6]. Ravald, N et al. [28], Balmer, M et al. [31], and Lorenz, J et al. [36] recorded PI based on individual implant surfaces (mesial, distal, buccal, and lingual). Buser, D. et al. [9] and Grassi, FR et al. [33], applied a modified plaque index (mPLI), where a score of 0 indicated no detection of plaque, a score of 1 indicated that plaque were only recognised by running a probe across the smooth marginal surface of the implant, a score of 2 indicated plaque could be seen by the naked eye, and a score of 3 indicated an abundance of soft matter. Brunello, G et al. [32] assessed PI as a dichotomous variable—0 indicating an absence of plaque, and 1 indicating presence of plaque.
Overall, zirconia implants exhibited a higher PI, with a mean value of 26.2 ± 27.5% [Balmer, M 2020 [31]].
3.3.7. Marginal Bone Loss (MBL)
MBL was assessed in ten studies [Koller 2020 [6], S. Ma DClinDent 2018 [26], N. Ravald 2013 [28], F. Müller 2015 [29], van Velzen FJ 2015 [30], Buser D 2012 [9], Balmer, M 2020 [31], Grassi, FR 2015 [33], Cionca, N 2021 [34], Lorenz, J 2019 [36]]. The majority defined it as the distance through the implant shoulder and the first bone-to-implant contact (DIB).
Buser, D et al. [9] and Cionca, N et al. [34] converted DIB values into a qualitative classification—<2.5 mm, no bone loss or even bone gain; [2.51–3.50 mm], no or minimal bone loss; [3.51–4.5 mm], moderate bone loss; and >4.51 mm, progressive bone loss, including the implants with peri-implant infections.
Titanium demonstrated superior performance in minimizing bone loss, which aligns with the PI results.
3.3.8. Aesthetic Score (PINK)
Koller et al. [6]’s study was the only study to provide results of the PINK aesthetic score. This score was assessed based on the evaluation of mesial and distal papilla; the integrity of alveolar process; and the contour, colour, and texture of peri-implant mucosa.
The PINK score did not show a significant difference between zirconia and titanium implants (p = 0.428). However, it is important to note that, after 30 months, zirconia implants exhibited higher scores, although the difference was not statistically significant. Conversely, after 80 months, titanium implants attained the highest scores.
3.4. Risk of Bias Assessment
The risk of bias (Table 4) was assessed using the ROB2 tool for four RCTs, all of which were classified as having “Some concern” [Koller 2020 [6], N. Ravald 2013 [28], F. Müller 2015 [29], Lorenz J 2019 [36]]. Upon reassessing the studies, we found that the outcomes remained clear and did not substantiate any significant issues. However, the identified concerns were related to three specific domains, classified as “Some concern” due to factors such as a lack of blinding or unclear randomisation procedures.

Table 4.
Risk of bias.
For the eleven nRCTs assessed with the ROBINS-I tool, three studies were flagged as having a high risk of bias due to issues related to the selection of reported results, including missing or incomplete outcome data [Balmer, M 2020 [31], BC Spies 2015 [35], Kohal RJ 2023 [41]]. Buser D et al. [9] exhibited low risk, supporting the robustness of the review.
4. Discussion
The purpose of this systematic review was to compare the survival rates of zirconia and titanium dental implants and evaluate the performance of zirconia implants as an alternative to titanium, given the latter’s biological properties. In contrast with the systematic reviews that have been published to date [42,43,44,45,46], we restricted the follow-up period to a minimum of five years after the implant surgery to properly evaluate the osseointegration and the wear rate of the implants. There was only one study [6] that compared titanium and zirconia directly, in accordance with our inclusion criteria. Therefore, this systematic review also included articles about titanium and zirconia implants individually to support Koller et al.’s findings and motivate the publication of new articles that compare both implants in the same investigation [6].
The analysis of this outcome requires a cautious interpretation due to the discrepancy in sample size of titanium and zirconia implants–1986 vs. 464, respectively. Given the small number of studies, the sample is heterogeneous in a range of aspects, such as the implant system (one-piece and two-pieces), rehabilitation type (SC and FDPs) and position (anterior and posterior; maxilla and mandible), implant brand, and implant characteristics. Regarding the rehabilitation type, zirconia implants were generally placed in SCs, while titanium implants were part of FDPs. The follow-up differences between titanium and zirconia studies—5.0 to 15.0 years vs. 5.0 to 9.0 years—should be considered. The study that compared both implants simultaneously had a follow-up of 6.7 years. These differences made it challenging to combine the results in a statistically meaningful way, as variations in study designs, patient populations, and outcome measures created a high degree of heterogeneity [6].
The recent introduction of zirconia in the marketplace justifies the lack of studies with longer follow-up periods. In addition, according to Roehling S et al. [16], a significant portion of the investigated zirconia implants, including the ones from recent studies, has been discontinued, which complicates the interpretation of zirconia implant behaviour. However, the decline in zirconia implant failure rates over the years and the improvement in its mechanical properties should be noted [16].
Studies with a 5-year follow-up, such as those conducted by Müller et al. [29] and Balmer et al. [31], which had the highest survival rates in each group, demonstrated similar results regardless of the implant material (titanium 97.8% and zirconia 98.4%). Both materials showed functional stability in partial edentulous scenarios. Zirconia implants, particularly one-piece designs like those used by Grassi et al. [33] and Balmer et al. [31], presented high success rates. However, studies indicated that one-piece zirconia implants might exhibit slightly higher peri-implant bone loss due to design limitations [47].
For intermediate follow-ups (8 to 10 years), a study by Kiechle et al. [37] on zirconia revealed a 100% survival rate. There are no studies on titanium implants with intermediate follow-ups. Zirconia implants, specifically two-pieces models seen by Brunello et al. [32], reported a success rate of approximately 96.7% at 9 years. However, zirconia’s susceptibility to micro-cracking and potential for earlier mechanical failure in one-piece designs was noted as a concern.
Studies extending beyond a decade, such as those by Ravald et al. [28], indicated that titanium implants retained high survival rates exceeding 94% in fully edentulous cases. In contrast, long-term data on zirconia implants are still limited, with no studies reporting follow-ups beyond the 10-year mark. The available data suggest that zirconia’s biocompatibility supports soft tissue integration well but may be compromised by material brittleness over extensive periods. This reflects an ongoing debate about the reliability of zirconia implants in sustaining similar durability as titanium counterparts, particularly in stress-bearing areas [47].
The design, whether one-piece or two-pieces, significantly influences outcomes. One-piece implants, common in zirconia models (e.g., Balmer et al. [31]), show ease of placement but limited reparability. Two-piece titanium implants, with a modular approach, enable better prosthetic flexibility and adaptation over long-term follow-ups, as seen in studies like Buser et al. [9] and van Velzen et al. [30]. Zirconia’s two-piece systems (Brunello et al. [32]) are emerging with improved success, yet concerns about material fractures persist. In two-piece implants, the load can be more effectively distributed across the entire implant system rather than being concentrated directly on the implant body, as is the case with one-piece implants. This feature of two-pieces implants provides greater biomechanical flexibility, allowing the abutment to absorb a significant portion of the force [48,49]. Another drawback of one-piece implants, which may impact the aesthetic advantages of zirconia implants, is the cementation process. When one-piece implants are cemented to the crown, excess cement can accumulate in the surrounding mucosa, potentially causing infections and increasing the risk of peri-implantitis [50,51,52,53].
The PINK score result leads us to conclude that the appearance of the implant is not the most important factor for the final aesthetics of the restoration but rather the properties of the implant itself, which facilitate proper osseointegration and soft tissue adaptation [6]. This conclusion contradicts the findings of previously published systematic reviews and complements those with shorter follow-up periods and highlights that zirconia, although chosen for its aesthetic potential, does not confer the long-term aesthetic benefits previously expected [44,46,54,55].
Although zirconia implants have demonstrated promising results, caution must be exercised in their use due to the limited evidence available, particularly with follow-up data beyond five years, making it difficult to confidently assess their benefits compared to titanium implants [56].
5. Conclusions
Despite the satisfactory results attained by zirconia implants, there is insufficient long-term data beyond five years to confidently conclude the benefits of this material when compared to titanium implants. However, while zirconia implants show great potential as an alternative to titanium implants, further research is essential to better understand its advantages and properties, refine surgical guidelines, and directly analyse peri-implant behaviour.
Author Contributions
The contributions of the authors are as follows: conceptualization, R.F.-A. and F.A.; methodology, F.A. and F.C.; software, F.A.; validation, F.A., T.C., F.C. and R.F.-A.; formal analysis, F.C. and R.F.-A.; investigation, F.A. and T.C.; resources, R.F.-A.; data curation, F.A.; writing—original draft preparation, F.A.; writing—review and editing, F.C. and R.F.-A.; visualization, F.A.; supervision, R.F.-A.; project administration, R.F.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Exclusion criteria per article—(1) follow-up <5 years; (2) reviews and clinical cases; (3) in vitro and animals; (4) abutments or/and crown material; (5) titanium–zirconia implants; (6) bone regeneration; (7) other science or thematic.
Table A1.
