Endocrine Toxicity of Micro- and Nanoplastics, and Advances in Detection Techniques for Human Tissues: A Comprehensive Review
Abstract
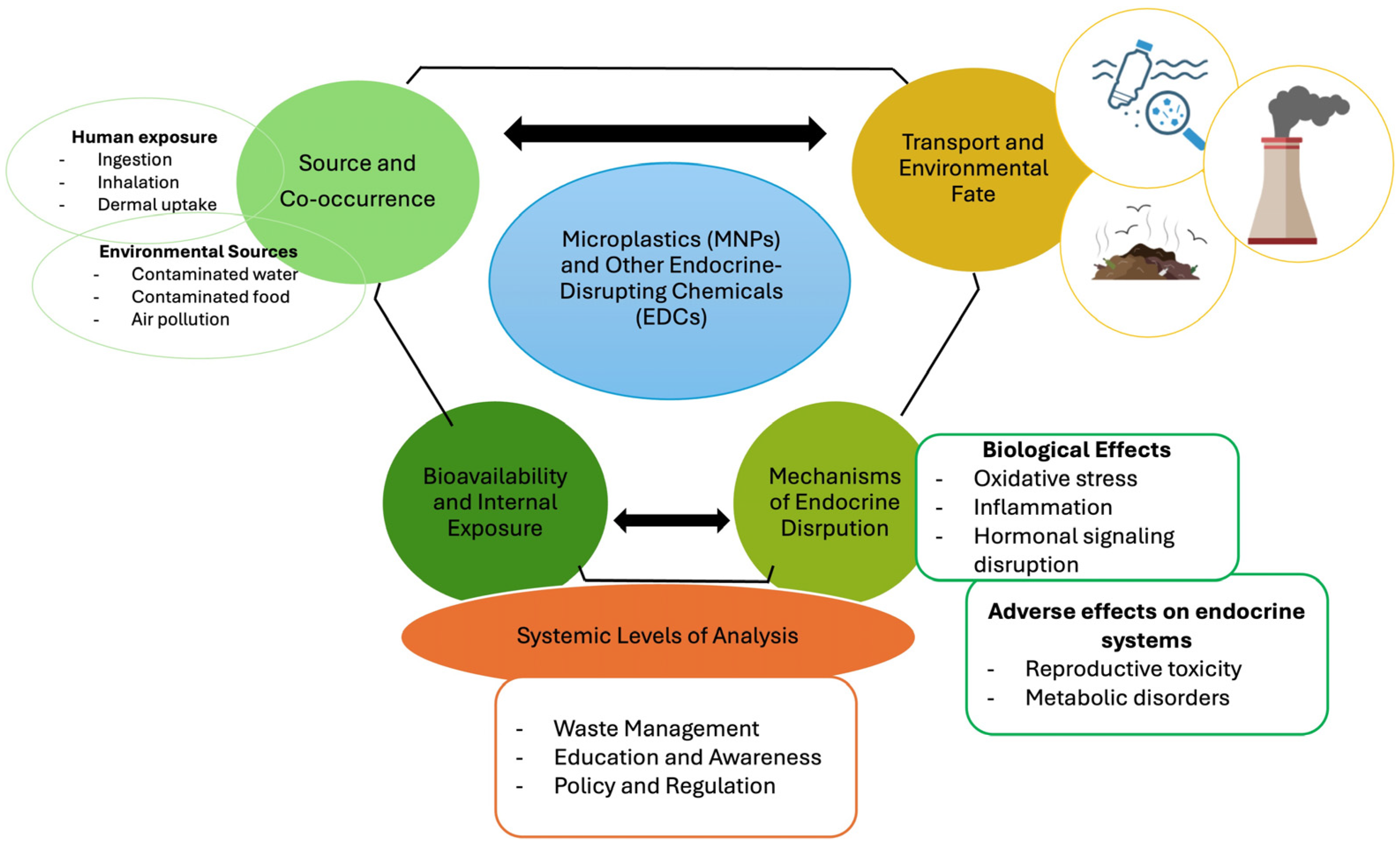
1. Introduction
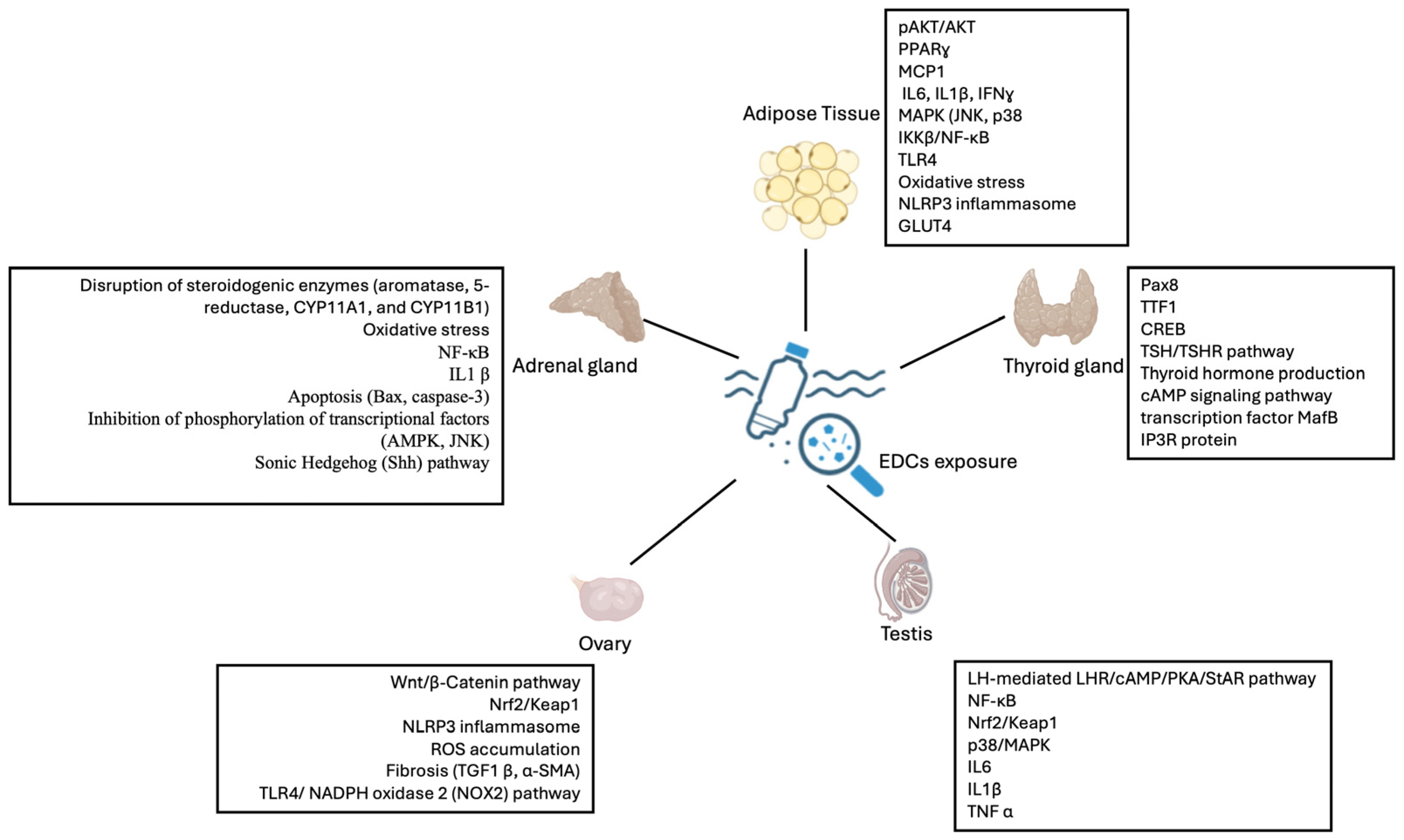
2. MNPs and the Reproductive System
3. MNPs and the Adrenal Gland
4. MNPs and the Thyroid and Parathyroid Glands
5. MNPs and Adipose Tissue: ECD’s Obesogenic Effects
6. Epigenetic Consequences of Endocrine Disruption Plasticity
7. Emerging Techniques to Efficaciously Detect the MNPs in Human Fluids and Tissues
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the Human Body: A Comprehensive Review of Exposure, Distribution, Migration Mechanisms, and Toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol a Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Usman, A.; Ikhlas, S.; Ahmad, M. Occurrence, Toxicity and Endocrine Disrupting Potential of Bisphenol-B and Bisphenol-F: A Mini-Review. Toxicol. Lett. 2019, 312, 222–227. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine Disrupting Chemicals: Exposure, Effects on Human Health, Mechanism of Action, Models for Testing and Strategies for Prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- Pinto Da Costa, J.; Avellan, A.; Mouneyrac, C.; Duarte, A.; Rocha-Santos, T. Plastic Additives and Microplastics as Emerging Contaminants: Mechanisms and Analytical Assessment. Trends Anal. Chem. 2023, 158, 116898. [Google Scholar] [CrossRef]
- López-Vázquez, J.; Rodil, R.; Trujillo-Rodríguez, M.J.; Quintana, J.B.; Cela, R.; Miró, M. Mimicking Human Ingestion of Microplastics: Oral Bioaccessibility Tests of Bisphenol A and Phthalate Esters under Fed and Fasted States. Sci. Total Environ. 2022, 826, 154027. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The Potential Impacts of Micro-and-Nano Plastics on Various Organ Systems in Humans. eBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A Review of the Endocrine Disrupting Effects of Micro and Nano Plastic and Their Associated Chemicals in Mammals. Front. Endocrinol. 2022, 13, 1084236. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.M. No-Threshold Dose-Response Curves for Nongenotoxic Chemicals: Findings and Applications for Risk Assessment. Environ. Res. 2006, 100, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.T.; Wareham, N.J.; et al. Urinary Bisphenol A Concentration and Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef]
