Aldehydes: What We Should Know About Them
Abstract
1. Introduction
2. Volatile Organic Compounds (VOCs)
3. Aldehydes
4. Aliphatic Aldehydes
4.1. Formaldehyde (Methanal)
4.2. Acetaldehyde (Ethanal)
4.3. n-Butanal (n-Butyraldehyde) and 2-Methylpropanal (Iso-Butyraldehyde or Iso-Butanal)
4.4. 2-Methylbutanal and 3-Methylbutanal (or Isovaleraldehyde)
4.5. Pentanal (Valeraldeheyde)
4.6. Hexanal (Caproaldehyde, Caproic Aldehyde, Capraldehyde, Capronaldehyde)
4.7. Heptanal (Enanthaldehyde)
4.8. Octanal (Caprylaldehyde)
4.9. Nonanal (Pelargonaldehyde)
4.10. Decanal (Caprinaldehyde)
4.11. Undecanal
4.12. Dodecanal (or Lauraldehyde or Lauric Aldehyde)
4.13. Tridecanal
4.14. Tetradecanal (or Myristyl Aldehyde)
4.15. Hexadecanal
4.16. Acrolein (or Propenal)
4.17. Citral
4.18. Other Aliphatic Aldehydes
5. Aromatic Aldehydes
5.1. Benzaldehyde
5.2. Furfural (2-Furaldehyde, Furan-2-Carboxaldehyde, Pyromucic Aldehyde, Pyromucic Aldehyde)
5.3. 4-Hydroxy-3-Methoxybenzaldehyde (Vanillin)
5.4. 4-Methoxybenzaldehyde (Anisaldehyde, para-Anisaldehyde, p-Anisaldehyde)
5.5. 2-Hydroxy-4-Methoxybenzaldehyde
5.6. Phenylpropanals—Floral Aromatic Aldehydes
5.6.1. 2-Phenylpropanal and 3-Phenylpropanal
5.6.2. 3-(4-Isopropylphenyl)propanal (PHCA) or 3-(p-Cumenyl)propionaldehyde
5.6.3. 3-(4-tert-Butylphenyl)propanal (BHCA, Bourgeonal, Lilional, Isolilial)
5.6.4. 3-(4-Isobutyl-2-Methylphenyl)propanal (Nympheal®)
5.6.5. 3-(4-Isopropylphenyl)-2-Methylpropanal (Cyclamen Aldehyde, PMHCA) or 3-p-Cumenyl-2-Methylpropionaldehyde (CPA)
5.6.6. 3-(4-Isobutylphenyl)-2-Methylpropanal (iBMHCA, Silvial®)
5.6.7. 2-(4-tert-Butylbenzyl)propionaldehyde (BMHCA, Lilial, Lysmeral)
5.6.8. 3-(4-Ethylphenyl)-2,2-Dimethylpropanal (Floralozone, Florone)
5.7. Cinnamaldehyde and Derivatives
5.7.1. Cinnamaldehyde (Cinnamic Aldehyde, Cinnamal, 3-Phenylacrolein, 3-Phenylpropenal)
5.7.2. Amyl Cinnamal (Jasminaldehyde, Amyl Cinnamaldehyde)
5.7.3. α-Hexylcinnamaldehyde (HCA, Hexyl Cinnamal)
6. Toxicity of Aldehydes and Mitigation of Their Toxic Effects in Humans
7. Methods for Removal of Aldehydes from Water and Air
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feron, V.J.; Kruysse, A.; Til, H.P.; Immel, H.R.; Cassee, F.R. Aldehydes: Occurrence, Carcinogenic Potential, Mechanism of Action and Risk Assessment. Mutat. Res. Rev. Genet. Toxicol. 1991, 259, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, R.; Wick, E.L.; Hornstein, I. Flavor Chemistry. In Flavor Chemistry: Thirty Years of Progress; Teranishi, R., Wick, E.L., Hornstein, I., Eds.; Springer: Boston, MA, USA, 1999; pp. 1–8. [Google Scholar]
- Takhar, M.; Li, Y.; Ditto, J.C.; Chan, A.W. Formation Pathways of Aldehydes from Heated Cooking Oils. Environ. Sci. Process. Impacts 2023, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Langton, K.; Patlewicz, G.Y.; Long, A.; Marchant, C.A.; Basketter, D.A. Structure-activity Relationships for Skin Sensitization: Recent Improvements to Derek for Windows. Contact Dermat. 2006, 55, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.L.; Shi, J.F.; Zhang, M.; Dalapati, R.; Tian, Q.Y.; Chen, S.; Wang, C.Y.; Zang, L. Optical Chemosensors for the Gas Phase Detection of Aldehydes: Mechanism, Material Design, and Application. Mater. Adv. 2021, 2, 6213–6245. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Hernandes, K.C.; Nicolli, K.P.; Souza-Silva, É.A.; Manfroi, V.; Zini, C.A.; Welke, J.E. Development of a Method for Determination of Target Toxic Carbonyl Compounds in Must and Wine Using HS-SPME-GC/MS-SIM after Preliminary GC× GC/TOFMS Analyses. Food Anal. Methods 2019, 12, 108–120. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; Alkhoori, M.A.; Hag-Ali, M.; Cheng, W.H.; Lim, S.H.; Loh, J.Y.; Lai, K.S. Contribution of Aldehydes and their Derivatives to Antimicrobial and Immunomodulatory Activities. Molecules 2022, 27, 3589. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential oils. In Essential Oil Research; Malik, S., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Schober, L.; Dobiašová, H.; Jurkaš, V.; Parmeggiani, F.; Rudroff, F.; Winkler, M. Enzymatic Reactions towards Aldehydes: An Overview. Flavour Fragr. J. 2023, 38, 221–242. [Google Scholar] [CrossRef]
- Floss, M.A.; Fink, T.; Maurer, F.; Volk, T.; Kreuer, S.; Müller-Wirtz, L.M. Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review. Molecules 2022, 27, 5258. [Google Scholar] [CrossRef]
- Magnano, M.C.; Ahmed, W.; Wang, R.; Marušič, M.B.; Fowler, S.J.; White, I.R. Exhaled Volatile Organic Compounds and Respiratory Disease: Recent Progress and Future Outlook. TrAC Trends Anal. Chem. 2024, 176, 117739. [Google Scholar] [CrossRef]
- Lv, J.J.; Li, X.Y.; Shen, Y.C.; You, J.X.; Wen, M.Z.; Wang, J.B.; Yang, X.T. Assessing Volatile Organic Compounds Exposure and Chronic Obstructive Pulmonary Diseases in US Adults. Front. Public Health 2023, 11, 1210136. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.S.; Calleja, L.F.; Moreno-Sánchez, R.; Yoval-Sánchez, B. Role of Aldehyde Dehydrogenases in Physiopathological Processes. Chem. Res. Toxicol. 2019, 32, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Stras, A.; Grassmann, A.; Van Campenhout, P.; Deconinck, E.; Vanhaecke, T.; Desmedt, B. Analysis of Preservatives and Fragrances in Topical Medical Devices: The Need for More Stringent Regulation. Contact Dermat. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Occupational and Environmental Health Team, Guidelines for Air Quality. 2000. Available online: https://apps.who.int/iris/handle/10665/66537 (accessed on 18 October 2024).
- U.S. Environmental Protection Agency. Fundamentals of Indoor Air Quality in Buildings. 2018. Available online: https://www.jm.com/en/blog/2019/november/fundamentals-of-indoor-air-quality-in-buildings/ (accessed on 18 June 2024).
- Zhu, G.; Xiao, Z. Flavors and Fragrances: Structure of Various Flavors with Food Ingredients. In Flavors and Fragrances in Food Processing: Preparation and Characterization Methods; ACS Publications: Washington, DC, USA, 2022; pp. 21–188. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S. Influence of Fragrances on Human Psychophysiological Activity: With Special Reference to Human Electroencephalographic Response. Sci. Pharm. 2016, 84, 724–751. [Google Scholar] [CrossRef] [PubMed]
- SCCNFP (Scientific Committee on Cosmetic Products and Non-Food Products). The First Update of the Inventory of Ingredients Employed in Cosmetic Products. Section II: Perfume and Aromatic Raw Materials. SCCNFP/0389. 2000. Available online: www.leffingwell.com/cosmetics/out131_en.pdf (accessed on 18 June 2024).
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Di Giacomo, S.; Pagano, E.; Borrelli, F.; Mazzanti, G. Genotoxicity assessment of some cosmetic and food additives. Regul. Toxicol. Pharm. 2014, 68, 16–22. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on Flavouring Group Evaluation 94, Revision 1 (FGE. 94Rev1): Consideration of aliphatic amines and amides evaluated in an addendum to the group of aliphatic and aromatic amines and amides evaluated by the JECFA (68th meeting). EFSA J. 2012, 10, 2747. [Google Scholar]
- Min, C.; Biyi, M.; Jianneng, L.; Yimin, L.; Yijun, L.M.; Long, C. Characterization of the Volatile Organic Compounds Produced from Green Coffee in Different Years by Gas Chromatography Ion Mobility Spectrometry. RSC Adv. 2022, 12, 15534–15542. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Ling, L.; Wang, Y.; Cheng, W.; Jiang, K.; Luo, H.; Pang, M.; Yue, R. Research Progress of Volatile Organic Compounds Produced by Plant Endophytic Bacteria in Control of Postharvest Diseases of Fruits and Vegetables. World J. Microbiol. Biotechnol. 2023, 39, 149. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Hashim, N.; Shamsudin, R.; Che Man, H. Rice for Food Security: Revisiting its Production, Diversity, Rice Milling Process and Nutrient Content. Agriculture 2022, 12, 741. [Google Scholar] [CrossRef]
- Theloke, J.; Friedrich, R. Compilation of a Database on the Composition of Anthropogenic VOC Emissions for Atmospheric Modeling in Europe. Atmospher. Environ. 2007, 41, 4148–4160. [Google Scholar] [CrossRef]
- Alabdulhadi, A.; Ramadan, A.; Devey, P.; Boggess, M.; Guest, M. Inhalation Exposure to Volatile Organic Compounds in the Printing Industry. J. Air Waste Manag. Assoc. 2019, 69, 1142–1169. [Google Scholar] [CrossRef]
- McDonald, B.C.; De Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile Chemical Products Emerging as Largest Petrochemical Source of Urban Organic Emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Gkatzelis, G.I.; Coggon, M.M.; McDonald, B.C.; Peischl, J.; Gilman, J.B.; Aikin, K.C.; Robinson, M.A.; Canonaco, F.; Prevot, A.S.H.; Trainer, M.; et al. Observations Confirm that Volatile Chemical Products Are a Major Source of Petrochemical Emissions in U.S. Cities. Environ. Sci. Technol. 2021, 55, 4332–4343. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Pervez, S.; Majumdar, D.; Chakrabarty, R.; Pervez, Y.F. Emission Estimation of Aromatic and Halogenated VOCs from Household Solid Fuel Burning Practices. Int. J. Environ. Sci. Technol. 2019, 16, 2683–2692. [Google Scholar] [CrossRef]
- Palmisani, J.; Nørgaard, A.W.; Kofoed-Sørensen, V.; Clausen, P.A.; de Gennaro, G.; Wolkoff, P. Formation of Ozone-Initiated VOCs and Secondary Organic Aerosol Following Application of a Carpet Deodorizer. Atmos. Environ. 2020, 222, 117149. [Google Scholar] [CrossRef]
- Davies, H.L.; O’Leary, C.; Dillon, T.; Shaw, D.R.; Shaw, M.; Mehra, A.; Phillips, G.; Carslaw, N. A measurement and modelling investigation of the indoor air chemistry following cooking activities. Environ. Sci. Proc. Imp. 2023, 25, 1532–1548. [Google Scholar] [CrossRef]
- Rádis-Baptista, G. Do Synthetic Fragrances in Personal Care and Household Products Impact Indoor Air Quality and Pose Health Risks? J. Xenobiotics 2023, 13, 121–131. [Google Scholar] [CrossRef]
- Asif, Z.; Chen, Z.; Haghighat, F.; Nasiri, F.; Dong, J. Estimation of Anthropogenic VOCs Emission Based on Volatile Chemical Products: A Canadian Perspective. Environ. Manag. 2022, 71, 685–703. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.C. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef]
