Oncocytic Adenoma in a Pediatric Patient: A Case Report and Literature Review
Abstract
1. Introduction
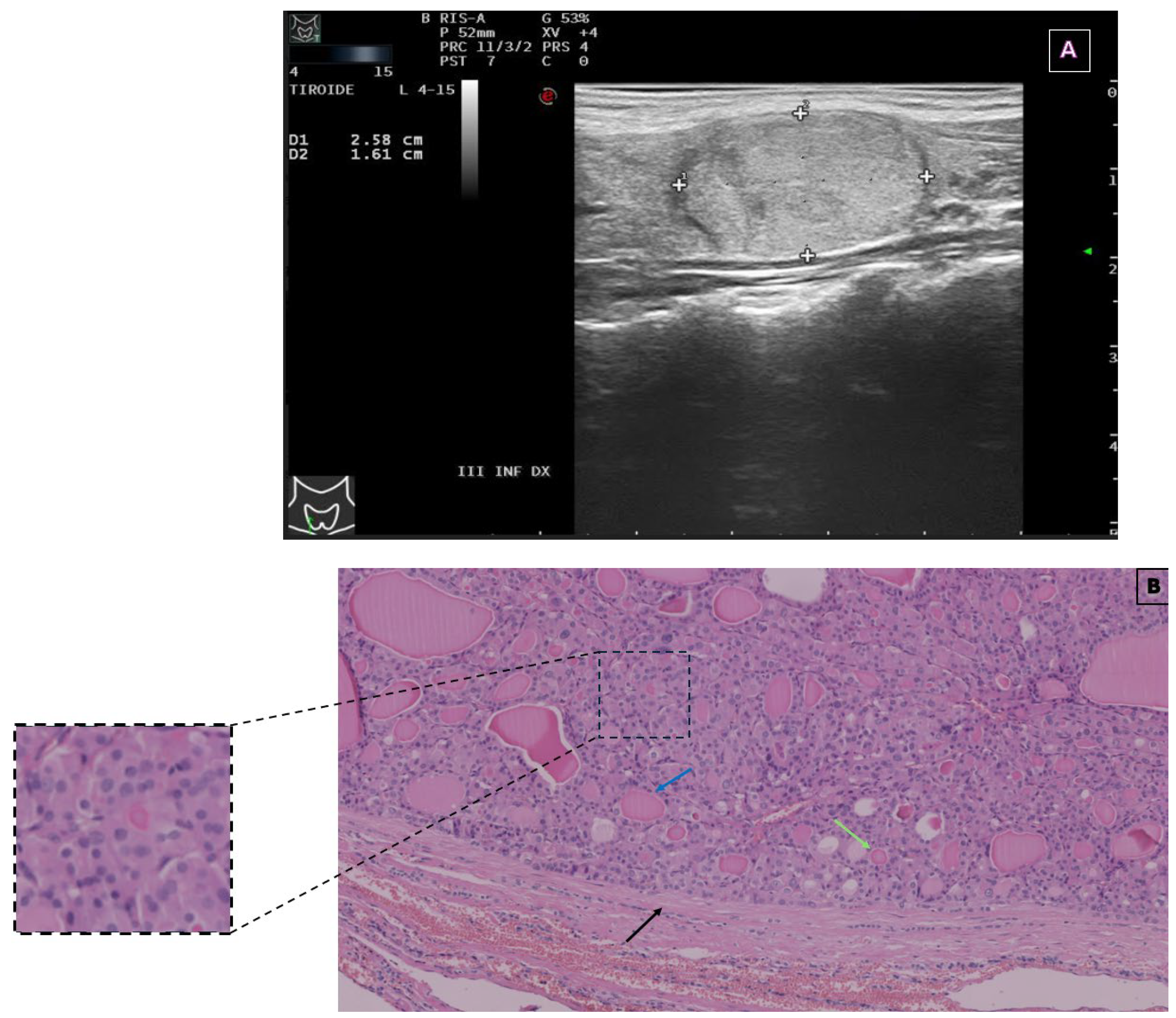
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EU-TIRADS | European Thyroid Imaging and Reporting Data System |
| FNA | Fine-needle aspiration |
| FT3 | Free tri-iodothyronine |
| FT4 | Free thyroxine |
| OA | Oncocytic adenoma |
| OC | Oncocytic carcinoma |
| SIAPEC | Italian Society of Anatomic Pathology and Diagnostic Cytopathology |
| TgAb | Anti-thyroglobulin antibodies |
| TPOAb | Thyroid peroxidase antibodies |
| TSH | Thyroid-stimulating hormone |
References
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for Thyroid Nodule Management. Eur. Thyroid. J. 2023, 12, e230067. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Papini, E.; Paschke, R.; Duick, D.S.; Valcavi, R.; Hegedüs, L.; Vitti, P. AACE/AME/ETA Task Force on Thyroid Nodules; American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules: Executive Summary of Recommendations. Endocr. Pract. 2010, 16, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Suzuki, S.; Fukushima, T.; Midorikawa, S.; Shimura, H.; Matsuzuka, T.; Ishikawa, T.; Takahashi, H.; Ohtsuru, A.; Sakai, A.; et al. Comprehensive Survey Results of Childhood Thyroid Ultrasound Examinations in Fukushima in the First Four Years After the Fukushima Daiichi Nuclear Power Plant Accident. Thyroid 2016, 26, 843–851. [Google Scholar] [CrossRef]
- Rallison, M.L.; Dobyns, B.M.; Keating, F.R.; Rall, J.E.; Tyler, F.H. Thyroid Nodularity in Children. JAMA 1975, 233, 1069–1072. [Google Scholar] [CrossRef]
- Creo, A.; Alahdab, F.; Al Nofal, A.; Thomas, K.; Kolbe, A.; Pittock, S.T. Ultrasonography and the American Thyroid Association Ultrasound-Based Risk Stratification Tool: Utility in Pediatric and Adolescent Thyroid Nodules. Horm. Res. Paediatr. 2018, 90, 93–101. [Google Scholar] [CrossRef]
- Hanley, P.; Lord, K.; Bauer, A.J. Thyroid Disorders in Children and Adolescents: A Review. JAMA Pediatr. 2016, 170, 1008–1019. [Google Scholar] [CrossRef]
- Carlomagno, F.; Minnetti, M.; Angelini, F.; Pofi, R.; Sbardella, E.; Spaziani, M.; Aureli, A.; Anzuini, A.; Paparella, R.; Tarani, L.; et al. Altered Thyroid Feedback Loop in Klinefelter Syndrome: From Infancy Through the Transition to Adulthood. J. Clin. Endocrinol. Metab. 2023, 108, e1329–e1340. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef]
- McFadden, D.G.; Sadow, P.M. Genetics, Diagnosis, and Management of Hürthle Cell Thyroid Neoplasms. Front. Endocrinol. 2021, 12, 696386. [Google Scholar] [CrossRef]