- Sheehan, D.M.; Willingham, E.; Gaylor, D.; Bergeron, J.M.; Crews, D. No Threshold Dose for Estradiol-Induced Sex Reversal of Turtle Embryos: How Little Is Too Much? Environ. Health Perspect. 1999, 107, 155–159. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Wang, S.; Xie, J.; Han, Q.; Chen, M. Comparing the Effects of Polystyrene Microplastics Exposure on Reproduction and Fertility in Male and Female Mice. Toxicology 2022, 465, 153059. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive Toxicity of Polystyrene Microplastics: In Vivo Experimental Study on Testicular Toxicity in Mice. J. Hazard Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene Microplastics Cause Granulosa Cells Apoptosis and Fibrosis in Ovary through Oxidative Stress in Rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic Exposure to Polystyrene Microplastics Induced Male Reproductive Toxicity and Decreased Testosterone Levels via the LH-Mediated LHR/CAMP/PKA/StAR Pathway. Part Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 Regulates ROS Production by Mitochondria and NADPH Oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Yu, H.; Yang, L.; Sun, Y.; Xu, N.; Wang, N.; Lei, Z.; Hou, J.; Jin, Y.; et al. Polystyrene Microplastics Induce Blood–Testis Barrier Disruption Regulated by the MAPK-Nrf2 Signaling Pathway in Rats. Environ. Sci. Pollut. Res. 2021, 28, 47921–47931. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to Polystyrene Microplastics Causes Reproductive Toxicity through Oxidative Stress and Activation of the P38 MAPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Duan, P.; Ha, M.; Huang, X.; Zhang, P.; Liu, C. Intronic MiR-140-5p Contributes to Beta-Cypermethrin-Mediated Testosterone Decline. Sci. Total Environ. 2022, 806, 150517. [Google Scholar] [CrossRef]
- Qu, J.; Wu, L.; Mou, L.; Liu, C. Polystyrene Microplastics Trigger Testosterone Decline via GPX1. Sci. Total Environ. 2024, 947, 174536. [Google Scholar] [CrossRef]
- He, Y.; Yin, R. The Reproductive and Transgenerational Toxicity of Microplastics and Nanoplastics: A Threat to Mammalian Fertility in Both Sexes. J. Appl. Toxicol. 2024, 44, 66–85. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Y.; Zhu, H.; Xu, P.; Zhang, J.; Hu, Y.; Ji, X.; Yan, R.; Yue, H.; Sang, N. Invisible Hand behind Female Reproductive Disorders: Bisphenols, Recent Evidence and Future Perspectives. Toxics 2023, 11, 1000. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhao, Y.; Zhao, J.; Yu, T.; Yao, Y.; Zhao, R.; Yu, R.; Liu, J.; Su, J. Reproductive Toxicity of Microplastics in Female Mice and Their Offspring from Induction of Oxidative Stress. Environ. Pollut. 2023, 327, 121482. [Google Scholar] [CrossRef]
- Balali, H.; Morabbi, A.; Karimian, M. Concerning Influences of Micro/Nano Plastics on Female Reproductive Health: Focusing on Cellular and Molecular Pathways from Animal Models to Human Studies. Reprod. Biol. Endocrinol. 2024, 22, 141. [Google Scholar] [CrossRef]
- Huang, J.; Zou, L.; Bao, M.; Feng, Q.; Xia, W.; Zhu, C. Toxicity of Polystyrene Nanoparticles for Mouse Ovary and Cultured Human Granulosa Cells. Ecotoxicol. Environ. Saf. 2023, 249, 114371. [Google Scholar] [CrossRef] [PubMed]