- Ancione, G.; Lisi, R.; Milazzo, M.F. Human health risk associated with emissions of volatile organic compounds due to the ship-loading of hydrocarbons in refineries. Atmos. Pollut. Res. 2021, 12, 432–442. [Google Scholar] [CrossRef]
- Mo, Z.; Lu, S.; Shao, M. Volatile organic compound (VOC) emissions and health risk assessment in paint and coatings industry in the Yangtze River Delta, China. Environ. Pollut. 2020, 269, 115740. [Google Scholar] [CrossRef] [PubMed]
- Mahilang, M.; Deb, M.K.; Pervez, S. Biogenic Secondary Organic Aerosols: A Review on Formation Mechanism, Analytical Challenges and Environmental Impacts. Chemosphere 2021, 262, 127771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, S.; Gao, B.; Bi, F.; Qiao, R.; Yang, Y.; Wu, M.; Zhang, X. A systematic review of intermediates and their characterization methods in VOCs degradation by different catalytic technologies. Sep. Purif. Technol. 2023, 314, 123510. [Google Scholar] [CrossRef]
- Moloney, M.G. Reactions of Aldehydes and Ketones and Their Derivatives. Org. React. Mech. 2020, 2024, 1–45. [Google Scholar] [CrossRef]
- da Silva, F.M.; Junior, J.J.; Hernández Muñoz, J.A. The Chemistry of Aldehydes and Ketones in the Synthesis of Heterocycles-Historical Reactions with a New and Green Perspective. Curr. Org. Chem. 2024, 28, 1023–1045. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, L.; Liu, L.; Jia, C.; Zheng, Y.; Shang, H.; Sun, H.; Cui, B. Recent Advances in Trifluoromethylation of Olefins, Aldehydes, and Ketones. Curr. Org. Chem. 2024, 28, 1229–1243. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavor Chemicals (Aroma Chemicals); Allured Publishing Corporation: Carol Stream, IL, USA, 1994. [Google Scholar]
- Kapadia, N.; Meyers, K.; Jain, S.; Modi, S. A Cross-Sectional Survey Analysis of the Human Olfactory Senses for Perfumes and its Alternations due to COVID-19. Int. J. Curr. Sci. Res. Rev. 2023, 6, 3870–3888. [Google Scholar] [CrossRef]
- Chen, T.; Xue, Y.; Li, C.; Zhao, Y.; Huang, H.; Feng, Y.; Xiang, H.; Chen, S. Identification of Key Volatile Compounds in Tilapia during Air Frying Process by Quantitative Gas Chromatography–Ion Mobility Spectrometry. Molecules 2024, 29, 4516. [Google Scholar] [CrossRef]
- Atamaleki, A.; Motesaddi Zarandi, S.; Massoudinejad, M.; Hesam, G.; Naimi, N.; Esrafili, A.; Fakhri, Y.; Mousavi Khaneghah, A. Emission of aldehydes from different cooking processes: A review study. Air Qual. Atmos. Health 2022, 15, 1183–1204. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Z.; Shi, L.; Son, J.H.; Li, L.; Wang, L.; Chen, J. Investigating Aldehyde and Ketone Compounds Produced from Indoor Cooking Emissions and Assessing their Health Risk to Human Beings. J. Environ. Sci. 2023, 127, 389–398. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, G.-H.; Yin, F.; Liu, Z.; Wang, J.; Zhou, D.; Shahidi, F.; Zhu, B. Effects of Roasting Temperature and Time on Aldehyde Formation Derived from Lipid Oxidation in Scallop (Patinopecten yessoensis) and the Deterrent Effect by Antioxidants of Bamboo Leaves. Food Chem. 2022, 369, 130936. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chan, A.W. Particulate Matter and Volatile Organic Compound Emissions Generated from a Domestic Air Fryer. Environ. Sci. Technol. 2023, 57, 17384–17392. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Silcock, P.; Bremer, P. Flavour Volatiles of Fermented Vegetable and Fruit Substrates: A Review. Molecules 2023, 28, 3236. [Google Scholar] [CrossRef]
- Albarri, R.; Vardara, H.F.; Al, S.; Önal, A. Chromatographic Methods and Sample Pretreatment Techniques for Aldehydes, Biogenic Amine, and Carboxylic Acids in Food Samples. Crit. Rev. Anal. Chem. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A Review of the Volatiles from the Healthy Human Body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Hossen, M.F.; Zahan1, M.K.; Haque, M.M.; Zamir, R.; Asraf, M.A. Synthesis, Characterization, Thermal Analysis and Antibacterial Activity of Cu(II) and Ni(II) Complexes with Thiosemicarbazone Derived from Thiophene-2-aldehyde. J. Mater. Sci. Res. Rev. 2020, 5, 15–25. [Google Scholar]
- Lin, J.; Meng, H.; Guo, X.; Tang, Z.; Yu, S. Natural Aldehyde-Chitosan Schiff Base: Fabrication, pH-Responsive Properties, and Vegetable Preservation. Foods 2023, 12, 2921. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, L.; Tan, W.; Li, Q.; Guo, Z. The Antioxidant and Antibacterial Activities of the Pyridine-4-aldehyde Schiff Bases Grafted Chloracetylchitosan Oligosaccharide Derivatives. Starch-Stärke 2023, 75, 2100268. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal Complexes with Schiff Bases: Data Collection and Recent Studies on Biological Activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef]
- Vera, S.; Landa, A.; Mielgo, A.; Ganboa, I.; Oiarbide, M.; Soloshonok, V. Catalytic Asymmetric α-Functionalization of α-Branched Aldehydes. Molecules 2023, 28, 2694. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Giuzio, F.; Saturnino, C.; Longo, P.; Sinicropi, M.S. Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics 2023, 11, 320. [Google Scholar] [CrossRef]
- Salem, H.; Cullumbine, H. Inhalation toxicities of some aldehydes. Toxicol. Appl. Pharmacol. 1960, 2, 183–187. [Google Scholar] [CrossRef]
- El-Maghrabey, M.H.; El-Shaheny, R.; El Hamd, M.A.; Al-Khateeb, L.A.; Kishikawa, N.; Kuroda, N. Aldehydes’ Sources, Toxicity, Environmental Analysis, and Control in Food. In Organic Pollutants: Toxicity and Solutions; Vasanthy, M., Sivasankar, V., Sunitha, T.G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 117–151. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef]
- Laskar, A.A.; Younus, H. Aldehyde Toxicity and Metabolism: The Role of Aldehyde Dehydrogenases in Detoxification, Drug Resistance and Carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef]
- Sinharoy, P.; Mcallister, S.L.; Vasu, M.; Gross, E.R. Environmental Aldehyde Sources and the Health Implications of Exposure. In Aldehyde Dehydrogenases. Advances in Experimental Medicine and Biology; Ren, J., Zhang, Y., Ge, J., Eds.; Springer: Singapore, 2019; Volume 1193, pp. 35–52. [Google Scholar] [CrossRef]
- Conklin, D.J.; Guo, Y.; Nystoriak, M.A.; Jagatheesan, G.; Obal, D.; Kilfoil, P.J.; Hoetker, J.D.; Guo, L.; Bolli, R.; Bhatnagar, A. TRPA1 Channel Contributes to Myocardial Ischemia-Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H889–H899. [Google Scholar] [CrossRef]
- Ribeaucourt, D.; Bissaro, B.; Lambert, F.; Lafond, M.; Berrin, J.G. Biocatalytic Oxidation of Fatty Alcohols into Aldehydes for the Flavors and Fragrances Industry. Biotechnol. Adv. 2022, 56, 107787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Z.; Wang, Y. Bioaldehydes and Beyond: Expanding the Realm of Bioderived Chemicals Using Biogenic Aldehydes as Platforms. Curr. Opin. Chem. Biol. 2020, 59, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kazimírová, V.; Rebroš, M. Production of Aldehydes by Biocatalysis. Int. J. Mol. Sci. 2021, 22, 4949. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.F.; He, W.M. Recent Advances in the Photocatalytic Synthesis of Aldehydes. Org. Chem. Front. 2023, 10, 4198–4210. [Google Scholar] [CrossRef]
- Dickey, R.M.; Forti, A.M.; Kunjapur, A.M. Advances in Engineering Microbial Biosynthesis of Aromatic Compounds and Related Compounds. Bioresourc. Bioprocess. 2021, 8, 91. [Google Scholar] [CrossRef]
- Liu, H.; Ma, L.; Chen, J.; Zhao, F.; Huang, X.; Dong, X.; Zhu, B.; Qin, L. Effect of Aliphatic Aldehydes on Flavor Formation in Glutathione–Ribose Maillard Reactions. Foods 2023, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Peng, W.Y.; Strand, C.L.; Hanson, R.K. Quantitative Measurements of Broad-band Mid-infrared Absorption Spectra of Formaldehyde, Acetaldehyde, and Acetone at Combustion-relevant Temperatures near 5.7 µm. J. Quantit. Spectrosc. Radiat. Transfer 2020, 248, 106981. [Google Scholar] [CrossRef]
- Dattilo, S.; Gugliuzzo, C.; Mirabella, E.F.; Puglisi, C.; Scamporrino, A.A.; Zampino, D.C.; Samperi, F. Characterization of VOCs and Additives in Italian PET Bottles and Studies on Potential Functional Aldehydes Scavengers. Eur. Food Res. Technol. 2022, 248, 1407–1420. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Liu, X.; Wang, Y.; Yang, W.; Zhao, W.; Zhao, G.; Cui, H.; Wen, J. Identification of Characteristic Aroma Compounds in Chicken Meat and their Metabolic Mechanisms Using Gas Chromatography-Olfactometry, Odor Activity Values, and Metabolomics. Food Res. Int. 2023, 175, 113782. [Google Scholar] [CrossRef] [PubMed]
- Wallington, T.J.; Anderson, J.E.; Dolan, R.H.; Winkler, S.L. Vehicle Emissions and Urban Air Quality: 60 Years of Progress. Atmosphere 2022, 13, 650. [Google Scholar] [CrossRef]
- Shi, B.; Chai, Y.; Qin, P.; Zhao, X.-X.; Li, W.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q.; Yao, H.; Qu, W.-J. Detection of Aliphatic Aldehydes by a Pillar[5]arene-based Fluorescent Supramolecular Polymer with Vaporchromic Behavior. Chem. Asian. J. 2020, 17, e202101421. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-J.; Liu, W.-C.; Lu, F.-N.; Tang, Y.; Yuan, Z.-Q. Recent Progress in Fluorescent Formaldehyde Detection Using Small Molecule Probes. J. Anal. Test. 2022, 6, 204–215. [Google Scholar] [CrossRef]
- Ahangar, R.M.; Farmanzadeh, D. O-doping Effects on the Adsorption and Detection of Acetaldehyde and Ethylene Oxide on Phosphorene Monolayer: A DFT Investigation. Chem. Phys. Lett. 2023, 813, 140315. [Google Scholar] [CrossRef]
- Pennings, J.L.A.; Cremers, J.W.J.M.; Becker, M.J.A.; Klerx, W.N.M.; Talhout, R. Aldehyde and Volatile Organic Compound Yields in Commercial Cigarette Mainstream Smoke Are Mutually Related and Depend on the Sugar and Humectant Content in Tobacco. Nicotine Tob. Res. 2020, 22, 1748–1756. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Bruze, M.; Cadby, P.; Calow, P.; Dagli, M.L.; Dekant, W.; Ellis, G.; Fryer, A.D.; Fukayama, M.; et al. Criteria for the Research Institute for Fragrance Materials, Inc. (RIFM) safety evaluation process for fragrance ingredients. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 82, S1–S19. [Google Scholar] [CrossRef]
- REACH 2006. Regulation (EC) No. 1907/2006 (REACH) Official Journal of the European Union. Available online: https://osha.europa.eu/it/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council (accessed on 18 October 2024).
