Abstract
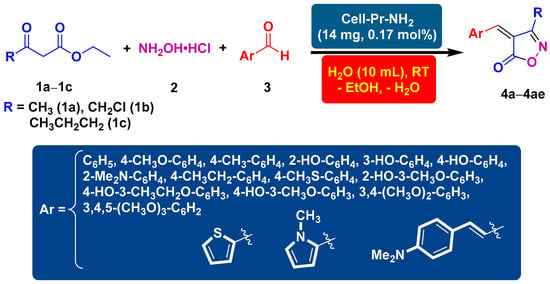
In this contribution, propylamine-functionalized cellulose (Cell-Pr-NH2) was employed as the catalyst in the three-component reaction between hydroxylamine hydrochloride and various types of aryl/heteroaryl aldehydes, ethyl acetoacetate/ethyl 4-chloroacetoacetate, or ethyl 3-oxohexanoate. The result of these experiments was the formation of 3,4-disubstituted isoxazol-5(4H)-one heterocycles. The desired five-membered heterocyclic compounds were obtained in good to high yields at room temperature. The investigation of different solvents led us to the conclusion that water is the best solvent to perform the current one-pot, three-component reactions. Attempts to find the optimal catalyst loading clearly showed that 14 mg of cell-Pr-NH2 seems to be sufficient to carry out the reactions. This method has highlighted some principles of green chemistry including less waste generation, atom economy, use of water as an environmentally friendly solvent, and energy saving. Purification without chromatographic methods, mild reaction conditions, simple work-up, low-cost reaction medium, saving time, and obtainable precursors are other notable features of this one-pot fashion.
1. Introduction
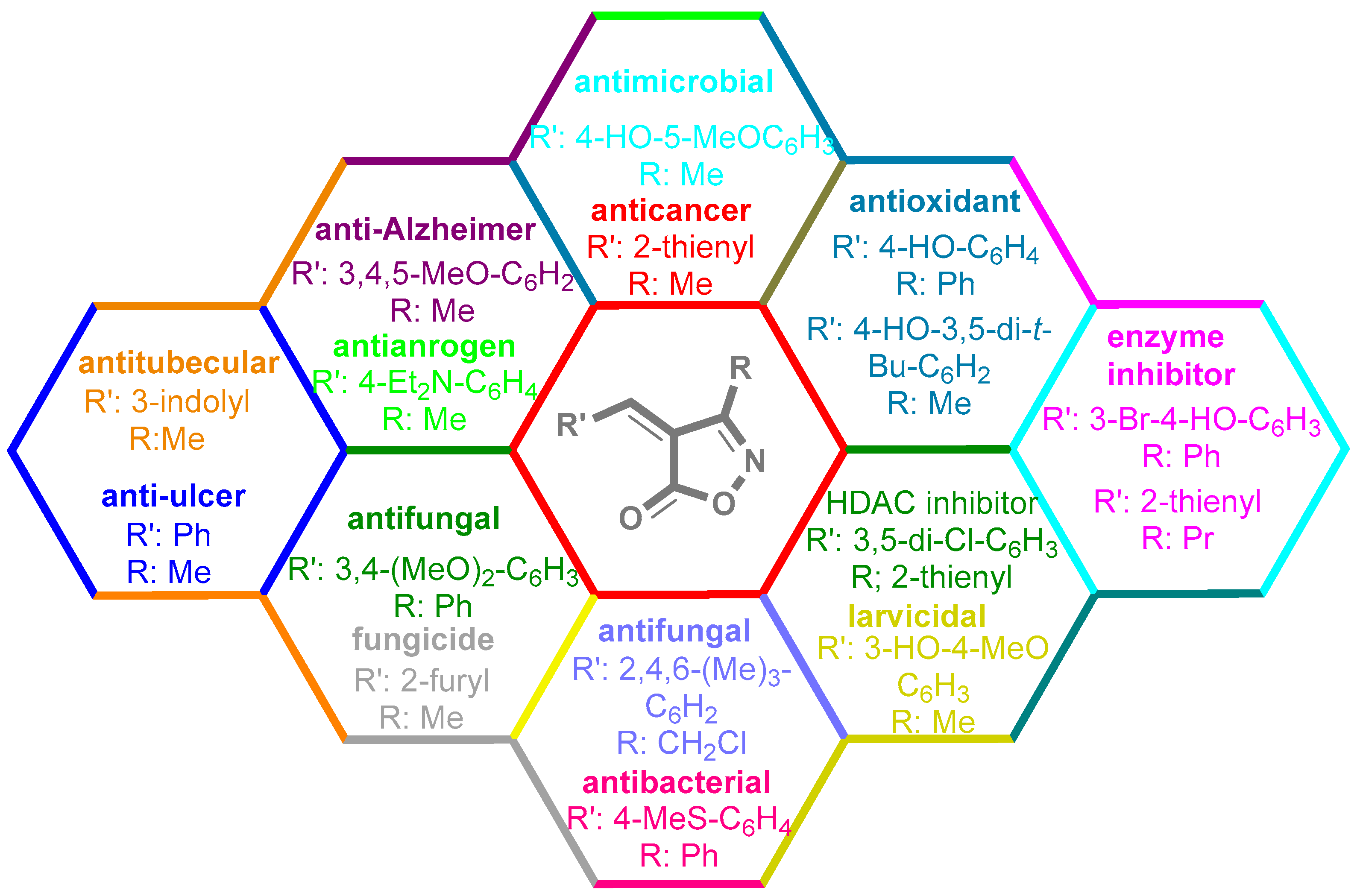
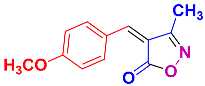
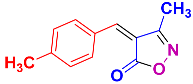
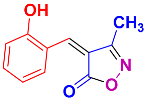
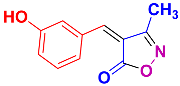
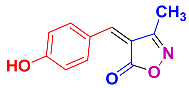
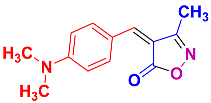
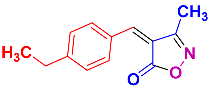
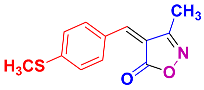
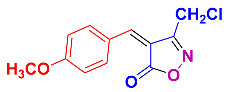
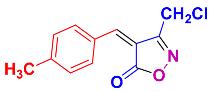
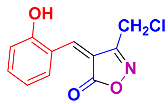
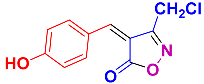
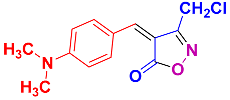
Isoxazoles, as one of the most important five-membered N,O-containing heterocyclic compounds, displays numerous pharmacological activities including anti-inflammatory, anti-obesity, antibacterial, anticonvulsant, antirheumatic, antifungal, antitumor, anti-mycobacterial, and antiviral [1,2,3,4]. Among the heterocyclic rings available in drugs, isoxazole ranks 33rd [5], and among the drugs available in the market that have this attractive heterocyclic ring we can mention oxacillin, cloxacillin, sulfisoxazole, sulfamethoxazole, isocarboxazid, leflunomide, valdecoxib, and dicloxacillin [6]. The arylideneisoxazol-5(4H)-one is present in a vast variety of bioactive compounds, agrochemicals, and nonlinear optical (NLO) materials [7]. Additionally, these heterocycles act as promising drug candidates for anti-ulcer [8], anti-obesity [9], antioxidant [10,11], anti-Alzheimer [12], antimicrobial [13,14], anticancer [15], antifungal [16], antitubercular [16], enzyme inhibitor [17], larvicidal [18], antibacterial [19], and fungicide [20] applications (Figure 1). Furthermore, arylidenesoxazol-5-ones have been used as flexible building blocks for the synthesis of various heterocycles [21,22,23,24], alkynes [25], β-amido-N-allylated products [26], γ-functionalized ketones [27], pyrrole-2-carboxylic acids [28], and other functionalized molecules [29,30]. Since arylideneisoxazol-5(4H)-one derivatives have various applications, accordingly, a variety of methods such as condensation of benzaldoximes with 1,3-dicarbonyls, cyclization of oxoesters with hydroxylamine under basic or acidic conditions, cyclization of O-propioloyl oximes, two-step reaction of 1,3-dicarbonyls with hydroxylamine followed by Knoevenagel condensation with various aldehydes, 1,3-dipolar cycloaddition reactions, and most importantly three-component reactions have been reported for their synthesis. In each of these methods, various reagents and catalysts have been used [29,30,31]. Developing efficient and green procedures in synthesizing these heterocycles remains attractive to organic and medicinal chemists. In recent years, numerous catalysts have been reported for their synthesis, some of which include lipase [32], Stiglich’s base [33], sodium acetate in EtOH [34], sodium malonate [35], guanidine hydrochloride [36], sodium acetate in EtOH under visible light [37], citrazinic acid [38], 2,2′-bipyridine]-1,1′-diium perchlorate [39], eosin-Y using visible light [40], acidic ionic liquid [41], Na2S2O3 [42], hydantoin potassium salt [43], NHC-precursor [44], malic acid [45], triphenylphosphine (TPP) [46], sulfamic acid [47], L-valine [48], sodium lauryl sulfate (SLS) [49], glutamic acid [50], succinic acid [51], eucalyptol [52], urea [53], vitamin B1 [54], nano-SiO2-H2SO4 [55], deep eutectic solvents (DESs) [56,57], nano-ZnO [58], nano-MgO [59], nano-SiO2 [60], potassium bromide (KBr) under microwave irradiation [61], gluconic acid aqueous solution (50 wt % GAAS) [62], NaCl [63], Fe3O4@SiPr@GDL MNPs [64], and magnetic DES [65].
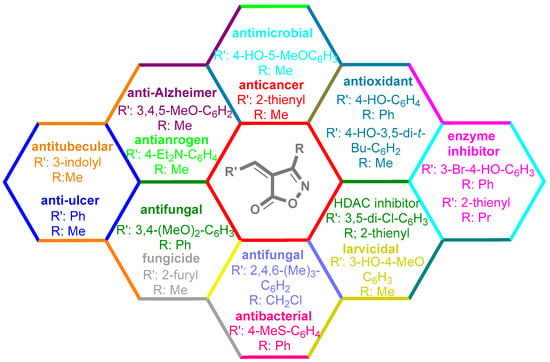
Figure 1.