- Charan, H.V.; Dwivedi, D.K.; Khan, S.; Jena, G. Mechanisms of NLRP3 Inflammasome-Mediated Hepatic Stellate Cell Activation: Therapeutic Potential for Liver Fibrosis. Genes Dis. 2023, 10, 480–494. [Google Scholar] [CrossRef]
- Dubey, I.; Khan, S.; Kushwaha, S. Developmental and Reproductive Toxic Effects of Exposure to Microplastics: A Review of Associated Signaling Pathways. Front. Toxicol. 2022, 4, 901798. [Google Scholar] [CrossRef]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene Microplastics Lead to Pyroptosis and Apoptosis of Ovarian Granulosa Cells via NLRP3/Caspase-1 Signaling Pathway in Rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef]
- Wu, H.; Xu, T.; Chen, T.; Liu, J.; Xu, S. Oxidative Stress Mediated by the TLR4/NOX2 Signalling Axis Is Involved in Polystyrene Microplastic-Induced Uterine Fibrosis in Mice. Sci. Total Environ. 2022, 838, 155825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of Microplastics in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wang, Y.; Xie, F.Q.; Li, Y.X.; Wan, X.L.; Ma, W.W.; Wang, D.C.; Wu, Y.H. Analysis of PAEs in Semen of Infertile Men. Int. J. Occup. Environ. Health 2015, 21, 40–48. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Farag, A.A.; Bayoumi, H.; Radwaan, S.E.; El Gazzar, W.B.; Youssef, H.S.; Nasr, H.E.; Badr, A.M.; Mansour, H.M.; Elalfy, A.; Sayed, A.E.-D.H.; et al. Melatonin Counteracts Polyethylene Microplastics Induced Adreno-Cortical Damage in Male Albino Rats. Ecotoxicol. Environ. Saf. 2024, 279, 116499. [Google Scholar] [CrossRef]
- Chen, X.; Mo, J.; Zhang, S.; Li, X.; Huang, T.; Zhu, Q.; Wang, S.; Chen, X.; Ge, R.-S. 4-Bromodiphenyl Ether Causes Adrenal Gland Dysfunction in Rats during Puberty. Chem. Res. Toxicol. 2019, 32, 1772–1779. [Google Scholar] [CrossRef]
- Medwid, S.; Guan, H.; Yang, K. Bisphenol A Stimulates Adrenal Cortical Cell Proliferation via ERβ-Mediated Activation of the Sonic Hedgehog Signalling Pathway. J. Steroid. Biochem. Mol. Biol. 2018, 178, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Pötzl, B.; Kürzinger, L.; Stopper, H.; Fassnacht, M.; Kurlbaum, M.; Dischinger, U. Endocrine Disruptors: Focus on the Adrenal Cortex. Horm. Metab. Res. 2024, 56, 78–90. [Google Scholar] [CrossRef]
- Olukole, S.G.; Lanipekun, D.O.; Ola-Davies, E.O.; Oke, B.O. Melatonin Attenuates Bisphenol A-Induced Toxicity of the Adrenal Gland of Wistar Rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 5971–5982. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Gungunes, A.; Durmaz, S.; Kisa, U.; Celik, Z.R. Nonfunctional Adrenal Incidentalomas May Be Related to Bisphenol-A. Endocrine 2021, 71, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Robaire, B. Effects of Endocrine-Disrupting Chemicals on Adrenal Function. Endocrinology 2025, 166, bqaf045. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of Thyroid Hormone Action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Andra, S.S.; Makris, K.C. Thyroid Disrupting Chemicals in Plastic Additives and Thyroid Health. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 107–151. [Google Scholar] [CrossRef]