- Brewer, T.F.; Chang, C.J. An Aza-Cope Reactivity-Based Fluorescent Probe for Imaging Formaldehyde in Living Cells. J. Am. Chem. Soc. 2015, 137, 10886–10889. [Google Scholar] [CrossRef] [PubMed]
- Dou, K.; Chen, G.; Yu, F.; Liu, Y.; Chen, L.; Cao, Z.; Chen, T.; Li, Y.; You, J. Bright and Sensitive Ratiometric Fluorescent Probe Enabling Endogenous FA Imaging and Mechanistic Exploration of Indirect Oxidative Damage Due to FA in Various Living Systems. Chem. Sci. 2017, 8, 7851–7861. [Google Scholar] [CrossRef]
- Blondel, A.; Plaisance, H. Screening of Formaldehyde Indoor Sources and Quantification of Their Emission Using a Passive Sampler. Build. Environ. 2011, 46, 1284–1291. [Google Scholar] [CrossRef]
- Tang, Y.; Kong, X.; Liu, Z.R.; Xu, A.; Lin, W. Lysosome-Targeted Turn-On Fluorescent Probe for Endogenous Formaldehyde in Living Cells. Anal. Chem. 2016, 88, 9359–9363. [Google Scholar] [CrossRef]
- Sutherland, B.W.; Toews, J.; Kast, J. Utility of Formaldehyde Cross-Linking and Mass Spectrometry in the Study of Protein–Protein Interactions. J. Mass Spectrom. 2008, 43, 699–715. [Google Scholar] [CrossRef]
- Zhu, H.B.; She, J.Y.; Zhou, M.L.; Fan, X.D. Rapid and Sensitive Detection of Formaldehyde Using Portable 2-Dimensional Gas Chromatography Equipped with Photoionization Detectors. Sens. Actuators B Chem. 2019, 283, 182–187. [Google Scholar] [CrossRef]
- International-Agency-for-Research-on-Cancer. Formaldehyde, 2-Butoxyethanol and 1-Tert-Butoxy-2-Propanol; World Health Organization: Lyon, France, 2006. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO: Geneva, Switzerland, 2006; Volume 88, pp. 39–325. [Google Scholar]
- International Agency for Research on Cancer. Chemical Agents and Related Occupations. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO: Geneva, Switzerland, 2012; Volume 100, pp. 9–562. [Google Scholar]
- Salthammer, T. The Formaldehyde Dilemma. Int. J. Hyg. Environ. Health 2015, 218, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Reingruber, H.; Pontel, L.B. Formaldehyde Metabolism and Its Impact on Human Health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- Hoffman, E.A.; Frey, B.L.; Smith, L.M.; Auble, D.T. Formaldehyde Crosslinking: A Tool for the Study of Chromatin Complexes. J. Biol. Chem. 2015, 290, 26404–26411. [Google Scholar] [CrossRef]
- Adamović, D.; Čepić, Z.; Adamović, S.; Stošić, M.; Obrovski, B.; Morača, S.; Miloradov, M.V. Occupational Exposure to Formaldehyde and Cancer Risk Assessment in an Anatomy Laboratory. Int. J. Environ. Res. Public Health 2021, 18, 11198. [Google Scholar] [CrossRef]
- Kang, D.S.; Kim, H.S.; Jung, J.H.; Lee, C.M.; Ahn, Y.S.; Seo, Y.R. Formaldehyde Exposure and Leukemia Risk: A Comprehensive Review and Network-based Toxicogenomic Approach. Genes Environ. 2021, 43, 13. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.S.; Labib, D.A.; Kamel, M.M. Carvedilol Can Attenuate Histamine-Induced Paw Edema and Formaldehyde-Induced Arthritis in Rats without Risk of Gastric Irritation. Int. Immunopharmacol. 2017, 50, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, L.; Barbosa, E.; Charão, M.F.; Brucker, N. Formaldehyde Toxicity Reports from In Vitro and In Vivo Studies: A Review and Updated Data. Drug. Chem. Toxicol. 2022, 45, 972–984. [Google Scholar] [CrossRef]
- Nishikawa, A.; Nagano, K.; Kojima, H.; Ogawa, K. A Comprehensive Review of Mechanistic Insights into Formaldehyde-induced Nasal Cavity Carcinogenicity. Regul. Toxicol. Pharmacol. 2021, 123, 104937. [Google Scholar] [CrossRef]
- Andersen, M.E.; Gentry, P.R.; Swenberg, J.A.; Mundt, K.A.; White, K.W.; Thompson, C.; Bus, J.; Sherman, J.H.; Greim, H.; Bolt, H.; et al. Considerations for Refining the Risk Assessment Process for Formaldehyde: Results from an Interdisciplinary Workshop. Reg. Toxicol. Pharmacol. 2019, 106, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Magaña, L.C.; Cui, H.; Huang, J.; McHale, C.M.; Yang, X.; Looney, M.R.; Li, R.; Zhang, L. Formaldehyde-induced Hematopoietic Stem and Progenitor Cell Toxicity in Mouse Lung and Nose. Arch. Toxicol. 2021, 95, 693–701. [Google Scholar] [CrossRef]
- Awad, J.; Jung, C. Evaluating the Indoor Air Quality after Renovation at the Greens in Dubai, United Arab Emirates. Buildings 2021, 11, 353. [Google Scholar] [CrossRef]
- WHO. Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization Regional Office for Europe: Bonn, Germany, 2010; ISBN 978 92 890 0213 4. [Google Scholar]
- van den Broek, J.; Cerrejon, D.K.; Pratsinis, S.E.; Güntner, A.T. Selective Formaldehyde Detection at ppb in Indoor Air with a Portable Sensor. J. Hazard. Mater. 2020, 399, 123052. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Nishio, G.; Sawai, M.; Saito, T.; Kudo, H.; Saito, H.; Otsuka, K.; Noguer, T.; Marty, J.L. A Bio-sniffer Stick with FALDH (formaldehyde dehydrogenase) for Convenient Analysis of Gaseous Formaldehyde. Sens. Actuat. B-Chem. 2008, 130, 32–37. [Google Scholar] [CrossRef]
- He, Q.; Li, J.; Feng, Q. Ppb-level Formaldehyde Detection System Based on a 3.6 µm Interband Cascade Laser and Mode-locked Cavity Enhanced Absorption Spectroscopy with Self-calibration of the Locking Frequency. Infrared Phys. Technol. 2020, 105, 103205. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Tan, Y.; Mo, R.; Zhang, Y. A CuO thin film type sensor via inkjet printing technology with high reproducibility for ppb-level formaldehyde detection. Sens. Actuators B Chem. 2022, 362, 131775. [Google Scholar] [CrossRef]
- Protano, C.; Antonucci, A.; De Giorgi, A.; Zanni, S.; Mazzeo, E.; Cammalleri, V.; Fabiani, L.; Mastrantonio, R.; Muselli, M.; Mastrangeli, G.; et al. Exposure and Early Effect Biomarkers for Risk Assessment of Occupational Exposure to Formaldehyde: A Systematic Review. Sustainability 2024, 16, 3631. [Google Scholar] [CrossRef]
- Arias-Pérez, I.; Sáenz-Navajas, M.P.; de-la-Fuente-Blanco, A.; Ferreira, V.; Escudero, A. Insights on the Role of Acetaldehyde and Other Aldehydes in the Odour and Tactile Nasal Perception of Red Wine. Food Chem. 2021, 361, 130081. [Google Scholar] [CrossRef] [PubMed]
- Majchrowicz, E.; Mendelsen, J.H. Blood Concentrations of Acetaldehyde and Ethanol in Chronic Alcoholics. Science 1970, 168, 1100–1102. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency: Research Triangle Park, NC, USA. Available online: https://nepis.epa.gov/Exe/ZyNET.EXE?ZyActionL=Register&User=anonymous&Password=anonymous&Client=EPA&Init=1 (accessed on 18 October 2024).
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley-VCH: Weinheim, Germany, 2001; p. 293. [Google Scholar]
- Sanz-Novo, M.; Belloche, A.; Rivilla, V.M.; Garrod, R.T.; Alonso, J.L.; Redondo, P.; Barrientos, C.; Kolesniková, L.; Valle, J.C.; Rodríguez-Almeida, L.; et al. Toward the Limits of Complexity of interstellar chemistry: Rotational spectroscopy and astronomical search for n-and i-butanal. Astron. Astrophys. 2022, 666, A114. [Google Scholar] [CrossRef]
- Chisega-Negrilă, C.G.; Diacon, A.; Călinescu, I.; Vînătoru, M.; Berger, D.; Matei, C.; Vasilievici, G. On the Ultrasound-assisted Preparation of Cu/SiO2 System as a Selective Catalyst for the Conversion of Biobutanol to Butanal. Chem. Pap. 2022, 76, 1443–1455. [Google Scholar] [CrossRef]
- Chang, C.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Deep-fried Flavor: Characteristics, Formation Mechanisms, and Influencing Factors. Crit. Rev. Food Sci. Nutr. 2020, 60, 1496–1514. [Google Scholar] [CrossRef]
- Rosati, J.A.; Krebs, K.A.; Liu, X. Emissions from Cooking Microwave Popcorn. Crit. Rev. Food Sci. Nutr. 2007, 47, 701–709. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Chen, J.; Xie, J.; Shen, S.; Deng, Y.; Zhu, J.; Yuan, H.; Jiang, Y. Characterization of the Key Aroma Compounds in Black Teas with Different Aroma Types by Using Gas Chromatography Electronic Nose, Gas Chromatography-Ion Mobility Spectrometry, and Odor Activity Value Analysis. LWT 2022, 163, 113492. [Google Scholar] [CrossRef]
- Del Toro-Gipson, R.S.; Rizzo, P.V.; Hanson, D.J.; Drake, M. Sensory Characterization of Specific Wood Smoke Aromas and their Contributions to Smoked Cheddar Cheese Flavor. J. Sens. Studies 2020, 35, e12564. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, J.; Yu, H.; Lou, X.; Wang, B.; Xu, Z.; Tian, H. Cloning, Purification, and Characterization of Branched-chain α-keto Acid Decarboxylases from Lactococcus lactis Strains with Different 3-Methylbutanal Production Abilities. Food Biosci. 2022, 47, 101713. [Google Scholar] [CrossRef]
- Du, W.; Zhao, M.; Zhen, D.; Tan, J.; Wang, T.; Xie, J. Key Aroma Compounds in Chinese Fried Food of Youtiao. Flav. Fragr. J. 2020, 35, 88–98. [Google Scholar] [CrossRef]
- Meng, H.Y.; Piccand, M.; Fuchsmann, P.; Dubois, S.; Baumeyer, A.; Tena Stern, M.; Von Ah, U. Formation of 3-Methylbutanal and 3-Methylbutan-1-ol Recognized as Malty during Fermentation in Swiss Raclette-Type Cheese, Reconstituted Milk, and de Man, Rogosa, and Sharpe Broth. J. Agric. Food Chem. 2021, 69, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xu, C.; Chen, L.; Yang, J.; Qiao, M.; Wu, Z. Effect of Different Cooking Methods on the Aroma and Taste of Chicken Broth. Molecules 2024, 29, 1532. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Z.; Yang, G.; Huang, S.; Liao, S.; Chen, K.; Du, M.; Zalan, Z.; Hegyi, F.; Kan, J. Biocontrol Potential of 1-Pentanal Emitted from Lactic Acid Bacteria Strains against Aspergillus flavus in Red Pepper (Capsicum annuum L.). Food Control 2022, 142, 109261. [Google Scholar] [CrossRef]
- Frankel, E.N. Volatile lipid oxidation products. Progr. Lip. Res. 1983, 22, 1–33. [Google Scholar] [CrossRef]
- Cho, Y.; Song, M.; Kim, T.S.; Ryu, J.C. DNA Methylome Analysis of Saturated Aliphatic Aldehydes in Pulmonary Toxicity. Sci. Rep. 2018, 8, 10497. [Google Scholar] [CrossRef]
- Majchrzak, T.; Marc, M.; Wasik, A. Understanding the Early-Stage Release of Volatile Organic Compounds from Rapeseed Oil During Deep-Frying of Tubers by Targeted and Omics-Inspired Approaches Using Ptr-Ms and Gas Chromatography. Food Res. Int. 2022, 160, 111716. [Google Scholar] [CrossRef]
- Müller-Wirtz, L.M.; Kiefer, D.; Maurer, F.; Floss, M.A.; Doneit, J.; Hüppe, T.; Shopova, T.; Wolf, B.; Sessler, D.I.; Volk, T.; et al. Volutrauma Increases Exhaled Pentanal in Rats: A Potential Breath Biomarker for Ventilator-Induced Lung Injury. Anesth. Analg. 2021, 133, 263–273. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, H.; Liu, Z.; Liu, M.; Qi, P.; Di, S.; Zhang, S.; Wang, X. Recent Advances on Mulberry Volatile Flavor: A Review. J. Food Composit. Anal. 2023, 124, 105665. [Google Scholar] [CrossRef]
- Ernstgård, L.; Iregren, A.; Sjögren, B.; Svedberg, U.; Johanson, G. Acute Effects of Exposure to Hexanal Vapors in Humans. J. Occup. Environ. Med. 2006, 48, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gao, Y.; Wang, K.; Sun, S.M.; Liu, Z.; Yan, P.; Feng, J.R.; Li, Q.S.; Li, L.W.; Wang, D.J. Dwarf Interstocks Improve Aroma Quality of ‘Huahong’ Apple (Malus × domestica). Agriculture 2022, 12, 1710. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, S.; Mao, J.; Li, W.; Li, W.; Zuo, C.; Chu, M.; Zhao, X.; Zhou, Q.; Chen, B. Effects of Shading on the Synthesis of Volatile Organic Compounds in ‘Marselan’ grape berries (Vitis vinifera L.). J. Plant Growth Regul. 2021, 40, 679–693. [Google Scholar] [CrossRef]
- Öz, A.T.; Kafkas, E. Volatile Compositions of Strawberry Fruit during Shelf Life Using Pre and Postharvest Hexanal Treatment. J. Food Process. Preserv. 2022, 46, e16464. [Google Scholar] [CrossRef]
- Furia, T.E.; Bellanca, N. Fenaroli’s Handbook of Flavor Ingredients; CRC Press, Inc.: Boca Raton, FL, USA, 1975; Volume 2. [Google Scholar]
- Thomas, S.L.; Myers, C.; Schug, K.A. Comparison of Fragrance and Flavor Components in Non-psilocybin and Psilocybin Mushrooms Using Vacuum-assisted Headspace High-capacity Solid-phase Microextraction and Gas Chromatography–mass Spectrometry. Adv. Sample Prep. 2023, 8, 100090. [Google Scholar] [CrossRef]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing Volatile Compounds of Wild Edible Nordic Mushrooms Analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef]
- Ashitha, G.N.; Sunny, A.C.; Nisha, R. Effect of Pre-harvest and Post-harvest Hexanal Treatments on Fruits and Vegetables: A Review. Agricult. Rev. 2020, 41, 124–131. [Google Scholar] [CrossRef]
- Paliyath, G.; Padmanabhan, P. Chapter 4: Preharvest and Postharvest Technologies Based on Hexanal: An Overview. In Postharvest Biology and Nanotechnology, 1st ed.; John Wiley & Sons, Inc.: Pondicherry, India, 2019; pp. 89–101. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, G.; Brar, J.S. Pre-Harvest Application of Hexanal Formulations for Improving Post-Harvest Life and Quality of Mango (Mangifera indica L.) Cv. Dashehari. J. Food Sci. Technol. 2020, 57, 4257–4264. [Google Scholar] [CrossRef] [PubMed]
- Öz, A.T.; Eryol, B.; Ali, M.A. Postharvest Hexanal Application Delays Senescence and Maintains Quality in Persimmon Fruit During Low-temperature Storage. J. Sci. Food Agricult. 2023, 103, 7653–7663. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, D.; Sundaresan, S.; Iyadurai, A.; Subramanian, K.S.; Janavi, G.J.; Paliyath, G.; Subramanian, J. Hexanal vapor induced resistance against major postharvest pathogens of banana (Musa acuminata L.). Plant Pathol. J. 2020, 36, 133–147. [Google Scholar] [CrossRef]
- Öz, A.T.; Ali, A. Retaining Overall Quality of Fresh Figs by Postharvest Hexanal Vapor Treatment during Cold Storage. Postharvest Biol. Technol. 2023, 205, 112539. [Google Scholar] [CrossRef]
- Cho, Y.; Song, M.K.; Jeong, S.C.; Lee, K.; Heo, Y.; Kim, T.S.; Ryu, J.C. MicroRNA Response of Inhalation Exposure to Hexanal in Lung Tissues from Fischer 344 Rats. Environ. Toxicol. 2016, 31, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in Exhaled Breath Condensate of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Tietje, O.; Wong, C. Prediction of Breast Cancer Using Volatile Biomarkers in the Breath. Breast Cancer Res. Treat. 2006, 99, 19–21. [Google Scholar] [CrossRef]
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The Application of Statistical Methods Using VOCs to Identify Patients with Lung Cancer. J. Breath Res. 2011, 5, 046008. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The Regulation of Key Flavor of Traditional Fermented Food by Microbial Metabolism: A Review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef]