Representative of some synthetically bioactive heterocycles with isoxazol-5(4H)-one skeleton.
On the other hand, multicomponent reactions (MCRs) are among the most extensive and diverse chemical transformations in organic synthesis and medicinal chemistry, enabling the rapid synthesis of various heterocyclic compounds from at least three starting materials [66]. The majority of the atoms from starting materials are incorporated into final products [67]. Due to the economic cost issue, high efficiency, mild conditions, and atom, step, and pot economy, as well as following principles of green chemistry, MCRs can be considered as significant, environmentally friendly, cost-effective, and the most successful processes in organic synthesis [68,69].
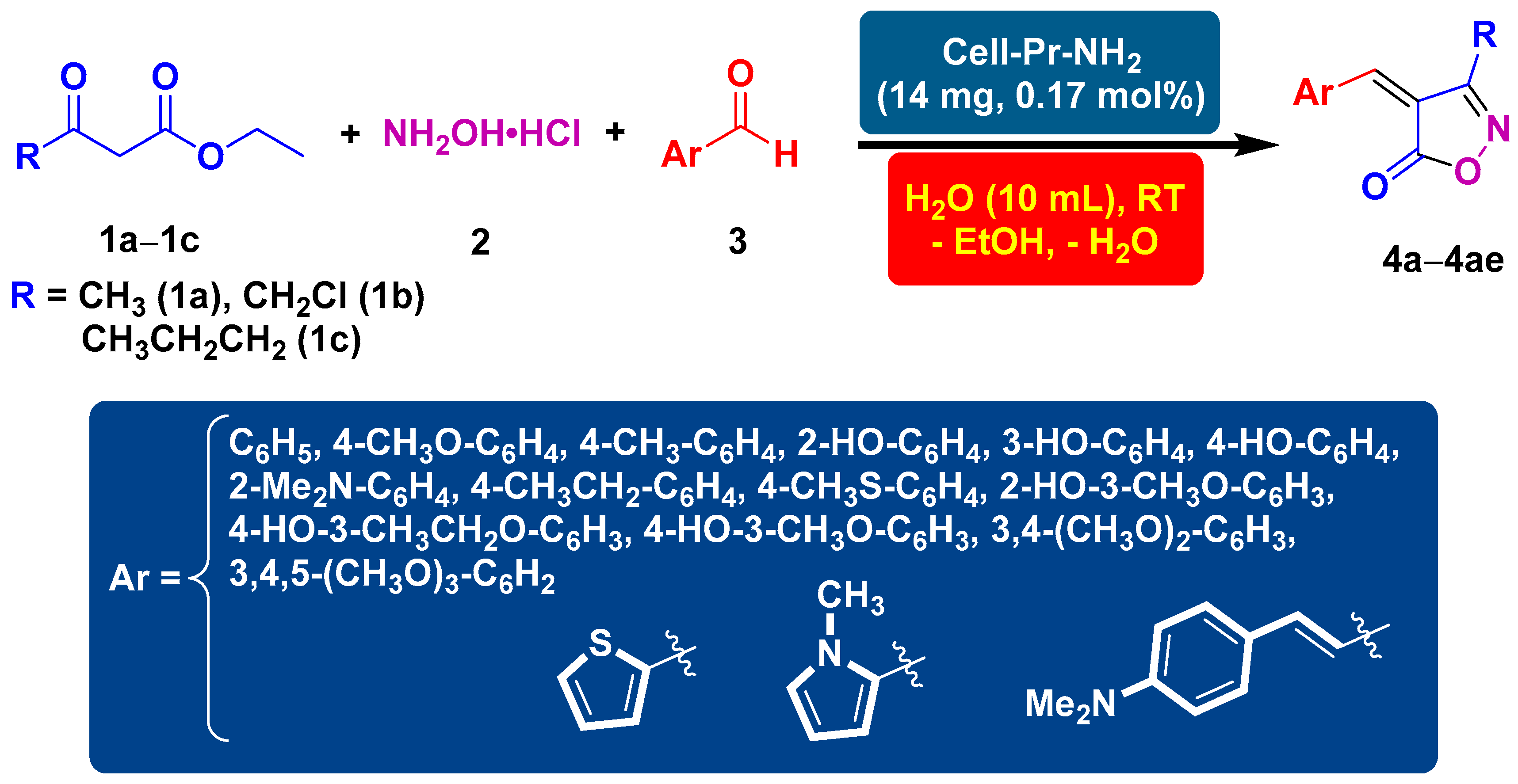
Cellulose-derived materials are used in numerous fields such as biology, medicine, tissue engineering, ultraviolet (UV) protection, and artificial blood vessels due to wide availability, biodegradability, biocompatibility, and inexpensiveness [70,71]. Nano-cellulose-grafted amines are also used in catalytic organic transformations [72,73]. Based on the considerations above, finding a sustainable and environmentally friendly catalyst for the synthesis of heterocycles is desirable for synthetic chemistry scholars, not only from the synthetic point of view but also for aligning with the principles of green chemistry. For this purpose, we tried to utilize a useful and efficient catalyst for the synthesis of isoxazol-5(4H)-one derivatives (Scheme 1).
Scheme 1.
Synthesis of isoxazol-5(4H)-ones (4a–4ae) using cell-Pr-NH2 (C) as the catalyst.
2. Materials and Methods
2.1. General
The aldehydes, hydroxylamine hydrochloride, β-keto esters, and solvents were purchased from commercial sources and were used without further purification. Thin layer chromatography (TLC) analysis was performed on a silica gel plate and thin layer spots were observed under UV lamp. Melting points were measured using the OIptimelt MPA100 melting-point apparatus. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance or Varian 300, 400, or 500 MHz spectrometer in DMSO-d6 or CDCl3.
2.2. General Procedure for the Synthesis of arylidenemethyleneisoxazole-5(4H)-ones (4a–4ae)
The mixture of β-keto esters (1a–1c, 1 mmol), hydroxylamine hydrochloride (2, 1 mmol), aldehyde derivatives (3, 1 mmol), and catalytic amount of cell-Pr-NH2 (C, 14 mg, 017 mol%) in water (10 mL) were stirred at room temperature. After the completion of the reaction, monitored through TLC analysis, the formed precipitates were filtered off, washed with ethanol (3 × 10 mL), and air-dried to provide the heterocyclic products (4a–4ae).
2.2.1. 4-(3-Hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4e) (Figures S1 and S2)
1H NMR (300 MHz, DMSO-d6): δ 2.28 (s, 3H, CH3), 7.08 (d, 1H, J = 8.0 Hz, Ar-H), 7.39 (t, 1H, J = 8.0 Hz, Ar-H), 7.79 (d, 1H, J = 7.6 Hz, Ar-H), 7.85 (s, 1H, H-vinyl), 7.95 (s, 1H, Ar-H), 9.96 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6): δ 11.7 (H-vinyl), 118.9, 119.9, 121.8, 125.8, 130.2, 134.1, 152.3, 157.8, 162.6 (C=N), 168.2 (C=O).
2.2.2. 3-Methyl-4-(4-(methylthio)benzylidene)isoxazol-5(4H)-one (4i) (Figures S7 and S8)
1H NMR (500 MHz, CDCl3): δ 2.30 (s, 3H, CH3), 2.55 (s, 3H, SCH3), 7.30 (d, J = 8.6 Hz, 2H, Ar-H), 7.33 (s, 1H, H-vinyl), 8.32 (d, J = 8.6 Hz, 2H, Ar-H); 13C NMR (CDCl3, 125 MHz): δ 11.6 (CH3), 14.5 (SCH3), 125.1, 125.3, 128.8, 134.2, 148.6, 148.9, 161.1 (C=N), 167.9 (C=O).
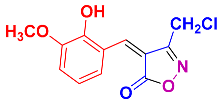
2.2.3. 4-(2-Hydroxy-3-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (4j) (Figures S9 and S10)
1H NMR (300 MHz, DMSO-d6): δ 2.24 (s, 3H, CH3), 3.86 (s, 3H, OCH3), 6.89 (t, J = 8.1 Hz, 1H, Ar-H), 7.26 (dd, J = 1.2, 8.1 Hz, 1H, Ar-H), 8.11 (s, 1H, H-vinyl), 8.32 (dd, J = 1.2, 8.4 Hz, Ar-H), 10.34 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6): δ 12.5 (CH3), 58.4 (OCH3), 116.8, 118.6, 119.8, 139.3, 145.0, 147.6, 149.5, 152.9, 162.2 (C=N, 176.1 (C=O)).
2.2.4. 4-(3-Ethoxy-4-hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4k) (Figures S11 and S12)
1H NMR (300 MHz, DMSO-d6): δ 1.41 (t, J = 6.9 Hz, 3H, CH3CH2), 2.27 (s, 3H, CH3), 4.14 (q, J = 6.9 Hz, 2H, CH2O), 6.99 (d, J = 8.4 Hz, 1H, Ar-H), 7.80 (s, 1H, H-vinyl), 7.90 (dd, J = 2.1, 8.4 Hz, 1H, Ar-H), 8.54 (d, J = 2.1 Hz, 1H, Ar-H), 10.72 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6): δ 11.8 (CH3), 15.1 (CH3CH2), 64.3 (CH2O), 114.1, 116.3, 118.1, 125.5, 132.0, 147.1, 152.4, 154.5, 162.8 (C=N), 169.5 (C=O).
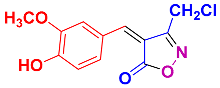
2.2.5. 4-(4-Hydroxy-3-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (4l) (Figures S13 and S14)
1H NMR (500 MHz, DMSO-d6): δ 2.28 (s, 3H, CH3), 3.89 (s, 3H, OCH3), 6.98 (d, J = 8.4 Hz, 1H, Ar-H), 7.90 (s, 1H, H-vinyl), 7.93 (dd, J = 1.8, 8.4 Hz, 1H, Ar-H), 8.56 (d, J = 1.8 Hz, 1H, Ar-H), 10.81 (s, 1H, OH); 13C NMR (125 MHz, DMSO-d6): δ 11.9 (CH3), 55.8 (OCH3), 114.1, 116.2, 117.3, 125.6, 132.2, 148.1, 152.3, 154.4, 162.9 (C=N), 169.6 (C=O).