- Saha, U.; Kumari, P.; Ghosh, A.; Sinha, A.; Jena, S.; Kirti, A.; Gupta, A.; Choudhury, A.; Simnani, F.Z.; Nandi, A.; et al. Detrimental Consequences of Micropolymers Associated Plasticizers on Endocrinal Disruption. Mater. Today Bio 2024, 27, 101139. [Google Scholar] [CrossRef]
- Calsolaro, V.; Pasqualetti, G.; Niccolai, F.; Caraccio, N.; Monzani, F. Thyroid Disrupting Chemicals. Int. J. Mol. Sci. 2017, 18, 2583. [Google Scholar] [CrossRef]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid Effects of Endocrine Disrupting Chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as Environmental Triggers of Thyroid Dysfunction: Clues and Evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef] [PubMed]
- Berto-Júnior, C.; Santos-Silva, A.P.; Ferreira, A.C.F.; Graceli, J.B.; de Carvalho, D.P.; Soares, P.; Romeiro, N.C.; Miranda-Alves, L. Unraveling Molecular Targets of Bisphenol A and S in the Thyroid Gland. Environ. Sci. Pollut. Res. Int. 2018, 25, 26916–26926. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Ren, X.-M.; Li, Y.-Y.; Yao, X.-F.; Li, C.-H.; Qin, Z.-F.; Guo, L.-H. Bisphenol A Alternatives Bisphenol S and Bisphenol F Interfere with Thyroid Hormone Signaling Pathway In Vitro and In Vivo. Environ. Pollut. 2018, 237, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Breous, E.; Wenzel, A.; Loos, U. The Promoter of the Human Sodium/Iodide Symporter Responds to Certain Phthalate Plasticisers. Mol. Cell. Endocrinol. 2005, 244, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.T.M.; van Zanden, J.J.; Luijks, E.A.C.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, Å.; Brouwer, A. Potent Competitive Interactions of Some Brominated Flame Retardants and Related Compounds with Human Transthyretin In Vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef]
- Du, Y.; Chen, C.; Zhou, G.; Cai, Z.; Man, Q.; Liu, B.; Wang, W.C. Perfluorooctanoic Acid Disrupts Thyroid-Specific Genes Expression and Regulation via the TSH-TSHR Signaling Pathway in Thyroid Cells. Environ. Res. 2023, 239, 117372. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Dai, X.; Li, B.; Zhang, S.; Yu, Y. Thyroid and Parathyroid Function Disorders Induced by Short-Term Exposure of Microplastics and Nanoplastics: Exploration of Toxic Mechanisms and Early Warning Biomarkers. J. Hazard. Mater. 2024, 476, 134960. [Google Scholar] [CrossRef]