- Yanru, X.U.; Qingzheng, W.A.N.G.; Guizhang, G.U.; Zhang, J.; Dalun, X.U. Study on the Function of Pepper Essential Oil in Endowing Flavor of Pepper-Salt Baked Shrimp. Shipin Gongye Ke-Ji. [CrossRef]
- de Flaviis, R.; Sacchetti, G.; Mastrocola, D. Wheat Classification According to Its Origin by an Implemented Volatile Organic Compounds Analysis. Food Chem. 2021, 341, 128217. [Google Scholar] [CrossRef]
- Basile, G.; De Maio, A.C.; Catalano, A.; Ceramella, J.; Iacopetta, D.; Bonofiglio, D.; Saturnino, C.; Sinicropi, M.S. Ancient Wheat as Promising Nutraceuticals for the Prevention of Chronic and Degenerative Diseases. Curr. Med. Chem. 2023, 30, 3384–3403. [Google Scholar] [CrossRef]
- Şavşatlı, Y. Identification of Volatile Compounds in Salep (Serapias vomeracea) Tubers and Effects of Harvest Time and Drying Method on Composition Variation. Ciênc. Agrotec. 2023, 47, e002223. [Google Scholar] [CrossRef]
- Catalano, A. COVID-19: Could Irisin Become the Handyman Myokine of the 21st Century? Coronaviruses 2020, 1, 32–41. [Google Scholar] [CrossRef]
- Zhu, A.; Luo, X. Detection of Covid-19 through a Heptanal Biomarker Using Transition Metal Doped Graphene. J. Phys. Chem. B 2022, 126, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, S.E.; Zhu, S.; Jia, W.; Sun, J.; Zhao, X.E.; Liu, H. 13-Plex UHPLC–MS/MS Analysis of Hexanal and Heptanal Using Multiplex Tags Chemical isotope Labeling Technology. J. Am. Soc. Mass Spectrom. 2020, 31, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Azorín, C.; López-Juan, A.L.; Aparisi, F.; Benedé, J.L.; Chisvert, A. Determination of Hexanal and Heptanal in Saliva Samples by an Adapted Magnetic Headspace Adsorptive Microextraction for Diagnosis of Lung Cancer. Anal. Chim. Acta 2023, 1271, 341435. [Google Scholar] [CrossRef]
- Reineccius, G. Flavoring Ingredients Classified As GRAS By the Flavor Extract Manufacturers Association. In Source Book of Flavors; Reineccius, G., Ed.; Springer: Boston, MA, USA, 1994; pp. 655–670. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and Biological Activities of Decanal, Linalool, Valencene, and Octanal from Sweet Orange Oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, B.; Parihar, N.; Ilaweibaphyrnai, M.; Panda, S.R.; Alexander, A.; Chella, N.; Murty, U.S.N.; Naidu, V.G.M.; Jagadeesh, K.G.; et al. Nutraceutical Prospects of Houttuynia cordata against the Infectious Viruses. Food Biosci. 2022, 50, 101977. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal Activity of Citral, Octanal and α-Terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Duan, B.; Zhang, Y.; Feng, Z.; Liu, Z.; Tao, N. Octanal Enhances Disease Resistance in Postharvest Citrus Fruit by the Biosynthesis and Metabolism of Aromatic Amino Acids. Pestic. Biochem. Physiol. 2024, 200, 105835. [Google Scholar] [CrossRef]
- Song, M.K.; Lee, H.S.; Choi, H.S.; Shin, C.Y.; Kim, Y.J.; Park, Y.K.; Ryu, J.C. Octanal-induced Inflammatory Responses in Cells Relevant for Lung Toxicity: Expression and Release of Cytokines in A549 Human Alveolar Cells. Human Exp. Toxicol. 2014, 33, 710–721. [Google Scholar] [CrossRef]
- Noordraven, L.E.; Petersen, M.A.; Van Loey, A.M.; Bredie, W.L. Flavour stability of sterilised chickpeas stored in pouches. Curr. Res. Food Sci. 2021, 4, 773–783. [Google Scholar] [CrossRef]
- Takakura, Y.; Osanai, H.; Masuzawa, T.; Wakabayashi, H.; Nishimura, T. Characterization of the Key Aroma Compounds in Pork Soup Stock by Using an Aroma Extract Dilution Analysis. Biosci. Biotechnol. Biochem. 2014, 78, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Wettasinghe, M.; Vasanthan, T.; Temelli, F.; Swallow, K. Volatile Flavour Composition of Cooked By-product Blends of Chicken, Beef and Pork: A Quantitative GC–MS Investigation. Food Res. Int. 2001, 34, 149–158. [Google Scholar] [CrossRef]
- Chang, H.; Wang, Y.; Xia, Q.; Pan, D.; He, J.; Zhang, H.; Cao, J. Characterization of the Physicochemical Changes and Volatile Compound Fingerprinting during the Chicken Sugar-smoking Process. Poult. Sci. 2021, 100, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Suh, J.H.; Gmitter, F.G.; Wang, Y. Differentiation between Flavors of Sweet Orange (Citrus sinensis) and Mandarin (Citrus reticulata). J. Agric. Food Chem. 2018, 66, 203–211. [Google Scholar] [CrossRef]
- Lan-Phi, N.T. Gas chromatography-–olfactometry and aroma--active components in citrus essential oils. In Citrus Essential Oils: Flavor and Fragrance; Wiley: Hoboken, NJ, USA, 2010; pp. 201–227. [Google Scholar]
- Boukouvalas, J.; Jean, M.A. Streamlined Biomimetic Synthesis of Paracaseolide A via Aerobic Oxidation of a 2-Silyloxyfuran. Tetrahedron Lett. 2014, 55, 4248–4250. [Google Scholar] [CrossRef]
- Varghese, C.P.; Murugaiyah, V.; Parasuraman, S.; Christina, A.J.M. Bioactive Phytoconstituents and Biological Activities of Polygonum minus Huds. In Bioactive Compounds in the Storage Organs of Plants; Springer: Cham, Switzerland, 2024; pp. 1–19. [Google Scholar] [CrossRef]
- Perkins, J.; Hayashi, T.; Peakall, R.; Flematti, G.R.; Bohman, B. The volatile chemistry of orchid pollination. Nat. Prod. Rep. 2023, 40, 819–839. [Google Scholar] [CrossRef]
- Mishor, E.; Amir, D.; Weiss, T.; Honigstein, D.; Weissbrod, A.; Livne, E.; Gorodisky, L.; Karagach, S.; Ravia, A.; Sobel, N. Sniffing the Human Body Volatile Hexadecanal Blocks Aggression in Men but Triggers Aggression in Women. Sci. Adv. 2021, 7, eabg1530. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, C.; Liu, F.; Zheng, J.; Ou, J.; Zhao, D.; Ou, S. Origin and Fate of Acrolein in Foods. Foods 2022, 11, 1976. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Some Industrial Chemicals. IARC Monographs on the Evaluation of Carcinogenic Risk for Chemicals to Humans; IARC: Lyon, France, 1994; Volume 60, p. 435.
- Daniali, G.; Jinap, S.; Hajeb, P.; Sanny, M. Acrylamide Formation in Vegetable Oils and Animal Fats during Heat Treatment. Food Chem. 2016, 212, 244–249. [Google Scholar] [CrossRef]
- Song, Y.; Ding, Z.; Peng, Y.; Wang, J.; Zhang, T.; Yu, Y.; Wang, Y. Acrylamide Formation and Aroma Evaluation of Fried Pepper Sauce under Different Exogenous Maillard Reaction Conditions. Food Chem. X 2022, 15, 100413. [Google Scholar] [CrossRef]
- Ambaw, A.; Zheng, L.; Tambe, M.A.; Strathearn, K.E.; Acosta, G.; Hubers, S.A.; Liu, F.; Herr, S.A.; Tang, J.; Truong, A.; et al. Acrolein-mediated Neuronal Cell Death and alpha-Synuclein Aggregation: Implications for Parkinson’s Disease. Mol. Cell. Neurosci. 2018, 88, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Girish Subbiah, K.; Kemparaju, K.; Thirunavukkarasu, C. Neutrophil Extracellular Traps in Acrolein Promoted Hepatic Ischemia Reperfusion Injury: Therapeutic Potential of NOX2 and P38MAPK Inhibitors. J. Cell. Physiol. 2017, 233, 3244–3261. [Google Scholar] [CrossRef] [PubMed]
- Burcham, P.C. Acrolein and Human Disease: Untangling the Knotty Exposure Scenarios Accompanying Several Diverse Disorders. Chem. Res. Toxicol. 2017, 30, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Hikisz, P.; Jacenik, D. The Tobacco Smoke Component, Acrolein, as a Major Culprit in Lung Diseases and Respiratory Cancers: Molecular Mechanisms of Acrolein Cytotoxic Activity. Cells 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, W.; Wu, Q.; Zhou, Q. Acrolein: Formation, health hazards and its controlling by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2023, 64, 9604–9617. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Y.; Zheng, B.; Chen, Y.; Xie, J.; Song, Y.; Ding, X.; Hu, X.; Hu, X.; Yu, Q. The Role of Acrolein in Neurodegenerative Diseases and Its Protective Strategy. Foods 2022, 11, 3203. [Google Scholar] [CrossRef]
- Guo, J.; Hecht, S.S. DNA damage in human oral cells induced by use of e-cigarettes. Drug Testing Anal. 2023, 15, 1189–1197. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Carcinogenic Components of Tobacco and Tobacco Smoke: A 2022 Update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Wu, J.C.; Pan, M.H.; Ho, C.T. Reactivity and Stability of Selected Flavor Compounds. J. Food Drug Anal. 2015, 23, 176–190. [Google Scholar] [CrossRef]
- Paoli, M.; Maroselli, T.; Casanova, J.; Bighelli, A. A Fast and Reliable Method to Quantify Neral and Geranial (Citral) in Essential Oils using 1H NMR Spectroscopy. Flav. Fragr. J. 2023, 38, 476–482. [Google Scholar] [CrossRef]
- Lu, W.C.; Huang, D.W.; Wang, C.C.R.; Yeh, C.H.; Tsai, J.C.; Huang, Y.T.; Li, P.H. Preparation, Characterization, and Antimicrobial Activity of Nanoemulsions Incorporating Citral Essential Oil. J. Food Drug Anal. 2018, 26, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.; Isman, M. Metabolism of Citral, the Major Constituent of Lemongrass Oil, in the Cabbage Looper, Trichoplusia ni, and Effects of Enzyme Inhibitors on Toxicity and Metabolism. Pestic. Biochem. Physiol. 2016, 133, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, C.M.; Ganev, E.G.; Mazzardo-Martins, L.; Martins, D.F.; Rocha, L.R.M.; Santos, A.R.S.; Hiruma-Lima, C.A. Citral: A Monoterpene with Prophylactic and Therapeutic Anti-Nociceptive Effects in Experimental Models of Acute and Chronic Pain. Eur. J. Pharmacol. 2014, 736, 16–25. [Google Scholar] [CrossRef]
- Bailly, C. Targets and Pathways Involved in the Antitumor Activity of Citral and its Stereo-isomers. Eur. J. Pharmacol. 2020, 871, 172945. [Google Scholar] [CrossRef]
- Zheng, Y.; Shang, Y.; Li, M.; Li, Y.; Ouyang, W. Antifungal Activities of cis-trans Citral Isomers against Trichophyton rubrum with ERG6 as a Potential Target. Molecules 2021, 26, 4263. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yin, Z.; Lu, X.; Shen, D.; Dou, D. Plant Secondary Metabolite Citral Interferes with Phytophthora Capsici Virulence by Manipulating the Expression of Effector Genes. Mol. Plant Pathol. 2023, 24, 932–946. [Google Scholar] [CrossRef]
- Cramer, G.M.; Ford, R.A.; Hall, R.L. Estimation of Toxic Hazard—A Decision Tree Approach. Food Cosmet. Toxicol. 1976, 16, 255–276. [Google Scholar] [CrossRef]
- Kroes, R.; Renwick, A.G.; Feron, V.; Galli, C.L.; Gibney, M.; Greim, H.; Guy, R.H.; Lhuguenot, J.C.; van de Sandt, J.J. Application of the Threshold of Toxicological Concern (TTC) to the Safety Evaluation of Cosmetic Ingredients. Food Chem. Toxicol. 2007, 45, 2533–2562. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann , J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM fragrance Ingredient Safety Assessment, 4-Tricyclodecylidene Butanal, CAS Registry Number 30168-23-1. Food Chem. Toxicol. 2022, 159, 112704. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Cancellieri, M.A.; Chon, H.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, β,4-Dimethylcyclohex-3-ene-1-propan-1-al, CAS Registry Number 6784-13-0. Food Chem. Toxicol. 2022, 165, 113174. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, 1-Methyl-4-(4-methyl-3-pentenyl)cyclohex-3-ene-1-carbaldehyde, CAS registry number 52475-86-2. Food Chem. Toxicol. 2022, 163, 113029. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, α,α,6,6-Tetramethylbicyclo[3.1.1]hept-2-ene-2-propionaldehyde, CAS Registry Number 33885-52-8. Food Chem. Toxicol. 2021, 153, 112364. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Xie, T.; Xie, J.R.; Chen, C.; Ai, L.Z.; Tian, H.X. Aroma Perceptual Interactions of Benzaldehyde, Furfural, and Vanillin and their Effects on the Descriptor Intensities of Huangjiu. Food Res. Int. 2020, 129, 108808. [Google Scholar] [CrossRef]
- Ramachanderan, R.; Schaefer, B. Lily-of-the-valley fragrances. Chemtexts 2019, 5, 11. [Google Scholar] [CrossRef]
- Ohrmann, E.; Chandrasekaran, V.; Hölscher, B.; Kraft, P. On the Structure–Odor Correlation of Muguet Aldehydes: Synthesis of 3-(4′-Isobutyl-2′-methylphenyl)propanal (Nympheal) and Four Novel Derivatives from a Hagemann’s Ester. Helv. Chim. Acta 2023, 106, e202300040. [Google Scholar] [CrossRef]
- Jordi, S.; Kraft, P. Crossing the Boundaries between Marine and Muguet: Discovery of Unusual Lily-of-the-Valley Odorants Devoid of Aldehyde Functions. Helvet. Chim. Acta 2018, 101, e1800048. [Google Scholar] [CrossRef]
- Chung, F.Y.; Lin, Y.Z.; Huang, C.R.; Huang, K.W.; Chen, Y.F. Crosslinking Kiwifruit-derived DNA with Natural Aromatic Aldehydes Generates Membranolytic Antibacterial Nanogels. Int. J. Biol. Macromol. 2024, 255, 127947. [Google Scholar] [CrossRef]
- Laue, H.; Kern, S.; Badertscher, R.P.; Ellis, G.; Natsch, A. p-Alkyl-benzoyl-CoA Conjugates as Relevant Metabolites of Aromatic Aldehydes with Rat Testicular Toxicity—Studies Leading to the Design of a Safer New Fragrance Chemical. Toxicol. Sci. 2017, 160, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Opgrande, J.L.; Brown, E.E.; Hesser, M.; Andrews, J. Benzaldehyde. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Verma, T.; Verma, P.; Singh, U.P. A Multi Responsive Phosphonic Acid Based Fluorescent Sensor for Sensing Fe3+, Benzaldehyde and Antibiotics. Microchem. J. 2023, 191, 108771. [Google Scholar] [CrossRef]
- Pepe, R.C.; Wenninger, J.A.; McEwen, G.N., Jr. , International Cosmetic Ingredient Dictionary and Handbook, 9th ed.; CTFA: Washington, DC, USA, 2002; Volume 1, p. 132. [Google Scholar]
- Jermnak, U.; Ngernmeesri, P.; Yurayart, C.; Poapolathep, A.; Udomkusonsri, P.; Poapolathep, S.; Phaochoosak, N. A New Benzaldehyde Derivative Exhibits Antiaflatoxigenic Activity against Aspergillus flavus. J. Fungi 2023, 9, 1103. [Google Scholar] [CrossRef]
- Andersen, A. Final Report on the Safety Assessment of Benzaldehyde. Int. J. Toxicol. 2006, 25 (Suppl. S1), 11–27. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.J.L.; Ramos, A.G.B.; Freitas, T.S.; Barbosa, C.; de Sousa Júnior, D.L.; Siyadatpanah, A.; Nejat, M.; Wilairatana, P.; Coutinho, H.D.M.; da Cunha, F.A.B. Evaluation of Benzaldehyde as an Antibiotic Modulator and Its Toxic Effect against Drosophila melanogaster. Molecules 2021, 26, 5570. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.L. Benzaldehyde Use to Protect Seeds from Foodborne Fungal Pathogens. Biol. Life Sci. Forum 2022, 18, 8873–8894. [Google Scholar] [CrossRef]
- Writer, C.I.R. Safety Assessment of Benzaldehyde as Used in Cosmetics. 2023. Available online: www.cir-safety.org (accessed on 18 October 2024).
- Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.D.; Zhou, Q.; Zhang, L. Furfural Production from Biomass Residues: Current Technologies, Challenges and Future Prospects. Biomass Bioenergy 2022, 161, 106458. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, X.; Ali, M.M.; Liang, J.; Du, Z. Analysis of the Evolution of Potential and Free Furfural Compounds in the Production Chain of Infant Formula and Risk Assessment. Food Chem. 2022, 368, 130814. [Google Scholar] [CrossRef]
- Mehrotra, S.; Rai, P.; Sharma, S.K. A Quick and Simple Paper-based Method for Detection of Furfural and 5-Hydroxymethylfurfural in Beverages and Fruit Juices. Food Chem. 2022, 377, 131532. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; NadeemZafar, M.; Dargahi, A.; Pirdadeh, F. Synthesis of Magnesium Oxide Nanoparticles and its Application for Photocatalytic Removal of Furfural from Aqueous Media: Optimization Using Response Surface Methodology. Arab. J. Chem. 2023, 16, 104998. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Zhang, Z.; Ayepa, E.; Xiang, Q.; Yu, X.; Zhao, K.; Zou, L.; Gu, Y.; Li, X.; et al. Discovery of New Strains for Furfural Degradation Using Adaptive Laboratory Evolution in Saccharomyces cerevisiae. J. Hazard. Mater. 2023, 459, 132090. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Arjan, N. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Feng, Y.; Sun, J.; Jiang, Y.; Zhang, W.; Xin, F.; Jiang, M. Current Status, Challenges, and Prospects for the Biological Production of Vanillin. Fermentation 2023, 9, 389. [Google Scholar] [CrossRef]
- Banerjee, G.; Chattopadhyay, P. Vanillin Biotechnology: The Perspectives and Future. J. Sci. Food Agric. 2019, 99, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liaqat, F.; Sun, J.; Khazi, M.I.; Xie, R.; Zhu, D. Advances in the Vanillin Synthesis and Biotransformation: A Review. Renew. Sustain. Energy Rev. 2024, 189, 113905. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Khazi, M.I.; Sun, J.; Zhu, D. Natural Deep Eutectic Solvents-based Green Extraction of Vanillin: Optimization, Purification, and Bioactivity Assessment. Front. Nutr. 2024, 10, 1279552. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.J.; Wickramasinghe, P.C.; Munafo, J.P., Jr. Key Odorants from Daldinia childiae. Flav. Fragr. J. 2020, 35, 722–733. [Google Scholar] [CrossRef]
- Xin, Y.; Peng, S.; Wei, S.; Lei, Y.; Zhang, S.; Hu, Y.; Lv, Y. Antimicrobial and Biofilm Inhibition Effects of p-Anisaldehyde against Vibrio parahaemolyticus. Food Control 2023, 154, 110021. [Google Scholar] [CrossRef]
- Adewunmi, Y.; Namjilsuren, S.; Walker, W.D.; Amato, D.N.; Amato, D.V.; Mavrodi, O.V.; Patton, D.L.; Mavrodi, D.V. Antimicrobial Activity of, and Cellular Pathways Targeted by, p-Anisaldehyde and Epigallocatechin Gallate in the Opportunistic Human Pathogen Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2020, 86, e02482-19. [Google Scholar] [CrossRef]
- Che, J.X.; Chen, X.M.; Ouyang, Q.L.; Tao, N.G. p-Anisaldehyde Exerts its Antifungal Activity against Penicillium digitatum and Penicillium italicum by Disrupting the Cell Wall Integrity and Membrane Permeability. J. Microbiol. Biotechnol. 2019, 30, 878–884. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, W.; Lei, Y.; Wei, S.; Zhang, S.; Li, N.; Hu, Y.; Lv, Y. Antifungal Mechanism of p-Anisaldehyde against Aspergillus flavus Based on Transcriptome Analysis. LWT 2024, 195, 115844. [Google Scholar] [CrossRef]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Basir, S.F.; Manzoor, N.; Khan, L.A. Exposure of Candida to p-Anisaldehyde Inhibits its Growth and Ergosterol Biosynthesis. J. General. App. Microbiol. 2011, 57, 129–136. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Meng, R.; Zhao, Z.; Liu, Z.; Zhao, X.; Shi, C.; Guo, N. Efficacy of a Combination of Nisin and p-Anisaldehyde against Listeria monocytogenes. Food Control 2016, 66, 100–106. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, X.; Meng, R.; Liu, Z.; Zhang, G.; Guo, N. Synergistic Antimicrobial Effects of Nisin and p-Anisaldehyde on Staphylococcus aureus in Pasteurized Milk. LWT 2017, 84, 222–230. [Google Scholar] [CrossRef]
- Dal-Ah, K.I.M.; Kong, K.H.; Hyun-Jeong, C.H.O.; Mi-Ran, L.E.E. Role of p-Anisaldehyde in the Differentiation of C2C12 Myoblasts. Korean J. Clin. Lab. Sci. 2023, 55, 184–194. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Scientific Opinion on Flavouring Group Evaluation 414 (FGE. 414): 2-hydroxy-4-methoxybenzaldehyde. EFSA 2021, 19, e06883. [Google Scholar] [CrossRef]
- Harohally, N.V.; Cherita, C.; Bhatt, P.; Anu Appaiah, K.A. Antiaflatoxigenic and Antimicrobial Activities of Schiff Bases of 2-Hydroxy-4-methoxybenzaldehyde, Cinnamaldehyde, and Similar Aldehydes. J. Agric. Food Chem. 2017, 65, 8773–8778. [Google Scholar] [CrossRef]
- Rathi, N.; Harwalkar, K.; Jayashree, V.; Sharma, A.; Rao, N.N. 2-Hydroxy-4-methoxybenzaldehyde, an Astounding Food Flavoring Metabolite: A Review. AJPCR 2017, 10, 105–110. [Google Scholar] [CrossRef][Green Version]
- Gangopadhyay, M.; Das, A.K.; Sahu, R.; Saha, A.; Dey, S.; Bandyopadhyay, S.; Mitra, A. Evaluation of Growth Response for Mass Production and Accumulation of 2-Hydroxy-4-methoxybenzaldehyde in Endangered Hemidesmus indicus by an Aeroponic System. Ind. Crops Prod. 2021, 172, 114072. [Google Scholar] [CrossRef]
- Rodrigues, V.; Kumar, A.; Prabhu, K.N.; Pragadheesh, V.S.; Shukla, A.K.; Sundaresan, V. Adventitious Root Cultures of Decalepis salicifolia for the Production of 2-Hydroxy-4-methoxybenzaldehyde, a Vanillin Isomer Flavor Metabolite. Appl. Microbiol. Biotechnol. 2021, 105, 3087–3099. [Google Scholar] [CrossRef]
- Andati, R.E.; Omolo, M.O.; Ndiege, I.O. Ovicidal Activity of 2-Hydroxy-4-Methoxybenzaldehyde, Derivatives and Structural Analogues on Anopheles gambiae eggs. bioRxiv 2021, 2021, 460396. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R.; Naidu, R.T. Establishment of In Vitro Cell Suspension Culture, Kinetics of Cell Growth, pH, Nutrient Uptake and Production of 2-Hydroxy-4-methoxybenzaldehyde from the Germinated Root of Decalepis hamiltonii Wight & Arn.-An Endangered Plant. Curr. Appl. Sci. Technol. 2022, 22, 10–55003. [Google Scholar] [CrossRef]
- Arunachalam, K.; Ravi, J.; Tian, X.; Shunmugiah, K.P.; Shanmugaraj, G.; Shi, C. Antibacterial Activity of 2-Hydroxy-4-methoxybenzaldehyde and its Possible Mechanism against Staphylococcus aureus. J. Appl. Microbiol. 2023, 134, lxad144. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, X.; Xie, Y.; Ren, S. 2-Hydroxy-4-methoxybenzaldehyde Inhibits the Growth of Aspergillus flavus via Damaging Cell Wall, Cell Membrane, Manipulating Respiration thus Creating a Promising Antifungal Effect on Corn Kernels. Int. J. Food Sci. Technol. 2020, 56, 178–184. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Xiao, H.; Zhang, Y.; Xie, Y. 2-Hydroxy-4-methoxybenzaldehyde, a More Effective Antifungal Aroma than Vanillin and its Derivatives against Fusarium graminearum, Destroys Cell Membranes, Inhibits DON Biosynthesis, and Performs a Promising Antifungal Effect on Wheat Grains. Front. Microbiol. 2024, 15, 1359947. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, D.; Rajaiah, A.; Swasthika, R.; Balu, P.; Gopalakrishnan, A.; Krishna Kumar, A.K.; Muthusamy, S.; Malayandi, J.; Durairaj, R.; Arumugam, V.R. Evaluation of Bcr/cflA Targeted Efflux Inhibitory Potential of 2-Hydroxy-4-Methoxybenzaldehyde against Proteus mirabilis. Ind. J. Microbiol. 2024, in press. [CrossRef]
- Durgam, M.K.; Bodiga, V.L.; Vemuri, P.K.; Aenugu, V.R.; Bodiga, S. 2-Hydroxy-4-methoxy benzaldehyde from Hemidesmus indicus Root Extract Suppresses Toll-like rRceptor2-mediated Migration and Invasive Mechanisms in Rheumatoid Arthritis. J. Herbal Med. 2023, 42, 100820. [Google Scholar] [CrossRef]
- Zviely, M. The Phenylpropanals–Floral Aromatic Aldehydes. Perf. Flav. 2012, 37, 52–55. [Google Scholar]
- Jin, R.; Xu, Z.; Feng, J.; Wang, M.; Yao, P.; Wu, Q.; Zhu, D. Stereocomplementary Synthesis of β-Aryl Propanamines by Enzymatic Dynamic Kinetic Resolution-Reductive Amination. Eur. J. Org. Chem. 2023, 26, e202300476. [Google Scholar] [CrossRef]
- Mosciano, G.; Fasano, M.; Cassidy, J.; Connelly, K.; Mazeiko, P.; Montenegro, A. Organoleptic Characteristics of Flavor Materials. Perf. Flav. 1994, 19, 53–55. [Google Scholar]
- Lozynskyi, A.; Karkhut, A.; Polovkovych, S.; Karpenko, O.; Holota, S.; Gzella, A.K.; Lesyk, R. 3-Phenylpropanal and Citral in the Multicomponent Synthesis of Novel Thiopyrano[2,3-d]thiazoles. Results Chem. 2022, 4, 100464. [Google Scholar] [CrossRef]
- Avila, E.; Nixarlidis, C.; Shon, Y.S. Water-Soluble Pd Nanoparticles for the Anti-Markovnikov Oxidation of Allyl Benzene in Water. Nanomaterials 2023, 13, 348. [Google Scholar] [CrossRef]
- Gorbachev, D.; Smith, E.; Argent, S.P.; Newton, G.N.; Lam, H.W. Synthesis of New Morphinan Opioids by TBADT-Catalyzed Photochemical Functionalization at the Carbon Skeleton. Chemistry 2022, 28, e202201478. [Google Scholar] [CrossRef]
- Hareng, L.; Schuster, P.; Haake, V.; Walk, T.; Herold, M.; Laue, H.; Natsch, A. Towards the Mechanism of Spermatotoxicity of p-tert-Butyl-alpha-methylhydrocinnamic Aldehyde: Inhibition of Late Stage Ex-Vivo Spermatogenesis in Rat Seminiferous Tubule Cultures by para-tert-Butyl-Benzoic Acid. Arch. Toxicol. 2023, 97, 279–294. [Google Scholar] [CrossRef] [PubMed]
- ECHA. European Chemicals Agency: Summary of Classification and Labeling, 3-(p-Cumenyl)propionaldehyde. 2016. Available online: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/84780 (accessed on 18 June 2024).