Exclusion criteria per article—(1) follow-up <5 years; (2) reviews and clinical cases; (3) in vitro and animals; (4) abutments or/and crown material; (5) titanium–zirconia implants; (6) bone regeneration; (7) other science or thematic.
| Author | Year | Title | Criteria |
|---|---|---|---|
| A. V. Lombardi, Jr.; K. R. Berend; B. E. Seng; I. C. Clarke; J. B. Adams | 2010 | Delta ceramic-on-alumina ceramic articulation in primary THA: prospective, randomized FDA-IDE study and retrieval analysis | 7 |
| M. M. Bornstein; J. G. Wittneben; U. Brägger; D. Buser | 2010 | Early loading at 21 days of non-submerged titanium implants with a chemically modified sandblasted and acid-etched surface: 3-year results of a prospective study in the posterior mandible | 1 |
| M. Todisco | 2010 | Early loading of implants in vertically augmented bone with non-resorbable membranes and deproteinised anorganic bovine bone. An uncontrolled prospective cohort study | 1 |
| C. Larsson; P. Vult von Steyern | 2010 | Five-year follow-up of implant-supported Y-TZP and ZTA fixed dental prostheses. A randomized, prospective clinical trial comparing two different material systems | 4 |
| P. O. Ostman; A. Wennerberg; T. Albrektsson | 2010 | Immediate occlusal loading of NanoTite PREVAIL implants: a prospective 1-year clinical and radiographic study | 4 |
| R. Crespi; P. Capparè; E. Gherlone | 2010 | Osteotome sinus floor elevation and simultaneous implant placement in grafted biomaterial sockets: 3 years of follow-up | 1 + 6 |
| Moschovitis A, Simon R, Seidenstücker A, Klauss V, Baylacher M, Lüscher TF, Moccetti T, Windecker S, Meier B, Hess OM | 2010 | Randomised comparison of titanium-nitride-oxide coated stents with bare metal stents: five year follow-up of the TiNOX trial | 7 |
| F. A. Quereshy; H. S. Dhaliwal; S. A. El; M. P. Horan; S. S. Dhaliwal | 2010 | Resorbable screw fixation for cortical onlay bone grafting: a pilot study with preliminary results | 7 |
| M. Veltri; M. Ferrari; P. Balleri | 2010 | Stability values of titanium dioxide-blasted dental implants in edentulous maxillas: A 3-year pilot study | 1 |
| J. Jofré; Y. Conrady; C. Carrasco | 2010 | Survival of splinted mini-implants after contamination with stainless steel | 7 |
| G. Liddelow; P. Henry | 2010 | The immediately loaded single implant-retained mandibular overdenture: a 36-month prospective study | 1 |
| M. Merli; F. Lombardini; M. Esposito | 2010 | Vertical ridge augmentation with autogenous bone grafts 3 years after loading: resorbable barriers versus titanium-reinforced barriers. A randomized controlled clinical trial | 6 |
| M. Esposito; G. Cannizarro; E. Soardi; G. Pellegrino; R. Pistilli; P. Felice | 2011 | A 3-year post-loading report of a randomised controlled trial on the rehabilitation of posterior atrophic mandibles: short implants or longer implants in vertically augmented bone? | 1 |
| B. Zerahn; L. Borgwardt; S. Ribel-Madsen; A. Borgwardt | 2011 | A prospective randomised study of periprosthetic femoral bone remodeling using four different bearings in hybrid total hip arthroplasty | 7 |
| U. Salihoglu; D. Boynuegri; D. Engin; A. N. Duman; P. Gokalp; K. Balos | 2011 | Bacterial adhesion and colonization differences between zirconium oxide and titanium alloys: an in vivo human study | 4 |
| I. J. De Kok; K. H. Chang; T. S. Lu; L. F. Cooper | 2011 | Comparison of three-implant-supported fixed dentures and two-implant-retained overdentures in the edentulous mandible: a pilot study of treatment efficacy and patient satisfaction | 1 |
| R. van Brakel; M. S. Cune; A. J. van Winkelhoff; C. de Putter; J. W. Verhoeven; W. van der Reijden | 2011 | Early bacterial colonization and soft tissue health around zirconia and titanium abutments: an in vivo study in man | 4 |
| S. M. Dogus; K. S. Kurtz; I. Watanabe; J. A. Griggs | 2011 | Effect of Engaging Abutment Position in Implant-Borne, Screw-Retained Three-Unit Fixed Cantilevered Prostheses | 4 |
| P. Magne; E. Oderich; L. L. Boff; A. C. Cardoso; U. C. Belser | 2011 | Fatigue resistance and failure mode of CAD/CAM composite resin implant abutments restored with type III composite resin and porcelain veneers | 4 |
| L. Hjalmarsson; J. I. Smedberg; M. Pettersson; T. Jemt | 2011 | Implant-level prostheses in the edentulous maxilla: A comparison with conventional abutment-level prostheses after 5 years of use | 4 |
| E. Bressan; G. Paniz; D. Lops; B. Corazza; E. Romeo; G. Favero | 2011 | Influence of abutment material on the gingival color of implant-supported all-ceramic restorations: a prospective multicenter study | 4 |
| T. Sornsuwan; A. Ellakwa; M. V. Swain | 2011 | Occlusal geometrical considerations in all-ceramic pre-molar crown failure testing | 4 |
| M. Suzuki; E. Bonfante; N. R. Silva; P. G. Coelho | 2011 | Reliability testing of indirect composites as single implant restorations | 4 |
| E. Nkenke; E. Vairaktaris; M. Spitzer; M. Kramer; M. Stamminger; L. Holbach; C. Knipfer; F. Stelzle | 2011 | Secondary reconstruction of posttraumatic enophthalmos: prefabricated implants vs. titanium mesh | 7 |
| M. Weissinger; A. Grübl; G. Pöll | 2011 | Serum-cobalt levels with metal-on-metal bearings in the cement-free total hip arthroplasty results covering two years; prospective study | 7 |
| L. den Hartog; H. J. Meijer; B. Stegenga; N. Tymstra; A. Vissink; G. M. Raghoebar | 2011 | Single implants with different neck designs in the aesthetic zone: a randomized clinical trial | 1 |
| M. Weinländer; V. Lekovic; S. Spadijer-Gostovic; B. Milicic; W. A. Wegscheider; E. Piehslinger | 2011 | Soft tissue development around abutments with a circular macro-groove in healed sites of partially edentulous posterior maxillae and mandibles: A clinical pilot study | 4 |
| M. Stilling; F. Madsen; A. Odgaard; L. Rømer; N. T. Andersen; O. Rahbek; K. Søballe | 2011 | Superior fixation of pegged trabecular metal over screw-fixed pegged porous titanium fiber mesh: a randomized clinical RSA study on cementless tibial components | 7 |
| D. Pakvis; J. Luites; G. van Hellemondt; M. Spruit | 2012 | A cementless, elastic press-fit socket with and without screws | 4 |
| B. Möller; H. Terheyden; Y. Açil; N. M. Purcz; K. Hertrampf; A. Tabakov; E. Behrens; J. Wiltfang | 2012 | A comparison of biocompatibility and osseointegration of ceramic and titanium implants: an in vivo and in vitro study | 1 |
| B. Al-Nawas; U. Brägger; H. J. Meijer; I. Naert; R. Persson; A. Perucchi; M. Quirynen; G. M. Raghoebar; T. E. Reichert; E. Romeo; H. J. Santing; M. Schimmel; S. Storelli; C. ten Bruggenkate; B. Vandekerckhove; W. Wagner; D. Wismeijer; F. Müller | 2012 | A double-blind randomized controlled trial (RCT) of Titanium-13Zirconium versus Titanium Grade IV small-diameter bone level implants in edentulous mandibles--results from a 1-year observation period | 1 |
| S. Vandeweghe; A. Ackermann; J. Bronner; A. Hattingh; A. Tschakaloff; H. De Bruyn | 2012 | A Retrospective, Multicenter Study on a Novo Wide-Body Implant for Posterior Regions | 7 |
| A. Acocella; C. Ercoli; A. Geminiani; C. Feng; M. Billi; G. Acocella; D. Giannini; R. Sacco | 2012 | Clinical evaluation of immediate loading of electroeroded screw-retained titanium fixed prostheses supported by tilted implant: a multicenter retrospective study | 4 |
| A. Örtorp; T. Jemt | 2012 | CNC-milled titanium frameworks supported by implants in the edentulous jaw: a 10-year comparative clinical study | 7 |
| H. J. Nickenig; K. A. Schlegel; M. Wichmann; S. Eitner | 2012 | Expression of interleukin 6 and tumor necrosis factor alpha in soft tissue over ceramic and metal implant materials before uncovering: a clinical pilot study | 1 |
| L. M. Martins; E. A. Bonfante; R. A. Zavanelli; A. C. Freitas Jr; N. R. F. A. Silva; L. Marotta; P. G. Coelho | 2012 | Fatigue reliability of 3 single-unit implant-abutment designs | 4 |
| C. Mangano; F. G. Mangano; J. A. Shibli; M. Ricci; V. Perrotti; S. d’Avila; A. Piattelli | 2012 | Immediate loading of mandibular overdentures supported by unsplinted direct laser metal-forming implants: results from a 1-year prospective study | 4 |
| R. A. Levine; P. Sendi; M. M. Bornstein | 2012 | Immediate restoration of nonsubmerged titanium implants with a sandblasted and acid-etched surface: five-year results of a prospective case series study using clinical and radiographic data | 7 |
| A. Pozzi; G. Sannino; A. Barlattani | 2012 | Minimally invasive treatment of the atrophic posterior maxilla: a proof-of-concept prospective study with a follow-up of between 36 and 54 months | 7 |
| A. E. Borgonovo; A. Fabbri; V. Vavassori; R. Censi; C. Maiorana | 2012 | Multiple teeth replacement with endosseous one-piece yttrium-stabilized zirconia dental implants | 1 |
| T. Kaneko; I. Masuda; N. Horie; T. Shimoyama | 2012 | New bone formation in nongrafted sinus lifting with space-maintaining management: a novel technique using a titanium bone fixation device | 7 |