- Gopal, R.K.; Kübler, K.; Calvo, S.E.; Polak, P.; Livitz, D.; Rosebrock, D.; Sadow, P.M.; Campbell, B.; Donovan, S.E.; Amin, S.; et al. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hürthle Cell Carcinoma. Cancer Cell 2018, 34, 242–255.e5. [Google Scholar] [CrossRef]
- Wong, K.S.; Angell, T.E.; Barletta, J.A.; Krane, J.F. Hürthle Cell Lesions of the Thyroid: Progress Made and Challenges Remaining. Cancer Cytopathol. 2021, 129, 347–362. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.S. Operative and Postoperative Management of the Patient with Follicular and Hürthle Cell Carcinoma. Do They Differ? Surg. Clin. N. Am. 1995, 75, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian Consensus for the Classification and Reporting of Thyroid Cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef]
- Corrias, A.; Mussa, A.; Baronio, F.; Arrigo, T.; Salerno, M.; Segni, M.; Vigone, M.C.; Gastaldi, R.; Zirilli, G.; Tuli, G.; et al. Diagnostic Features of Thyroid Nodules in Pediatrics. Arch. Pediatr. Adolesc. Med. 2010, 164, 714–719. [Google Scholar] [CrossRef]
- Gupta, A.; Ly, S.; Castroneves, L.A.; Frates, M.C.; Benson, C.B.; Feldman, H.A.; Wassner, A.J.; Smith, J.R.; Marqusee, E.; Alexander, E.K.; et al. A Standardized Assessment of Thyroid Nodules in Children Confirms Higher Cancer Prevalence Than in Adults. J. Clin. Endocrinol. Metab. 2013, 98, 3238–3245. [Google Scholar] [CrossRef]
- Green, O.; Keisling, M.; Kambalapalli, M.; McDaniel, J.; Boulanger, S.; Fornwalt, B.E.; Jeyakumar, A. Unusual Thyroid Mass in an Adolescent Patient. Ear Nose Throat J. 2022, 101, 654–656. [Google Scholar] [CrossRef]
- Kochummen, E.; Tong, S.; Umpaichitra, V.; Chin, V.L. A Unique Case of Bilateral Hürthle Cell Adenoma in an Adolescent. Horm. Res. Paediatr. 2017, 87, 136–142. [Google Scholar] [CrossRef]
- Bremer, A.A.; Feldman, B.J.; Iezza, G.; Clark, O.H.; Rosenthal, S.M. Report of a Hürthle Cell Neoplasm in a Peripubertal Girl. Thyroid 2007, 17, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Roggli, V.L.; Estrada, R.; Fechner, R.E. Thyroid Neoplasia Following Irradiation for Medulloblastoma: Report of Two Cases. Cancer 1979, 43, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Nagamachi, Y.; Nakamura, T.; Yamada, T. Hürthle Cell Adenoma of the Thyroid in Identical Twins with 13 Year Follow-Up. Jpn. J. Surg. 1973, 3, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Doerfler, W.R.; Nikitski, A.V.; Morariu, E.M.; Ohori, N.P.; Chiosea, S.I.; Landau, M.S.; Nikiforova, M.N.; Nikiforov, Y.E.; Yip, L.; Manroa, P. Molecular Alterations in Hürthle Cell Nodules and Preoperative Cancer Risk. Endocr. Relat. Cancer 2021, 28, 301–309. [Google Scholar] [CrossRef]