- Kehinde, S.A.; Fatokun, T.P.; Olajide, A.T.; Praveena, S.M.; Sokan-Adeaga, A.A.; Adekunle, A.P.; Fouad, D.; Papadakis, M. Impact of Polyethylene Microplastics Exposure on Kallikrein-3 Levels, Steroidal-Thyroidal Hormones, and Antioxidant Status in Murine Model: Protective Potentials of Naringin. Sci. Rep. 2024, 14, 23664. [Google Scholar] [CrossRef]
- Huang, P.-C.; Kuo, P.-L.; Guo, Y.-L.; Liao, P.-C.; Lee, C.-C. Associations between Urinary Phthalate Monoesters and Thyroid Hormones in Pregnant Women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef]
- Meeker, D.J.; Ferguson, K.K. Relationship between Urinary Phthalate and Bisphenol A Concentrations and Serum Thyroid Measures in U.S. Adults and Adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ. Health Perspect. 2011, 119, 1396–1402. [Google Scholar] [CrossRef]
- Sriphrapradang, C.; Chailurkit, L.; Aekplakorn, W.; Ongphiphadhanakul, B. Association between Bisphenol A and Abnormal Free Thyroxine Level in Men. Endocrine 2013, 44, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Saunders, N.; Safley, S.; Smith, M.R.; Liang, Y.; Tran, V.; Sharma, J.; Jones, D.P.; Weber, C.J. Environmental Chemicals and Metabolic Disruption in Primary and Secondary Human Parathyroid Tumors. Surgery 2021, 169, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Nappi, F.; Barrea, L.; Di Somma, C.; Savanelli, M.C.; Muscogiuri, G.; Orio, F.; Savastano, S. Endocrine Aspects of Environmental “Obesogen” Pollutants. Int. J. Environ. Res. Public Health 2016, 13, 765. [Google Scholar] [CrossRef]
- Janesick, A.S.; Blumberg, B. Obesogens: An Emerging Threat to Public Health. Am. J. Obs. Gynecol. 2016, 214, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S. Persistent Organic Pollutants and Concern Over the Link with Insulin Resistance Related Metabolic Diseases. Rev. Environ. Contam. Toxicol. 2016, 238, 69–89. [Google Scholar] [CrossRef]
- Stel, J.; Legler, J. The Role of Epigenetics in the Latent Effects of Early Life Exposure to Obesogenic Endocrine Disrupting Chemicals. Endocrinology 2015, 156, 3466–3472. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Schaffert, A.; Krieg, L.; Weiner, J.; Schlichting, R.; Ueberham, E.; Karkossa, I.; Bauer, M.; Landgraf, K.; Junge, K.M.; Wabitsch, M.; et al. Alternatives for the Worse: Molecular Insights into Adverse Effects of Bisphenol a and Substitutes During Human Adipocyte Differentiation. Environ. Int. 2021, 156, 106730. [Google Scholar] [CrossRef]
- González-Casanova, J.E.; Bermúdez, V.; Caro Fuentes, N.J.; Angarita, L.C.; Caicedo, N.H.; Rivas Muñoz, J.; Rojas-Gómez, D.M. New Evidence on BPA’s Role in Adipose Tissue Development of Proinflammatory Processes and Its Relationship with Obesity. Int. J. Mol. Sci. 2023, 24, 8231. [Google Scholar] [CrossRef]