2.2.6. 4-(3,4-Dimethoxybenzylidene)-3-methylisoxazol-5(4H)-one (4m) (Figures S15 and S16)
1H NMR (300 MHz, DMSO-d6): δ 2.29 (s, 3H, CH3), 3.86 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 7.22 (d, J = 9.0 Hz, 1H, Ar-H), 7.87 (s, 1H, Ar-H), 8.03 (d, J = 9.0 Hz, 1H, Ar-H), 8.51 (s, 1H, H-vinyl); 13C NMR (75 MHz, DMSO-d6): δ 11.8 (CH3), 55.9 (OCH3), 56.5 (OCH3), 112.1, 115.5, 116.1, 126.5, 131.5, 148.8, 152.2, 154.8, 162.8 (C=N), 169.3 (C=O).
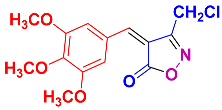
2.2.7. 3-Methyl-4-(3,4,5-trimethoxybenzylidene)isoxazol-5-(4H)-one (4n) (Figures S17 and S18)
1H NMR (500 MHz, CDCl3): δ 2.30 (s, 3H, CH3), 3.96 (s, 6H, OCH3), 3.99 (s, 3H, OCH3), 7.32 (s, 1H, H-vinyl), 7.84 (s, 2H, Ar-H); 13C NMR (125 MHz, DMSO-d6): δ 11.8 (CH3), 56.6 (OCH3), 60.8 (OCH3), 112.4, 117.5, 128.5, 143.5, 152.3, 152.8, 162.6 (C=N), 168.8 (C=O).
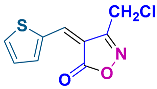
2.2.8. 3-Methyl-4-(thiophen-2-ylmethylene)isoxazol-5(4H)-one (4o) (Figures S19 and S20)
1H NMR (300 MHz, CDCl3): δ 2.29 (s, 3H, CH3), 7.39–7.42 (m, 1H, Ar-H), 8.24 (d, J = 3.0 Hz, 1H, Ar-H), 8.27 (s, 1H, H-vinyl), 8.34 (d, J = 6.0 Hz, 1H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 11.6 (CH3), 113.5, 129.5, 136.7, 141.6, 142.1, 143.5, 162.1 (C=N), 169.0 (C=O).
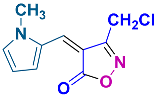
2.2.9. 3-Methyl-4-((1-methyl-1H-pyrrol-2-yl)methylene)isoxazol-5(4H)-one (4p) (Figures S21 and S22)
1H NMR (500 MHz, CDCl3): δ 2.26 (s, 3H, CH3), 3.84 (s, 3H, N-CH3), 6.41 (dd, J = 2.3, 4.5 Hz, 1H, Ar-H), 7.13 (t, J = 2.0 Hz, 1H, Ar-H), 7.16 (s, 1H, H-vinyl), 8.56 (dd, J = 1.5, 4.5 Hz, 1H, Ar-H); 13C NMR (125 MHz, CDCl3 + DMSO-d6): δ 11.1 (CH3), 34.2 (N-CH3), 112.2, 126.0, 129.2, 131.6, 134.9, 160.9 (C=N), 169.2 (C=O).
2.2.10. 3-(Chloromethyl)-4-(4-(dimethylamino)benzylidene)isoxazol-5(4H)-one (4v) (Figures S23 and S24)
1H NMR (400 MHz, CDCl3): δ 3.22 (s, 6H, N(CH3)2), 4.54 (s, 2H, CH2Cl), 6.78 (d, J = 9.2 Hz, 2H, Ar-H), 7.55 (s, 1H, H-vinyl), 8.47 (d, J = 7.2 Hz, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ 35.7 (N(CH3)2), 40.3 (CH2Cl), 107. 3, 111.7, 121.6, 125.5, 138.3, 150.4, 154.8, 160.6 (C=N), 169.8 (C=O).
2.2.11. 3-(Chloromethyl)-4-(2-hydroxy-3-methoxybenzylidene)isoxazol-5(4H)-one (4w) (Figures S25 and S26)
1H NMR (300 MHz, DMSO-d6): δ 3.85 (s, 3H, OCH3), 4.89 (s, 2H, CH2Cl), 6.87 (t, J = 8.1 Hz, 1H, Ar-H), 7.26 (d, J = 8.1 Hz, 1H, Ar-H), 8.33 (d, J = 8.2 Hz, 1H, Ar-H), 8.41 (s, 1H, H-vinyl), 10.46 (s, 1H, OH); 13C NMR (75 MHz, DMSO-d6): δ 35.3 (CH2Cl), 56.2 (OCH3), 113.1, 118.2, 118.8, 119.6, 123.4, 146.8, 147.8, 150.1, 161.5 (C=N), 167.7 (C=O).
2.2.12. 3-(Chloromethyl)-4-((1-methyl-1H-pyrrol-2-yl)methylene)isoxazol-5(4H)-one (4aa) (Figures S27 and S28)
1H NMR (500 MHz, DMSO-d6): δ 3.92 (s, 3H, N-CH3), 4.94 (s. 2H, CH2Cl), 6.53–6.54 (m, 1H, Ar-H), 7.72 (t, J = 1.6 Hz, 1H, Ar-H), 7.78 (s, 1H, H-vinyl), 8.44 (dd, J = 1.6, 4.5 Hz, 1H, Ar-H); 13C NMR (125 MHz, DMSO-d6): δ 34.8 (N-CH3), 35.9 (CH2Cl), 103.2, 113.7, 127.0, 130.1, 134.8, 138.5, 161.8 (C=N), 169.6 (C=O).
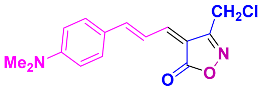
2.2.13. 3-(Chloromethyl)-4-(3-(4(dimethylamino)phenyl)allylidene)isoxazol-5(4H)-one (4ab) (Figures S29 and S30)
1H NMR (500MHz, CDCl3): δ 3.11 (s, 6H, N(CH3)2), 4.49 (s, 2H, CH2Cl), 6.69 (d, J = 8.6 Hz, 2H, Ar-H), 7.34 (d, J = 3.5, 6.9 Hz, 1H, =CH), 7.55 (dd, J = 3.5, 6.7 Hz, 1H, =CH), 7.61 (d, J = 8.6 Hz, 2H, Ar-H), 8.90 (m, 1H, =CH); 13C NMR (125 MHz, CDCl3): δ 35.5 (CH2Cl), 40.1 (N(CH3)2), 112.0, 118.1, 123.1, 132.3, 150.4, 153.3, 155.4, 159.2, 164.1 (C=N), 169.0 (C=O).
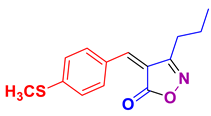
2.2.14. 4-(4-(Methylthio)benzylidene)-3-propylisoxazol-5(4H)-one (4ac) (Figures S31 and S32)
1H NMR (500 MHz, CDCl3): δ 1.01 (t, J = 7.2 Hz, 3H, CH3CH2CH2), 1.81 (sex, J = 7.5 Hz, 2H, CH3CH2CH2), 2.59 (s, 3H, SCH3), 2.68 (t, J = 7.5 Hz, 2H, CH3CH2CH2), 7.45 (d, J = 8.7 Hz, 2H, Ar-H), 7.92 (s. 1H, H-vinyl), 8.42 (d, J = 8.7 Hz, 1H, Ar-H); 13C NMR (125 MHz, CDCl3): δ 13.9 (CH3CH2CH2), 14.5 (SCH3), 19.8 (CH3CH2CH2), 27.6 (CH3CH2CH2), 112.2, 114.9, 116.2, 126.4, 131.4, 148.7, 166.3 (C=N), 169.1 (C=O).
2.2.15. (4-(Dimethylamino)phenyl)allylidene)-3-propylisoxazol-5(4H)-one (4ad) (Figures S33 and S34)
1H NMR( 500 MHz, CDCl3): δ 1.04 (t, J = 7.5 Hz, 3H, CH3CH2CH2), 1.74 (sex, J = 7.3 Hz, 2H, CH3CH2CH2), 2.55 (t, J = 7.3 Hz, 2H, CH3CH2CH2), 3.11 (s, 6H, N(CH3)2), 6.68 (d, J = 8.9 Hz, 2H, Ar-H), 7.22–7.27 (m, 1H, =CH), 7.46–7.51 (m, 1H, =CH), 7.56 (d, J = 8.9 Hz, 2H, Ar-H), 8.09–8.15 (m, 1H, =CH); 13C NMR (125 MHz, CDCl3): δ 13.9 (CH3CH2CH2), 20.2 (CH3CH2CH2), 27.9 (CH3CH2CH2), 40.1 (N(CH3)2), 111.9, 118.2, 123.2, 131.2, 131.7, 148.5, 152.8, 153.3, 163.0 (C=N), 170.5 (C=O).
2.2.16. 4-((1-Methyl-1H-pyrrol-2-yl)methylene)-3-propylisoxazol-5(4H)-one (4ae) (Figures S35 and S36)
1H NMR (500 MHz, DMSO-d6): δ 0.98 (t, J =7.2 Hz, 3H, CH3CH2CH2), 1.68 (sex, J = 7.4, 2H, CH3CH2CH2), 2.67 (t, J = 7.3, 2H, CH3CH2CH2), 3.91 (s, 3H, N-CH3), 6.45 (dd, J = 1.6, 2.4 Hz, 1H, Ar-H), 7.51 (s, 1H, H-vinyl), 7.58 (t, J = 1.7 Hz, 1H, Ar-H), 8.38 (dd, J = 1.6 Hz, 1H, Ar-H); 13C NMR (125 MHz, DMSO-d6): δ 14.2 (CH3CH2CH2), 19.9 (CH3CH2CH2), 27.4 (CH3CH2CH2), 34.7 (N-CH3), 106.5, 112.7, 125.8, 129.7, 133.7, 164.8 (C=N), 170.0 (C=N).