| R. J. Kohal; M. Knauf; B. Larsson; H. Sahlin; F. Butz | 2012 | One-piece zirconia oral implants: one-year results from a prospective cohort study. 1. Single tooth replacement | 1 |
| L. Barbier; J. Abeloos; C. de Clercq; R. Jacobs | 2012 | Peri-implant bone changes following tooth extraction, immediate placement and loading of implants in the edentulous maxilla | 7 |
| P. Felice; R. Pistilli; M. Piattelli; E. Soardi; V. Corvino; M. Esposito | 2012 | Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a novel nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. Preliminary results from a randomised controlled trial | 7 |
| M. Sasse; S. Eschbach; M. Kern | 2012 | Randomized clinical trial on single retainer all-ceramic resin-bonded fixed partial dentures: Influence of the bonding system after up to 55 months | 4 |
| R. Oliveira; M. El Hage; J. P. Carrel; T. Lombardi; J. P. Bernard | 2012 | Rehabilitation of the edentulous posterior maxilla after sinus floor elevation using deproteinized bovine bone: a 9-year clinical study | 6 |
| R. van Brakel; G. J. Meijer; J. W. Verhoeven; J. Jansen; C. de Putter; M. S. Cune | 2012 | Soft tissue response to zirconia and titanium implant abutments: an in vivo within-subject comparison | 4 |
| T. Grandi; G. Garuti; P. Guazzi; L. Tarabini; A. Forabosco | 2012 | Survival and success rates of immediately and early loaded implants: 12-month results from a multicentric randomized clinical study | 1 |
| A. E. Büttel; H. Lüthy; P. Sendi; C. P. Marinello | 2012 | Wear of ceramic and titanium ball attachments in subjects with an implant-retained overdenture: a controlled clinical trial | 4 |
| M. Hosseini; N. Worsaae; M. Schiødt; K. Gotfredsen | 2013 | A 3-year prospective study of implant-supported, single-tooth restorations of all-ceramic and metal-ceramic materials in patients with tooth agenesis | 1 + 4 |
| K. Akca; Y. Cavusoglu; S. Uysal; M. C. Cehreli | 2013 | A prospective, open-ended, single-cohort clinical trial on early loaded Titanium-zirconia alloy implants in partially edentulous patients: up-to-24-month results | 5 |
| M. Naumann; C. Hohmann; A. Happe; F. Beuer; R. Frankenberger; R. Seemann; M. Rosentritt | 2013 | Are implants more reliable than severely compromised endodontically treated teeth as abutments for zirconia-based FPDs?: In vitro results of long-term preclinical load simulation | 3 + 4 |
| A. E. Borgonovo; V. Vavassori; R. Censi; J. L. Calvo; D. Re | 2013 | Behavior of endosseous one-piece yttrium stabilized zirconia dental implants placed in posterior areas | 1 |
| H. T. Faber; C. A. J. Dun; R. C. Nelissen; E. A. M. Mylanus; C. W. R. J. Cremers; M. K. S. Hol | 2013 | Bone-anchored hearing implant loading at 3 weeks: Stability and tolerability after 6 months | 1 |
| M. Sasse; M. Kern | 2013 | CAD/CAM single retainer zirconia-ceramic resin-bonded fixed dental prostheses: clinical outcome after 5 years | 4 + 5 |
| A. Pozzi; M. Tallarico; F. Mangani; A. Barlattani | 2013 | Different implant impression techniques for edentulous patients treated with CAD/CAM complete-arch prostheses: a randomised controlled trial reporting data at 3 year post-loading | 7 |
| A. Zembic; A. Bösch; R. E. Jung; C. H. Hämmerle; I. Sailer | 2013 | Five-year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single-implant crowns in canine and posterior regions | 4 |
| J. K. Foong; R. B. Judge; J. E. Palamara; M. V. Swain | 2013 | Fracture resistance of titanium and zirconia abutments: an in vitro study | 3 + 4 |
| R. M. de Freitas; C. Susin; R. Spin-Neto; C. Marcantonio; U. M. Wikesjö; L. A. Pereira; E. Marcantonio, Jr. | 2013 | Horizontal ridge augmentation of the atrophic anterior maxilla using rhBMP-2/ACS or autogenous bone grafts: a proof-of-concept randomized clinical trial | 7 |
| K. S. Choi; H. C. Yoon; Y. S. Cho | 2013 | Immediate provisionalization of mini-implants with friction-engaging abutments in the mandibular anterior region: A 1-year retrospective study | 4 |
| F. Mangano; S. Pozzi-Taubert; P. A. Zecca; G. Luongo; R. L. Sammons; C. Mangano | 2013 | Immediate restoration of fixed partial prostheses supported by one-piece narrow-diameter selective laser sintering implants: a 2-year prospective study in the posterior jaws of 16 patients | 7 |
| C. do Nascimento; M. S. Pita; V. Pedrazzi; R. F. de Albuquerque Junior; R. F. Ribeiro | 2013 | In vivo evaluation of Candida spp. adhesion on titanium or zirconia abutment surfaces | 4 |
| G. W. Omlor; J. P. Kretzer; J. Reinders; M. R. Streit; T. Bruckner; T. Gotterbarm; P. R. Aldinger; C. Merle | 2013 | In vivo serum titanium ion levels following modular neck total hip arthroplasty--10 year results in 67 patients | 7 |
| C. do Nascimento; C. da Rocha Aguiar; M. S. Pita; V. Pedrazzi; R. F. de Albuquerque, Jr.; R. F. Ribeiro | 2013 | Oral biofilm formation on the titanium and zirconia substrates | 1 |
| R. Pistilli; P. Felice; M. Piattelli; M. Gessaroli; E. Soardi; C. Barausse; J. Buti; V. Corvino | 2013 | Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a novel nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. One-year results from a randomised controlled trial | 7 |
| K. Fischer; T. Stenberg | 2013 | Prospective 10-year cohort study based on a randomized, controlled trial (RCT) on implant-supported full-arch maxillary prostheses. Part II: Prosthetic outcomes and maintenance | 4 |
| K. Patel; N. Mardas; N. Donos | 2013 | Radiographic and clinical outcomes of implants placed in ridge preserved sites: a 12-month post-loading follow-up | 1 |
| M. Esposito; I. Dojcinovic; L. Germon; N. Lévy; R. Curno; S. Buchini; P. Péchy; B. O. Aronsson | 2013 | Safety and efficacy of a biomimetic monolayer of permanently bound multi-phosphonic acid molecules on dental implants: 1 year post-loading results from a pilot quadruple-blinded randomised controlled trial | 7 |
| L. den Hartog; G. M. Raghoebar; J. J. Slater; K. Stellingsma; A. Vissink; H. J. Meijer | 2013 | Single-tooth implants with different neck designs: a randomized clinical trial evaluating the aesthetic outcome | 1 |
| A. Happe; V. Schulte-Mattler; S. Fickl; M. Naumann; J. E. Zöller; D. Rothamel | 2013 | Spectrophotometric assessment of peri-implant mucosa after restoration with zirconia abutments veneered with fluorescent ceramic: a controlled, retrospective clinical study | 4 |
| T. Koutouzis; G. Koutouzis; H. Gadalla; R. Neiva | 2013 | The effect of healing abutment reconnection and disconnection on soft and hard peri-implant tissues: A short-term randomized controlled clinical trial | 4 |
| G. I. Benic; G. O. Gallucci; M. Mokti; C. H. Hämmerle; H. P. Weber; R. E. Jung | 2013 | Titanium-zirconium narrow-diameter versus titanium regular-diameter implants for anterior and premolar single crowns: 1-year results of a randomized controlled clinical study | 5 |
| D. Lops; E. Bressan; M. Chiapasco; A. Rossi; E. Romeo | 2013 | Zirconia and titanium implant abutments for single-tooth implant prostheses after 5 years of function in posterior regions | 4 |
| A. Carrillo de Albornoz; F. Vignoletti; L. Ferrantino; E. Cárdenas; M. De Sanctis; M. Sanz | 2014 | A randomized trial on the aesthetic outcomes of implant-supported restorations with zirconia or titanium abutments | 4 |
| R. B. Osman; M. V. Swain; M. Atieh; S. Ma; W. Duncan | 2014 | Ceramic implants (Y-TZP): are they a viable alternative to titanium implants for the support of overdentures? A randomized clinical trial | 1 |
| N. A. Smith; I. Turkyilmaz | 2014 | Evaluation of the sealing capability of implants to titanium and zirconia abutments against Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum under different screw torque values | 4 |
| M. Rosentritt; A. Hagemann; S. Hahnel; M. Behr; V. Preis | 2014 | In vitro performance of zirconia and titanium implant/abutment systems for anterior application | 3 |
| M. Karl; T. D. Taylor | 2014 | Parameters determining micromotion at the implant-abutment interface | 4 |
| R. B. Osman; S. Ma | 2014 | Prosthodontic maintenance of overdentures on zirconia implants: 1-year results of a randomized controlled trial | 1 |
| J. F. Esquivel-Upshaw; A. E. Clark; J. J. Shuster; K. J. Anusavice | 2014 | Randomized clinical trial of implant-supported ceramic-ceramic and metal-ceramic fixed dental prostheses: preliminary results | 4 |
| R. van Brakel; G. J. Meijer; C. de Putter; J. W. Verhoeven; J. Jansen; M. S. Cune | 2014 | The association of clinical and microbiologic parameters with histologic observations in relatively healthy peri-implant conditions- a preliminary short-term in vivo study | 1 + 4 |
| T. Borges; T. Lima; A. Carvalho; C. Dourado; V. Carvalho | 2014 | The influence of customized abutments and custom metal abutments on the presence of the interproximal papilla at implants inserted in single-unit gaps: A 1-year prospective clinical study | 4 |
| W. C. Jennings; S. W. Galt; S. Shenoy; S. Wang; E. D. Ladenheim; M. H. Glickman; P. Kathuria; B. J. Browne | 2014 | The Venous Window Needle Guide, a hemodialysis cannulation device for salvage of uncannulatable arteriovenous fistulas | 7 |