- Leiva, M.; Matesanz, N.; Pulgarín-Alfaro, M.; Nikolic, I.; Sabio, G. Uncovering the Role of P38 Family Members in Adipose Tissue Physiology. Front. Endocrinol. 2020, 11, 572089. [Google Scholar] [CrossRef]
- Lolescu, B.M.; Furdui-Lința, A.V.; Ilie, C.A.; Sturza, A.; Zară, F.; Muntean, D.M.; Blidișel, A.; Crețu, O.M. Adipose Tissue as Target of Environmental Toxicants: Focus on Mitochondrial Dysfunction and Oxidative Inflammation in Metabolic Dysfunction-Associated Steatotic Liver Disease. Mol. Cell. Biochem. 2024, 480, 2863–2879. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial Oxidative Stress Causes Insulin Resistance Without Disrupting Oxidative Phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef] [PubMed]
- Clare, K.; Dillon, J.F.; Brennan, P.N. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J. Clin. Transl. Hepatol. 2022, 10, 939–946. [Google Scholar] [CrossRef]
- Nettore, I.C.; Franchini, F.; Palatucci, G.; Macchia, P.E.; Ungaro, P. Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Obesity. Biomedicines 2021, 9, 1716. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Nigro, C.; Oriente, F.; Formisano, P.; Miele, C.; Beguinot, F. Low-Dose Bisphenol-A Promotes Epigenetic Changes at Pparγ Promoter in Adipose Precursor Cells. Nutrients 2020, 12, 3498. [Google Scholar] [CrossRef]
- Meruvu, S.; Zhang, J.; Choudhury, M. Butyl Benzyl Phthalate Promotes Adipogenesis in 3T3-L1 Cells via the MiRNA-34a-5p Signaling Pathway in the Absence of Exogenous Adipogenic Stimuli. Chem. Res. Toxicol. 2021, 34, 2251–2260. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, E.; Kim, Y. The Association between Bisphenol A Exposure and Obesity in Children—A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2521. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.J.; Sun, Y.; Xu, G.; Sun, Q.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab. J. 2019, 43, 59–75. [Google Scholar] [CrossRef]
- Haverinen, E.; Fernandez, M.F.; Mustieles, V.; Tolonen, H. Metabolic Syndrome and Endocrine Disrupting Chemicals: An Overview of Exposure and Health Effects. Int. J. Environ. Res. Public Health 2021, 18, 13047. [Google Scholar] [CrossRef]
- Ashapkin, V.; Suvorov, A.; Pilsner, J.R.; Krawetz, S.A.; Sergeyev, O. Age-Associated Epigenetic Changes in Mammalian Sperm: Implications for Offspring Health and Development. Hum. Reprod. Update 2023, 29, 24–44. [Google Scholar] [CrossRef]
- Skinner, M.K. Endocrine Disruptor Induction of Epigenetic Transgenerational Inheritance of Disease. Mol. Cell. Endocrinol. 2014, 398, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, M.; Matarazzo, M.R. Epigenetic Effects of Environmental Chemicals on Reproductive Biology. Curr. Drug Targets 2016, 18, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Servy, E. Advanced Paternal Age and Endocrine Disruptors: Two Causes of Psychiatric Disorders in Children, with DNA Methylation Dys-Regulation as a Common Biochemical Mechanis. Psychiatr. Disord. 2017, 20, 1–12. [Google Scholar]