3. Results and Discussion
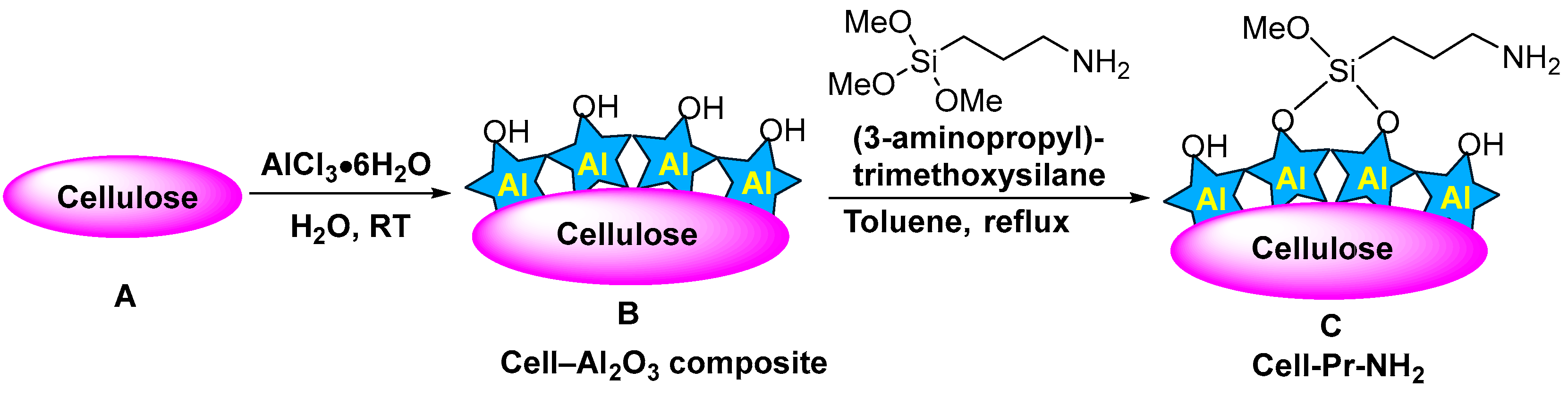
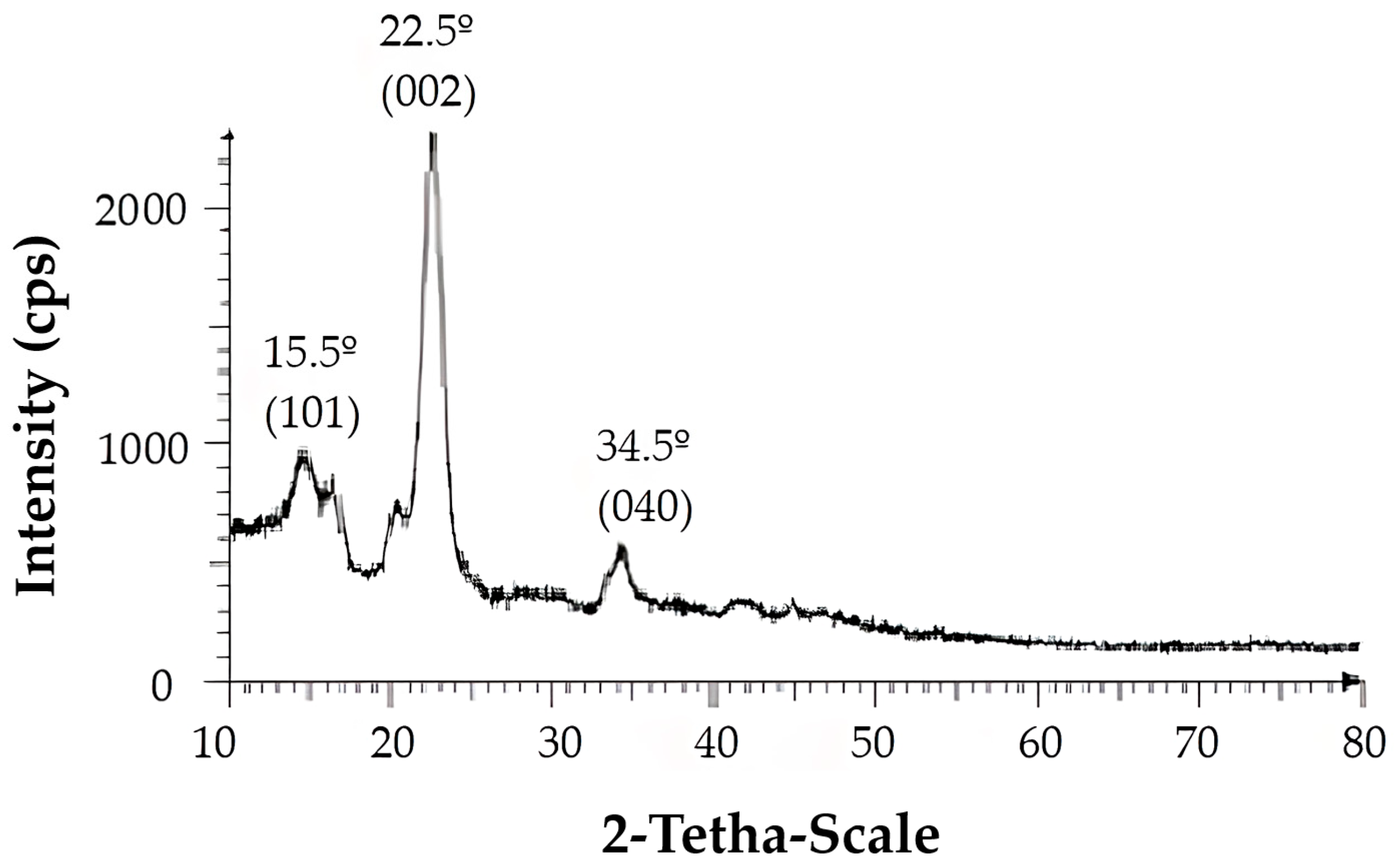
Propylamine-functionalized cellulose (cell-Pr-NH2, C) was synthesized according to the literature [74,75]. Briefly, microcrystalline cellulose (A, 10 g) and AlCl3·6H2O (10 g) in water (100 mL) was stirred at RT for 12 h. Then, the mixture was filtered, the residue was exposed to ammonia, washed with water, and dried in vacuum to produce the cell-alumina (cell-Al2O3) composite (B). Cell-Al2O3 composite (B, 10.0 g) and (3-aminopropyl)- trimethoxysilane (6.98 mL, 40.0 mmol) were mixed in refluxing toluene for 24 h. After cooling, the mixture was filtered off and washed with toluene. Finally, drying at RT in vacuum led to the formation of the cell-Pr-NH2 (C) (Figure 2). The X-ray diffraction (XRD) pattern of the Cell-Pr-NH2 (C) is shown in Figure 3. The three peaks at 2θ = 15.5°, 22.5° and 34.5°, which are related to crystallographic planes (101), (002), and (040) are due to microcrystalline cellulose [76]. These peaks are also observed in the XRD pattern of other cellulose sources containing an NH2 functional group [77]. Other peaks at 2θ = 16.7° and 21.5° can be attributed to other parts of the catalyst (probably due to incorporation of amino groups on the cellulose surface.) (Figure 3). Of course, the peak located at 2θ = 16.4° is also found in microcrystalline cellulose [78].
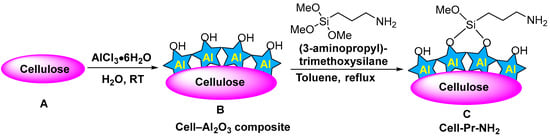
Figure 2.
Preparation of cellulose propylamine (cell-Pr-NH2) (C).
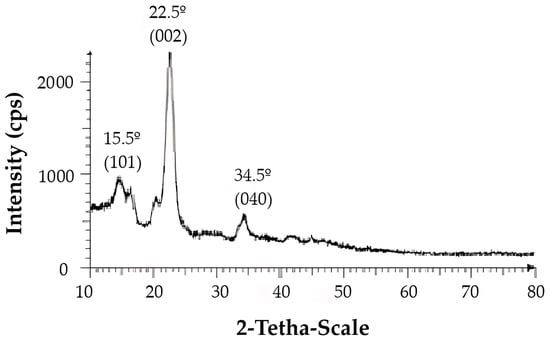
Figure 3.
XRD pattern of aminopropyl cellulose Cell-Pr-NH2 (C).
The field emission scanning electron microscopy image (FE-SEM) shows the rough structure of cellulose and the presence of new parts. In parts of the micrographs, nano-sized spots can be seen (Figure 4, left) approximately 28 to 36 nm in size.

Figure 4.
Field emission scanning electron microscopy (FESEM) micrographs of Cell-Pr-NH2 (C).
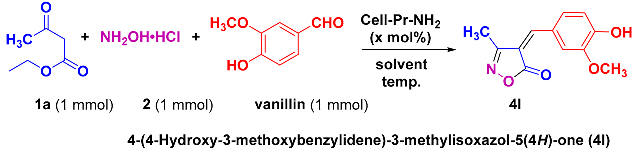
The one-pot, three-component reaction of ethyl acetoacetate (1a), hydroxylamine hydrochloride (2), and vanillin was initially designated for investigation with the aim of achieving the best conditions for carrying out the reaction. The experimental results of these studies are listed in Table 1. When the reaction was carried out without a catalyst in water at room temperature (RT), a yellow solid product was obtained after 40 min with a yield of 50% (Table 1, Entry 1). Continuation of the reaction did not change the efficiency appreciably. After purification, after measuring the melting point (214–216 °C; Lit. [53] 215–217 °C), and recording the 1H and 13C nuclear magnetic resonance (NMR) spectra, it was determined that the compound formed is the expected heterocycle, i.e., 4-(4-hydroxy-3-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (4l). The 1H NMR spectrum of this compound showed the peaks of CH3, OCH3, H-vinyl, OH as four characteristic singlet signals at δ 2.28, 3.89, 7.90, and 10.81 ppm, respectively. Furthermore, two doublet peaks at δ 6.98 (J = 8.4 Hz) and 8.56 ppm, as well as a doublet of doublets peak at δ 7.93 ppm, were attributed to three protons of the benzene ring. The 1H NMR decoupled spectrum of 4l showed 12 distinct signals in agreement with the desired product. The characteristic signals of CH3, OCH3, C=N, and C=O carbons were observed at δ 11.9, 55.8, 162.9, and 169.6 ppm, respectively. Six carbons of the benzene ring, one carbon of the vinyl group, and the 4-position of the isoxazolone ring appeared in the expected regions (114.1–154.4 ppm). After observing this favorable result, we decided to continue the experiments in order to find the best reaction conditions. In this context, important parameters such as the amount of catalyst, the type of solvent, and reaction temperature were investigated. The reactions were performed under aqueous conditions at RT. Various amounts of cell-Pr-NH2 (C) as the catalyst (2–14 mg) were loaded into the reaction mixture in a water solvent. It was found that using 14 mg (0.17 mol%) of cell-Pr-NH2 (C) led to the best results in terms of reaction time (25 min) and isolated reaction yield (97%) (Table 1, Entry 8). Due to this excellent result, other catalyst loading values were not checked and 14 mg of cell-Pr-NH2 (C) as the catalyst was used to check other parameters. After testing various catalyst loadings in water at RT, some solvents available in our laboratory were investigated. Solvents including ethanol (EtOH), acetone (CH3COCH3), chloroform (CHCl3), dimethylformamide (DMF), n-hexane, and a mixture of EtOH-H2O (1:1, v:v) were screened at room temperature (Table 1, Entries 9–14). It was observed that these solvents did not have a significant and acceptable effect on the reaction times or yields. In some solvents, such as CHCl3 and DMF, small amount of the product 4l was seen (checked with the help of TLC analysis) and the reactants remained almost intact (Table 1, Entries 11 and 12). When the reaction was carried out in solvent-free conditions and using the optimal amount of catalyst at RT, no favorable results were obtained (Table 1, Entry 15). Considering the temperature parameter led us to explore other temperatures in addition to room temperature. The reaction was investigated at different temperatures in water solvent and using the optimal amount of the catalyst. Two results are given in the optimization table (Table 1, Entries 16 and 17). No significant results were obtained in any of these studies. With all this controversy, it can be concluded that the best conditions for carrying out the reaction are water as a solvent, 14 mg of cell-Pr-NH2 (C) as the catalyst, and RT (Table 1, Entry 8).