- ECHA Substance Information. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.038.182 (accessed on 18 June 2024).
- Movalli, P.; Biesmeijer, K.; Gkotsis, G.; Alygizakis, N.; Nika, M.C.; Vasilatos, K.; Kostakis, M.; Thomaidis, N.S.; Oswald, P.; Oswaldova, M.; et al. High Resolution Mass Spectrometric Suspect Screening, Wide-scope Target Analysis of Emerging Contaminants and Determination of Legacy Pollutants in Adult Black-tailed Godwit Limosa limosa limosa in the Netherlands–A Pilot Study. Chemosphere 2023, 321, 138145. [Google Scholar] [CrossRef]
- Goeke, A.; Kraft, P.; Laue, H.; Zou, Y.; Voirol, F. Int. Pat. Appl. WO 2014180945 A1, 13 November 2014. [Google Scholar]
- Goeke, A.; Kraft, P.; Lelievre, D.; Alchenberger, A.E. Discovery of Nympheal: The Definitive Muguet Aldehyde. Perfum. Flavor. 2018, 43, 25–40. [Google Scholar]
- West, T.F. Synthetic perfumes. Sci. Progr. (1933-) 1948, 36, 38–54. [Google Scholar]
- Yokowo, Y.; Matuwura, A.; Saburi, M.; Yoshikawa, S. Optical Activation of Some Perfume of Chiral Aldehydes via Enamines. J. Japan Oil Chemists’ Soc. 1981, 30, 109–115. [Google Scholar] [CrossRef][Green Version]
- Beghetto, V.; Matteoli, U.; Scrivanti, A.; Bertoldini, M. Asymmetric Catalysis in Fragrance Chemistry: A New Catalytic Approach to Non Racemic Cyclamen-aldehyde. Sci. Ca’ Foscari 2012, 1, 20–24. [Google Scholar] [CrossRef]
- Natsch, A.; Nordone, A.; Adamson, G.M.; Laue, H. A Species Specific Metabolism Leading to Male Rat Reprotoxicity of Cyclamen Aldehyde: In Vivo and In Vitro Evaluation. Food Chem. Toxicol. 2021, 153, 112243. [Google Scholar] [CrossRef]
- Givaudan Health and Nutrition Hub. Available online: https://healthnutritionhub.givaudan.com/?gad_source=1&gclid=CjwKCAjw68K4BhAuEiwAylp3khI7wkT_6bkOixr83NdfZ-HGuL8YRlhfb_ORS16-6rgB_UHtxRs7nRoCQkUQAvD_BwE (accessed on 10 June 2024).
- Fragrance Ingredients Compendium. Available online: https://www.iff.com/portfolio/products/fragrance-ingredients/online-compendium/ (accessed on 10 June 2024).
- Beghetto, V.; Scrivanti, A.; Bertoldini, M.; Aversa, M.; Zancanaro, A.; Matteoli, U. A Practical, Enantioselective Synthesis of the Fragrances Canthoxal and Silvial®, and Evaluation of their Olfactory Activity. Synthesis 2015, 47, 272–288. [Google Scholar] [CrossRef]
- Gil, A.; Savchuk, S.; Appolonova, S.; Nadezhdin, A.; Kakorina, E. The Composition of Nonbeverage Alcohols Consumed in Russia in 2015–2017. Rev. D’épidémiologie St. Publique 2018, 66, S355–S356. [Google Scholar] [CrossRef]
- Scherer, M.; Koch, H.M.; Schütze, A.; Pluym, N.; Krnac, D.; Gilch, G.; Leibold, E.; Scherer, G. Human Metabolism and Excretion Kinetics of the Fragrance Lysmeral after a Single Oral Dosage. Int. J. Hyg. Environ. Health 2017, 220, 123–129. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Celleno, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.; et al. SCCS Preliminary OPINION ON the Safety of Butylphenyl Methylpropional (p-BMHCA) in Cosmetic Products”-Submission II, Ref SCCS/1591/17-Preliminary Version. 2017. hal-01669154. Available online: https://www.academia.edu/68263682/SCCS_preliminary_OPINION_ON_the_safety_of_Butylphenyl_methylpropional_p_BMHCA_in_cosmetic_products_Submission_II_ref_CCS_1591_17_Preliminary_version (accessed on 18 October 2024).
- Armanino, N.; Charpentier, J.; Flachsmann, F.; Goeke, A.; Liniger, M.; Kraft, P. What’s Hot, What’s Not: The Trends of the Past 20 Years in the Chemistry of Odorants. Angew. Chem. 2020, 59, 16310–16344. [Google Scholar] [CrossRef] [PubMed]
- de Sá, L.D.M. Farmácia 5 de Outubro, Porto e Serviços Farmacêuticos do Hospital CUF, Porto. 2022. Available online: https://sigarra.up.pt/ffup/pt/PUB_GERAL.PUB_VIEW?pi_pub_base_id=588952 (accessed on 18 October 2024).
- Commission Regulation (EU) 2021/1902 of 29 October 2021 Amending Annexes II, III and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regards the Use in Cosmetic Products of Certain Substances Classified as Carcinogenic. Available online: https://eur-lex.europa.eu/eli/reg/2021/1902/oj (accessed on 18 October 2024).
- Commission Regulation (EU) 2017/1410 of 2 August 2017 Amending Annexes II and III to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R1410 (accessed on 18 October 2024).
- UNION, P. Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 18 June 2024).
- European Commission. Commission Delegated Regulation (EU) 2020/1182 of 19 May 2020 Amending, for the Purposes of Its Adaptation to Technical and Scientific Progress, Part 3 of Annex VI to Regulation (EC) no 1272/2008 of the European Parliament and of the Council on Classification, Labelling and Packaging of Substances and Mixtures. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32020R1182 (accessed on 18 June 2024).
- Soriano, L.F.; Soriano, S.K.; Buckley, D.A. Ironing water: An under-recognized source of contact allergens. Contact Dermat. 2023, 88, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Da Lycia a Nivea, Passando per Infasil e Palmolive, Ecco Tutti i Prodotti Richiamati per la Presenza di Sostanza Vietata (LISTA AGGIORNATA). Available online: https://www.greenme.it/lifestyle/sai-cosa-compri/da-lycia-a-nivea-passando-per-infasil-e-palmolive-ecco-tutti-i-prodotti-richiamati-per-la-presenza-di-sostanza-vietata-lista-aggiornata/ (accessed on 18 October 2024).
- LIlial, Nuovi Ritiri: L’Elenco Aggiornato con Oltre 130 Prodotti Nocivi Bloccati. Available online: https://ilsalvagente.it/2023/10/15/lilial-nuovi-ritiri-lelenco-aggiornato-con-oltre-130-prodotti-nocivi-bloccati/ (accessed on 18 October 2024).
- Safety Gate: The EU Rapid Alert System for Dangerous Non-Food Products. Available online: https://ec.europa.eu/safety-gate-alerts/screen/search (accessed on 18 June 2024).
- Pigłowski, M. Notifications in European Rapid Alert system for dangerous products (RAPEX). In Safety and Reliability of Systems and Processes. Summer Safety and Reliability Seminar 2023; Gdynia Maritime University: Gdynia, Poland, 2023; pp. 187–198. [Google Scholar] [CrossRef]
- Ferreira, M.; Matos, A.; Couras, A.; Marto, J.; Ribeiro, H. Overview of Cosmetic Regulatory Frameworks around the World. Cosmetics 2022, 9, 72. [Google Scholar] [CrossRef]
- Canada.ca. Cosmetic Ingredient Hotlist: Prohibited and Restricted Ingredients. 15 January 2020. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients.html (accessed on 18 June 2024).
- U.S. Food & Drug Administration. Prohibited & Restricted Ingredients in Cosmetics. 25 February 2022. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/prohibited-restricted-ingredients-cosmetics (accessed on 18 June 2024).
- U.S. Food & Drug Administration. Product Testing of Cosmetics. 25 February 2022. Available online: https://www.fda.gov/cosmetics/cosmetics-science-research/product-testing-cosmetics (accessed on 18 June 2024).
- Cosmetic Ingredient Review. About the Cosmetic Ingredient Review. Available online: https://www.cir-safety.org/about (accessed on 18 June 2024).
- Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Pellegrino, M.; Saturnino, C.; Longo, P.; Aquaro, S. Triclosan: A Small Molecule with Controversial Roles. Antibiotics 2022, 11, 735. [Google Scholar] [CrossRef]
- ECHA Disseminated Dossier. 2-(4-tert-Butylbenzyl)propionaldehyde. 2023. Available online: https://echa.europa.eu/it/substance-information/-/substanceinfo/100.001.173 (accessed on 7 March 2024).
- ECHA. Substance Infocard: 2-(4-tert-Butylbenzyl)propionaldehyde. 2023. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.001.173 (accessed on 18 June 2024).
- ECHA. European Chemicals Agency: REACH Registration Dossier, 3-(4-tert-Butylphenyl)-2-Methylpropanal. 2016. Available online: http://echa.europa.eu/registration-dossier/-/registered-dossier/13572 (accessed on 18 June 2024).