- Skinner, M.K.; Manikkam, M.; Haque, M.M.; Zhang, B.; Savenkova, M.I. Epigenetic Transgenerational Inheritance of Somatic Transcriptomes and Epigenetic Control Regions. Genome Biol. 2012, 13, R91. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef]
- Tricotteaux-Zarqaoui, S.; Lahimer, M.; Abou Diwan, M.; Corona, A.; Candela, P.; Cabry, R.; Bach, V.; Khorsi-Cauet, H.; Benkhalifa, M. Endocrine Disruptor Chemicals Exposure and Female Fertility Declining: From Pathophysiology to Epigenetic Risks. Front. Public Health 2024, 12, 1466967. [Google Scholar] [CrossRef]
- Biswas, S.; Ghosh, S.; Das, S.; Maitra, S. Female Reproduction: At the Crossroads of Endocrine Disruptors and Epigenetics. Proc. Zool. Soc. 2021, 74, 532–545. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Santiago-Toledo, G.; Barrón, V.; Espinosa-García, A.M.; García-García, J.A.; García-Arrazola, R. Environmental Epigenomics: Current Approaches to Assess Epigenetic Effects of Endocrine Disrupting Compounds (EDC’s) on Human Health. Environ. Toxicol. Pharmacol. 2017, 51, 94–99. [Google Scholar] [CrossRef]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA Methyltransferase Inhibitors Combination Therapy for the Treatment of Solid Tumor: Mechanism and Clinical Application. Clin. Epigenetics 2021, 13, 166. [Google Scholar] [CrossRef]
- Brunetti, L.S.; Piersante, C.; La Russa, M.F.; Cellini, E.; Bolea, E.; Laborda, F.; Ruffolo, S.A. Examining Microplastics Along the Calabrian Coastline: Analysis of Key Characteristics and Metal Contamination. Environments 2025, 12, 4. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, B.; Lu, Y.; Yin, Y.; Zong, Y.; Chen, M.-T.; O’Donnell, Z. A Review of Microplastics in Sediments: Spatial and Temporal Occurrences, Biological Effects, and Analytic Methods. Quat. Int. 2019, 519, 274–281. [Google Scholar] [CrossRef]
- Roslan, N.S.; Lee, Y.Y.; Ibrahim, Y.S.; Tuan Anuar, S.; Yusof, K.M.K.K.; Lai, L.A.; Brentnall, T. Detection of Microplastics in Human Tissues and Organs: A Scoping Review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Liu, S.; Lin, G.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of Various Microplastics in Placentas, Meconium, Infant Feces, Breastmilk and Infant Formula: A Pilot Prospective Study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, J.; Yu, H.; Su, H.; Yang, Y.; Cao, Y.; Zhang, Q.; Ren, Y.; Hollert, H.; Shi, H.; et al. An Emerging Role of Microplastics in the Etiology of Lung Ground Glass Nodules. Environ. Sci. Eur. 2022, 34, 25. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, J.; Zuo, R.; Xu, Q.; Qian, Y.; An, L. Identification of Microplastics in Human Placenta Using Laser Direct Infrared Spectroscopy. Sci. Total Environ. 2023, 856, 159060. [Google Scholar] [CrossRef]