- Goswami, P.; Patel, T.; Dave, R.; Singh, G.; Singh, A.; Kalonia, T. WHO 2022 Updates on Follicular Cell and C-Cell Derived Thyroid Neoplasm. J. Med. Life 2024, 17, 15–23. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Caturegli, P.; Ruggere, C. Karl Hürthle! Now, Who Was He? Thyroid 2005, 15, 121–123. [Google Scholar] [CrossRef]
- Auger, M. Hürthle Cells in Fine-needle Aspirates of the Thyroid: A Review of Their Diagnostic Criteria and Significance. Cancer Cytopathol. 2014, 122, 241–249. [Google Scholar] [CrossRef]
- Asa, S.L. My Approach to Oncocytic Tumours of the Thyroid. J. Clin. Pathol. 2004, 57, 225–232. [Google Scholar] [CrossRef]
- Montone, K.T.; Baloch, Z.W.; LiVolsi, V.A. The Thyroid Hürthle (Oncocytic) Cell and Its Associated Pathologic Conditions: A Surgical Pathology and Cytopathology Review. Arch. Pathol. Lab. Med. 2008, 132, 1241–1250. [Google Scholar] [CrossRef]
- Elliott, D.D.; Pitman, M.B.; Bloom, L.; Faquin, W.C. Fine-Needle Aspiration Biopsy of Hurthle Cell Lesions of the Thyroid Gland: A Cytomorphologic Study of 139 Cases with Statistical Analysis. Cancer 2006, 108, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Okere, P.; Olusina, D.; Enyinnah, M. Hurthle Cell Tumor of the Thyroid Gland: Report of a Rare Case and Review of Literature. Niger. J. Clin. Pract. 2014, 17, 375–377. [Google Scholar] [CrossRef]
- Barnabei, A.; Ferretti, E.; Baldelli, R.; Procaccini, A.; Spriano, G.; Appetecchia, M. Hurthle Cell Tumours of the Thyroid. Personal Experience and Review of the Literature. ACTA Otorhinolaryngol. Ital. 2009, 29, 305–311. [Google Scholar] [PubMed]
- Zirilli, G.; Santucci, S.; Cuzzupè, C.; Corica, D.; Pitrolo, E.; Salzano, G. Peculiarities of Autoimmune Polyglandular Syndromes in Children and Adolescents. Acta Bio-Medica Atenei Parm. 2017, 88, 271–275. [Google Scholar]
- Zirilli, G.; Valenzise, M.; Dionigi, G.; Tuccari, G.; Romeo, C.; Campennì, A.; Corrias, A.; Tuli, G.; Ieni, A.; Pajno, G.B.; et al. Hurthle Cell Carcinoma in Childhood: A Retrospective Analysis of Five Cases and Review of Pediatric Literature. Pediatr. Blood Cancer 2020, 67, e28300. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Micangeli, G.; Menghi, M.; Profeta, G.; Paparella, R.; Tarani, F.; Petrella, C.; Barbato, C.; Minni, A.; Greco, A.; Ferraguti, G.; et al. Malignant and Benign Head and Neck Tumors of the Pediatric Age: A Narrative Review. Curr. Pediatr. Rev. 2024, 212, 118–132. [Google Scholar] [CrossRef]
| Thyroid Function Test | Result | Reference Range |
|---|---|---|
| FT3 | 3.4 pg/mL | 2.3–4.2 |
| FT4 | 1.09 ng/dL | 0.8–1.6 |
| TSH | 1.81 mIU/mL | 0.4–3.5 |
| Calcitonin | <0.5 pg/mL | <6.4 |
| TPOAb | 30 U/mL | <60 |
| TgAb | <10 U/mL | <4.5 |