Table 1.
Optimization of the reaction conditions.
After optimizing the reaction conditions, the substrate scope of the reaction was explored using ethyl acetoacetate (1a), hydroxylamine hydrochloride (2), and substituted benzaldehydes. Consequently, using 14 mg of cell-Pr-NH2 (C) in H2O at RT, the reaction between ethyl acetoacetate (1a), hydroxylamine hydrochloride (2), benzaldehyde, or structurally diverse substituted benzaldehydes produced a broad spectrum of arylideneisoxzol-5(4H)-ones (4a–4n). The results indicate that aryl aldehydes, with the electron-donating groups, participate better in this three-component reaction and produce the corresponding heterocyclic products in good to excellent yields (Table 2, Entries 1–14). When heteroaryl aldehydes, such as thiophene-2-carboxaldehyde and N-methyl-2-pyrrolecarboxaldehyde were used, the cyclocondensation reaction proceeded well and the corresponding products (4o and 4p) were obtained in high isolated yields and reasonable reaction times (Table 2, Entries 15 and 16). In addition, the scope of the substrate was explored further. In this context, ethyl 4-chloroacetoacetate (1b) was employed instead of ethyl acetoacetate (1a). A good result was obtained when benzaldehyde was used as a precursor (Table 2, Entry 17). When the substituted benzaldehydes containing electron-donating functional groups, including OCH3, CH3, N(CH3)2, and OH, at different positions on the aryl ring were used in this three-component catalytic reaction, the processes proceeded well and the corresponding 3-chloromethyl substituted isoxazole-5(4H)-ones (4r–4y) were obtained in good to excellent yields and in satisfactory reaction times (Table 2, Entries 18–25). We also tested two five-membered heteroaryl aldehydes, thiophene-2-carbaldehyde and 1-methyl-1H-pyrrole-2-carbaldehyde, in a reaction with ethyl 4-chloroacetoacetate (1b) and hydroxylamine hydrochloride (2). Fortunately, the two desired heterocyclic products (4z and 4aa) were synthesized with excellent isolated reaction yields (Table 2, Entries 26 and 27). Under the optimized reaction conditions (as described in Table 1, Entry 8), the use of the α,β-unsaturated aldehyde containing a phenyl ring (4-(dimethylamino)cinnamaldehyde) was also fruitful and the desired heterocyclic product (4ab) was formed in an excellent isolated yield of reaction and short reaction time (Table 2, Entry 28). Moreover, under optimal conditions, the attempt to use ethyl 3-oxohexanoate (1c) as a β-keto ester precursor was fruitful. In this regard, three heterocycles (4ac–4ae), were successfully synthesized and the target compounds were isolated with satisfactory yields along with rational reaction times (Table 2, Entries 29–31).

Table 2.
Substrate scope of aldehydes and β-keto esters for the synthesis of isoxazole-5(4H)-ones (4a–4ae) a.
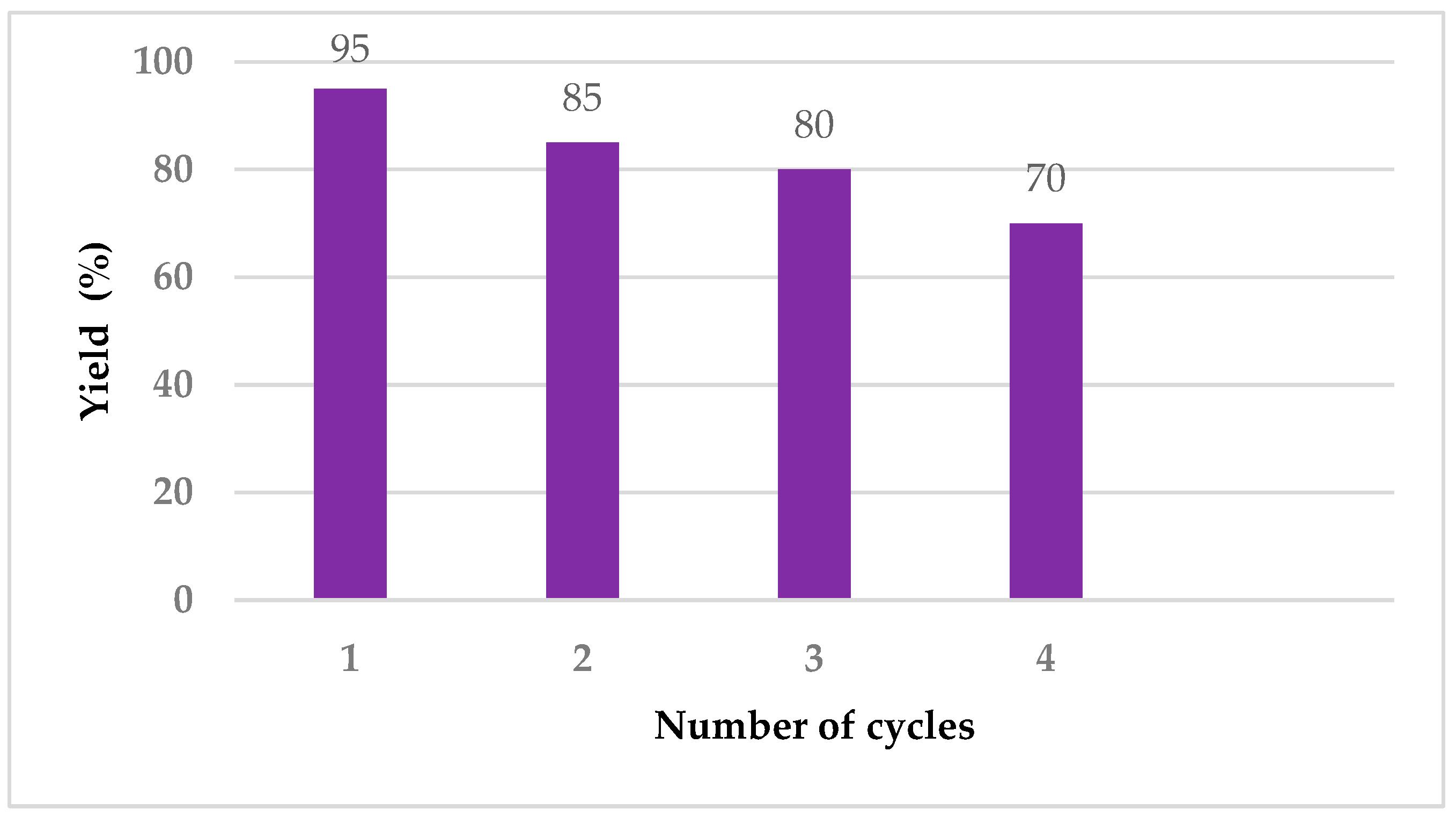
Cellulose is partially soluble in ethanol [78]. Since the amount of catalyst containing cellulose is very small, it dissolves in ethanol during the washing process. The filtrate containing the catalyst can be reused in subsequent reactions. The catalyst was reused four times (Figure 5).
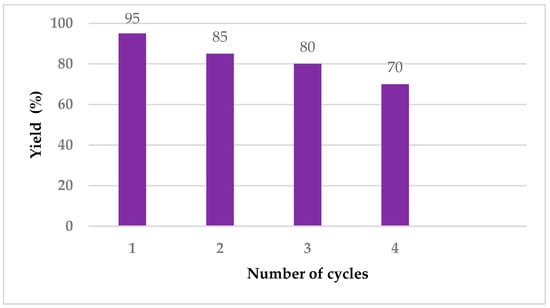
Figure 5.
Recycle investigation of catalyst in the model reaction.
This reaction is comparable to other similar reactions in several aspects (Table 3). Although each of the methods revealed has its own merits, they frequently suffer from some problems. This cyclocondensation process is comparable with other similar chemical transformations in several aspects such as reaction yield, reaction time, type of reaction medium, amount of catalyst, and reaction temperature. From the efficiency point of view, it is superior to entries 1–4 and 7–10. From the aspect of the reaction medium, it is better than cases 9 and 10 because no organic solvent was used. In comparison with methods 1–4 and 6–10, which are included in Table 3, the current reaction has been implemented in a shorter reaction time. It should also be noted that this reaction was carried out at RT, but when 2,2′-bpy (Table 3, Entry 4), [H2-BiPyr][ClO4]2 (Table 3, Entry 5), 50 wt % aq. GAAS (Table 3, Entry 8), PPTS (Table 3, Entry 10), Na2S2O3 (Table 3, Entry 9), and sulfamic acid (Table 3, Entry 10) were used as catalysts, harsh reaction conditions (70 °C, reflux, 65 °C, and reflux, respectively) were needed.

Table 3.
Comparison of various catalysts applied in synthesis of 4-(4-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (4b).