- Laue, H.; Badertscher, R.P.; Hostettler, L.; Weiner-Sekiya, Y.; Haupt, T.; Nordone, A.; Adamson, G.M.; Natsch, A. Benzoyl-CoA Conjugate Accumulation as an Initiating Event for Male Reprotoxic Effects in the Rat? Structure–Activity Analysis, Species Specificity, and In vivo Relevance. Arch. Toxicol. 2020, 94, 4115–4129. [Google Scholar] [CrossRef]
- Hunter, C.G.; Chambers, P.L.; Stevenson, D.E. Studies on the Oral Toxicity of p-tert-Butyl Benzoic Acid in Rats. Food Cosmet. Toxicol. 1965, 3, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Whorton, M.D.; Stubbs, H.A.; Obrinsky, A.; Milby, T.H. Testicular Function of Men Occupationally exposed to para-tertiary Butyl Benzoic Acid. Scand. J. Work Environ. Health 1981, 7, 204–213. [Google Scholar] [CrossRef]
- Bernard, A. Dermal exposure to hazardous chemicals in baby diapers: A re-evaluation of the quantitative health risk assessment conducted by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). Int. J. Environ. Res. Public Health 2022, 19, 4159. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. RIFM Fragrance Ingredient Safety Assessment, p-t-butyl-α-methylhydrocinnamic aldehyde, CAS Registry Number 80-54-6. Food Chem. Toxicol. 2020, 141, 111430. [Google Scholar] [CrossRef]
- Jablonská, E.; Míchal, Z.; Křížkovská, B.; Strnad, O.; Tran, V.N.; Žalmanová, T.; Petr, J.; Lipov, J.; Viktorová, J. Toxicological Investigation of Lilial. Sci. Rep. 2023, 13, 18536. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Franchini, A.; De Luca, M.; Saturnino, C.; Andreu, I.; Sinicropi, M.S.; Catalano, A. A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Appl. Sci. 2022, 12, 10909. [Google Scholar] [CrossRef]
- Murawski, A.; Fiedler, N.; Schmied-Tobies, M.I.H.; Rucic, E.; Schwedler, G.; Stoeckelhuber, M.; Scherer, G.; Pluym, N.; Scherer, M.; Kolossa-Gehring, M. Metabolites of the Fragrance 2-(4-tert-butylbenzyl)propionaldehyde (Lysmeral) in Urine of Children and Adolescents in Germany—Human Biomonitoring Results of the German Environmental Survey 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2020, 229, 113594. [Google Scholar] [CrossRef] [PubMed]
- Fichter, S.C.; Groth, K.; Fiedler, N.; Kolossa-Gehring, M.; Dębiak, M.; INGER Study Group. Lysmeral Exposure in Children and Adolescences Participating in the German Environmental Survey (2012–2015): Integrating Sex/Gender into Analysis. Int. J. Environ. Res. Public Health 2022, 19, 17072. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Rose, J.; Ellison, C.; Mudd, A.M.; Zhang, X.; Wu, S. Refine and Strengthen SAR-Based Read-Across by Considering Bioactivation and Modes of Action. Chem. Res. Toxicol. 2023, 36, 1532–1548. [Google Scholar] [CrossRef]
- Song, Y.T.; Li, S.S.; Chao, C.Y.; Guo, S.; Chen, G.Z.; Wang, S.X.; Zhang, M.X.; Yin, Y.L.; Li, P. Floralozone Regulates MiR-7a-5p Expression through AMPKα2 Activation to Improve Cognitive Dysfunction in Vascular Dementia. Exp. Neurol. 2024, 376, 114748. [Google Scholar] [CrossRef]
- Huang, N.; Qiu, Y.; Liu, Y.; Liu, T.; Xue, X.; Song, P.; Xu, J.; Fu, Y.; Sun, R.; Yin, Y.; et al. Floralozone Protects Endothelial Function in Atherosclerosis by Ameliorating NHE1. Acta Biochim. Biophys. Sin. 2021, 53, 1310–1320. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Dong, J.; Cao, L.; Huang, Z. A New Potential Strategy for Treatment of Ischemic Stroke: Targeting TRPM2–NMDAR Association. Neurosci. Bull. 2023, 39, 703–706. [Google Scholar] [CrossRef]
- Weng, X.; Ho, C.T.; Lu, M. The biological fate, health benefits and novel delivery strategies for cinnamaldehyde. Food Funct. 2024, 15, 6217–6231. [Google Scholar] [CrossRef]
- Nussbaum, L.; Hogea, L.M.; Călina, D.; Andreescu, N.; Grădinaru, R.; Ștefănescu, R.; Puiu, M. Modern Treatment Approaches in Psychoses. Pharmacogenetic, neuroimagistic and Clinical Implications. Farmacia 2017, 65, 75–81. [Google Scholar]
- Khare, P.; Jagtap, S.; Jain, Y.; Baboota, R.K.; Mangal, P.; Boparai, R.K.; Bhutani, K.K.; Sharma, S.S.; Premkumar, L.S.; Kondepudi, K.K.; et al. Cinnamaldehyde Supplementation Prevents Fasting-induced Hyperphagia, Lipid Accumulation, and Inflammation in High-fat Diet-fed Mice. Biofactors 2016, 2, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Hancı, D.; Altun, H.; Çetinkaya, E.A.; Muluk, N.B.; Cengiz, B.P.; Cingi, C. Cinnamaldehyde Is an Effective Anti-inflammatory Agent for Treatment of Allergic Rhinitis in a Rat Model. Int. J. Pediatr. Otorhinolaryngol. 2016, 84, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, S.; Kooshki, A.; Hormozi, A.; Akbari, A.; Mehrpour, O.; Farrokhfall, K. Cinnamon and Cognitive Function: A Systematic Review of Preclinical and Clinical Studies. Nutr. Neurosci. 2024, 27, 132–146. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, Z.; Jiang, W.; Zhou, W. Antimicrobial Activity of Cinnamaldehyde on Streptococcus mutans Biofilms. Front. Microbiol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; D’Amato, A.; Lauria, G.; Saturnino, C.; Andreu, I.; Longo, P.; Sinicropi, M.S. Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries. Antibiotics 2023, 12, 112. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, S. Anticancer Potential and Molecular Mechanisms of Cinnamaldehyde and Its Congeners Present in the Cinnamon Plant. Physiologia 2023, 3, 173–207. [Google Scholar] [CrossRef]
- Vezzani, B.; Perrone, M.; Carinci, M.; Palumbo, L.; Tombolato, A.; Tombolato, D.; Daminato, C.; Gentili, V.; Rizzo, R.; Campo, G.; et al. SARS-CoV-2 Infection as a Model to Study the Effect of Cinnamaldehyde as Adjuvant Therapy for Viral Pneumonia. J. Inflamm. 2023, 20, 40. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Maio, A.C.; Basile, G.; Giuzio, F.; Bonomo, M.G.; Aquaro, S.; Walsh, T.J.; Sinicropi, M.S.; et al. Are Nutraceuticals Effective in COVID-19 and Post-COVID Prevention and Treatment? Foods 2022, 11, 2884. [Google Scholar] [CrossRef]
- Guo, X.R.; Zhang, X.G.; Wang, G.S.; Wang, J.; Liu, X.J.; Deng, J.H. Effect of Cinnamaldehyde on Systemic Candida albicans Infection in Mice. Chin. J. Integr. Med. 2024, in press. [Google Scholar] [CrossRef]
- Pereira, W.; Pereira, C.; Assunção, R.; da Silva, I.; Rego, F.; Alves, L.; Santos, J.; Nogueira, F.; Zagmignan, A.; Thomsen, T.; et al. New Insights into the Antimicrobial Action of Cinnamaldehyde towards Escherichia coli and Its Effects on Intestinal Colonization of Mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef]
- Topa, S.H.; Palombo, E.A.; Kingshott, P.; Blackall, L.L. Activity of Cinnamaldehyde on Quorum Sensing and Biofilm Susceptibility to Antibiotics in Pseudomonas aeruginosa. Microorganisms 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Usai, F.; Di Sotto, A. Trans-cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies. Antibiotics 2023, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Pellegrino, M.; Giuzio, F.; Marra, M.; Rosano, C.; Saturnino, C.; Sinicropi, M.S.; Aquaro, S. Antibiotic-Resistant ESKAPE Pathogens and COVID-19: The Pandemic beyond the Pandemic. Viruses 2023, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef]
- Honma, M.; Yamada, M.; Yasui, M.; Horibata, K.; Sugiyama, K.; Masumura, K. In Vivo and in Vitro Mutagenicity of Perillaldehyde and Cinnamaldehyde. Genes Environ. 2021, 43, 30. [Google Scholar] [CrossRef]
- Alves, D.D.N.; Martins, R.X.; Ferreira, E.D.S.; Alves, A.F.; Andrade, J.C.D.; Batista, T.M.; Lazarini, J.G.; Amorim, L.S.; Rosalen, P.M.; Castro, R.D.D. Toxicological Parameters of a Formulation Containing Cinnamaldehyde for Use in Treatment of oral fungal infections: An In Vivo Study. BioMed Res. Int. 2021, 2021, 2305695. [Google Scholar] [CrossRef]
- Na, M.; O’Brien, D.; Lavelle, M.; Lee, I.; Gerberick, G.F.; Api, A.M. Weight of Evidence Approach for Skin Sensitization Potency Categorization of Fragrance Ingredients. Dermatitis 2022, 33, 161–175. [Google Scholar] [CrossRef]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; De Giorgi, M.; Guido, M.; Congedo, M.; De Donno, A. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, E50–E57. [Google Scholar] [CrossRef]
- Velambath, K.B.; Mali, S.N.; Pratap, A.P. Greener Synthesis of Jasminaldehyde via Cross Aldol Condensation Reaction Using Recyclable Phase Transfer Catalysis and its Cosmetic Application. Lett. Appl. NanoBioSci. 2023, 12, 104. [Google Scholar] [CrossRef]
- Meng, Y.; Fang, G.; Hu, Y.; Qin, Y.; Xu, R.; Yang, F.; Lei, J.; Zhang, C. Study on the Effect of Different Aldehyde Modifiers on the Fume Suppression Effect, Mechanism and Road Performance of SBS Modified Asphalt. Sci. Tot. Environ. 2024, 912, 169162. [Google Scholar] [CrossRef] [PubMed]
- Pacholczyk-Sienicka, B.; Ciepielowski, G.; Albrecht, Ł. The First Application of 1H NMR Spectroscopy for the Assessment of the Authenticity of Perfumes. Molecules 2021, 26, 3098. [Google Scholar] [CrossRef]
- EFSA. Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on List of Alpha, Beta-unsaturated Aldehydes and Ketones Representative of FGE.19 Substances for Genotoxicity Testing. EFSA J. 2008, 910, 1–7. [Google Scholar]
- Lu, J.; Zeng, X.; Feng, Y.; Li, S.; Wang, Y.; Liu, Y.; Chen, F.; Guan, Z.; Chen, T.; Wei, F. Inhibitory Effects of Jasminum grandiflorum L. Essential Oil on Lipopolysaccharide-induced Microglia Activation-integrated Characteristic Analysis of Volatile Compounds, Network Pharmacology, and BV-2 Cell. Front. Pharmacol. 2023, 14, 1180618. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ji, K. Identification of Combinations of Endocrine Disrupting Chemicals in Household Chemical Products that Require Mixture Toxicity Testing. Ecotoxicol. Environ. Saf. 2022, 240, 113677. [Google Scholar] [CrossRef]
- Matveychuk, D.; Dursun, S.M.; Wood, P.L.; Baker, G.B. Reactive Aldehydes and Neurodegenerative Disorders. Klin. Psikofarmakol. Bülteni Bull. Clin. Psychopharmacol. 2016, 21, 277–288. [Google Scholar] [CrossRef]
- Chen, C.; Lu, J.; Peng, W.; Mak, M.S.; Yang, Y.; Zhu, Z.; Wang, S.; Hou, J.; Zhou, X.; Xin, W.; et al. Acrolein, an Endogenous Aldehyde Induces Alzheimer’s Disease-like Pathologies in Mice: A New Sporadic AD Animal Model. Pharmacol. Res. 2022, 175, 106003. [Google Scholar] [CrossRef]
- Sanotra, M.R.; Kao, S.H.; Lee, C.K.; Hsu, C.H.; Huang, W.C.; Chang, T.C.; Tu, F.Y.; Hsu, I.U.; Lin, Y.F. Acrolein Adducts and Responding Autoantibodies Correlate with Metabolic Disturbance in Alzheimer’s Disease. Alzheimers Res. Ther. 2023, 15, 115. [Google Scholar] [CrossRef]
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting Breast Cancer: An Overlook on Current Strategies. Int. J. Mol. Sci. 2023, 24, 3643. [Google Scholar] [CrossRef]
- Vijayraghavan, S.; Saini, N. Aldehyde-Associated Mutagenesis-Current State of Knowledge. Chem. Res. Toxicol. 2023, 36, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, J. The Cumulative and Single Effect of 12 Aldehydes Concentrations on Cardiovascular Diseases: An Analysis Based on Bayesian Kernel Machine Regression and Weighted Logistic Regression. Rev. Cardiovasc. Med. 2024, 25, 206. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Scali, E.; Scumaci, D.; Pellegrino, M.; Aquaro, S.; Saturnino, C.; Sinicropi, M.S. Impact of Cytochrome P450 Enzymes on the Phase I Metabolism of Drugs. Appl. Sci. 2023, 13, 6045. [Google Scholar] [CrossRef]
- Jackson, B.; Brocker, C.; Thompson, D.C.; Black, W.; Vasiliou, K.; Nebert, D.W.; Vasiliou, V. Update on the Aldehyde Dehydrogenase Gene (ALDH) Superfamily. Hum. Genom. 2011, 5, 283–303. [Google Scholar] [CrossRef]
- Marchitti, S.A.; Brocker, C.; Stagos, D.; Vasiliou, V. Non-P450 Aldehyde Oxidizing Enzymes: The Aldehyde Dehydrogenase Superfamily. Expert Opin. Drug Metab. Toxicol. 2008, 4, 697–720. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Zhao, X.; Pang, J.; Pan, C.; Wang, J.; Wei, S.; Yu, X.; Zhang, C.; Chen, Y.; et al. The Role of Aldehyde Dehydrogenase 2 in Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Batsika, C.S.; Koutsilieris, C.; Koutoulogenis, G.S.; Kokotou, M.G.; Kokotos, C.G.; Kokotos, G. Light-promoted Oxidation of Aldehydes to Carboxylic Acids under Aerobic and Photocatalyst-free Conditions. Green Chem. 2022, 24, 6224–6231. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Cai, H.; He, S.; Shan, Q.; Zhao, H.; Chen, Y.; Wang, B. Catalyst-free Aerobic Oxidation of Aldehydes into Acids in Water under Mild Conditions. Green Chem. 2017, 19, 5708–5713. [Google Scholar] [CrossRef]
- Peng, W.X.; Yue, X.; Chen, H.; Ma, N.L.; Quan, Z.; Yu, Q.; Wei, Z.; Guan, R.; Lam, S.S.; Rinklebe, J.; et al. A Review of Plants Formaldehyde Metabolism: Implications for Hazardous Emissions and Phytoremediation. J. Hazard. Mater. 2022, 436, 129304. [Google Scholar] [CrossRef]
- Ono, Y.; Sekiguchi, K.; Sankoda, K.; Nii, S.; Namiki, N. Improved Ultrasonic Degradation of Hydrophilic and Hydrophobic Aldehydes in Water by Combined Use of Atomization and UV Irradiation onto the Mist Surface. Ultrason. Sonochem. 2019, 60, 104766. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Salari, M.; Talaei, M.R.; Bagheri, M.; Eskandari, Z. Formaldehyde Removal from Wastewater and Air by Using UV, Ferrate(VI) and Uv/Ferrate(VI). J. Environ. Manag. 2016, 184, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Fan, B.M.; Wen, B.Y.; Jiang, C. Experimental and Theoretical Studies on the Removal Mechanism of Formaldehyde from Water by Mesoporous Calcium Silicate. Sci. China Technol. Sci. 2020, 63, 2098–2112. [Google Scholar] [CrossRef]
- Salehi, E.; Shafie, M. Adsorptive Removal of Acetaldehyde from Water Using Strong Anionic Resins Pretreated with Bisulfite: An Efficient Method for Spent Process Water Recycling in Petrochemical Industry. J. Water Process Eng. 2020, 33, 101025. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, X.; Roberts, C.G. Method of Use for Aldehyde Removal (US7041220 B2). 2006. Available online: https://patents.google.com/patent/US7041220B2/en (accessed on 18 October 2024).