| M. Borg; P. Vult von Steyern; C. Larsson | 2014 | Titanium- and zirconia-based implant-supported fixed dental prostheses. A randomized, prospective clinical pilot study | 1 |
| K. Bechara; A. M. Dottore; P. Y. Kawakami; S. A. Gehrke; P. G. Coelho; A. Piattelli; G. Iezzi; J. A. Shibli | 2015 | A histological study of non-ceramic hydroxyapatite as a bone graft substitute material in the vertical bone augmentation of the posterior mandible using an interpositional inlay technique: A split mouth evaluation | 7 |
| M. Payer; A. Heschl; M. Koller; G. Arnetzl; M. Lorenzoni; N. Jakse | 2015 | All-ceramic restoration of zirconia two-piece implants--a randomized controlled clinical trial | 1 |
| C. A. Barwacz; K. A. Brogden; C. M. Stanford; D. V. Dawson; E. N. Recker; D. Blanchette | 2015 | Comparison of pro-inflammatory cytokines and bone metabolism mediators around titanium and zirconia dental implant abutments following a minimum of 6 months of clinical function | 4 |
| M. Ferrari; M. C. Cagidiaco; F. Garcia-Godoy; C. Goracci; F. Cairo | 2015 | Effect of different prosthetic abutments on peri-implant soft tissue. A randomized controlled clinical trial | 4 |
| A. Lons; A. Arnould; T. Pommepuy; E. Drumez; J. Girard | 2015 | Excellent short-term results of hip resurfacing in a selected population of young patients | 7 |
| K. Kuhn; H. Rudolph; M. Graf; M. Moldan; S. Zhou; M. Udart; A. Böhmler; R. G. Luthardt | 2015 | Interaction of titanium, zirconia and lithium disilicate with peri-implant soft tissue: study protocol for a randomized controlled trial | 4 |
| B. Bloch; S. Brown; D. Angadi; E. Crawfurd | 2015 | Long-term follow-up of a cemented titanium stem | 7 |
| R. Cosgarea; C. Gasparik; D. Dudea; B. Culic; B. Dannewitz; A. Sculean | 2015 | Peri-implant soft tissue colour around titanium and zirconia abutments: a prospective randomized controlled clinical study | 4 |
| M. Quirynen; B. Al-Nawas; H. J. Meijer; A. Razavi; T. E. Reichert; M. Schimmel; S. Storelli; E. Romeo | 2015 | Small-diameter titanium Grade IV and titanium-zirconium implants in edentulous mandibles: three-year results from a double-blind, randomized controlled trial | 1 |
| T. Joda; U. Brägger | 2015 | Time-Efficiency Analysis Comparing Digital and Conventional Workflows for Implant Crowns: A Prospective Clinical Crossover Trial | 4 |
| S. P. Lyngstadaas; A. Verket; E. M. Pinholt; C. Mertens; H. R. Haanaes; G. Wall; M. Wallström; L. Rasmusson | 2015 | Titanium Granules for Augmentation of the Maxillary Sinus—A Multicenter Study | 7 |
| M. Ollivier; S. Parratte; A. Galland; A. Lunebourg; X. Flecher; J. N. Argenson | 2015 | Titanium-titanium modular neck for primary THA. Result of a prospective series of 170 cemented THA with a minimum follow-up of 5 years | 4 |
| A. Ioannidis; G. O. Gallucci; R. E. Jung; S. Borzangy; C. H. Hämmerle; G. I. Benic | 2015 | Titanium-zirconium narrow-diameter versus titanium regular-diameter implants for anterior and premolar single crowns: 3-year results of a randomized controlled clinical study | 5 |
| D. Spinelli; G. De Vico; R. Condò; L. Ottria; C. Arcuri | 2015 | Transcrestal guided sinus lift without grafting materials: A 36 months clinical prospective study | 7 |
| M. Ferrari; M. G. Tricarico; M. C. Cagidiaco; A. Vichi; E. F. Gherlone; F. Zarone; R. Sorrentino | 2016 | 3-Year Randomized Controlled Prospective Clinical Trial on Different CAD-CAM Implant Abutments | 4 |
| R. Zita Gomes; A. Paraud Freixas; C. H. Han; S. Bechara; I. Tawil | 2016 | Alveolar Ridge Reconstruction with Titanium Meshes and Simultaneous Implant Placement: A Retrospective, Multicenter Clinical Study | 1 + 6 |
| P. King; C. Maiorana; R. G. Luthardt; K. Sondell; J. Øland; P. Galindo-Moreno; P. Nilsson | 2016 | Clinical and Radiographic Evaluation of a Small-Diameter Dental Implant Used for the Restoration of Patients with Permanent Tooth Agenesis (Hypodontia) in the Maxillary Lateral Incisor and Mandibular Incisor Regions: A 36-Month Follow-Up | 1 |
| G. Fabbri; M. Fradeani; G. Dellificorelli; M. De Lorenzi; F. Zarone; R. Sorrentino | 2016 | Clinical evaluation of the influence of connection type and restoration height on the reliability of zirconia abutments: A retrospective study on 965 abutments with a mean 6-year follow-up | 4 |
| S. Mistry; R. Roy; B. Kundu; S. Datta; M. Kumar; A. Chanda; D. Kundu | 2016 | Clinical Outcome of Hydroxyapatite Coated, Bioactive Glass Coated, and Machined Ti6Al4V Threaded Dental Implant in Human Jaws: A Short-Term Comparative Study | 4 |
| F. Nejatidanesh; H. Moradpoor; O. Savabi | 2016 | Clinical outcomes of zirconia-based implant- and tooth-supported single crowns | 4 |
| J. H. Lee; C. B. Kong; J. J. Yang; H. J. Shim; K. H. Koo; J. Kim; C. K. Lee; B. S. Chang | 2016 | Comparison of fusion rate and clinical results between CaO-SiO(2)-P(2)O(5)-B(2)O(3) bioactive glass ceramics spacer with titanium cages in posterior lumbar interbody fusion | 7 |
| L. F. Cooper; D. Tarnow; S. Froum; J. Moriarty; I. J. De Kok | 2016 | Comparison of Marginal Bone Changes with Internal Conus and External Hexagon Design Implant Systems: A Prospective, Randomized Study | 7 |
| C. A. Barwacz; C. M. Stanford; U. A. Diehl; F. Qian; L. F. Cooper; J. Feine; M. McGuire | 2016 | Electronic assessment of peri-implant mucosal esthetics around three implant-abutment configurations: a randomized clinical trial | 4 |
| N. Baldini; C. D’Elia; M. Clementini; A. Carrillo de Albornoz; M. Sanz; M. De Sanctis | 2016 | Esthetic Outcomes of Single-Tooth Implant-Supported Restorations Using Metal-Ceramic Restorations with Zirconia or Titanium Abutments: A Randomized Controlled Clinical Study | 4 |
| R. E. Jung; P. Grohmann; I. Sailer; Y. N. Steinhart; A. Fehér; C. Hämmerle; J. R. Strub; R. Kohal | 2016 | Evaluation of a one-piece ceramic implant used for single-tooth replacement and three-unit fixed partial dentures: a prospective cohort clinical trial | 1 |
| A. Pozzi; M. Tallarico; P. K. Moy | 2016 | Four-implant overdenture fully supported by a CAD-CAM titanium bar: A single-cohort prospective 1-year preliminary study | 7 |
| P. E. de Lacerda; A. A. Pelegrine; M. L. Teixeira; V. A. Montalli; H. Rodrigues; M. H. Napimoga | 2016 | Homologous transplantation with fresh frozen bone for dental implant placement can induce HLA sensitization: a preliminary study | 7 |
| A. Kammermeier; M. Rosentritt; M. Behr; S. Schneider-Feyrer; V. Preis | 2016 | In vitro performance of one- and two-piece zirconia implant systems for anterior application | 3 |
| P. P. Karjalainen; W. Nammas; A. Ylitalo; B. de Bruyne; J. Lalmand; A. de Belder; F. Rivero-Crespo; K. Kervinen; J. K. E. Airaksinen | 2016 | Long-term clinical outcome of titanium-nitride-oxide-coated stents versus everolimus-eluting stents in acute coronary syndrome: Final report of the BASE ACS trial | 7 |
| L. Tolentino; F. Sukekava; J. Garcez-Filho; M. Tormena; L. A. Lima; M. G. Araújo | 2016 | One-year follow-up of titanium/zirconium alloy X commercially pure titanium narrow-diameter implants placed in the molar region of the mandible: a randomized controlled trial | 1 |
| D. S. Thoma; F. Brandenberg; V. Fehmer; D. L. E. Büchi; C. H. F. Hämmerle; I. Sailer | 2016 | Randomized Controlled Clinical Trial of All-Ceramic Single Tooth Implant Reconstructions Using Modified Zirconia Abutments: Radiographic and Prosthetic Results at 1 Year of Loading | 1 |
| R. C. Nelissen; C. A. den Besten; E. A. Mylanus; M. K. Hol | 2016 | Stability, survival, and tolerability of a 4.5-mm-wide bone-anchored hearing implant: 6-month data from a randomized controlled clinical trial | 1 |
| T. Joda; U. Brägger | 2016 | Time-efficiency analysis of the treatment with monolithic implant crowns in a digital workflow: a randomized controlled trial | 4 |
| E. Chong; R. J. Mobbs; M. H. Pelletier; W. R. Walsh | 2016 | Titanium/Polyetheretherketone Cages for Cervical Arthrodesis with Degenerative and Traumatic Pathologies: Early Clinical Outcomes and Fusion Rates | 7 |
| F. Martínez-Rus; M. Prieto; M. P. Salido; C. Madrigal; M. Özcan; G. Pradíes | 2017 | A Clinical Study Assessing the Influence of Anodized Titanium and Zirconium Dioxide Abutments and Peri-implant Soft Tissue Thickness on the Optical Outcome of Implant-Supported Lithium Disilicate Single Crowns | 4 |
| M. S. Gil; S. Ishikawa-Nagai; H. W. Elani; J. D. Da Silva; D. M. Kim; D. Tarnow; U. Schulze-Späte; N. Bittner | 2017 | A prospective clinical trial to assess the optical efficacy of pink neck implants and pink abutments on soft tissue esthetics | 4 |
| C. Cacaci; F. Cantner; T. Mücke; P. Randelzhofer; J. Hajtó; F. Beuer | 2017 | Clinical performance of screw-retained and cemented implant-supported zirconia single crowns: 36-month results | 1 + 4 |
| M. Esposito; E. Bressan; M. G. Grusovin; F. D’Avenia; K. Neumann; L. Sbricoli; G. Luongo | 2017 | Do repeated changes of abutments have any influence on the stability of peri-implant tissues? One-year post-loading results from a multicentre randomised controlled trial | 4 |