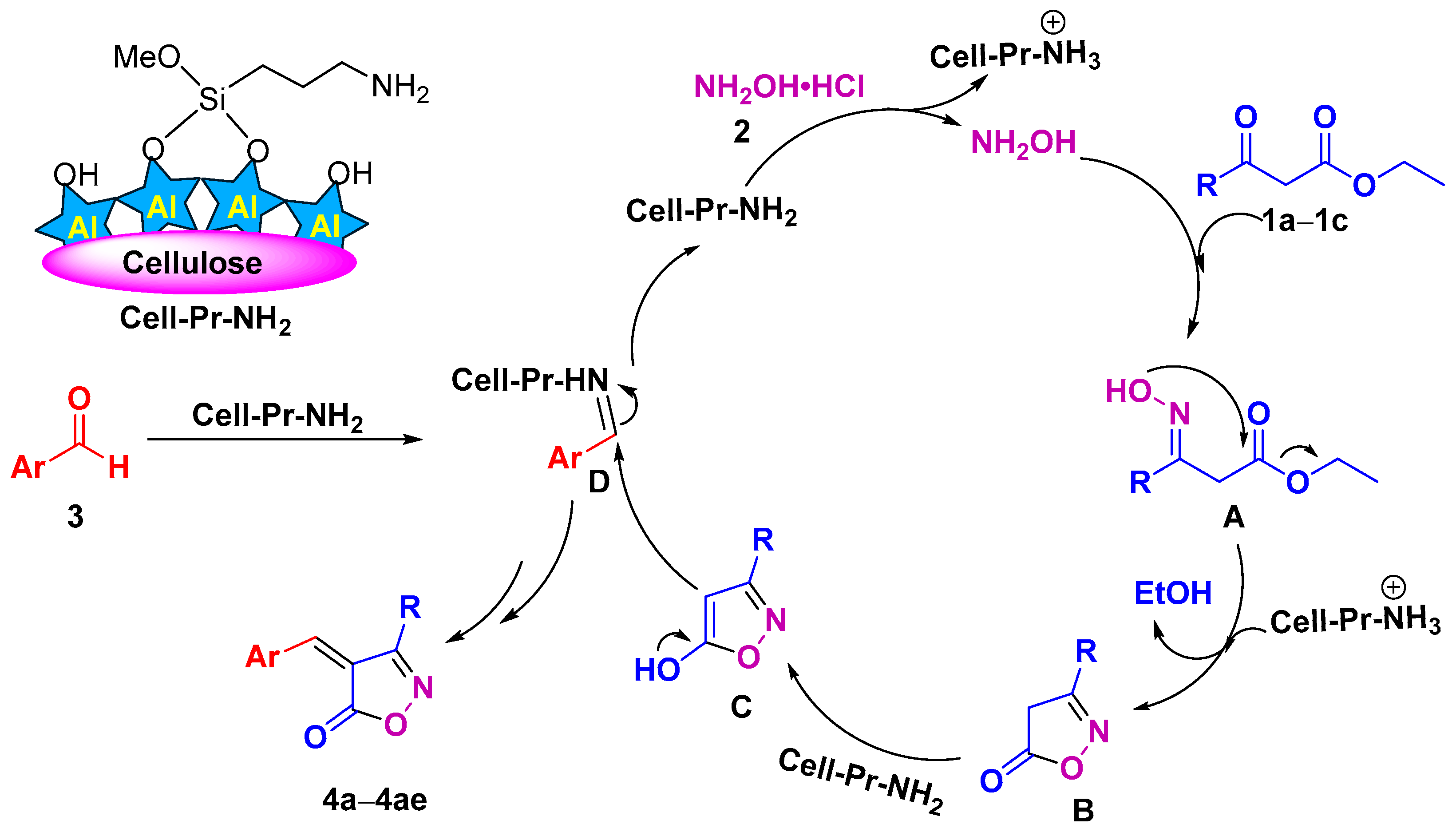
Although we did not carry out mechanistic investigations, based on the literature [79], the mechanism drawn in Scheme 2 can be proposed for the reaction. The Cell-Pr-NH2 catalyst helps the released hydroxylamine to initiate the oximation reaction and nucleophilic attack of the NH2OH on the carbonyl carbon of the β-keto ester (1a–1c) leading to the formation of oxime intermediate A. Intermediate A was cyclized to generate 3-substituted-isoxazole-5(4H)-ones (B). In the presence of the catalyst, cyclic intermediates B are enolized to intermediates C. Finally, the Knoevenagel condensation reaction between enolized 3-substituted-isoxazole-5(4H)-ones (C) and D leads to the formation of the corresponding heterocycles (4a–4ae) (Scheme 2).
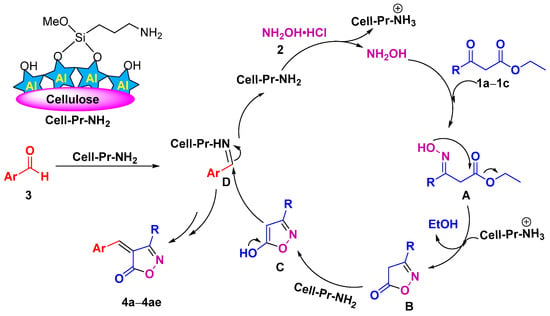
Scheme 2.
A plausible mechanism for the formation of isoxazol-5(4H)-ones (4a–4ae).
4. Conclusions
In conclusion, this work introduces an efficient and green procedure to synthesize 3,4-disubstituted isoxzol-5(4H)-ones, heterocyclic compounds with potential biological activity, using commercially available starting materials. The reactions have been successfully implemented under aqueous conditions in the presence of cell-Pr-NH2 as a catalyst at RT. Employing this method for the synthesis of isoxazole-5(4H)-one heterocycles has several advantages, including ease of reaction implementation, simplicity of product purification, reasonable yields, acceptable reaction times, performing the reactions in non-organic solvents, as well as the low cost, availability, and abundance of the reaction solvent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org5040020/s1 and contains the 1H and 13C NMR spectra for compounds.
Author Contributions
Conceptualization, H.K.; investigation, S.G.; data curation, S.G.; writing—original draft preparation, H.K. and S.G.; writing—review and editing, H.K.; supervision, H.K.; project administration, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data of this study can be found in the Supplementary Materials section.
Acknowledgments
We would to express our thankfulness to the Shahrekord University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dengale, S.G.; Akolkar, H.N.; Darekar, N.R.; Shaikh, M.H.; Deshmukh, K.K.; Mhaske, S.D.; Karale, B.K.; Raut, D.N.; Khedkar, V.M. Synthesis and Biological Evaluation of 2-(4,5,6,7-Tetrahydrobenzo[c]Isoxazol-3-yl)-4H-Chromen-4-Ones. Polycycl. Aromat. Comp. 2002, 42, 6337–6351. [Google Scholar] [CrossRef]
- Thakur, A.; Verma, M.; Bharti, R.; Sharma, R. Oxazole and isoxazole: From one-pot synthesis to medical applications. Tetrahedron 2022, 119, 132813. [Google Scholar] [CrossRef]
- Arya, G.C.; Kaur, K.; Jaitak, V. Isoxazole derivatives as anticancer agent: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2021, 221, 113511. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Ngaini, Z. Synthesis of Benzalacetophenone-based Isoxazoline and Isoxazole Derivatives. Curr. Org. Chem. 2022, 26, 679–692. [Google Scholar] [CrossRef]
- Pattanayak, P.; Chatterjee, T. Synthesis of (4-Trifluoromethyl)isoxazoles through a Tandem Trifluoromethyloximation/Cyclization/Elimination Reaction of α,β-Unsaturated Carbonyls. J. Org. Chem. 2023, 88, 5420–5430. [Google Scholar] [CrossRef]
- Pandhurnekar, C.P.; Pandhurnekar, H.C.; Mungole, A.J.; Butoliya, S.S.; Yadao, B.G. A review of recent synthetic strategies and biological activities of isoxazole. J. Heterocycl. Chem. 2023, 60, 536–565. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Zhang, X.; Hu, Z.; Wu, Y. Synthesis and characterization of a new isoxazolone-based nonlinear optical crystal: MPMOI. CrystEngComm 2023, 25, 1313–1318. [Google Scholar] [CrossRef]
- Razzaq, S.; Minhas, A.M.; Qazi, N.G.; Nadeem, H.; Khan, A.U.; Ali, F.; Hassan, S.S.; Bungau, S. Novel Isoxazole Derivative Attenuates Ethanol-Induced Gastric Mucosal Injury through Inhibition of H+/K+-ATPase Pump, Oxidative Stress and Inflammatory Pathways. Molecules 2022, 27, 5065. [Google Scholar] [CrossRef]
- Kafle, B.; Aher, N.G.; Khadka, D.; Park, H.; Cho, H. Isoxazol-5(4H)one Derivatives as PTP1B Inhibitors Showing an Anti-Obesity Effect. Chem. Asian J. 2011, 6, 2073–2079. [Google Scholar] [CrossRef]
- Sukanya, S.H.; Venkatesh, T.; Kumar, R.S.; Bodke, Y.D. Facile TiO2 NPs catalysed synthesis of substituted-4-Hydroxy/methoxy benzylidene derivatives as potent antioxidant and anti-tubercular agents. Chem. Data Collect. 2021, 33, 100713. [Google Scholar] [CrossRef]
- Kuchana, M.; Bethapudi, D.R.; Ediga, R.K.; Sisapuram, Y. Synthesis, in-Vitro Antioxidant Activity and In-Silico Prediction of Drug-Likeness Properties of a Novel Compound: 4-(3,5-Di-Tert-Butyl-4-Hydroxybenzylidene)-3-Methylisoxazol-5(4H)-One. J. Appl. Pharm. Sci. 2019, 9, 105–110. [Google Scholar] [CrossRef]
- Ali, M.; Saleem, U.; Anwar, F.; Imran, M.; Nadeem, H.; Ahmad, B.; Ali, T.; Atta-ur-rehman; Ismail, T. Screening of Synthetic Isoxazolone Derivative Role in Alzheimer’s Disease: Computational and Pharmacological Approach. Neurochem. Res. 2021, 46, 905–920. [Google Scholar] [CrossRef]
- Kadam, H.K.; Salkar, K.; Naik, A.P.; Naik, M.M.; Salgaonkar, L.N.; Charya, L.; Pinto, K.C.; Mandrekar, V.K.; Vaz, T. Silica Supported Synthesis and Quorum Quenching Ability of Isoxazolones Against Both Gram Positive and Gram-Negative Bacterial Pathogens. ChemistrySelect 2021, 6, 11718–11728. [Google Scholar] [CrossRef]
- Wang, Y.; Du, D.M. Highly Diastereo- and Enantioselective Synthesis of Isoxazolone-Spirooxindoles via Squaramide-Catalyzed Cascade Michael/Michael Addition Reactions. J. Org. Chem. 2020, 85, 15325–15336. [Google Scholar] [CrossRef] [PubMed]
- Badiger, K.B.; Khatavi, S.Y.; Kamanna, K. Green synthesis of 3-methyl-4-(hetero)aryl methylene isoxazole-5(4H)-ones using WEOFPA/glycerol: Evaluation of anticancer and electrochemical behaviour properties. RSC Med. Chem. 2022, 13, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Nongrum, R.; Nongkhlaw, R.; Majaw, S.P.; Kumari, J.; Sriram, D.; Nongkhlaw, R. A nano-organo catalyst mediated approach towards the green synthesis of 3-methyl-4-(phenyl)methylene-isoxazole-5(4H)-one derivatives and biological evaluation of the derivatives as a potent anti-fungal and anti-tubercular agent. Sustain. Chem. Pharm. 2023, 32, 100967. [Google Scholar] [CrossRef]
- Saleem, A.; Farooq, U.; Bukhari, S.M.; Khan, S.; Zaidi, A.; Wani, T.A.; Shaikh, A.J.; Sarwar, R.; Mahmud, S.; Israr, M.; et al. Isoxazole Derivatives against Carbonic Anhydrase: Synthesis, Molecular Docking, MD Simulations, and Free Energy Calculations Coupled with In Vitro Studies. Acs Omega 2022, 7, 30359–30368. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.B.S.; Mori, M.S.S.; Albernaz, L.C.; Espindola, L.S.; Salvador, C.E.M.; Andrade, C.K.Z. Continuous Flow Photochemical Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones through Organic Photoredox Catalysis and Investigation of Their Larvicidal Activity. Catalysts 2023, 13, 518. [Google Scholar] [CrossRef]