- Adeniyi, A.A.; Adeniyi, J.N.; Olumayede, E.G. The Theoretical Study of the Oxidation Reaction of Hydroxyl Radical for the Removal of Volatile Organic Aliphatic and Aromatic Aldehydes from the Atmosphere. Struct. Chem. 2023, 34, 1355–1368. [Google Scholar] [CrossRef]
- Kim, K.J.; Khalekuzzaman, M.; Suh, J.N.; Kim, H.J.; Shagol, C.; Kim, H.-H.; Kim, H.J. Phytoremediation of Volatile Organic Compounds by Indoor Plants: A Review. Hortic. Environ. Biotechnol. 2018, 59, 143–157. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Sano, N.; Yamamoto, D.; Kanki, T.; Charinpanitkul, T.; Tanthapanichakoon, W. Removal of Acetaldehyde in Air Using a Wetted—Wall Corona Discharge Reactor. Chem. Eng. J. 2004, 103, 115–122. [Google Scholar] [CrossRef]
- Sung, M.; Kato, S.; Kawanami, F.; Sudo, M. Evaluation of an Air-cleaning Unit Having Photocatalytic Sheets to Remove Acetaldehyde from Indoor Air. Build. Environ. 2010, 45, 2002–2007. [Google Scholar] [CrossRef]
- Peng, S.; Deng, Y.; Li, W.; Chen, J.; Liuac, H.; Chen, Y. Aminated Mesoporous Silica Nanoparticles for the Removal of Low-concentration Malodorous Aldehyde Gases. Environ. Sci. Nano 2018, 5, 2663–2671. [Google Scholar] [CrossRef]
- Wu, T.-C.; Peng, C.-Y.; Hsieh, H.-M.; Pan, C.-H.; Wu, M.-T.; Lin, P.-C.; Wu, C.-F.; Hsieh, T.-J. Reduction of Aldehyde Emission and Attribution of Environment Burden in Cooking Fumes from Food Stalls Using a Novel Fume Collector. Environ. Res. 2021, 195, 110815. [Google Scholar] [CrossRef]
- Vikrant, K.; Qu, Y.; Szulejko, J.E.; Kumar, V.; Vellingiri, K.; Boukhvalov, D.W.; Kim, T.; Kim, K.H. Utilization of Metal–organic Frameworks for the Adsorptive Removal of an Aliphatic Aldehyde Mixture in the Gas Phase. Nanoscale 2020, 12, 8330–8343. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.; Hajizadeh, Y.; Amin, M.M.; Kiani, G.; Haidari, R.; Falahi-Nejad, K.; Parseh, I. Biofiltration of Formaldehyde, Acetaldehyde, and Acrolein from Polluted Airstreams Using a Biofilter. J. Chem. Technol. Biotechnol. 2018, 93, 1328–1337. [Google Scholar] [CrossRef]
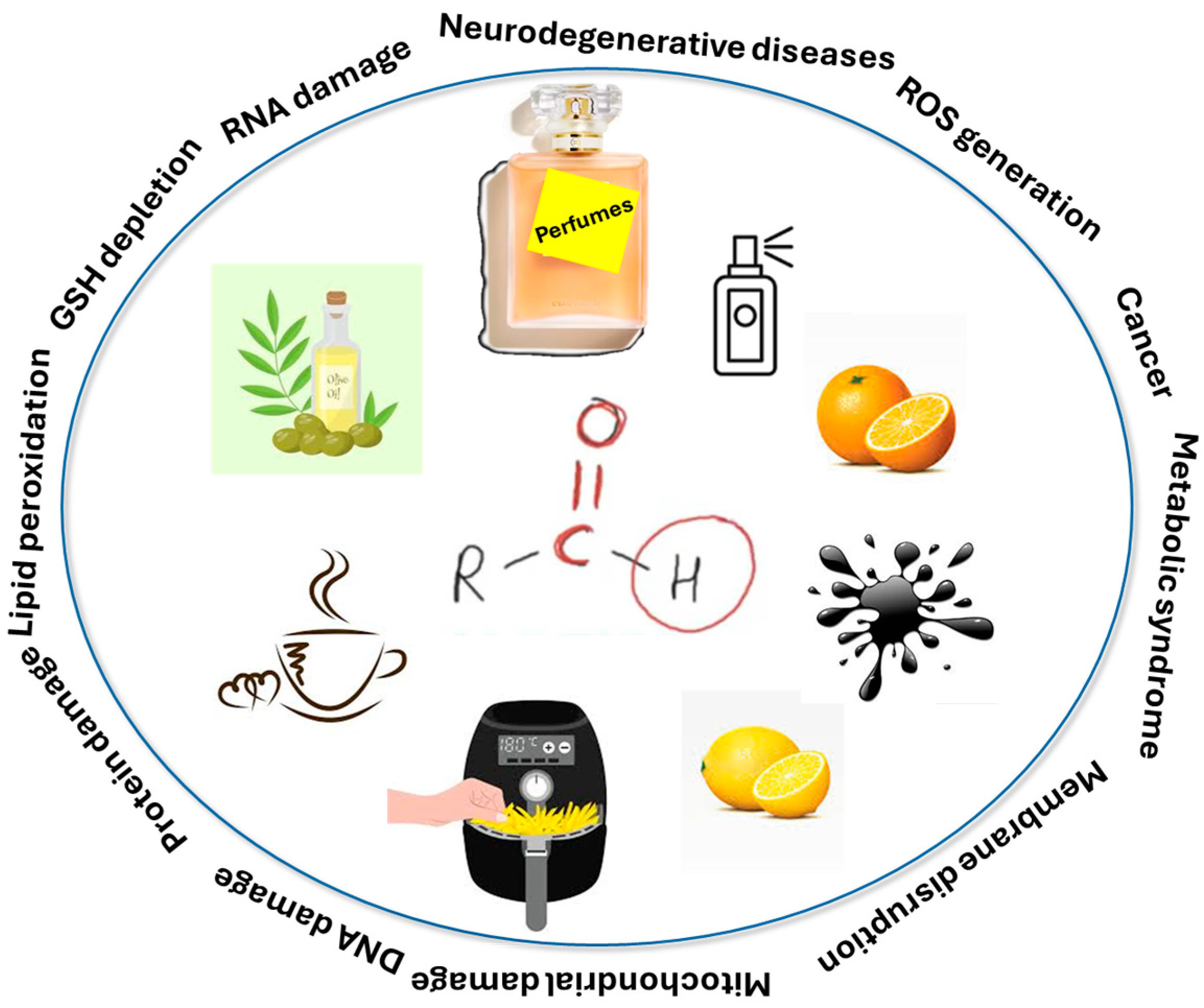
| Structure | Formula | CAS Number | Name |
|---|---|---|---|
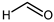 | HCOH CH2O | [50-00-0] | Formaldehyde (or methanal) |
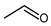 | C2H3OH C2H4O | [75-07-0] | Acetaldehyde (or ethanal) |
 | C4H7OH C4H8O | [123-72-8] | n-Butanal (or n-butyraldehyde) |
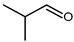 | C4H7OH C4H8O | [78-84-2] | i-Butanal or i-butyraldehyde or 2-methylpropanal |
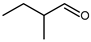 | C5H9OH C5H10O | [96-17-3] | 2-Methylbutanal |
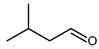 | C5H9OH C5H10O | [590-86-3] | 3-Methylbutanal or isovaleraldehyde |
 | C5H9OH C5H10O | [110-62-3] | Pentanal or valeraldehyde |
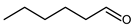 | C6H11OH C6H12O | [66-25-1] | Hexanal or caproaldehyde or caproic aldehyde or capraldehyde or capronaldehyde |
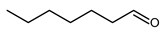 | C7H13OH C7H14O | [111-71-7] | Heptanal (or enanthaldehyde) |
 | C8H15OH C8H16O | [124-13-0] | Octanal (or caprylaldehyde) |
 | C9H17OH C9H18O | [124-19-6] | Nonanal (or pelargonaldehyde) |
 | C10H19OH C10H20O | [112-31-2] | Decanal (or caprinaldehyde) |
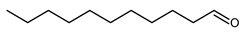 | C11H21OH C11H22O | [112-44-7] | Undecanal |
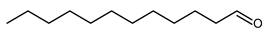 | C12H21OH C12H22O | [112-54-9] | Dodecanal (or lauraldehyde or lauric aldehyde) |
 | C13H23OH C13H24O | [10486-19-8] | Tridecanal |
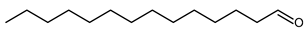 | C14H25OH C14H26O | [124-25-4] | Tetradecanal (or myristyl aldehyde) |
 | C16H29OH C16H30O | [629-80-1] | Hexadecanal |
 | C3H2OH C3H3O | [107-02-8] | Acrolein |
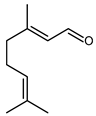 | C10H15OH C10H16O | [141-27-5] | Geranial or Z-citral or cis-citral or citral α or citral A |
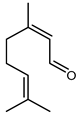 | C10H15OH C10H16O | [106-26-3] | Neral or E-citral or trans-citral or citral β or citral B |
| Structure | Name | Acronym and Trade Name | |
|---|---|---|---|
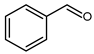 | Benzaldehyde | [588-68-1] | |
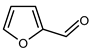 | Furfural 2-Furaldehyde Furan-2-carboxaldehyde Pyromucic aldehyde | [98-01-1] | |
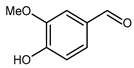 | 4-Hydroxy-3-methoxybenzaldehyde Vanillin | [121-33-5] | |
 | 4-Methoxybenzaldehyde Anisaldehyde para-anisaldehyde, p-anisaldehyde | [123-11-5] | |
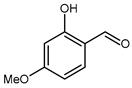 | 2-Hydroxy-4-methoxybenzaldehyde | [673-22-3] | HMB, HMBA |
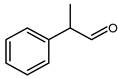 | 2-Phenylpropanal | [93-53-8] | |
 | 3-Phenylpropanal | [104-53-0] | |
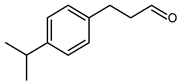 | 3-(4-Isopropylphenyl)propanal 3-(p-Cumenyl)propionaldehyde | [7775-00-0] | PHCA |
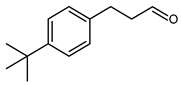 | 3-(4-tert–Butylphenyl)propanal | [18127-01-0] | BHCA Bourgeonal Lilional Isolilial |
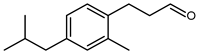 | 3-(4-Isobutyl-2-methylphenyl)propanal | [1637294-12-2] | Nympheal |
 | 3-(4-Isopropylphenyl)-2-methylpropanal Cyclamen aldehyde Cyclamal 3-p-Cumenyl-2-methylpropionaldehyde | [103-95-7] | PMHCA CPA Floral |
 | 3-(4-Isobutylphenyl)-2-methylpropanal iso-Butyl-α-methylhydrocinnamic aldehyde | [6658-48-6] | iBMHCA Silvial® |
 | 2-(4-tert-Butylbenzyl)propionaldehyde. p-t-Butyl-α-methylhydrocinnamic aldehyde | [80-54-6]: (RS) [75166-30-2]: (S)-isomer [75166-31-3]: (R)-isomer | BMHCA Lilial, Lismeral |
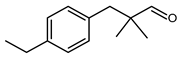 | 3-(4-Ethylphenyl)-2,2-dimethylpropanal Ethyl-α-dimethylhydrocnamicaldehyde | [67634-15-5] | Floralozone Florone |
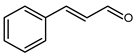 | Cinnamaldehyde, Cinnamic aldehyde Cinnamal | [14371-10-9]: trans [57194-69-1]: cis | |
 | Amyl cinnamal, Amyl cinnamaldehyde Jasminaldehyde | [122-40-7] | |
 | α-Hexylcinnamaldehyde | [101-86-0] | HCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, A.; Mariconda, A.; D’Amato, A.; Iacopetta, D.; Ceramella, J.; Marra, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Aldehydes: What We Should Know About Them. Organics 2024, 5, 395-428. https://doi.org/10.3390/org5040021
Catalano A, Mariconda A, D’Amato A, Iacopetta D, Ceramella J, Marra M, Saturnino C, Sinicropi MS, Longo P. Aldehydes: What We Should Know About Them. Organics. 2024; 5(4):395-428. https://doi.org/10.3390/org5040021
Chicago/Turabian StyleCatalano, Alessia, Annaluisa Mariconda, Assunta D’Amato, Domenico Iacopetta, Jessica Ceramella, Maria Marra, Carmela Saturnino, Maria Stefania Sinicropi, and Pasquale Longo. 2024. "Aldehydes: What We Should Know About Them" Organics 5, no. 4: 395-428. https://doi.org/10.3390/org5040021
APA StyleCatalano, A., Mariconda, A., D’Amato, A., Iacopetta, D., Ceramella, J., Marra, M., Saturnino, C., Sinicropi, M. S., & Longo, P. (2024). Aldehydes: What We Should Know About Them. Organics, 5(4), 395-428. https://doi.org/10.3390/org5040021