| S. Acham; P. Rugani; A. Truschnegg; A. Wildburger; W. A. Wegscheider; N. Jakse | 2017 | Immediate loading of four interforaminal implants supporting a locator-retained mandibular overdenture in the elderly. Results of a 3-year randomized, controlled, prospective clinical study | 4 |
| P. P. Karjalainen; W. Nammas; K. Kervinen; A. de Belder; F. Rivero-Crespo; A. Ylitalo; J. K. Airaksinen | 2017 | Impact of Calcified Target Lesions on the Outcome of Percutaneous Coronary Intervention for Acute Coronary Syndrome: Insights From the BASE ACS Trial | 7 |
| D. Lops; E. Stellini; L. Sbricoli; N. Cea; E. Romeo; E. Bressan | 2017 | Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: a multicentric prospective study | 4 |
| Y. Kumar; V. Jain; S. S. Chauhan; V. Bharate; D. Koli; M. Kumar | 2017 | Influence of different forms and materials (zirconia or titanium) of abutments in peri-implant soft-tissue healing using matrix metalloproteinase-8: A randomized pilot study | 4 |
| T. H. Lanman; J. K. Burkus; R. G. Dryer; M. F. Gornet; J. McConnell; S. D. Hodges | 2017 | Long-term clinical and radiographic outcomes of the Prestige LP artificial cervical disc replacement at 2 levels: results from a prospective randomized controlled clinical trial | 7 |
| W. Nammas; A. de Belder; M. Niemelä; J. Sia; H. Romppanen; M. Laine; P. P. Karjalainen | 2017 | Long-term clinical outcome of elderly patients with acute coronary syndrome treated with early percutaneous coronary intervention: Insights from the BASE ACS randomized controlled trial: Bioactive versus everolimus-eluting stents in elderly patients | 7 |
| T. Joda; M. Ferrari; U. Brägger | 2017 | Monolithic implant-supported lithium disilicate (LS2) crowns in a complete digital workflow: A prospective clinical trial with a 2-year follow-up | 1 |
| A. Marković; A. Đinić; J. L. Calvo Guirado; A. Tahmaseb; M. Šćepanović; B. Janjić | 2017 | Randomized clinical study of the peri-implant healing to hydrophilic and hydrophobic implant surfaces in patients receiving anticoagulants | 7 |
| F. D. Brandenberg; I. Sailer; V. Fehmer; D. L. Büchi; C. H. Hämmerle; D. S. Thoma | 2017 | Randomized controlled clinical pilot study of all-ceramic single-tooth implant reconstructions: clinical and microbiological outcomes at one year of loading | 1 |
| G. O. Alrabeah; P. Brett; J. C. Knowles; H. Petridis | 2017 | The effect of metal ions released from different dental implant-abutment couples on osteoblast function and secretion of bone resorbing mediators | 4 |
| E. Bressan; M. G. Grusovin; F. D’Avenia; K. Neumann; L. Sbricoli; G. Luongo; M. Esposito | 2017 | The influence of repeated abutment changes on peri-implant tissue stability: 3-year post-loading results from a multicentre randomised controlled trial | 4 |
| A. Trbakovic; P. Hedenqvist; T. Mellgren; C. Ley; J. Hilborn; D. Ossipov; S. Ekman; C. B. Johansson; M. Jensen-Waern; A. Thor | 2018 | A new synthetic granular calcium phosphate compound induces new bone in a sinus lift rabbit model | 3 + 7 |
| C. Y. Lee; C. A. Johnson, Jr.; J. A. Siordia; J. M. Lehoux; P. A. Knight | 2018 | Comparison of Automated Titanium Fasteners to Hand-Tied Knots in Open Aortic Valve Replacement | 7 |
| D. Bordin; L. Witek; V. P. Fardin; E. A. Bonfante; P. G. Coelho | 2018 | Fatigue Failure of Narrow Implants with Different Implant-Abutment Connection Designs | 4 |
| M. Øilo; D. Arola | 2018 | Fractographic analyses of failed one-piece zirconia implant restorations | 1 |
| T. Sampatanukul; P. Serichetaphongse; A. Pimkhaokham | 2018 | Histological evaluations and inflammatory responses of different dental implant abutment materials: A human histology pilot study | 4 |
| M. Erhan Çömlekoğlu; N. Nizam; M. D. Çömlekoğlu | 2018 | Immediate definitive individualized abutments reduce peri-implant bone loss: a randomized controlled split-mouth study on 16 patients | 4 |
| R. Davó; P. Felice; R. Pistilli; C. Barausse; C. Marti-Pages; A. Ferrer-Fuertes; D. R. Ippolito; M. Esposito | 2018 | Immediately loaded zygomatic implants vs. conventional dental implants in augmented atrophic maxillae: 1-year post-loading results from a multicentre randomised controlled trial | 7 |
| M. Esposito; R. Davó; C. Marti-Pages; A. Ferrer-Fuertes; C. Barausse; R. Pistilli; D. R. Ippolito; P. Felice | 2018 | Immediately loaded zygomatic implants vs. conventional dental implants in augmented atrophic maxillae: 4 months post-loading results from a multicentre randomised controlled trial | 7 |
| T. Linkevicius; R. Linkevicius; J. Alkimavicius; L. Linkeviciene; P. Andrijauskas; A. Puisys | 2018 | Influence of titanium base, lithium disilicate restoration and vertical soft tissue thickness on bone stability around triangular-shaped implants: A prospective clinical trial | 4 |
| U. T. Kalyoncuoglu; B. Yilmaz; S. G. Koc; Z. Evis; P. U. Arpaci; G. Kansu | 2018 | Investigation of surface structure and biocompatibility of chitosan-coated zirconia and alumina dental abutments | 4 |
| G. Gastaldi; P. Felice; V. Pistilli; C. Barausse; D. R. Ippolito; M. Esposito | 2018 | Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. 3-year results from a randomised controlled trial | 7 |
| P. Felice; C. Barausse; R. Pistilli; D. R. Ippolito; M. Esposito | 2018 | Short implants versus longer implants in vertically augmented posterior mandibles: result at 8 years after loading from a randomised controlled trial | 7 |
| A. Bösch; R. E. Jung; I. Sailer; B. Goran; C. H. Hämmerle; D. S. Thoma | 2018 | Single-Tooth Replacement Using Dental Implants Supporting All-Ceramic and Metal-Based Reconstructions: Results at 18 Months of Loading | 1 |
| C. L. Sikora; M. F. Alfaro; J. C. C. Yuan; V. A. Barao; C. Sukotjo; M. T. Mathew | 2018 | Wear and Corrosion Interactions at the Titanium/Zirconia Interface: Dental Implant Application | 4 |
| S. S. Ghazal; G. Huynh-Ba; T. Aghaloo; S. Dibart; S. Froum; R. O’Neal; D. Cochran | 2019 | A Randomized, Controlled, Multicenter Clinical Study Evaluating The Crestal Bone Level Change Of SLActive Bone Level Ø 3.3 mm Implants Compared To SLActive Bone Level Ø 4.1 mm Implants For Single-Tooth Replacement | 5 |
| P. Korovessis; V. Syrimpeis; V. Tsekouras; A. Baikousis; K. Vardakastanis; P. Fennema | 2019 | A unilateral less invasive posterolateral approach for disc debridement and titanium cage insertion supplemented by contralateral transfascial screw fixation for high-morbidity patients suffering from septic thoracolumbosacral spondylodiscitis | 7 |
| T. Wang; L. Wang; Q. Lu; Z. Fan | 2019 | Changes in the esthetic, physical, and biological properties of a titanium alloy abutment treated by anodic oxidation | 4 |
| T. N. Pansani; F. G. Basso; I. D. R. Souza; J. Hebling; C. A. de Souza Costa | 2019 | Characterization of titanium surface coated with epidermal growth factor and its effect on human gingival fibroblasts | 4 |
| M. S. Gil; S. Ishikawa-Nagai; H. W. Elani; J. D. Da Silva; D. M. Kim; D. Tarnow; U. Schulze-Späte; C. Silva; N. Bittner | 2019 | Comparison of the Color Appearance of Peri-implant Soft Tissue with Natural Gingiva Using Anodized Pink-Neck Implants and Pink Abutments: A Prospective Clinical Trial | 4 |
| S. Roffel; G. Wu; I. Nedeljkovic; M. Meyer; T. Razafiarison; S. Gibbs | 2019 | Evaluation of a novel oral mucosa in vitro implantation model for analysis of molecular interactions with dental abutment surfaces | 3 + 4 |
| R. Reis; P. Nicolau; N. Calha; A. Messias; F. Guerra | 2019 | Immediate versus early loading protocols of titanium-zirconium narrow-diameter implants for mandibular overdentures in edentulous patients: 1-year results from a randomized controlled trial | 1 |
| D. Edelhoff; J. Schweiger; O. Prandtner; M. Stimmelmayr; J. F. Güth | 2019 | Metal-free implant-supported single-tooth restorations. Part I: Abutments and cemented crowns | 4 |
| M. Esposito; C. Barausse; R. Pistilli; M. Piattelli; S. Di Simone; D. R. Ippolito; P. Felice | 2019 | Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. Five-year results from a randomised controlled trial | 7 |
| B. K. AlZarea | 2019 | Randomized controlled clinical investigation on the association between personality profiles and the impacts of two types of maxillary anterior implant-supported crown restorations on daily living and dental satisfaction | 4 |
| C. W. Cheng; C. H. Chien; C. J. Chen; P. Papaspyridakos | 2019 | Randomized Controlled Clinical Trial to Compare Posterior Implant-Supported Modified Monolithic Zirconia and Metal-Ceramic Single Crowns: One-Year Results | 1 + 4 |
| F. L. Guljé; G. M. Raghoebar; A. Vissink; H. J. A. Meijer | 2019 | Single crowns in the resorbed posterior maxilla supported by either 11-mm implants combined with sinus floor elevation or 6-mm implants:A 5-year randomised controlled trial | 6 |