- Oraby, A.K.; Abdellatif, K.R.A.; Abdelgawad, M.A.; Attia, K.M.; Dawe, L.N.; Georghiou, P.E. 2,4-Disubstituted Phenylhydrazonopyrazolone and Isoxazolone Derivatives as Antibacterial Agents: Synthesis, Preliminary Biological Evaluation and Docking Studies. ChemistrySelect 2018, 3, 3295–3301. [Google Scholar] [CrossRef]
- Gadkari, Y.U.; Jadhav, N.L.; Hatvate, N.T.; Telvekar, V.N. Concentrated Solar Radiation Aided Green Approach for Preparative Scale and Solvent-Free Synthesis of 3-Methyl-4-(hetero)arylmethylene Isoxazole-5(4H)-ones. ChemistrySelect 2020, 5, 12320–12323. [Google Scholar] [CrossRef]
- Uvarova, E.S.; Kutasevich, A.V.; Lipatov, E.S.; Mityanov, V.S. Assembly of isoxazol-5-one with 2-unsubstituted imidazole N-oxides and aldehydes. Org. Biomol. Chem. 2023, 21, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Galenko, E.E.; Linnik, S.A.; Khoroshilova, O.V.; Novikov, M.S.; Khlebnikov, A.F. Isoxazole Strategy for the Synthesis of α-Aminopyrrole Derivatives. J. Org. Chem. 2019, 84, 11275–11285. [Google Scholar] [CrossRef]
- Galenko, E.E.; Kryukova, M.A.; Novikov, M.S.; Khlebnikov, A.F. An Isoxazole Strategy for the Synthesis of Fully Substituted Nicotinates. J. Org. Chem. 2021, 86, 6888–6896. [Google Scholar] [CrossRef]
- Galenko, E.E.; Novikov, M.S.; Khlebnikov, A.F. [2 + 2] Cycloaddition/Retro-Electrocyclization/Decarboxylation Reaction Sequence: Access to 4-Aminopyridines from Methylideneisoxazolones and Ynamines. J. Org. Chem. 2023, 88, 8854–8864. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, G.; Reddy, M.M.; Archana, B.; Yadav, J.S. A Convenient Synthesis of Benzopyranacetylenes. Synth. Commun. 1998, 28, 573–581. [Google Scholar] [CrossRef]
- Wan, S.H.; Li, X.A.; Liu, Y.H.; Liu, S.T. N-Allylation versus C-allylation of intermediates from aza-Michael adducts of arylideneisoxazol-5-ones. Org. Biomol. Chem. 2020, 18, 9516–9525. [Google Scholar] [CrossRef] [PubMed]
- Macchia, A.; Summa, F.F.; Monaco, G.; Eitzinger, A.; Ofial, A.R.; Di Mola, A.; Massa, A. Access to β-Alkylated γ-Functionalized Ketones via Conjugate Additions to Arylideneisoxazol-5-ones and Mo(CO)6-Mediated Reductive Cascade Reactions. Acs Omega 2022, 7, 8808–8818. [Google Scholar] [CrossRef]
- Li, M.J.; Xiao, H.J.; Xu, P.; Wu, L.T.; Chen, S.Q.; Zhang, Z.; Xu, H. Mechanosynthesis of Pyrrole-2-carboxylic Acids via Copper-Catalyzed Spiroannulation/Ring-Opening Aromatization of 4-Arylidene Isoxazol-5-ones with Enamino Esters. Org. Lett. 2024, 26, 4189–4193. [Google Scholar] [CrossRef]
- MacChia, A.; Eitzinger, A.; Brière, J.F.; Waser, M.; Massa, A. Asymmetric Synthesis of Isoxazol-5-ones and Isoxazolidin-5-ones. Synthesis 2021, 53, 107–122. [Google Scholar] [CrossRef]
- Titov, G.D.; Rostovskii, N.V. (1RS,3SR)-1-(4-Methylbenzyl)-7-phenyl-5-oxa-6-azaspiro [2.4]hept-6-en-4-one. Molbank 2024, 2024, M1799. [Google Scholar] [CrossRef]
- Newar, U.D.; Kumar, S.; Borah, A.; Borra, S.; Manna, P.; Gokulnath, S.; Maurya, R.A. Access to Isoxazoles via Photo-oxygenation of Furan Tethered α-Azidoketones. J. Org. Chem. 2024, 89, 12378–12386. [Google Scholar] [CrossRef] [PubMed]
- Kapale, S.S.; Gadkari, Y.U.; Chaudhari, H.K. Lipase Catalyzed One-Pot Synthesis of 3-Methyl-4-(Hetero) Arylmethyleneisoxazole-5(4H)-Ones under Aqueous Conditions. Polycycl. Aromat. Comp. 2022, 43, 4856–4865. [Google Scholar] [CrossRef]
- Faramarzi, Z.; Kiyani, H. Steglich’s Base Catalyzed Three-Component Synthesis of Isoxazol-5-Ones. Polycycl. Aromat. Comp. 2023, 43, 3099–3121. [Google Scholar] [CrossRef]
- Parveen, M.; Aslam, A.; Ahmad, A.; Alam, M.; Silva, M.R.; Silva, P.S.P. A facile & convenient route for the stereoselective synthesis of Z-isoxazol-5(4H)-ones derivatives catalysed by sodium acetate: Synthesis, multispectroscopic properties, crystal structure with DFT calculations, DNA-binding studies and molecular docking studies. J. Mol. Struct. 2020, 1200, 127067. [Google Scholar] [CrossRef]
- Gharehassanlou, S.; Kiyani, H. A Catalytic Three-Component Synthesis of Isoxazol-5(4H)-Ones under Green Conditions. Indian J. Chem. 2022, 61, 515–520. [Google Scholar] [CrossRef]
- Barkule, A.B.; Gadkari, Y.U.; Telvekar, V.N. One-Pot Multicomponent Synthesis of 3-Methyl-4-(Hetero)Arylmethylene Isoxazole-5(4H)-Ones Using Guanidine Hydrochloride as the Catalyst under Aqueous Conditions. Polycycl. Aromat. Comp. 2022, 42, 5870–5881. [Google Scholar] [CrossRef]
- Saikh, F.; Das, J.; Ghosh, S. Synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones by visible light in aqueous ethanol. Tetrahedron Lett. 2013, 54, 4679–4682. [Google Scholar] [CrossRef]
- Ostadzadeh, H.; Kiyani, H. Synthesis of Isoxazole-5(4H)-Ones Using Citrazinic Acid as an Organocatalyst in Aqueous Conditions. Org. Prep. Proced. Int. 2023, 55, 538–548. [Google Scholar] [CrossRef]
- Aleaba, G.; Asadi, S.K.; Daneshvar, N.; Shirini, F. Introduction of [2,2’-Bipyridine]-1,1’-Diium Perchlorate as a Novel and Highly Efficient Dicationic Brönsted Acidic Organic Salt for the Synthesis of 3-Methyl-4-Arylmethylene Isoxazole-5(4H)-one Derivatives in Water. Polycycl. Aromat. Comp. 2022, 42, 7569–7581. [Google Scholar] [CrossRef]
- Kumar, R.; Garima, K.; Srivastava, V.; Singh, P.P.; Singh, P.K. Visible light mediated eosin-Y catalysed synthesis of benzylidene-methylisoxazolone. Tetrahedron Lett. 2023, 133, 154841. [Google Scholar] [CrossRef]
- Queiroz, Y.B.; de Freitas, Y.A.; Lima, J.G.M.; Ribeiro, L.; Ramos, L.M. Ionic Liquid-Catalyzed Multicomponent Synthesis of Isoxazole-5(4H)-ones: In vitro Activities and Principal Component Analysis. J. Braz. Chem. Soc. 2024, 35, e-20230188. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, J.; Bian, X.; Chen, M.; Zhu, G.; Zhang, Y.; Gu, Q. Na2S2O3 Catalyzed Three-Component Synthesis and Anti-Bacterial Activity of 3-Methyl-4-(hetero)arylmethylene isoxazole-5(4H)-ones. Chem. Biodivers. 2024, 21, e202400073. [Google Scholar] [CrossRef] [PubMed]
- Reihani, N.; Kiyani, H. Three-Component Synthesis of 4-Arylidene-3-Alkylisoxazol-5(4H)-ones in the Presence of Potassium 2,5-Dioxoimidazolidin-1-ide. Curr. Org. Chem. 2021, 25, 950–962. [Google Scholar] [CrossRef]
- Delfani, A.M.; Kiyani, H.; Zamani, M. An Expeditious Synthesis of Ethyl-2-(4-(arylmethylene)-5-oxo-4,5-dihydroisoxazol-3-yl)acetate Derivatives. Curr. Org. Chem. 2022, 26, 1575–1584. [Google Scholar] [CrossRef]
- Tahmasabi, S.Z.; Kiyani, H.; Samimi, H.A. Efficient Synthesis of 4-Arylmethylene-3-methylisoxazol-5(4H)-one Derivatives Catalyzed by Malic Acid. Lett. Org. Chem. 2023, 20, 167–174. [Google Scholar] [CrossRef]
- Daroughehzadeh, Z.; Kiyani, H. Arylideneisoxazole-5(4H)-one Synthesis by Organocatalytic Three-Component Hetero-Cyclization. Polycycl. Aromat. Comp. 2024, 44, 3200–3221. [Google Scholar] [CrossRef]
- Boureghda, C.; Krid, A.; Dems, M.A.; Boutebdja, M.; Boulcina, R.; Debache, A. Facile synthesis, crystal structure, Hirshfeld surface analysis, DFT calculations, IR and UV–visible spectra analyzes, ADMET and molecular docking studies of arylideneisoxazolone derivatives. J. Mol. Struct. 2024, 1317, 139005. [Google Scholar] [CrossRef]
- Kour, P.; Ahuja, M.; Sharma, P.; Kumar, A.; Kumar, A. An Improved Protocol for the Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-ones through L-Valine-Mediated Domino Three-Component Strategy. J. Chem. Sci. 2020, 132, 108. [Google Scholar] [CrossRef]