- Seeley, M.E.; Lynch, J.M. Previous Successes and Untapped Potential of Pyrolysis-GC/MS for the Analysis of Plastic Pollution. Anal. Bioanal. Chem. 2023, 415, 2873–2890. [Google Scholar] [CrossRef]
- Brits, M.; van Velzen, M.J.M.; Sefiloglu, F.Ö.; Scibetta, L.; Groenewoud, Q.; Garcia-Vallejo, J.J.; Vethaak, A.D.; Brandsma, S.H.; Lamoree, M.H. Quantitation of Micro and Nanoplastics in Human Blood by Pyrolysis-Gas Chromatography–Mass Spectrometry. Microplastics Nanoplastics 2024, 4, 12. [Google Scholar] [CrossRef]
- Lin, X.; Gowen, A.A.; Pu, H.; Xu, J.-L. Microplastic Contamination in Fish: Critical Review and Assessment of Data Quality. Food Control 2023, 153, 109939. [Google Scholar] [CrossRef]
- Shi, B.; Patel, M.; Yu, D.; Yan, J.; Li, Z.; Petriw, D.; Pruyn, T.; Smyth, K.; Passeport, E.; Miller, R.J.D.; et al. Automatic Quantification and Classification of Microplastics in Scanning Electron Micrographs via Deep Learning. Sci. Total Environ. 2022, 825, 153903. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of Microplastics in Human Colectomy Specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Austin, C.; Chapman, E.; Atherall, C.A.; Liddle, C.R.; Dunstan, T.S.; Blackburn, B.; Mead, A.; Filart, K.; Beeby, E.; et al. Microplastics in Human Urine: Characterisation Using ΜFTIR and Sampling Challenges Using Healthy Donors and Endometriosis Participants. Ecotoxicol. Environ. Saf. 2024, 274, 116208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossio, S.; Ruffolo, S.A.; Lofaro, D.; Perri, A.; La Russa, M.F. Endocrine Toxicity of Micro- and Nanoplastics, and Advances in Detection Techniques for Human Tissues: A Comprehensive Review. Endocrines 2025, 6, 23. https://doi.org/10.3390/endocrines6020023
Bossio S, Ruffolo SA, Lofaro D, Perri A, La Russa MF. Endocrine Toxicity of Micro- and Nanoplastics, and Advances in Detection Techniques for Human Tissues: A Comprehensive Review. Endocrines. 2025; 6(2):23. https://doi.org/10.3390/endocrines6020023
Chicago/Turabian StyleBossio, Sabrina, Silvestro Antonio Ruffolo, Danilo Lofaro, Anna Perri, and Mauro Francesco La Russa. 2025. "Endocrine Toxicity of Micro- and Nanoplastics, and Advances in Detection Techniques for Human Tissues: A Comprehensive Review" Endocrines 6, no. 2: 23. https://doi.org/10.3390/endocrines6020023
APA StyleBossio, S., Ruffolo, S. A., Lofaro, D., Perri, A., & La Russa, M. F. (2025). Endocrine Toxicity of Micro- and Nanoplastics, and Advances in Detection Techniques for Human Tissues: A Comprehensive Review. Endocrines, 6(2), 23. https://doi.org/10.3390/endocrines6020023