| T. S. de Oliveira Silva; A. R. de Freitas; R. F. de Albuquerque; V. Pedrazzi; R. F. Ribeiro; C. do Nascimento | 2020 | A 3-year longitudinal prospective study assessing microbial profile and clinical outcomes of single-unit cement-retained implant restorations: Zirconia versus titanium abutments | 1 + 4 |
| J. H. Lee; S. K. Kim; S. S. Kang; S. J. Han; C. K. Lee; B. S. Chang | 2020 | A Long-Term Follow-up, Multicenter, Comparative Study of the Radiologic, and Clinical Results Between a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramics (BGS-7) Intervertebral Spacer and Titanium Cage in 1-Level Posterior Lumbar Interbody Fusion | 7 |
| J. G. Wittneben; J. Gavric; I. Sailer; D. Buser; D. Wismeijer | 2020 | Clinical and esthetic outcomes of two different prosthetic workflows for implant-supported all-ceramic single crowns-3 year results of a randomized multicenter clinical trail | 1 + 4 |
| G. E. Salvi; R. Moëne; B. Wallkamm; S. P. Hicklin; M. Bischof; R. Nedir; A. Mombelli; A. Sculean | 2020 | Clinical and radiographic changes at tissue level implants with either a machined or a modified transmucosal neck surface: A 3-year multicentre randomized controlled proof-of-concept study | 1 + 4 |
| N. Bittner; U. Schulze-Späte; C. Silva; J. D. Da Silva; D. M. Kim; D. Tarnow; S. Ishikawa-Nagai; M. S. Gil | 2020 | Comparison of Peri-implant Soft Tissue Color with the Use of Pink-Neck vs. Gray Implants and Abutments Based on Soft Tissue Thickness: A 6-Month Follow-up Study | 4 |
| Esquivel-Upshaw JF, Mecholsky JJ Jr, Clark AE, Jenkins R, Hsu SM, Neal D, Ren F | 2020 | Factors influencing the survival of implant-supported ceramic-ceramic prostheses: A randomized, controlled clinical trial | 4 |
| A. Azizi; F. Zamparini; A. Spinelli; C. Pirani; M. G. Gandolfi; C. Prati | 2020 | Maryland-bridge application as a suitable technique to preserve marginal bone level of not-submerged supracrestal implants | 7 |
| S. A. Barbosa-Júnior; G. K. R. Pereira; K. S. Dapieve; P. S. Machado; L. F. Valandro; C. Schuh; R. L. X. Consani; A. Bacchi | 2020 | Mechanical Fatigue Analysis of PEEK as Alternative to Zirconia for Definitive Hybrid Abutments Supporting All-Ceramic Crowns | 4 |
| M. Arts; B. Torensma; J. Wolfs | 2020 | Porous titanium cervical interbody fusion device in the treatment of degenerative cervical radiculopathy; 1-year results of a prospective controlled trial | 7 |
| S. Mühlemann; T. Lakha; R. E. Jung; C. H. F. Hämmerle; G. I. Benic | 2020 | Prosthetic outcomes and clinical performance of CAD-CAM monolithic zirconia versus porcelain-fused-to-metal implant crowns in the molar region: 1-year results of a RCT | 7 |
| Cigerim L, Kaplan V. | 2020 | The Effect of Age of Titanium Dental Implants on Implant Survival and Marginal Bone Resorption: A 5-Year Retrospective Follow-Up Study | 1 |
| Jacobs R, Gu Y, Quirynen M, De Mars G, Dekeyser C, van Steenberghe D, Vrombaut D, Shujaat S, Naert I | 2021 | A 20-year split-mouth comparative study of two screw-shaped titanium implant systems | 7 |
| S. Wolfart; A. Rittich; K. Groß; O. Hartkamp; A. von der Stück; S. Raith; S. Reich | 2021 | Cemented versus screw-retained posterior implant-supported single crowns: A 24-month randomized controlled clinical trial | 1 + 4 |
| S. P. Bienz; M. Hilbe; J. Hüsler; D. S. Thoma; C. H. F. Hämmerle; R. E. Jung | 2021 | Clinical and histological comparison of the soft tissue morphology between zirconia and titanium dental implants under healthy and experimental mucositis conditions-A randomized controlled clinical trial | 1 |
| L. Wang; T. Wang; Y. Lu; Z. Fan | 2021 | Comparing the Clinical Outcome of Peri-implant Hard and Soft Tissue Treated with Immediate Individualized CAD/CAM Healing Abutments and Conventional Healing Abutments for Single-Tooth Implants in Esthetic Areas Over 12 Months: A Randomized Clinical Trial | 4 |
| B. Yilmaz; B. Batak; R. Seghi; W. M. Johnston; L. A. Lang | 2021 | Effect of Crown Height on the Screw Stability of Titanium Screw-Retained Crowns | 4 |
| M. Toia; M. Stocchero; E. Corrà; J. P. Becktor; A. Wennerberg; D. Cecchinato | 2021 | Fixed full-arch maxillary prostheses supported by four versus six implants with a titanium CAD/CAM milled framework: 3-year multicentre RCT | 1 |
| F. Rathe; R. Junker; S. Gröger; J. Meyle; M. Schlee | 2021 | Inflammatory effects of individualized abutments bonded onto titanium base on peri-implant tissue health: A randomized controlled clinical trial | 4 |
| J. Pitta; J. Hjerppe; F. Burkhardt; V. Fehmer; P. Mojon; I. Sailer | 2021 | Mechanical stability and technical outcomes of monolithic CAD/CAM fabricated abutment-crowns supported by titanium bases: An in vitro study | 4 |
| T. Guo; K. Gulati; H. Arora; P. Han; B. Fournier; S. Ivanovski | 2021 | Race to invade: Understanding soft tissue integration at the transmucosal region of titanium dental implants | 2 |
| P. A. Ruiz Henao; L. Caneiro Queija; S. Mareque; A. Tasende Pereira; A. Liñares González; J. Blanco Carrión | 2021 | Titanium vs. ceramic single dental implants in the anterior maxilla: A 12-month randomized clinical trial | 1 |
| A. Cucchi; E. Vignudelli; D. Franceschi; E. Randellini; G. Lizio; A. Fiorino; G. Corinaldesi | 2021 | Vertical and horizontal ridge augmentation using customized CAD/CAM titanium mesh with versus without resorbable membranes. A randomized clinical trial | 6 |
| L. Stucki; A. G. Asgeirsson; R. E. Jung; I. Sailer; C. H. F. Hämmerle; D. S. Thoma | 2021 | Zirconia Restorations Cemented onto Nonoriginal Titanium Bases May Result in Increased Bleeding on Probing, Probing Depth Values, and Varying Mean Marginal Bone Levels | 4 |
| M. Hosseini; N. Worsaae; K. Gotfredsen | 2022 | A 5-year randomized controlled trial comparing zirconia-based versus metal-based implant-supported single-tooth restorations in the premolar region | 4 |
| K. Vazouras; H. Gholami; M. Margvelashvili-Malament; Y. J. Kim; M. Finkelman; H. P. Weber | 2022 | An Esthetic Evaluation of Different Abutment Materials in the Anterior Maxilla: A Randomized Controlled Clinical Trial Using a Crossover Design | 4 |
| S. T. Lamperti; K. Wolleb; C. H. F. Hämmerle; R. E. Jung; J. Hüsler; D. S. Thoma | 2022 | Cemented versus screw-retained zirconia-based single-implant restorations: 5-year results of a randomized controlled clinical trial | 4 |
| M. T. Salem; M. El-Layeh; S. A. A. El-Farag; A. S. Salem; A. Attia | 2022 | Clinical assessment of different implant-supported esthetic crown systems fabricated with semi-digital workflow: Two-year prospective study | 1 + 4 |
| A. Happe; G. S. von Glasser; J. Neugebauer; K. Strick; R. Smeets; R. Rutkowski | 2022 | Clinical performance of zirconia implant abutments luted to a titanium base–a retrospective cross-sectional study | 5 |
| K. W. Hsu; C. H. Liang; Y. C. Peng; C. C. Hsiao | 2022 | Comparison of the residual cement on custom computer-aided design and computer-aided manufacturing titanium and zirconia abutments: A preliminary cohort study | 4 |
| T. Linkevicius; J. Alkimavicius; R. Linkevicius; E. Gineviciute; L. Linkeviciene | 2022 | Effect of Ti-Base Abutment Gingival Height on Maintenance of Crestal Bone in Thick Biotype Patients: A Randomized Clinical Trial with 1-Year Follow-up | 4 |
| M. B. Knudsen; J. K. Thillemann; P. B. Jørgensen; S. S. Jakobsen; H. Daugaard; K. Søballe; M. Stilling | 2022 | Electrochemically applied hydroxyapatite on the cementless porous surface of Bi-Metric stems reduces early migration and has a lasting effect: an efficacy trial of a randomized five-year follow-up radiostereometric study | 7 |
| F. A. Spitznagel; E. A. Bonfante; F. Vollmer; P. C. Gierthmuehlen | 2022 | Failure Load of Monolithic Lithium Disilicate Implant-Supported Single Crowns Bonded to Ti-base Abutments versus to Customized Ceramic Abutments after Fatigue | 4 |
| P. Atalay; D. D. Öztaş | 2022 | Fatigue resistance and fracture strength of narrow-diameter one-piece zirconia implants with angled abutments | 3 |
| R. D. Kraus; C. Espuelas; C. H. F. Hämmerle; R. E. Jung; I. Sailer; D. S. Thoma | 2022 | Five-year randomized controlled clinical study comparing cemented and screw-retained zirconia-based implant-supported single crowns | 4 |
| C. R. Leles; M. S. de Paula; T. F. F. Curado; J. R. Silva; J. L. R. Leles; G. McKenna; M. Schimmel | 2022 | Flapped versus flapless surgery and delayed versus immediate loading for a four mini implant mandibular overdenture: A RCT on post-surgical symptoms and short-term clinical outcomes | 7 |
| I. Milinkovic; A. D. Krasavcevic; S. Jankovic; J. Sopta; Z. Aleksic | 2022 | Immunohistochemical analysis of soft tissue response to polyetheretherketone (PEEK) and titanium healing abutments on dental implants: a randomized pilot clinical study | 4 |
| R. Lang; K. A. Hiller; L. Kienböck; K. Friedl; K. H. Friedl | 2022 | Influence of autoclave sterilization on bond strength between zirconia frameworks and Ti-base abutments using different resin cements | 4 |