- Bhowmik, D.; Kamandar, P.; Gupta, J.; Mete, S.; Hatvate, N. An efficient, green and micellar catalyzed preparative-scale synthesis of 3,4-Disubstituted isoxazole-5(4H)-ones in the water. Sustain. Chem. Environ. 2024, 5, 100070. [Google Scholar] [CrossRef]
- Mane, R.; Yaraguppi, D.A.; Chandrakala, K.B. Kamanna, Synthesis, Computational, Anticancer, and Electrochemical Behavior Studies of 3-Methyl-4-(hetero)aryl Methylene Isoxazole-5(4H)-One. ChemistrySelect 2024, 9, e202401158. [Google Scholar] [CrossRef]
- Ghogare, R.S.; Patankar-Jain, K.; Momin, S.A.H. A Simple and Efficient Protocol for the Synthesis of 3,4-Disubstituted Isoxazol-5(4H)-ones Catalyzed by Succinic Acid Using Water as Green Reaction Medium. Lett. Org. Chem. 2021, 18, 83–87. [Google Scholar] [CrossRef]
- Kundu, T.; Mitra, B.; Ghosh, P. Eucalyptol: An Efficient, Unexplored, Green Media for Transition Metal Free Synthesis of 2,3-Dihydroquinazolin-4(1H)-one Derivatives and Isoxazolone Derivatives. Synth. Commun. 2023, 53, 779–794. [Google Scholar] [CrossRef]
- Haydari, F.; Kiyani, H. Urea-Catalyzed Multicomponent Synthesis of 4-Arylideneisoxazol-5(4H)-one Derivatives under Green Conditions. Res. Chem. Intermed. 2023, 49, 837–858. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, C.; Ren, L.; Li, W.; Luan, B.; Zhang, Y. Vitamin B1-Catalyzed Multicomponent Reaction for Efficient Synthesis of an Isoxazolone Compound by Using Ultrasound in a Water and Its Selective Identification of Metal Ions. ChemistrySelect 2023, 8, e202204658. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kiyani, H.; Pourmousavi, S.A. Facile and Expedient Synthesis of α,β-Unsaturated Isoxazol-5(4H)-ones under Mild Conditions. Res. Chem. Intermed. 2020, 46, 943–959. [Google Scholar] [CrossRef]
- Atharifar, H.; Keivanloo, A.; Maleki, B. Greener Synthesis of 3,4-Disubstituted Isoxazole-5(4H)-ones in a Deep Eutectic Solvent. Org. Prep. Proced. Int. 2020, 52, 517–523. [Google Scholar] [CrossRef]
- Zadem, A.; Cheraiet, Z.; Chahra, B.H. (Betaine: Citric Acid: H2O) a Natural Deep Eutectic Solvent (NaDES)-Mediated Efficient and Green Synthesis of 4-Benzylidene Isoxazol-5-one Derivatives. Polycycl. Aromat. Comp. 2023, in press. [Google Scholar] [CrossRef]
- Aslanpour, S.; Kiyani, H. Rapid Synthesis of Fully Substituted Arylideneisoxazol-5(4H)-one Using Zinc Oxide Nanoparticles. Res. Chem. Intermed. 2023, 49, 4603–4619. [Google Scholar] [CrossRef]
- Kiyani, H.; Ghorbani, F. Expeditious Green Synthesis of 3,4-Disubstituted Isoxazole-5(4H)-Ones Catalyzed by Nano-MgO. Res. Chem. Intermed. 2016, 42, 6831–6844. [Google Scholar] [CrossRef]
- Mosallanezhad, A.; Kiyani, H. Green Synthesis of Arylideneisoxazol-5-Ones Catalyzed by Silicon Dioxide Nanoparticles. Polycycl. Aromat. Comp. 2023, 44, 5022–5037. [Google Scholar] [CrossRef]
- Kulkarni, P. An efficient solvent-free synthesis of 3,4-disubstituted isoxazole-5(4H)-ones using microwave irradiation. J. Indian Chem. Soc. 2021, 98, 100013. [Google Scholar] [CrossRef]
- Shitre, G.V.; Patel, A.R.; Ghogare, R.S. Green synthesis of 3,4-disubstituted isoxazol-5(4H)-one using Gluconic acid aqueous solution as an efficient recyclable medium. Org. Commun. 2023, 16, 87–97. [Google Scholar] [CrossRef]
- Haidary, F.; Kiyani, H. Green and clean synthesis of 4-arylideneisoxazol-5-ones using NaCl aqueous solution. Sustain. Chem. Environ. 2024, 5, 100066. [Google Scholar] [CrossRef]
- Gharib, M.; Nikpassand, M.; Mokhtary, M.; Zare Fekri, L. “Glucono-delta-lactone” as a substrate for preparation of new magnetically recoverable nanocatalyst for the environmentally benign synthesis of novel azo linked 3-methyl-isoxazol-5-ones. Synth. Commun. 2023, 53, 1935–1953. [Google Scholar] [CrossRef]
- Atharifar, H.; Keivanloo, A.; Maleki, B.; Baghayeri, M.; Alinezhad, H. Magnetic nanoparticle supported choline chloride-glucose (deep eutectic solvent) for the one-pot synthesis of 3,4-disubstituted isoxazol-5(4H)-ones. Res. Chem. Intermed. 2024, 50, 281–296. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Zheng, Q.; Kurpiewska, K.; Kalinowska-Tluscik, J.; Dömling, A. Access to Isoquinolin-2(1H)-yl-acetamides and Isoindolin-2-ylacetamides from a Common MCR Precursor. J. Org. Chem. 2022, 87, 14463–14475. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Zheng, Q.; Patil, P.; Dömling, A. A Bifurcated Multicomponent Synthesis Approach to Polycyclic Quinazolinones. J. Org. Chem. 2022, 87, 13023–13033. [Google Scholar] [CrossRef] [PubMed]
- Kamalifar, S.; Kiyani, H. Facile and Efficient Synthesis of 9-Aryl-1,8-Dioxo-Octahydroxanthenes Catalyzed by Sulfacetamide. Polycycl. Aromat. Comp. 2022, 42, 3675–3693. [Google Scholar] [CrossRef]
- Kiyani, H.; Samimi, H.; Ghorbani, F.; Esmaieli, S. One-pot, four-component synthesis of pyrano[2,3-c]pyrazoles catalyzed by sodium benzoate in aqueous medium. Curr. Chem. Lett. 2013, 2, 197–206. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Lavilla, I.; de la Calle, I.; Romero, V.; Bendicho, C. Detection of gases and organic vapours by cellulose-based sensors. Anal. Bioanal. Chem. 2023, 415, 4039–4060. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef] [PubMed]
- Karhale, S.; Bhenki, C.; Rashinkar, G.; Helavi, V. Covalently anchored sulfamic acid on cellulose as heterogeneous solid acid catalyst for the synthesis of structurally symmetrical and unsymmetrical 1,4-dihydropyridine derivatives. New J. Chem. 2017, 41, 5133–5141. [Google Scholar] [CrossRef]
- Karhale, S. Grafting of sulphamic acid on functionalized sawdust: A novel solid acid catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes. Res. Chem. Intermed. 2020, 46, 3085–3096. [Google Scholar] [CrossRef]
- Pharande, P.S.; Rashinkar, G.S.; Pore, D.M. Cellulose Schiff base-supported Pd(II): An efficient heterogeneous catalyst for Suzuki Miyaura cross-coupling. Res. Chem. Intermed. 2021, 47, 4457–4476. [Google Scholar] [CrossRef]
- Bezerra, R.D.S.; Leal, R.C.; da Silva, S.M.; Morais, I.S.A.; Marques, H.C.T.; Osajima, A.J.; Meneguin, B.A.; Barud, H.D.S.; da Silva Filho, C.E. Direct Modification of Microcrystalline Cellulose with Ethylenediamine for Use as Adsorbent for Removal Amitriptyline Drug from Environment. Molecules 2017, 22, 2039. [Google Scholar] [CrossRef]
- Qiao, Y.; Teng, J.; Wang, S.; Ma, H. Amine-Functionalized Sugarcane Bagasse: A Renewable Catalyst for Efficient Continuous Flow Knoevenagel Condensation Reaction at Room Temperature. Molecules 2018, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.M.; Tahir, P.M.; Liyana, R.; Javahershenas, R. A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties. Molecules 2018, 23, 2470. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, N.; Akhtar, J.; Ahmad, N.M.; Ali, A.; Zia, M. Management of citrus waste by switching in the production of nanocellulose. IET Nanobiotechnol. 2016, 10, 395–399. [Google Scholar] [CrossRef]
- Safari, J.; Ahmadzadeh, M.; Zarnegar, Z. Sonochemical synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones by amine-modified montmorillonite nanoclay. Catal. Commun. 2016, 86, 91–95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).