| Study | Age (Years)/Gender | Nodule Size | Presentation | FNA Cytology | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Current case | 13/Female | 26 × 16 mm | Enlarging neck mass | Bethesda IV (follicular neoplasm with oncocytic features) | Hemithyroidectomy | No recurrence |
| Green et al., 2020 [19] | 18/Female | 5.9 × 4.4 × 3.7 cm | Incidentally identified neck mass, mild hyperthyroid symptoms | Rare clusters of cells with cytological atypia, negative for nuclear features of papillary carcinoma | Left hemithyroidectomy | No recurrence |
| Kochummen et al., 2017 [20] | 14/Female | Bilateral nodules (sizes not specified) | Enlarging thyroid nodules | Not specified | Sequential hemithyroidectomy | No recurrence |
| Bremer et al., 2007 [21] | 12/Female | 20 mm | Palpable thyroid nodule | Suspicious for follicular neoplasm | Hemithyroidectomy | No recurrence |
| Roggli et al., 1979 [22] 1 | 22/Female | Multiple nodules (largest: 4 cm) | Neck mass 18 years after medulloblastoma treatment | Not specified | Subtotal thyroidectomy (95%) | No recurrence (8.5-month follow-up) |
| Nagamachi et al., 1973 [23] | 12/Female (Twin 1) | Left lobe: single cystic tumor (~egg-sized); right lobe: three nodules (~10 mm each) | Enlarging cervical mass | Not specified | Local removal (First surgery), hemithyroidectomy (Second surgery) | No recurrence (13-year follow-up) |
| Nagamachi et al., 1973 [23] | 12/Female (Twin 2) | Left lobe: 1 cm nodule; right lobe: 3 cm cystic nodule | Enlarging cervical mass | Not specified | Local removal (First surgery), hemithyroidectomy (Second surgery) | No recurrence (13-year follow-up) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparella, R.; Bellone, G.; Rizza, L.; Veccia, N.; Ricci, G.; Calvani, M.; Scommegna, S. Oncocytic Adenoma in a Pediatric Patient: A Case Report and Literature Review. Endocrines 2025, 6, 22. https://doi.org/10.3390/endocrines6020022
Paparella R, Bellone G, Rizza L, Veccia N, Ricci G, Calvani M, Scommegna S. Oncocytic Adenoma in a Pediatric Patient: A Case Report and Literature Review. Endocrines. 2025; 6(2):22. https://doi.org/10.3390/endocrines6020022
Chicago/Turabian StylePaparella, Roberto, Giulia Bellone, Laura Rizza, Norman Veccia, Gabriele Ricci, Mauro Calvani, and Salvatore Scommegna. 2025. "Oncocytic Adenoma in a Pediatric Patient: A Case Report and Literature Review" Endocrines 6, no. 2: 22. https://doi.org/10.3390/endocrines6020022
APA StylePaparella, R., Bellone, G., Rizza, L., Veccia, N., Ricci, G., Calvani, M., & Scommegna, S. (2025). Oncocytic Adenoma in a Pediatric Patient: A Case Report and Literature Review. Endocrines, 6(2), 22. https://doi.org/10.3390/endocrines6020022