| M. Â. Gouveia; S. I. V. Sousa; P. Fonseca; P. T. B. S. Branco; J. M. S. Quintanilla | 2022 | Marginal Bone Loss and Pink Esthetic Evaluation of Narrow-Diameter Dental Implants for Single Crowns: 1-Year Prospective Clinical Study | 1 |
| Gahlert M, Kniha H, Laval S, Gellrich NC, Bormann KH. | 2022 | Prospective Clinical Multicenter Study Evaluating the 5-Year Performance of Zirconia Implants in Single-Tooth Gaps | 6 |
| P. Ayyadanveettil; V. Thavakkara; N. Latha; M. Pavanan; A. Saraswathy; M. S. Kuruniyan | 2022 | Randomized clinical trial of zirconia and polyetheretherketone implant abutments for single-tooth implant restorations: A 5-year evaluation | 4 |
| N. Enkling; M. Marder; S. Bayer; W. Götz; M. Stoilov; D. Kraus | 2022 | Soft tissue response to different abutment materials: A controlled and randomized human study using an experimental model | 4 |
| S. Mühlemann; S. T. Lamperti; L. Stucki; C. H. F. Hämmerle; D. S. Thoma | 2022 | Time efficiency and efficacy of a centralized computer-aided-design/computer-aided-manufacturing workflow for implant crown fabrication: A prospective controlled clinical study | 4 |
| F. Zamparini; A. Spinelli; A. Buonavoglia; M. G. Gandolfi; C. Prati | 2023 | 10-year Historical Prospective Cohort Study of Calcium Phosphate–Blasted Acid-Etched Titanium Implants Placed in Different Ridges | 4 |
| V. L. Humm; I. Sailer; D. S. Thoma; C. H. F. Hämmerle; R. E. Jung; A. Zembic | 2023 | 13-year follow-up of a randomized controlled study on zirconia and titanium abutments | 4 |
| M. Strasding; S. P. Hicklin; A. Todorovic; V. Fehmer; P. Mojon; I. Sailer | 2023 | A multicenter randomized controlled clinical pilot study of buccally micro-veneered lithium-disilicate and zirconia crowns supported by titanium base abutments: 1-year outcomes | 4 |
| V. Thakare; S. Chaware; V. Kakatkar; A. Darekar | 2023 | An insight performance of zirconia implant abutment: A systematic review and meta-analysis of randomized controlled clinical trial | 4 |
| M. R. Norton | 2023 | Biologic and Mechanical Stability of Screw-Retained Layered Zirconia Crowns Bonded to CAD/CAM Titanium Abutments Using Angulated Screw Access: A Prospective Closed Cohort Study | 4 |
| S. R. Sherigar; J. S. Feine; L. F. Cooper; C. M. Stanford; C. A. Barwacz; M. McGuire; S. Abi Nader; R. F. de Souza | 2023 | Can patients detect peri-implant mucosal inflammation? Results from a multicentre randomized trial | 4 |
| V. Valantijiene; A. Mazeikiene; J. Alkimavicius; L. Linkeviciene; E. Alkimaviciene; T. Linkevicius | 2023 | Clinical and immunological evaluation of peri-implant tissues around ultra-polished and conventionally-polished zirconia abutments. A 1-year follow-up randomized clinical trial | 1 + 4 |
| Y. Zhang; D. Wei; J. Tian; Y. Zhao; Y. Lin; P. Di | 2023 | Clinical evaluation and quantitative occlusal change analysis of posterior implant-supported all-ceramic crowns: A 3-year randomized controlled clinical trial | 1 + 4 |
| A. Di Fiore; S. Granata; C. Monaco; E. Stellini; B. Yilmaz | 2023 | Clinical performance of posterior monolithic zirconia implant-supported fixed dental prostheses with angulated screw channels: A 3-year prospective cohort study | 1 |
| T. C. Bittencourt; N. M. Souza Picorelli Assis; C. G. Ribeiro; C. F. Ferreira; B. S. Sotto-Maior | 2023 | Evaluation of the peri-implant tissues in the esthetic zone with prefabricated titanium or zirconia abutments: A randomized controlled clinical trial with a minimum follow-up of 7 years | 4 |
| L. Ferrantino; A. Carrillo de Albornoz; M. Sanz | 2023 | Five-year outcomes of a randomized controlled clinical trial comparing single-tooth implant-supported restoration with either zirconia or titanium abutments | 4 |
| R. Takano; J. Honda; T. Kobayashi; K. Kubochi; H. Takata; F. Komine | 2023 | Fracture strength of implant-supported hybrid abutment crowns in premolar region fabricated using different restorative CAD/CAM materials | 4 |
| M. Pisano; D. Melodia; M. Tallarico; A. M. I. Lumbau; E. Baldoni; G. Spano; A. Demartis; B. Fornaca; S. M. Meloni | 2023 | FULLY DIGITAL WORKFLOW FOR IMMEDIATE LOADING OF SCREW-RETAINED TITANIUM-RESIN PROSTHESES ON MORSE CONE TISSUE-LEVEL CONNECTORS: 1-YEAR POST-LOADING RESULTS OF A CASE SERIES | 3 + 4 |
| W. Derksen; T. Joda; J. Chantler; V. Fehmer; G. O. Gallucci; P. C. Gierthmuehlen; A. Ioannidis; D. Karasan; A. Lanis; K. Pala; B. E. Pjetursson; M. Roccuzzo; I. Sailer; F. J. Strauss; T. C. Sun; S. Wolfart; N. U. Zitzmann | 2023 | Group 2 ITI Consensus Report: Technological developments in implant prosthetics | 2 |
| B. Al-Nawas; F. Lambert; S. W. M. Andersen; M. M. Bornstein; M. Gahlert; A. Jokstad; J. Jung; Y. D. Kwon; I. Laleman; G. Oteri; S. Roehling; E. Schiegnitz; Y. Takeda; H. Terheyden | 2023 | Group 3 ITI Consensus Report: Materials and antiresorptive drug-associated outcomes in implant dentistry | 2 |
| G. B. Menchini-Fabris; S. Cosola; P. Toti; M. Hwan Hwang; R. Crespi; U. Covani | 2023 | Immediate Implant and Customized Healing Abutment for a Periodontally Compromised Socket: 1-Year Follow-Up Retrospective Evaluation | 4 |
| T. F. F. Curado; J. R. Silva; L. N. Nascimento; J. L. R. Leles; G. McKenna; M. Schimmel; C. R. Leles | 2023 | Implant survival/success and peri-implant outcomes of titanium-zirconium mini implants for mandibular overdentures: Results from a 1-year randomized clinical trial | 1 + 3 |
| A. Pozzi; L. Arcuri; G. Fabbri; G. Singer; J. Londono | 2023 | Long-term survival and success of zirconia screw-retained implant-supported prostheses for up to 12 years: A retrospective multicenter study | 4 |
| J. Ravi; S. Duraisamy; K. Rajaram; R. Kannan; E. Arumugam | 2023 | Survival rate and stability of surface-treated and non-surface-treated orthodontic mini-implants: a randomized clinical trial | 7 |
| W. Derksen; D. Wismeijer | 2023 | Three-Year Follow-up of a Randomized Clinical Trial on Screw-Retained Monolithic Zirconia Restorations on Ti-Base Abutments Based on Digital or Conventional Impression Techniques | 4 |
References
- Freitag, L.; Spinell, T.; Kröger, A.; Würfl, G.; Lauseker, M.; Hickel, R.; Kebschull, M. Dental implant material related changes in molecular signatures in peri-implantitis—A systematic review and integrative analysis of omics in-vitro studies. Dent. Mater. 2023, 39, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wachi, T.; Shuto, T.; Shinohara, Y.; Matono, Y.; Makihira, S. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology 2015, 327, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Matsuya, S.; Udoh, K. Effects of fluoride and dissolved oxygen concentrations on the corrosion behavior of pure titanium and titanium alloys. Dent. Mater. J. 2002, 21, 83–92. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Lu, Q.; Fan, Z. Changes in the esthetic, physical, and biological properties of a titanium alloy abutment treated by anodic oxidation. J. Prosthet. Dent. 2019, 121, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Oztug, N.A.K.; Han, P.; Ivanovski, S.; Gulati, K. Untwining the topography-chemistry interdependence to optimize the bioactivity of nano-engineered titanium implants. Appl. Surf. Sci. 2021, 570, 151083. [Google Scholar] [CrossRef]
- Koller, M.; Steyer, E.; Theisen, K.; Stagnell, S.; Jakse, N.; Payer, M. Two-piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clin. Oral Implant. Res. 2020, 31, 388–396. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V.; Atieh, M.; Ma, S.; Duncan, W. Ceramic implants (Y-TZP): Are they a viable alternative to titanium implants for the support of overdentures? A randomized clinical trial. Clin. Oral Implant. Res. 2014, 25, 1366–1377. [Google Scholar] [CrossRef]
- Ruiz Henao, P.A.; Caneiro Queija, L.; Mareque, S.; Tasende Pereira, A.; Liñares González, A.; Blanco Carrión, J. Titanium vs ceramic single dental implants in the anterior maxilla: A 12-month randomized clinical trial. Clin. Oral Implant. Res. 2021, 32, 951–961. [Google Scholar] [CrossRef]
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Noronha Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Stolzer, C.; Müller, M.; Gosau, M.; Henningsen, A.; Fuest, S.; Aavani, F.; Smeets, R. Do Titanium Dioxide Particles Stimulate Macrophages to Release Proinflammatory Cytokines and Increase the Risk for Peri-implantitis? J. Oral Maxillofac. Surg. 2023, 81, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.G.; Nalli, G.; Verdú, S.; Paparella, M.L.; Cabrini, R.L. Exfoliative cytology and titanium dental implants: A pilot study. J. Periodontol. 2013, 84, 78–83. [Google Scholar] [CrossRef]
- Chen, L.; Tong, Z.; Luo, H.; Qu, Y.; Gu, X.; Si, M. Titanium particles in peri-implantitis: Distribution, pathogenesis and prospects. Int. J. Oral Sci. 2023, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Vallés, G.; González-Melendi, P.; González-Carrasco, J.L.; Saldaña, L.; Sánchez-Sabaté, E.; Munuera, L.; Vilaboa, N. Differential inflammatory macrophage response to rutile and titanium particles. Biomaterials 2006, 27, 5199–5211. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Roehling, S.; Gahlert, M.; Bacevic, M.; Woelfler, H.; Laleman, I. Clinical and radiographic outcomes of zirconia dental implants-A systematic review and meta-analysis. Clin. Oral Implant. Res. 2023, 34 (Suppl. S26), 112–124. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef]
- Kavashima, L.; Gerlin Neto, V.; Bastos, N.; Fortulan, C.; Borges, A.; Pintão, C.; Foschini, C. Measuring the Static and Dynamic Elastic Modulus in Y-TZP Ceramic Applied to Dentistry. J. Test. Eval. 2020, 48, 20180579. [Google Scholar] [CrossRef]
- Leib, E.W.; Vainio, U.; Pasquarelli, R.M.; Kus, J.; Czaschke, C.; Walter, N.; Janssen, R.; Müller, M.; Schreyer, A.; Weller, H.; et al. Synthesis and thermal stability of zirconia and yttria-stabilized zirconia microspheres. J. Colloid. Interface Sci. 2015, 448, 582–592. [Google Scholar]
- Kohal, R.J.; Finke, H.C.; Klaus, G. Stability of prototype two-piece zirconia and titanium implants after artificial aging: An in vitro pilot study. Clin. Implant. Dent. Relat. Res. 2009, 11, 323–329. [Google Scholar] [CrossRef]
- Gahlert, M.; Burtscher, D.; Grunert, I.; Kniha, H.; Steinhauser, E. Failure analysis of fractured dental zirconia implants. Clin. Oral Implant. Res. 2012, 23, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; Ma, S.; Duncan, W.; De Silva, R.K.; Siddiqi, A.; Swain, M.V. Fractured zirconia implants and related implant designs: Scanning electron microscopy analysis. Clin. Oral Implant. Res. 2013, 24, 592–597. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; El-dridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tawse-Smith, A.; Brown, S.D.K.; Duncan, W. Immediately restored single implants in the aesthetic zone of the max-illa using a novel design: 5-year results from a prospective single-arm clinical trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 344–351. [Google Scholar] [CrossRef]
- Cochran, D.L.; Jackson, J.M.; Jones, A.A.; Jones, J.D.; Kaiser, D.A.; Taylor, T.D.; Weber, H.P.; Higginbottom, F.L.; Richardson, J.R.; Oates, T. A 5-year prospective multicenter clinical trial of non-submerged dental implants with a titanium plas-ma-sprayed surface in 200 patients. J. Periodontol. 2011, 82, 990–999. [Google Scholar] [CrossRef]
- Ravald, N.; Dahlgren, S.; Teiwik, A.; Gröndahl, K. Long-term evaluation of Astra Tech and Brånemark implants in patients treated with full-arch bridges. Results after 12–15 years. Clin. Oral Implant. Res. 2013, 24, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Al-Nawas, B.; Storelli, S.; Quirynen, M.; Hicklin, S.; Castro-Laza, J.; Bassetti, R.; Schimmel, M. Small-diameter titanium grade IV and titanium-zirconium implants in edentulous mandibles: Five-year results from a double-blind, ran-domized controlled trial. BMC Oral Health 2015, 15, 123. [Google Scholar] [CrossRef]
- van Velzen, F.J.; Ofec, R.; Schulten, E.A.; Ten Bruggenkate, C.M. 10-year survival rate and the incidence of peri-implant dis-ease of 374 titanium dental implants with a SLA surface: A prospective cohort study in 177 fully and partially edentulous patients. Clin. Oral Implant. Res. 2015, 26, 1121–1128. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Kohal, R.J.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Rauch, N.; Becker, K.; Hakimi, A.R.; Schwarz, F.; Becker, J. Two-piece zirconia implants in the posterior man-dible and maxilla: A cohort study with a follow-up period of 9 years. Clin. Oral Implant. Res. 2022, 33, 1233–1244. [Google Scholar] [CrossRef]
- Grassi, F.R.; Capogreco, M.; Consonni, D.; Bilardi, G.; Buti, J.; Kalemaj, Z. Immediate occlusal loading of one-piece zirconia implants: Five-year radiographic and clinical evaluation. Int. J. Oral Maxillofac. Implant. 2015, 30, 671–680. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: Six-year results of a prospective cohort study. Clin. Oral Implant. Res. 2021, 32, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Spies, B.C.; Stampf, S.; Kohal, R.J. Evaluation of Zirconia-Based All-Ceramic Single Crowns and Fixed Dental Prosthesis on Zirconia Implants: 5-Year Results of a Prospective Cohort Study. Clin. Implant. Dent. Relat. Res. 2015, 17, 1014–1028. [Google Scholar] [CrossRef]
- Lorenz, J.; Giulini, N.; Hölscher, W.; Schwiertz, A.; Schwarz, F.; Sader, R. Prospective controlled clinical study investigating long-term clinical parameters, patient satisfaction, and microbial contamination of zirconia implants. Clin. Implant. Dent. Relat. Res. 2019, 21, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, S.; Liebermann, A.; Mast, G.; Heitzer, M.; Möhlhenrich, S.C.; Hölzle, F.; Kniha, H.; Kniha, K. Evaluation of one-piece zirconia dental implants: An 8-year follow-up study. Clin. Oral Investig. 2023, 27, 3415–3421. [Google Scholar] [CrossRef]
- Kohal, R.J.; Burkhardt, F.; Chevalier, J.; Patzelt, S.B.M.; Butz, F. One-Piece Zirconia Oral Implants for Single Tooth Replace-ment: Five-Year Results from a Prospective Cohort Study. J. Funct. Biomater. 2023, 14, 116. [Google Scholar] [CrossRef]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar]
- Albrektsson, T.; Donos, N. Implant survival and complications. The Third EAO consensus conference 2012. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 63–65. [Google Scholar] [CrossRef]
- Kohal, R.J.; Knauf, M.; Larsson, B.; Sahlin, H.; Butz, F. One-piece zirconia oral implants: One-year results from a prospective cohort study. 1. Single tooth replacement. J. Clin. Periodontol. 2012, 39, 590–597. [Google Scholar] [CrossRef]
- Elnayef, B.; Lazaro, A.; Suárez-López del Amo, F.; Galindo-Moreno, P.; Wang, H.L.; Gargallo-Albiol, J.; Hernandez-Alfaro, F. Zirconia Implants as an Alternative to Titanium: A Systematic Review and Meta-Analysis. Int. J. Oral. Maxillofac. Implant. 2017, 32, e125–e134. [Google Scholar] [CrossRef] [PubMed]
- Haimov, E.; Sarikov, R.; Haimov, H.; Juodzbalys, G. Differences in Titanium, Titanium-Zirconium, Zirconia Implants Treatment Outcomes: A Systematic Literature Review and Meta-Analysis. J. Oral. Maxillofac. Res. 2023, 14, e1. [Google Scholar] [CrossRef]
- Padhye, N.M.; Calciolari, E.; Zuercher, A.N.; Tagliaferri, S.; Donos, N. Survival and success of zirconia compared with titanium implants: A systematic review and meta-analysis. Clin. Oral. Investig. 2023, 27, 6279–6290. [Google Scholar] [CrossRef] [PubMed]
- da Hora Sales, P.H.; Barros, A.W.P.; de Oliveira-Neto, O.B.; de Lima, F.J.C.; Carvalho, A.D.A.T.; Leao, J.C. Do zirconia dental implants present better clinical results than titanium dental implants? A systematic review and meta-analysis. J. Stomatol. Oral. Maxillofac. Surg. 2023, 124, 101324. [Google Scholar]
- Morena, D.; Leitão-Almeida, B.; Pereira, M.; Resende, R.; Fernandes, J.C.H.; Fernandes, G.V.O.; Borges, T. Comparative Clinical Behavior of Zirconia versus Titanium Dental Implants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 4488. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Bethke, A.; Pieralli, S.; Kohal, R.J.; Burkhardt, F.; von Stein-Lausnitz, M.; Vach, K.; Spies, B.C. Fracture Resistance of Zirconia Oral Implants In Vitro: A Systematic Review and Meta-Analysis. Materials 2020, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Monzavi, M.; Li, M.; Čokić, S.; Manesh, A.; Nowzari, H.; Vleugels, J.; Van Meerbeek, B. Fracture analysis of one/two-piece clinically failed zirconia dental implants. Dent. Mater. 2022, 38, 1633–1647. [Google Scholar] [CrossRef]
- Dini, C.; Borges, G.A.; Costa, R.C.; Magno, M.B.; Maia, L.C.; Barão, V.A.R. Peri-implant and esthetic outcomes of cemented and screw-retained crowns using zirconia abutments in single implant-supported restorations-A systematic review and meta-analysis. Clin. Oral Implant. Res. 2021, 32, 1143–1158. [Google Scholar] [CrossRef]
- Korsch, M.; Robra, B.P.; Walther, W. Cement-associated signs of inflammation: Retrospective analysis of the effect of excess cement on peri-implant tissue. Int. J. Prosthodont. 2015, 28, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M.; Robra, B.P.; Walther, W. Predictors of excess cement and tissue response to fixed implant-supported dentures after cementation. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S1), e45–e53. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G., Jr. The positive relationship between excess cement and peri-implant disease: A prospective clinical endoscopic study. J. Periodontol. 2009, 80, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 135–153. [Google Scholar] [CrossRef]
- Comisso, I.; Arias-Herrera, S.; Gupta, S. Zirconium dioxide implants as an alternative to titanium: A systematic review. J. Clin. Exp. Dent. 2021, 13, e511–e519. [Google Scholar] [CrossRef]
- Morton, D.; Gallucci, G.; Lin, W.S.; Pjetursson, B.; Polido, W.; Roehling, S.; Sailer, I.; Aghaloo, T.; Albera, H.; Bohner, L.; et al. Group 2 ITI Consensus Report: Prosthodontics and implant dentistry. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 215–223. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).







